Abstract
This study explores the potential of modified shrimp-based chitosan (MSC) as an innovative adsorbent for eliminating heavy metals (HMs) from contaminated water sources. The modifications encompassed various chemical treatments, surface functionalization, and structural optimization to enhance the chitosan’s adsorption capabilities. Comprehensive analyses using FT-IR and SEM-EDS were conducted to evaluate the properties of the chitosan. The adsorption capacity of MSC was assessed using ICP-MS before and after the adsorption process. Moreover, the study investigated the efficiency of HM removal by MSC under different conditions, including variations in pH, adsorbent dosage, and contact time. Under neutral pH conditions, the highest adsorption rates of copper, zinc, cadmium, and lead were determined as 99.72%, 84.74%, 91.35%, and 99.92%, respectively, with corresponding adsorption capacities of 20.30 mg/g for copper, 7.50 mg/g for zinc, 15.00 mg/g for cadmium, and 76.34 mg/g for lead. Analysis based on the Langmuir and Freundlich isotherm models revealed highly significant adsorption of HMs, supported by strong correlation coefficients (r2 > 0.98) obtained from the data. The pseudo-second-order kinetic model with linear coefficients (r2) greater than 0.97 effectively explained the kinetic studies of metal adsorption employing modified shrimp shells. These coefficients indicate a robust fit of the models to the experimental adsorption data for heavy metals. Further confirmation of the effectiveness of the adsorbent was obtained through FT-IR spectroscopy, which confirmed the presence of specific functional groups on the adsorbent, such as N–H joined with –COO−, H–O, C−O−C, and C–H. Additionally, the SEM-EDS analysis detected the presence of elements on the surface of MSC chitosan. The results emphasize that MSC is a highly effective and cost-efficient adsorbent for eliminating Cu, Zn, Cd, and Pb from wastewater, making it a promising eco-friendly choice.
1. Introduction
Essentially, life depends on water. However, water is seriously threatened by massive pollution and the associated, harmful effects on aquatic habitats [1,2]. In recent years, the issue of water pollution due to heavy metal contamination has garnered significant attention worldwide [3]. The World Health Organization (WHO) and the United States Environmental Protection Agency (EPA) have set permissible limits for various metal ions in drinking water to ensure public health and safety. For instance, the WHO has established guidelines for a range of metal ions in drinking water. These guidelines include a maximum allowable concentration of 0.01 mg/L for arsenic, 0.003 mg/L for cadmium, 0.01 mg/L lead, 3 mg/L for zinc, and 0.01 mg/L for mercury [4].
A massive volume of wastewater that contains heavy metals is discharged into water bodies in industrial areas in developing countries worldwide, causing severe harm to aquatic ecosystems. With industrial, agricultural, and residential development, the environmental problems produced by toxic metals in lakes, rivers, soils, or sediments have become progressively worse [5]. Additionally, every system and setup produces wastewater that contains various heavy metal derivatives, for example, arsenic (As), lead (Pb), cobalt (Co), chromium (Cr), copper (Cu), nickel (Ni), zinc (Zn), silver (Ag), manganese (Mn), mercury (Hg), cadmium (Cd), and others. These heavy metal ions have lengthy biological half-lives, are persistent, and are not biodegradable [6]. Consequently, they have the capability to inhibit the particular biological activities of specific biomolecules like proteins and enzymes [7]. Continual release of these metals into the environment significantly impairs aquatic habitats [8], leading to their accumulation in crucial organs like the brain, muscles, kidneys, and liver, and ultimately causing various deadly illnesses and conditions, including hepatitis, renal disease, nervous system disorders, anemia, encephalopathy, or nephritis, and can result in death [9,10,11,12,13]. Moreover, their accumulation within living tissues has been linked to the development of numerous diseases, including cancer [14]. Determining which metal ion is the most hazardous compared with others can depend on various factors, including its toxicity, bioaccumulation potential, and the likelihood and severity of health effects resulting from exposure [15].
Lead is often considered one of the most hazardous among the commonly found heavy metals. This is because it is known to cause severe neurological damage, especially in children, even at low levels of exposure. Lead exposure can result in developmental delays, learning difficulties, and behavioral problems [16]. Furthermore, Pb, in particular, causes various health issues, including malaise, high blood pressure, abdominal discomfort, digestive problems, emesis, queasiness, speech impediments, and more [17]. Moreover, cadmium accumulates in animals and plants with a long half-life of 25–30 years [18]. Prolonged exposure to cadmium has been associated with various cancer types, including those affecting the kidneys, lungs, breasts, pancreas, prostate, and more [18]. Copper (Cu) is well known as a vital trace element, whereas cadmium, lead, mercury, arsenic, etc., are highly toxic metals.
Cu is classified as a member of prevalent environmental pollutants widely used in various industries. Prolonged exposure to copper produces anemia, hepatotoxicity, and severe neurological defects [19]. In addition to being a necessary mineral, zinc is a metal. On the other hand, zinc can be combined with various materials to manufacture industrial products, like paint and dyes, due to its versatile properties and ability to enhance corrosion resistance and provide effective coating adhesion. These composite compounds can be notably hazardous. Excessive absorption of zinc can hinder the uptake of iron and copper. Although a trace amount of zinc is essential for biological systems, an excess of free zinc ions in solution is highly toxic to plants, bacteria, invertebrates, and even vertebrate fish [20]. Other metal ions like mercury can cause serious health problems to accumulate in the body, and its profound impact on neurological development makes it particularly hazardous, especially for vulnerable populations such as children and pregnant women. Thus, it is essential that wastewater recycling be undertaken at the point of generation. As a result, heavy metal removal from water bodies is crucial to ensure that human activities and water consumption are safe [21].
The sustainable removal of heavy metals from wastewater has posed a significant challenge for experts. Therefore, various techniques have been discovered to remove contaminants from industrial wastewater, including heavy metals and others [22]. Among such methods are coagulation, electrodeposition, bioaccumulation, chemical precipitation, ion exchange, evaporation, electro-floatation, membrane filtration, reverse osmosis, electro-dialysis, solvent extraction, and electrocoagulation, and studies on microbial degradation for heavy metal removal from aquatic environments have been conducted [23,24,25,26,27,28]. Nonetheless, these methods come with specific disadvantages, such as the generation of secondary waste, substantial slug formation, inadequate elimination of trace-level heavy metals, extensive use of chemical reagents and energy, production of toxic byproducts necessitating additional treatment, and elevated operational costs [29].
Among the various methods employed for toxic metal elimination from polluted water sources, adsorption has emerged as a promising and ecologically friendly approach [23]. Adsorbents are materials capable of selectively capturing toxic metals from wastewater, thus offering an effective means of water remediation. Considering the above factors, bio-technological removal methods have been found to have specific benefits for heavy metal removal as they are environmentally friendly, abundant, low-cost, highly efficient, readily available, easy to handle, widely suitable, possess substantial surface area, and have different surface groups. Therefore, massive and abandoned agro-wastes may be considered possible resources for the manufacturing of bioadsorbents beyond the high-cost adsorbents for heavy metal remediation [30,31,32,33,34,35]. A recent study has explored the cleanup of oil spills using gel-based modified chitosan [36].
Globally, there is a growing demand for seafood, particularly in coastal towns or nations [37]. As a result, substantial amounts of waste shrimp shells are produced annually from shrimp processing and consumption [38]. Shrimp-derived chitosan products are rich in minerals, proteins, polysaccharides, as well as in functional groups containing oxygen and nitrogen, such as carboxyls, hydroxyls, amines, imidazole, amides, and phosphate, that demonstrate efficacy in the adsorption of metal ions [38,39,40]. Despite this, the use of modified shrimp-based chitosan for heavy metal removal in wastewater treatment remains underutilized in the current literature. This paper explores the innovative use of modified shrimp-based chitosan as a highly efficient adsorbent for eliminating toxic metals. The most significant shrimp shell byproducts are chitin and chitosan, the second most common natural polysaccharide after cellulose [41,42]. Chitosan derived from waste shrimp shells demonstrates eco-friendly cleanup technologies [43]. Following the process of deacetylation, chitin can be transformed into valuable chitosan. This positively charged polysaccharide, abundant in amino groups, is frequently employed to remove anionic impurities from aqueous solutions. A low pH facilitates the adsorption of anionic pollutants [38].
The heavy metal adsorption method using shrimp-based chitosan presents a promising approach for removing heavy metal ions from various environmental matrices. However, this method also poses specific challenges that need to be addressed to ensure its effective implementation. Some challenges include kinetics and efficiency optimization, regeneration and reusability of chitosan material, and selectivity for the targeting of heavy metals. In addition, there are challenges in terms of environmental impact and the proper disposal of used chitosan materials.
The primary objective of this study was to investigate and optimize the effectiveness of a novel and environmentally friendly process for the removal of heavy metals, specifically copper, zinc, cadmium, and lead, from wastewater. This was achieved through the utilization of MSC, exploring its potential as a sustainable and effective alternative for the mitigation of water pollution, and promoting eco-friendly wastewater treatment practices. The modified form of chitosan exhibits a strong affinity for adsorbing heavy metals, enhancing its adsorption capacity and selectivity and thereby providing a cost-effective and sustainable solution by which to address the persistent challenge of heavy metal pollution in aquatic environments.
2. Materials and Method
2.1. Chemicals
Except where explicitly specified, all research reagents were obtained from Wako Chemical Corporation, Osaka, Japan. These substances met the quality standards of ACS grade and were utilized as received without further purification. Stock solutions of 1 M of copper sulfate (CuSO4), zinc sulfate (ZnSO4), lead acetate Pb(C2H3O2)2, and cadmium chloride (CdCl2) were prepared and stored at 4 °C. Standard working solutions were made from the stock solutions by successive dilution, first to 100 mM and then to 1.0 mM, using Milli-Q water with a resistivity of 18.2 Ω cm. Amounts of 0.1 M NaOH or 0.1 M HCl were used to regulate the pH of the metal solutions. All adsorption experiments were conducted at a constant temperature of 28 °C.
2.2. Processing of Shrimp Shells and Chitosan Preparation
The waste shrimp shells were collected from a shrimp processing facility in Saga, Japan. The shells were cleaned in boiling water to eliminate any remaining lipids, flesh, and additional debris. Subsequently, the shrimp shells were washed with distilled water, filtered with a net, and dried at 70 °C. The production of chitosan from shrimp shells encompasses several stages, primarily deproteinization, demineralization, and deacetylation, as described previously [44].
2.2.1. Demineralization
The demineralization of shrimp shells was carried out using a previously described method, with certain adjustments [45]. Dried shrimp shells were soaked in 1.5% HCl for 20 h at room temperature in a ratio of 1:30 (weight to volume) to remove calcium ions. Afterward, the shells were thoroughly washed with deionized (DI) water multiple times to remove CaCl2 and other water-soluble impurities until the solution reached a neutral pH of 7 ± 0.1. The resulting chitin containing filtrate was collected for the subsequent steps.
2.2.2. Deproteinization
The deproteinization of shells was conducted with certain modifications following earlier protocols [39,45]. The shells were submerged in 5% NaOH for 24 h at 90 °C with a shell-to-solvent ratio of 1:12 (w/v) to eliminate any residual proteins and additional organic substances. The shrimp shell sample was neutralized by DI water washing and then dried at 60 °C. The obtained product was chitin [45].
2.2.3. Decolorization
Chitin was decolorized in acetone solution for 24 h at room temperature. Subsequently, the shells were washed with deionized water until a neutral pH was reached. The shells were dried for 12 h at 60 °C to obtain pure chitin chitosan.
2.2.4. Deacetylation
The deacetylation process of the recovered chitosan was conducted in 50% NaOH at 60 °C for 8 h at the ratio of 15%, w/v. The remaining material was purified with deionized water until a neutral pH was reached, then dried at 80 °C until a consistent weight was achieved. Afterward, the material was finely ground and passed through 180 mesh sieves to obtain the chitosan. Equation (1) was used to obtain the chitosan yield in terms of percentage [46]. The dried chitosan was stored in a dry, airtight container away from direct sunshine and moisture.
2.3. Determination of Functional Group by FT-IR Spectroscopy
FT-IR spectroscopy, a powerful analytical technique, was employed to recognize and examine the chemical composition of substances based on their adsorption and emission of infrared light [47]. The functional groups in the treated and untreated chitosan were characterized using FT-IR spectroscopy (model: VERTEX 70v) employing an attenuated total reflectance (ATR) attachment. The FT-IR spectrometer provides extensive insights into the functional groups present in the MSC samples. FT-IR spectra of MSC subjected to treatment with four different metal ions (Cu2+, Zn2+, Cd2+, and Pb2+) and untreated MSC were recorded in absorbance mode in the 400–4000 cm−1 range.
2.4. SEM-EDS Analyses
Scanning electron microscopy (SEM), combined with energy dispersive spectroscopy (EDS), was used as a powerful technique for the high-resolution imaging and elemental analysis of materials [48]. The essential standards for calibration in EDS analysis included gold (Au), a single-element standard. The surface of the MSC was further examined using SEM-EDS (SEM Hitachi 3400N, Tokyo, Japan) both before and after exposure to metal ions. The MSC was exposed to a 1.0 mM solution of four metals (Cu2+, Zn2+, Cd2+, and Pb2+) and continuously agitated at 160 rpm for 2 h at room temperature. Subsequently, the MSC was extracted using centrifugation and lightly rinsed with DI water. Before imaging, the MSC was coated with platinum. MSC samples for SEM analysis were mounted on an aluminum stub using double-sided carbon tape and coated with 10 nm of gold-palladium using a vacuum device called a Magnetron Sputtering Equipment MSP-1S (Magnetron Sputter, RT1195006, Tokyo, Japan). Samples for EDS were covered with 15 nm of carbon using an Emitech 500X carbon coater attachment on the sputter coater. Thermo Fisher Scientific’s AVANTAGE software (Thermo Fisher Scientific, XPS, Wilmington, DE, USA) was used to fit and quantify high-resolution spectra. The concentration of an element was represented by the weight percentage (Wt%). While carbon (C) and oxygen (O) were retained as the signal elements, the additional elements were excluded from the EDS investigation. In this study, the system derived information regarding the quantitative percentages of elements, displaying the weight percentage of each element of interest. EDS elemental maps were overlaid onto SEM images to visualize the elemental distribution in space.
2.5. ICP-MS Analyses
ICP-MS, a potent analytical method for the qualitative analysis of elements in various materials, was utilized to detect the concentration of metal ions in the liquid solution following the adsorption of metals by MSC [49]. The MSC was exposed to a combination of four metal ions, Cu2+, Zn2+, Cd2+, and Pb2+, for 120 min with continuous agitation at 160 rpm at 28 °C. Similarly, the control samples were shaken in a shaker incubator but without MSC adsorbent. Centrifugation was employed to separate the MSC-free supernatant (10,000 rpm for 10 min). To remove the tiny particles, a 0.45 µm filter was used to filter all of the samples. Before injection into ICP-MS, the required dilutions were made using supra pure 2M HNO3 for all of the liquid samples and were made to a constant volume. The concentration of metal ions in the cellulose-free liquid was determined using an Agilent Technologies 7900 quadrupole ICP-MS (Santa Clara, CA, USA). The results of each experiment were accomplished in triplicate and stated as the mean standard error of the mean (S.E.). Bioadsorption was quantified through the application of the subsequent equations:
where C0 represents the initial concentration, Ct represents the concentration at a time ‘t’ (ppm) and Cad represents the concentration adsorbed (ppm).
Cad = C0 − Ct
The adsorption capacity qe (mg/g) after equilibrium was calculated using the following equation
where W represents the quantity (in grams) of MSC utilized, V stands for the volume (in mL) of the metal solution, and qe represents the adsorption reached at equilibrium [50,51].
2.6. Adsorption Isotherm
An adsorption isotherm is a crucial tool providing insights into the binding attraction, adsorption capability, and surface properties of the adsorbent. This information aids in understanding both the adsorptive capability and the interaction mechanism between the adsorbate and adsorbent [52]. Adsorption isotherm was performed to show the relationship between the amount of adsorbate (substance being adsorbed) and the concentration of the adsorbate [53]. In this study, the Langmuir adsorption isotherm and Freundlich isotherm were employed to elucidate the equilibrium adsorption behavior of chitosan derived from shrimp for metal adsorption as previously described [44].
To thoroughly assess the adsorption kinetics parameters, the following models were introduced: the pseudo-first-order model, represented by Equation (5) and the pseudo-second-order model, represented by Equation (6). These models are widely recognized and utilized for analyzing adsorption kinetics [38].
The equations for the pseudo-first-order and pseudo-second-order models are as follows:
Pseudo-first-order model:
where k1 is the rate constant of pseudo-first-order adsorption (min−1).
Pseudo-second-order model:
where k2 is the rate constant of pseudo-second-order adsorption (g mg−1 min −1).
The fitting of experimental data to these models allows for a comprehensive evaluation of the kinetic characteristics of the adsorption process. The obtained parameters provide valuable insights into the underlying mechanisms of the adsorption phenomenon [54].
2.7. Influence of pH on Adsorption
The pH of a solution can significantly influence the adsorption capacity and efficiency of biosorbents, such as microorganisms, algae, or agricultural waste, in eliminating toxic metals from solutions [55]. The influence of pH on the biosorption of metal ions by MSC was assessed at different pHs. In a 3 mL tube, 10 mg of MSC was added into a 1.0 mL solution combining 1.0 mM concentrations of each metal. The pH of each metal solution was individually adjusted from 1.0 to 8.0 ± 0.1. Some metals are precipitated at a basic pH solution [56]. Therefore, pH levels of more than 7 were discounted in this investigation. The adsorption process was conducted at 28 °C for two hours with continuous stirring at 160 rpm. The World Health Organization recommends a pH of 7 for drinking water. MSC could be used to purify sewage and drinkable water. Therefore, all of the tests were carried out in a solution of the heavy metal ions at pH 7. All of the tests were performed in triplicate and ICP-MS was used to determine the metal ion concentrations in the solution after adsorption.
2.8. Influence of Time on Adsorption
The contact time between the adsorbent and the metal ions affects the amount of time available for adsorption to occur. The longer the contact time, the greater the amount of adsorption that can occur. However, after a certain amount of time, the adsorption rate reaches equilibrium, and no further adsorption occurs. Understanding the influence of contact duration on heavy metal adsorption is crucial as it helps determine the time required for a given adsorbent to reach equilibrium and effectively remove heavy metals from a solution [57]. To study the impact of exposure duration on adsorption, 10 mg of MSC was supplemented with 1 mL of a mixed solution containing four ions (Cu2+, Zn2+, Cd2+, and Pb2+), each at a final concentration of 1.0 mM. The adsorption study was conducted at 28 °C for seven different time points ranging from 0 to 180 min at 30 min intervals with nonstop agitation at 160 rpm. The samples were removed at each time point, prepared, and measured using ICP-MS.
2.9. Variation of Bioadsorbent Quantity
The effects of bio-adsorbent dosages on the elimination of toxic metals from solutions are essential factors to the optimization of the adsorption process [2]. The adsorption of heavy metals greatly depends on the volume of adsorbent employed. Therefore, increasing dosages of MSC (2.5, 5.0, 7.5, 10, and 12.5 mg) were added to a 1 mL cocktail solution containing four metal ions (Cu2+, Zn2+, Cd2+, and Pb2+) at 1.0 mM concentration each. The metal solutions were adjusted to pH 7.0 ± 0.2, and incubation was accomplished at 160 rpm at 28 °C for 24 h.
2.10. Statistical Analysis
Statistical analysis in this study utilized OriginPro 2021 developed by OriginLab Corporation, based in Northampton, MA, USA. The findings were accessed by calculating the mean values, accompanied by error bars denoting the standard deviation, derived from three repeated tests (n = 3). The suitability of the data for a bio-adsorption model was evaluated through the determination coefficient (r2). A linear correlation analysis was conducted to discover the connection between metal concentration and adsorbent quantity. Remarkably, a highly significant positive correlation was observed between the adsorbent amount and metal concentration, yielding an r2 value of 0.999. The statistical significance of the slope was confirmed at the 0.05 significance level.
3. Results and Discussion
3.1. Yield of MSC
To determine the yield percentages and consistent weight of chitosan, 100 g of wet shrimp shells were subjected to drying, resulting in a measurement of 35 g. Consequently, the moisture content in the wet shrimp shells was calculated to be 65%. According to the data obtained, the chitosan content constitutes 24% of the dry weight after undergoing the demineralization, deproteinization, decolorization, and deacetylation described previously [44]. The reduction in chitosan content is attributed to the removal of water-soluble contaminants, calcium carbonate, and additional mineral components using a high concentration of HCl and NaOH is responsible for decreasing chitosan content. However, it is essential to note that the extensive washing steps involved in the modification processes may impact the final chitosan yield. Previous studies on chitosan extraction have reported varying yield percentages [45,58,59]. The variations in yield percentages observed in this study were primarily attributed to differences in conditions, including the specific washing procedures and variations in the concentrations of acids and bases employed.
3.2. Elemental Analysis Using SEM-EDS
Shrimp shells comprise approximately 20–30% protein, 20–30% chitin, 30–40% calcium carbonate, and various minor components [60]. SEM was applied to examine the surface properties of MSC, while energy dispersive spectrometry (EDS) was used to unveil the elemental compositions of MSC. Figure 1 demonstrates the results of the EDS elemental analysis conducted on MSC, revealing the presence of elements such as C, O, N, Na, Mg, and Ca, among others. The findings indicate that the primary constituents of MSC are C (47.41%), O (25.45%), and N (23.94%), while trace elements such as Na, Mg, I, P, Ca, and S in shrimp shells constitute less than one percent. Additionally, EDS results demonstrate that the surface composition of shrimp-derived chitosan predominantly comprises carbon (C), nitrogen (N), and oxygen (O). However, other inorganic elements like Al, Ca, P, Mg, and S continue to be present in MSC due to their strong resistance to acidic conditions (Figure 1). Furthermore, modifying shrimp shells using strong acids and bases (1.5% HCl and 5% NaOH, respectively) significantly reduces the mineral content, particularly calcium.
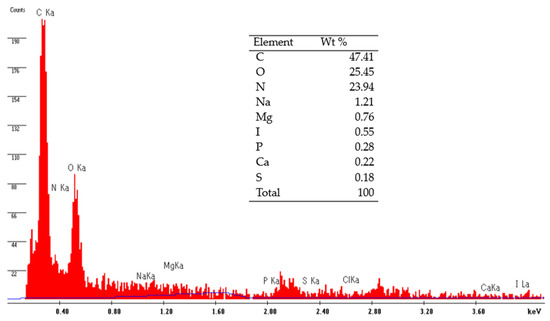
Figure 1.
EDS spectrograms of adsorbent MSC before exposure to metal solutions and the percentage amount of elements present in MSC.
SEM was also employed to observe the surface characteristics of MSC. The SEM images depicted surfaces that were not uniform and lacked smoothness, as illustrated in Figure 2.

Figure 2.
The images were captured using scanning electron microscopy (SEM). Images show the surface of MSC before the adsorption micrograph at (a) 250×, (b) 500×, and (c) 1000×.
The MSC specimen exhibited an irregular surface featuring valleys and hills that created dimples of various sizes, as shown in Figure 2. This surface heterogeneity suggests that the adsorption capabilities of this chitosan are likely superior to those of a smooth chitosan surface. Upon closer inspection at magnifications of 250×, 500×, and 1000×, MSC exhibited irregular shapes, featuring numerous small fragments and an uneven texture, marked by multiple small peaks and valleys of varying forms (Figure 2a–c). Moreover, the structure of MSC exhibited a distinctive network pattern and exhibited evident porous structures, indicating the removal of most surface proteins and fats [58] and enhancing its adsorption capacity [61].
Similarly, the surface analysis was investigated using SEM-EDS after exposure to a solution containing four metals (Cu2+, Zn2+, Cd2+, and Pb2+) to determine the distribution and physical morphology of MSC as adsorbents. The EDS findings indicate that following exposure to metals, the surface composition of each specimen of chitin and chitosan derived from shrimp predominantly comprised nitrogen (N), carbon (C), and oxygen (O). Remarkably, elements other than C, N, and O were not detected in the EDS spectra of MSC chitosan before adsorption. However, subsequent to adsorption, metal ions, such as Pb, Cd, Cu, and Zn, were observed across the entire surface (Figure 3). EDS spectra serve as consistent qualitative measurements but do not provide a quantifiable assessment of bioadsorption efficacy.
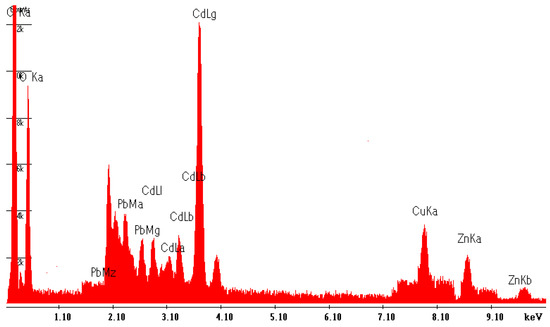
Figure 3.
EDS spectrograms of MSC of the top surface of MSC after adsorption.
Image mapping and overlapping techniques were further conducted to confirm the distribution of heavy metals on the surface. Image mapping involved 64 images corresponding to the respective metals, each comprising 256 pixels. This approach enabled visualization of how the heavy metals were spread on the surface of MSC. The results reveal distinct colors for different metal ions, as illustrated in Figure 4. The combined use of image mapping in conjunction with EDS confirmed that, when MSC was exposed to the metal solution, it effectively adsorbed all four metals onto its surface. Notably, the intensity levels of all these metals remained consistent throughout the mapping process. This image mapping approach validated the findings from ion imaging, affirming the presence and distribution patterns of metal ions on the MSC surface. Image mapping effectively detected the presence and spatial arrangement of heavy metals on the surface of adsorbents.
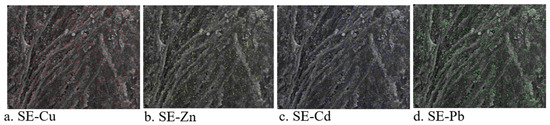
Figure 4.
SEM-EDS images of mapping of MSC after adsorption of metal ions. The metals are spread on the surface of chitosan. Different colors indicate different metal ions. Scanning electron micrograph with (a) Cu, (b) Zn, (c) Cd, and (d) Pb.
3.3. FT-IR Analysis
The surface of MSC was analyzed for its functional groups using FT-IR spectroscopy. The FT-IR spectra of the sorbent were obtained in the range of 400 to 4000 cm−1 to determine the presence of oxygen-containing functional groups, including carbonyl, phenolic hydroxyl, carboxyl, and hydroxyl groups. These functional groups exhibit a high affinity for adsorbing metal ions [62]. Figure 5 displays the FT-IR spectra of MSC, revealing specific details about these functional groups. This information supports the evaluation of the probable binding of adsorbate substances to the adsorbent’s surface [63].
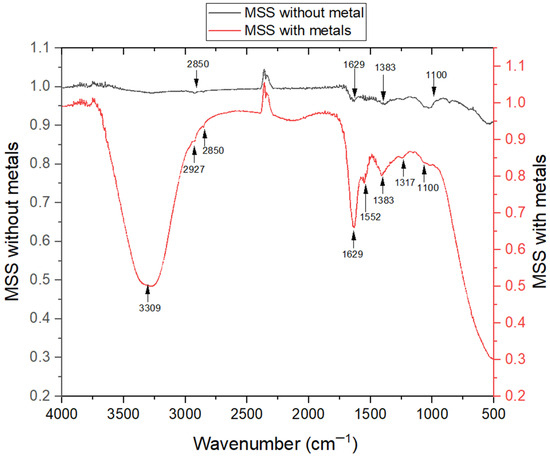
Figure 5.
The FT-IR spectra of MSC both prior to and following treatment with a combination of four metals (Cu2+, Zn2+, Cd2+, and Pb2+).
The functional groups show a vital role in facilitating the binding of metal ions in an aqueous solution [62,64]. Another highly noticeable change in peak intensity occurred at 1629 cm−1, pointing to the existence of carboxylate anions, as shown in Figure 5. The distinctive peak attribute of chitosan polysaccharides strongly implies the process of deacetylation [65]. The peak detected at 1100 cm−1 is attributed to the stretching vibrations of C–O–C [66]. The small peak observed at 1317 cm−1 indicates the minimal presence of the C–O stretching vibration group [67], while the 1383 cm−1 peak could be attributed to the symmetric stretching of –COO– in pectin [64].
Furthermore, the vibrational peaks at 2850 cm−1 and 2927 cm−1 can be linked to the C–H asymmetric and symmetric stretching vibrations, respectively, as elucidated by Zhang et al. [68]. These vibrations result from aliphatic structures originating from lipids present in MSC [69]. The arrangement of metal ions occurs through the binding of deprotonated carboxyl and hydroxyl groups [66]. These alterations are linked to the involvement of carboxylate and hydroxylate ions in facilitating metal adsorption [64]. FT-IR analysis has revealed that increased carboxylate ligands amplify the binding capacity to adsorbent with metals, as reported by Abd-Talib et al. in 2020 [70]. Through chemical modification, the presence of hydroxyl, polysaccharides, carbonyl, and carboxyl groups has been found to enhance the adsorption process [70]. The ability of MSC to eliminate metal ions can be attributed to the higher concentration of functional groups found on the modified shrimp adsorbent.
3.4. Effect of pH
The initial pH of the aqueous sources significantly influences the removal efficiency and plays a critical role as a parameter that affects the protonation of functional groups and the chemical interactions involved in the adsorption of metal ions using biological materials [38,71]. To investigate this, the adsorption process was individually studied at various pH levels within the range of 1 to 8, as depicted in Figure 6.
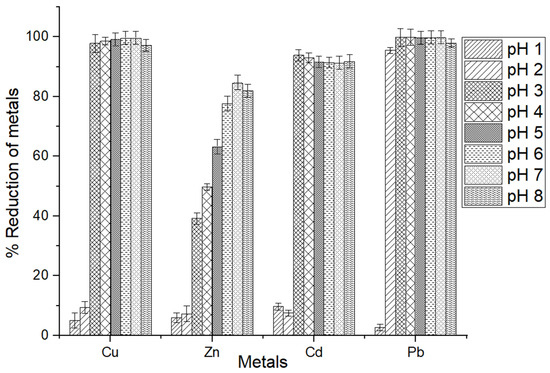
Figure 6.
Results of initial pH on elimination percentage of metals by the MSC of Cu2+, Zn2+, Cd2+, and Pb2+ ions. The error bars indicate the mean value ± standard error (SE) based on three replications of each treatment.
The highest removal of copper ions occurred at pH levels of 6 and 7, while the highest zinc ion removal efficiency was observed at a pH of 7. At the same time, the maximum adsorption of cadmium and lead ions was observed at pH 3. Bioadsorption was notably lower at pH 1 and 2 but exhibited a linear increase from pH 3 to pH 7 (Figure 6). The sudden increase in the adsorption of metal ions at pH 3, compared with pH 1 and 2, using shrimp chitosan, can be attributed to several factors by considering the specific properties of chitosan and the behavior of metal ions at different pH levels. A similar observation has been shown for copper ion adsorption using a modified shrimp shell [58]. At pH levels close to neutral, the amine groups of chitosan readily bind to metal cations [59].
Moreover, chitosan becomes more protonated at lower pH values (pH 1 and 2), and the functional groups on the chitosan, such as amino and hydroxyl groups, are more likely to be protonated, thereby reducing the electrostatic attraction between the chitosan and the metal ions. Consequently, the adsorption capacity of chitosan for metal ions may be relatively lower at these acidic pH levels due to reduced electrostatic interactions and fewer accessible binding sites.
However, as the pH increases from 3 to 7, the degree of protonation of chitosan decreases, resulting in a higher positive charge density on the chitosan molecules. This increased positive charge enhances the electrostatic attraction between the chitosan and the metal ions, leading to a higher adsorption capacity compared with pH 1 and 2. The more favorable electrostatic interactions and increased availability of binding sites at pH 3 contribute to the sudden increase in the adsorption of metal ions using shrimp chitosan at this specific pH. The adsorption capacity rapidly increased at pH levels exceeding 3, reaching a maximum of 99.72%, 84.74%, 93.87%, and 99.93% removal for Cu, Zn, Cd, and Pb, respectively. This represents a noteworthy nearly eleven-to-twelve-fold increase. The lower reduction of zinc could be attributed to the chemical affinity between the MSC and zinc ions, which might be weaker compared with other metal ions, resulting in a lower rate of adsorption [72]. The MSC may form stronger surface complexes with other metal ions compared with zinc.
More specifically, at a lower pH, the -NH2 groups would undergo protonation, forming positively charged -NH3+ groups. This led to electrostatic repulsion between the Cu2+ ions and MSC, which hindered the adsorption of Cu2+ [58]. Conversely, at higher pH, the concentration of H+ ions in the solution significantly decreased, leading to a substantial reduction in the protonation of -NH2 groups. Consequently, this led to a significant increase in the adsorption capacity for metal ions [58].
Several other studies have reported varying optimal pH values for the highest biosorption of metals and obtained different results depending on the adsorbent used [39,40,73,74,75,76,77,78]. These differences may be attributed to variations in sample preparation procedures compared with the present study.
3.5. Effect of Contact Time
The effect of contact time on metal bioadsorption is the most critical parameter for the remediation of metals. Figure 7 illustrates the effect of contact time on % removal at 7 time points from 0 to 180 min at 30 min intervals.
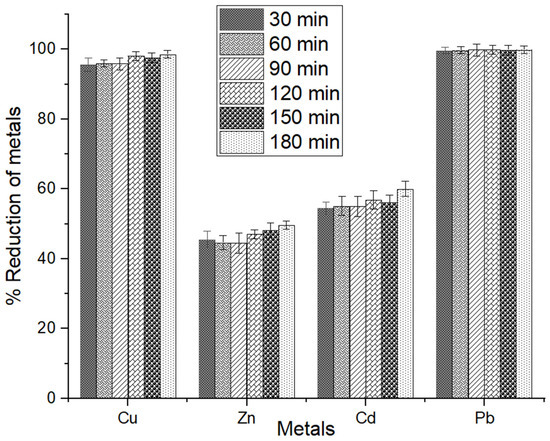
Figure 7.
Results of contact time on metal elimination percentage by MSC of Cu2+, Zn2+, Cd2+, and Pb2+ ions. The error bars indicate the mean value ± standard error (SE) based on three replications of each treatment.
The results show that the bioadsorption of metals was nearly identical for Cu2+ over the time points. A similar adsorption pattern was observed for Pb2+ throughout the time points. On the other hand, the adsorption of Zn2+ and Cd2+ was gradually increased in all time intervals from 30 to 180 min (Figure 7). Figure 7 indicates that the optimum adsorption of Pb was recorded at 30 min, and that the concentration remained relatively stable afterward. Typically, the % removal of Pb increases slowly until 120 min, reaching the maximum 99.93% removal. Several other studies have demonstrated that the percentage removal (89%) of Cu equilibrium was achieved at 360 min [79], 92% of Zn equilibrium was reached at 180 min [79], removal percentage (99.88%) of Pb reached an equilibrium at 90 min [80] and 90% of Cd reached at equilibrium at 50 min [73]. It has been observed that the biosorption rate was higher in the initial stages. The rapid initial removal rate, followed by slower adsorption of metal ions, can be attributed to the occupancy of required sites to bind on the MSC surface during the initial phases, limiting accessibility [81]. Hence, 30 min is the ideal contact time for removing Cu2+, Zn2+, Cd2+, and Pb2+ using MSC in an aqueous solution.
3.6. Langmuir and Freundlich Isotherm Analysis
The Langmuir model assumes monolayer adsorption on a uniform surface with a limited number of identical adsorption sites, while the Freundlich model is more versatile and applicable to heterogeneous surfaces and multilayer adsorption [53]. The Langmuir model indicates the highest adsorption capacities for each metal, while the Freundlich model suggests the possibility of multilayer adsorption or heterogeneous surfaces [82]. The results from the Langmuir and Freundlich isotherm models provide valuable insights into the adsorption behavior of copper, zinc, cadmium, and lead onto the MSC adsorbent in this study. The results for the Langmuir and Freundlich isotherm are shown in Figure 8 and Table 1.
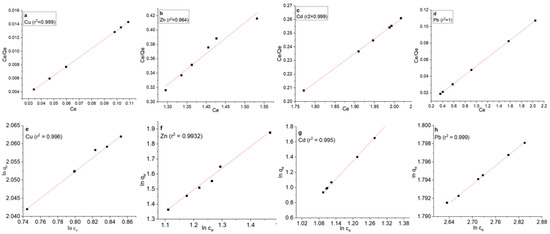
Figure 8.
Adsorption isotherm analysis for heavy metal ions (copper, zinc, cadmium, and lead). Langmuir models (a–d) and Freundlich models (e–h).

Table 1.
Langmuir and Freundlich isotherm model parameters for removing metals using MSC.
3.7. Kinetic Modeling of Adsorption Process
The adsorption kinetics were analyzed using the pseudo-first-order and pseudo-second-order models at six distinct times. The fitting curves for both models are illustrated in Figure 9, while the corresponding kinetic parameters are presented in Table 1. The overall correlation coefficients (r2) for the pseudo-first-order kinetic model were found to be 0.9230 for Cu, 0.9918 for Zn, 0.9495 for Cd, and 0.9949 for Pb. In comparison, the correlation coefficients for the pseudo-second-order kinetic model were notably higher, with values of 0.9999 for Cu, 0.9809 for Zn, 0.9931 for Cd, and 0.9994 for Pb (Table 1).
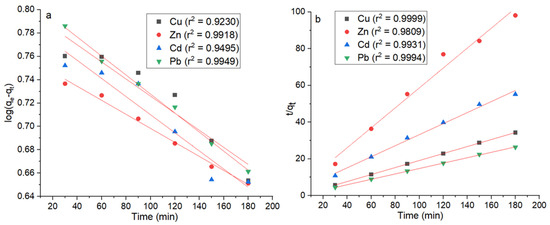
Figure 9.
Fitting curves of kinetic models. The plots depict the fitting curves of the pseudo-first-order kinetic model (a) and the pseudo-second-order kinetic model (b) at six different times for the adsorption of Cu, Zn, Cd, and Pb.
The data revealed that the pseudo-second-order kinetic model exhibited superior fitting performance compared with the pseudo-first-order model. With overall higher correlation coefficients, the pseudo-second-order model was deemed more suitable for accurately describing the adsorption process. This result suggests that the adsorption mechanism is better understood when employing the pseudo-second-order kinetic model, emphasizing its applicability and robustness in studying the adsorption dynamics of the investigated metals. These findings align with similar observations in related studies, including the adsorption of cadmium [83] and nickel [21] on dead and live biomass of Bacillus subtilis, respectively; arsenic adsorption on chitosan biosorbent [52] and shrimp shells [54]; and methyl orange adsorption on shrimp-shell-derived hydrochar [38]. This consistency underscores the robustness and applicability of the pseudo-second-order kinetic model when characterizing diverse adsorption phenomena across various substrates and target substances.
Figure 8 and Table 1 demonstrate the results of the Langmuir and Freundlich isotherm analyses for the adsorption of copper, zinc, cadmium, and lead onto the studied adsorbent. The highest adsorption capacity for copper was observed, with a value of 20.30 mg/g. This indicates that the adsorbent has a significant affinity for copper ions, and that the adsorption process tends to reach a saturation point where further adsorption becomes limited. The adsorption capacity for zinc was 7.50 mg/g, according to the Langmuir model. This suggests that the adsorbent has a moderate affinity for zinc ions, but that the adsorption capacity is lower compared with copper. The Langmuir isotherm analysis resulted in an adsorption capacity of 15.00 mg/g for cadmium. The highest adsorption capacity was found for lead, with a value of 76.34 mg/g, suggesting a strong affinity of the adsorbent for lead ions, and the adsorption process is highly efficient for lead removal.
According to the Freundlich model, the adsorption capacity for copper was 18.78 mg/g. This value is slightly lower than that obtained from the Langmuir model, suggesting that the adsorption process may not strictly follow monolayer adsorption. The adsorption capacity of 5.98 mg/g for zinc and 13.87 mg/g for cadmium is also lower compared with the Langmuir model, indicating that zinc and cadmium adsorption may involve multiple layers or heterogeneous surfaces. The Freundlich isotherm analysis showed an adsorption capacity of 71.57 mg/g for lead. While this value is slightly lower than that obtained from the Langmuir model, it still indicates a strong affinity of the adsorbent for lead ions, and the adsorption process may involve multilayer adsorption.
Overall, the Langmuir isotherm results suggest that the adsorbent has a higher affinity for copper, cadmium, and lead, with lead having the highest affinity. The Freundlich isotherm results specify that the adsorption behavior may be more complex, potentially involving multilayer adsorption or heterogeneous surfaces for all metals.
3.8. Impact of Varying the Quantity of Adsorbent
The quantity of adsorbent significantly affects the adsorption process, encompassing factors such as removal efficiency and adsorption capacity, among the other parameters under investigation. The adsorbent dosages of MSC investigated in this study were 2.5–12.5 mg at a 1.0 mM concentration of four metal ions (Cu2+, Zn2+, Cd2+, and Pb2+). Results are presented in Figure 10.
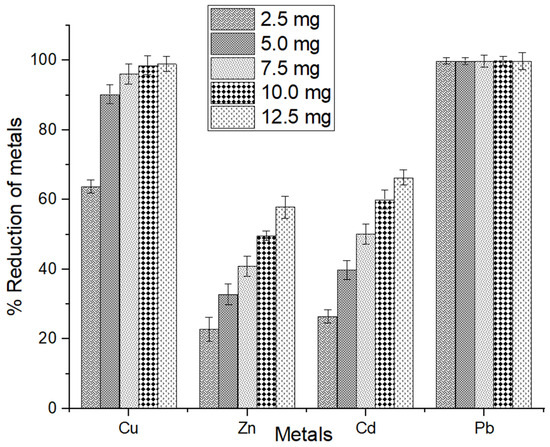
Figure 10.
Impact of initial biosorbent dosages of MSC on adsorption. The error bars indicate the mean value ± standard error (SE) based on three replications of each treatment.
The findings revealed a gradual increase in the adsorption of metal ions as the biosorbent quantity increased from 2.5 to 12.5 mg for all ions, with the exception of lead. The measurement reached 98.97%, 57.88%, 66.39%, and 99.83% removal of Cu, Zn, Cd, and Pb, respectively, at 12.5 mg of adsorbent. Several researchers have shown that the adsorption increased with the increased amount of adsorbent, which aligns with the findings of the present study [45,84]. The adsorption capabilities of shrimp shells depend on their surface activity, specifically the available surface area for interactions with metals. Typically, as the concentration of the adsorbent rises, there is an increase in the number of active sites on the surface of MSC that can adsorb metal ions.
Furthermore, for a given mass of adsorbent, the more concentrated the solution or effluent, the smaller the volume it can purify [85]. Hence, 10 mg of MSC was designated as the optimum amount of adsorbent to remove metal ions from the aqueous solution. As the quantity of MSC was raised from 2.5 mg to 12.5 mg, the removal efficiency for Cu2+ increased from 63.81% to 98.97%. A related study demonstrated that increased shrimp shells from 1.0 g to 3.0 g resulted in an increase in removal capacity from 76.39% to 91.47% [84]. Likewise, within this investigation, the removal capacity demonstrated an increase of Zn2+, Cd2+, and Pb2+ from 22.76% to 57.88%, 26.45% to 66.39%, and 99.81% to 99.83%, respectively (Figure 10). Several other research studies have noted that an elevated adsorbent dosage results in an increased removal efficiency [51,76,77,86,87].
However, the higher adsorbent dosage led to increased adsorption due to the greater surface area and additional functional groups available on the adsorbent. Increasing the amount of adsorbent led to a decrease in unit adsorption, which is the amount of metal ions adsorbed per unit mass of the adsorbent. For example, for Cu, unit adsorption decreased from 20.30 to 6.30 mg/g as the adsorbent dosage increased from 2.5 to 12.5 mg/mL. This trend was also observed for Zn and Cd (Figure 11). The adsorption of Pb was reached at the maximum, even at a low amount of adsorbent. The decrease in unit adsorption can be attributed to the possible overlapping or aggregation of the adsorbent’s surface area that interacts with ions in the solution. This means that as more adsorbent is added, the availability of metal ions for adsorption can be reduced, leading to lower unit adsorption values.
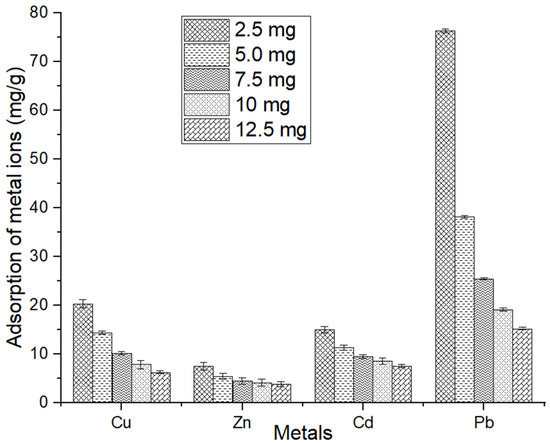
Figure 11.
The adsorbed amount of metals per unit of adsorbents. The error bars indicate the mean value ± standard error (SE) based on three replications of each treatment.
On the other hand, at the optimal amount of adsorbent, sufficient sites are ready to engage with metal ions in the solution. Hence, the use of additional adsorbent is not suitable for bioadsorption. The ability of chitosan to adsorb specific heavy metal ions exhibits variability in its capacity. In their respective research studies, many researchers have chosen the ideal dosage for various biosorbent materials to eliminate metal ions from polluted water [43,45,84,88,89]. However, the dosages utilized in this study exhibit disparities compared with the findings in this work, primarily due to the use of diverse biosorbents, various metal ions, and even dissimilar metal concentrations in prior studies.
The maximum adsorption percentage was documented at a level of copper, zinc, cadmium, and lead and was 99.72%, 84.74%, 91.35%, and 99.92%, respectively, after 120 min of 10 mg of MSC contact separately at neutral pH (Figure 12). Copper and lead adsorption is relatively higher than zinc and cadmium. The observed differences in affinity for these metal ions can be attributed to the specific interactions between the MSC and each metal ion. Several factors contribute to the affinity of the adsorbent, including chemical speciation, electrostatic interactions, and the coordination chemistry of metal ions with the functional groups present in the chitosan [90]. In the case of lead and copper, the higher affinity could be influenced by the formation of stronger and more stable complexes due to the unique electronic configuration and coordination preferences of lead ions. The chelation process involving copper and lead may lead to more favorable interactions, resulting in a higher adsorption capacity [72].
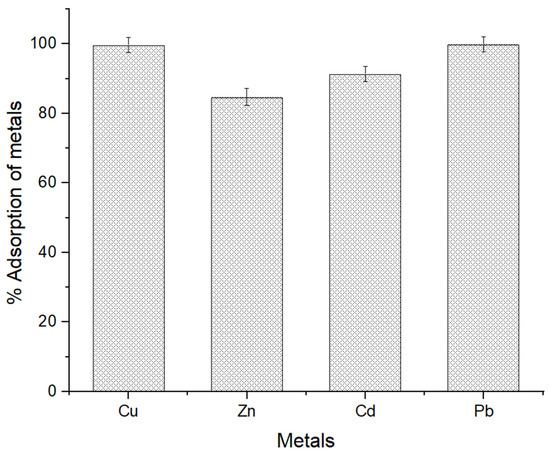
Figure 12.
Percentage adsorption of heavy metals after 120 min of MSC exposure. The error bars indicate the mean value ± standard error (SE) based on three replications of each treatment.
Conversely, the lower affinity for zinc and cadmium might be attributed to differences in coordination chemistry and electrostatic interactions. Zinc ions may form weaker complexes with the chitosan or compete less effectively for available binding sites compared with lead and copper ions. As a result, chitosan’s action in adsorbing heavy metals differed significantly [91].
Table 2 presents a comprehensive overview of the maximum adsorption capacities documented for the absorption of various metals, highlighting the potential of different chitosan-based adsorbents for Cu, Zn, Cd, and Pb removal from aqueous solutions. The data reflect the significant variability in the adsorption capabilities, with reported capacities ranging from 0.198 mg/g for Cd and 0.059 mg/g for Pb using shrimp-based chitosan [39] to 1.81 mg/g for Cd and 1.24 mg/g for Pb using chitin polymer materials [92]. Notably, the diverse range of adsorption capacities underscores the importance of selecting the appropriate adsorbent based on the target metal species. Moreover, the provided references serve as valuable resources for further investigation and optimization of adsorption processes for efficient metal removal in water treatment and environmental remediation applications. The inconsistency of metal adsorption among different adsorbents might be attributed to several factors contributing to the observed variations in metal adsorption. The surface charge and structure could impact the adsorption capacity for specific metal ions. The modified chitosan may have different affinities for specific metal ion species, resulting in variations in adsorption capacity. The modified chitosan may exhibit different responses to variations in these environmental conditions compared to shrimp chitosan. Further studies are warranted to explore the feasibility of scaling up these promising adsorption techniques for practical implementation in industrial settings. The adsorption mechanisms of heavy metals using chitosan have been proposed in different ways with diagrams as published previously [93].

Table 2.
Different chitosan and their maximum adsorption capacity.
4. Conclusions
In summary, this study highlights the significant potential of modified shrimp-based chitosan as a highly efficient adsorbent for eliminating heavy metals, specifically copper (Cu), zinc (Zn), cadmium (Cd) and lead (Pb) from polluted water sources. Environmental contamination by heavy metals causes major aquatic and terrestrial life risks, underscoring the importance of effective removal methods. Shrimp-based chitosan, derived from abundant natural resources like shrimp shells, offers a cost-effective and environmentally friendly solution. The innovative procedures employed in this study, encompassing acid washing, alkaline pretreatment, acetone rinsing, and deacetylation, resulted in a remarkable adsorbent with enhanced metal-binding capabilities. The comprehensive analyses, encompassing FT-IR spectroscopy and SEM-EDS, provided a detailed understanding of the structural and chemical properties of the modified chitosan. The Langmuir and Freundlich isotherm models, supported by high correlation coefficients (r2 > 0.98), demonstrated the highly significant adsorption of heavy metals. Furthermore, the pseudo-second-order kinetic model with linear coefficients (r2 > 0.97) effectively elucidated the kinetic studies of metal adsorption, confirming the robust fit of these models to the experimental data. The adsorption experiments were conducted individually for each metal over a pH range of 1 to 8, revealing distinct optimal pH values for copper, zinc, cadmium, and lead removal. The effective range of MSC varied from 2.5 mg to 12.5 mg for different metals, and the optimal amount differed accordingly. Similarly, diverse optimal durations were observed for each metal. In the case of a metal mixture, the comprehensive analysis identified the optimal conditions as pH 7, the adsorbent dosage of 10 mg, and an adsorption time of 120 min. The findings of this research contribute to the field of adsorption science and provide a practical, eco-friendly approach by which to combat heavy metal pollution in water bodies. The modified shrimp-based adsorbent demonstrated superior performance in removing metals from wastewater sources, suggesting its potential for sustainable water remediation technologies that benefit both the environment and human health.
Funding
The author would like to thank the Deputyship for Research and Innovation at King Faisal University, Saudi Arabia (grant number GRANT 4264), and The Japan Society for the Promotion of Science (JSPS, ID. P20766) for their support.
Data Availability Statement
The data used in this study are available from the corresponding author upon reasonable request.
Acknowledgments
The author is grateful to Noriko Ryuda for supporting SEM-EDS imaging and to Tsuge and Kazuhiro Yoshida for serving in ICP-MS analysis. The author extends his appreciation to Genta Kobayashi for assisting the project.
Conflicts of Interest
The author declares no conflict of interest.
References
- Lin, L.; Yang, H.; Xu, X. Effects of Water Pollution on Human Health and Disease Heterogeneity: A Review. Front. Environ. Sci. 2022, 10, 880246. [Google Scholar] [CrossRef]
- Zaimee, M.Z.A.; Sarjadi, M.S.; Rahman, M.L. Heavy Metals Removal from Water by Efficient Adsorbents. Water 2021, 13, 2659. [Google Scholar] [CrossRef]
- Afzaal, M.; Hameed, S.; Liaqat, I.; Khan, A.A.A.; Manan, H.A.; Shahid, R.; Altaf, M. Heavy Metals Contamination in Water, Sediments and Fish of Freshwater Ecosystems in Pakistan. Water Pract. Technol. 2022, 17, 1253–1272. [Google Scholar] [CrossRef]
- Cobbina, S.J.; Duwiejuah, A.B.; Quansah, R.; Obiri, S.; Bakobie, N. Comparative Assessment of Heavy Metals in Drinking Water Sources in Two Small-Scale Mining Communities in Northern Ghana. Int. J. Environ. Res. Public Health 2015, 12, 10620–10634. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Nahar, N.; Nawani, N.N.; Jass, J.; Hossain, K.; Saud, Z.A.; Saha, A.K.; Ghosh, S.; Olsson, B.; Mandal, A. Bioremediation of Hexavalent Chromium (VI) by a Soil-Borne Bacterium, Enterobacter Cloacae B2-DHA. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2015, 50, 1136–1147. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy Metal Pollution in the Environment and Their Toxicological Effects on Humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- Krstić, V.; Urošević, T.; Pešovski, B. A Review on Adsorbents for Treatment of Water and Wastewaters Containing Copper Ions. Chem. Eng. Sci. 2018, 192, 273–287. [Google Scholar] [CrossRef]
- Rahman, A.; Nahar, N.; Nawani, N.N.; Jass, J.; Desale, P.; Kapadnis, B.P.; Hossain, K.; Saha, A.K.; Ghosh, S.; Olsson, B.; et al. Isolation and Characterization of a Lysinibacillus Strain B1-CDA Showing Potential for Bioremediation of Arsenics from Contaminated Water. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2014, 49, 1349–1360. [Google Scholar] [CrossRef]
- Zia, Z.; Hartland, A.; Mucalo, M.R. Use of Low-Cost Biopolymers and Biopolymeric Composite Systems for Heavy Metal Removal from Water. Int. J. Environ. Sci. Technol. 2020, 17, 4389–4406. [Google Scholar] [CrossRef]
- Singh, K.K.; Talat, M.; Hasan, S.H. Removal of Lead from Aqueous Solutions by Agricultural Waste Maize Bran. Bioresour. Technol. 2006, 97, 2124–2130. [Google Scholar] [CrossRef]
- Win, D.T.; Than, M.M.; Tun, S. Lead Removal from Industrial Waters by Water Hyacinth. ThaiScience 2003, 6, 187–192. [Google Scholar]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of Heavy Metals-Concepts and Applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Said Mohd, N.; Mohamed, R.M. The Initial Ion Effect of Heavy Metals Adsorption By Using Hydrothermal Carbonization Banana Peels. Environ. Contam. Rev. 2020, 4, 08–10. [Google Scholar] [CrossRef]
- Lim, J.T.; Tan, Y.Q.; Valeri, L.; Lee, J.; Geok, P.P.; Chia, S.E.; Ong, C.N.; Seow, W.J. Association between Serum Heavy Metals and Prostate Cancer Risk—A Multiple Metal Analysis. Environ. Int. 2019, 132, 105109. [Google Scholar] [CrossRef] [PubMed]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, Mechanism and Health Effects of Some Heavy Metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead Toxicity: A Review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Carrott, P.J.M.; Ribeiro Carrott, M.M.L. Suhas Low-Cost Adsorbents: Growing Approach to Wastewater Treatmenta Review. Crit. Rev. Environ. Sci. Technol. 2009, 39, 783–842. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- Karim, N. Copper and Human Health—A Review. J. Bahria Univ. Med. Dental. 2018, 8, 117–122. [Google Scholar] [CrossRef]
- Hussain, S.; Khan, M.; Sheikh, T.M.M.; Mumtaz, M.Z.; Chohan, T.A.; Shamim, S.; Liu, Y. Zinc Essentiality, Toxicity, and Its Bacterial Bioremediation: A Comprehensive Insight. Front. Microbiol. 2022, 13, 900740. [Google Scholar] [CrossRef]
- Prithviraj, D.; Deboleena, K.; Neelu, N.; Noor, N.; Aminur, R.; Balasaheb, K.; Abul, M. Biosorption of Nickel by Lysinibacillus Sp. BA2 Native to Bauxite Mine. Ecotoxicol. Environ. Saf. 2014, 107, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Yoshida, K.; Islam, M.M.; Kobayashi, G. Investigation of Efficient Adsorption of Toxic Heavy Metals (Chromium, Lead, Cadmium) from Aquatic Environment Using Orange Peel Cellulose as Adsorbent. Sustainability 2023, 15, 4470. [Google Scholar] [CrossRef]
- Babu Poudel, M.; Shin, M.; Joo Kim, H. Interface Engineering of MIL-88 Derived MnFe-LDH and MnFe2O3 on Three-Dimensional Carbon Nanofibers for the Efficient Adsorption of Cr(VI), Pb(II), and As(III) Ions. Sep. Purif. Technol. 2022, 287, 120463. [Google Scholar] [CrossRef]
- Al-Shannag, M.; Al-Qodah, Z.; Bani-Melhem, K.; Qtaishat, M.R.; Alkasrawi, M. Heavy Metal Ions Removal from Metal Plating Wastewater Using Electrocoagulation: Kinetic Study and Process Performance. Chem. Eng. J. 2015, 260, 749–756. [Google Scholar] [CrossRef]
- Elbana, T.A.; Magdi Selim, H.; Akrami, N.; Newman, A.; Shaheen, S.M.; Rinklebe, J. Freundlich Sorption Parameters for Cadmium, Copper, Nickel, Lead, and Zinc for Different Soils: Influence of Kinetics. Geoderma 2018, 324, 80–88. [Google Scholar] [CrossRef]
- Altıntıg, E.; Yenigun, M.; Sarı, A.; Altundag, H.; Tuzen, M.; Saleh, T.A. Facile Synthesis of Zinc Oxide Nanoparticles Loaded Activated Carbon as an Eco-Friendly Adsorbent for Ultra-Removal of Malachite Green from Water. Environ. Technol. Innov. 2021, 21, 101305. [Google Scholar] [CrossRef]
- Badmus, S.O.; Oyehan, T.A.; Saleh, T.A. Synthesis of a Novel Polymer-Assisted AlNiMn Nanomaterial for Efficient Removal of Sulfate Ions from Contaminated Water. J. Polym. Environ. 2021, 29, 2840–2854. [Google Scholar] [CrossRef]
- Saleh, T.A. Protocols for Synthesis of Nanomaterials, Polymers, and Green Materials as Adsorbents for Water Treatment Technologies. Environ. Technol. Innov. 2021, 24, 101821. [Google Scholar] [CrossRef]
- Abdel Salam, O.E.; Reiad, N.A.; ElShafei, M.M. A Study of the Removal Characteristics of Heavy Metals from Wastewater by Low-Cost Adsorbents. J. Adv. Res. 2011, 2, 297–303. [Google Scholar] [CrossRef]
- Lim, A.P.; Aris, A.Z. A Review on Economically Adsorbents on Heavy Metals Removal in Water and Wastewater. Rev. Environ. Sci. Biotechnol. 2014, 13, 163–181. [Google Scholar] [CrossRef]
- He, J.; Chen, J.P. A Comprehensive Review on Biosorption of Heavy Metals by Algal Biomass: Materials, Performances, Chemistry, and Modeling Simulation Tools. Bioresour. Technol. 2014, 160, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Munagapati, V.S.; Yarramuthi, V.; Kim, Y.; Lee, K.M.; Kim, D.S. Removal of Anionic Dyes (Reactive Black 5 and Congo Red) from Aqueous Solutions Using Banana Peel Powder as an Adsorbent. Ecotoxicol. Environ. Saf. 2018, 148, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Saeed, K.; Mabood, F. Removal of Chromium (VI) from Aqueous Medium Using Chemically Modified Banana Peels as Efficient Low-Cost Adsorbent. Alexandria Eng. J. 2016, 55, 2933–2942. [Google Scholar] [CrossRef]
- Khademian, E.; Salehi, E.; Sanaeepur, H.; Galiano, F.; Figoli, A. A Systematic Review on Carbohydrate Biopolymers for Adsorptive Remediation of Copper Ions from Aqueous Environments-Part A: Classification and Modification Strategies. Sci. Total Environ. 2020, 738, 139829. [Google Scholar] [CrossRef] [PubMed]
- Salehi, E.; Daraei, P.; Arabi Shamsabadi, A. A Review on Chitosan-Based Adsorptive Membranes. Carbohydr. Polym. 2016, 152, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Yudaev, P.; Semenova, A.; Chistyakov, E. Gel Based on Modified Chitosan for Oil Spill Cleanup. J. Appl. Polym. Sci. 2023, 141, e54838. [Google Scholar] [CrossRef]
- Begum, S.; Yuhana, N.Y.; Md Saleh, N.; Kamarudin, N.H.N.; Sulong, A.B. Review of Chitosan Composite as a Heavy Metal Adsorbent: Material Preparation and Properties. Carbohydr. Polym. 2021, 259, 117613. [Google Scholar] [CrossRef]
- He, C.; Lin, H.; Dai, L.; Qiu, R.; Tang, Y.; Wang, Y.; Duan, P.G.; Ok, Y.S. Waste Shrimp Shell-Derived Hydrochar as an Emergent Material for Methyl Orange Removal in Aqueous Solutions. Environ. Int. 2020, 134, 105340. [Google Scholar] [CrossRef]
- Marzuki, I.; Alwi, R.S.; Erniati; Mudyawati; Sinardi; Iryani, A.S. Chitosan Performance of Shrimp Shells in The Biosorption Ion Metal of Cadmium, Lead and Nickel Based on Variations pH Interaction. Adv. Eng. Res. 2019, 165, 5–11. [Google Scholar] [CrossRef]
- Liang, X.; Fan, X.; Li, R.; Li, S.; Shen, S.; Hu, D. Efficient Removal of Cr(VI) from Water by Quaternized Chitin/Branched Polyethylenimine Biosorbent with Hierarchical Pore Structure. Bioresour. Technol. 2018, 250, 178–184. [Google Scholar] [CrossRef]
- Singh, R.; Shitiz, K.; Singh, A. Chitin and Chitosan: Biopolymers for Wound Management. Int. Wound J. 2017, 14, 1276–1289. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Gómez, C.P.; Cecilia, J.A. Chitosan: A Natural Biopolymer with a Wide and Varied Range of Applications. Molecules 2020, 25, 3981. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Gómez, D.; Rodrigues, C.; Lapolli, F.R.; Lobo-Recio, M.Á. Adsorption of Heavy Metals from Coal Acid Mine Drainage by Shrimp Shell Waste: Isotherm and Continuous-Flow Studies. J. Environ. Chem. Eng. 2019, 7, 102787. [Google Scholar] [CrossRef]
- Rahman, A.; Haque, A.; Ghosh, S.; Shinu, P.; Attimarad, M. Modified Shrimp-Based Chitosan as an Emerging Adsorbent Removing Heavy Metals (Chromium, Nickel, Arsenic, and Cobalt) from Polluted Water. Sustainability 2023, 15, 2431. [Google Scholar] [CrossRef]
- Shahabuddin, S.; Nor, S.; Baharin, A.; Farahin, N.; Yunus, N. Proceedings Preparation of Shrimp-Based Chitin Blend with Polyaniline for Chromium (VI) Removal from Aqueous Solution. Mater. Today Proc. 2021, 62, 6940–6944. [Google Scholar] [CrossRef]
- Alagesan, C.M.; Panneerselvam, A.; Rathinam, K.M.S. Extraction, Optimization and Characterization of Chitosan from Penicillium Chrysogenum. Int. J. Curr. Microbiol. App. Sci. 2016, 3, 19–26. [Google Scholar]
- Fadlelmoula, A.; Pinho, D.; Carvalho, V.H.; Catarino, S.O.; Minas, G. Fourier Transform Infrared (FTIR) Spectroscopy to Analyse Human Blood over the Last 20 Years: A Review towards Lab-on-a-Chip Devices. Micromachines 2022, 13, 187. [Google Scholar] [CrossRef]
- Girão, A.V.; Caputo, G.; Ferro, M.C. Application of Scanning Electron Microscopy–Energy Dispersive X-Ray Spectroscopy (SEM-EDS). Compr. Anal. Chem. 2017, 75, 153–168. [Google Scholar] [CrossRef]
- Al-Hakkani, M.F. Guideline of Inductively Coupled Plasma Mass Spectrometry “ICP–MS”: Fundamentals, Practices, Determination of the Limits, Quality Control, and Method Validation Parameters. SN Appl. Sci. 2019, 1, 791. [Google Scholar] [CrossRef]
- Al-Qahtani, K.M. Water Purification Using Different Waste Fruit Cortexes for the Removal of Heavy Metals. J. Taibah Univ. Sci. 2016, 10, 700–708. [Google Scholar] [CrossRef]
- Nathan, R.J.; Barr, D.; Rosengren, R.J. Six Fruit and Vegetable Peel Beads for the Simultaneous Removal of Heavy Metals by Biosorption. Environ. Technol. 2020, 43, 1935–1952. [Google Scholar] [CrossRef]
- Ayub, A.; Irfan, M.; Rizwan, M.; Irfan, A. Development of Sustainable Magnetic Chitosan Biosorbent Beads for Kinetic Remediation of Arsenic Contaminated Water. Int. J. Biol. Macromol. 2020, 163, 603–617. [Google Scholar] [CrossRef] [PubMed]
- Kalam, S.; Abu-Khamsin, S.A.; Kamal, M.S.; Patil, S. Surfactant Adsorption Isotherms: A Review. ACS Omega 2021, 6, 32342–32348. [Google Scholar] [CrossRef] [PubMed]
- Chio, C.; Lin, M.; Liao, C. Low-Cost Farmed Shrimp Shells Could Remove Arsenic from Solutions Kinetically. J. Hazard. Mater. 2009, 171, 859–864. [Google Scholar] [CrossRef]
- Raji, Z.; Karim, A.; Karam, A.; Khalloufi, S. Adsorption of Heavy Metals: Mechanisms, Kinetics, and Applications of Various Adsorbents in Wastewater Remediation—A Review. Waste 2023, 1, 775–805. [Google Scholar] [CrossRef]
- Król, A.; Mizerna, K.; Bożym, M. An Assessment of PH-Dependent Release and Mobility of Heavy Metals from Metallurgical Slag. J. Hazard. Mater. 2020, 384, 121502. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Ke, T.; Zhu, H.; Xu, P.; Wang, H. Efficient Removal of Heavy Metals from Aqueous Solution Using Licorice Residue-Based Hydrogel Adsorbent. Gels 2023, 9, 559. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wen, H.; Chen, K.; Chen, Y. A Simple One-Step Modification of Shrimp Shell for the Efficient Adsorption and Desorption of Copper Ions. Molecules 2021, 26, 5690. [Google Scholar] [CrossRef]
- Mishra, V.M.M. Studies on Heavy Metal Removal Efficiency and Antibacterial Activity of Chitosan Prepared from Shrimp Shell Waste. 3 Biotech 2014, 4, 167–175. [Google Scholar] [CrossRef]
- Mathew, G.M.; Mathew, D.C.; Sukumaran, R.K.; Sindhu, R.; Huang, C.-C.; Binod, P.; Sirohi, R. Sustainable and eco-friendly strategies for shrimp shell valorization. J. Environ. Pollut. 2020, 267, 115656. [Google Scholar] [CrossRef]
- Shoueir, K.; El-Sheshtawy, H.; Misbah, M.; El-Hosainy, H.; El-Mehasseb, I.; El-Kemary, M. Fenton-like Nanocatalyst for Photodegradation of Methylene Blue under Visible Light Activated by Hybrid Green DNSA@Chitosan@MnFe2O4. Carbohydr. Polym. 2018, 197, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Bae, J.S.; Hwang, I.; Kim, S.H.; Jeon, K.W. Superior Heavy Metal Ion Adsorption Capacity in Aqueous Solution by High-Density Thiol-Functionalized Reduced Graphene Oxides. Molecules 2023, 28, 3998. [Google Scholar] [CrossRef] [PubMed]
- Belskaya, O.B.; Danilova, I.G.; Kazakov, M.O.; Mironenko, R.M.; Lavrenov, A.V.; Likholobov, V.A. FTIR Spectroscopy of Adsorbed Probe Molecules for Analyzing the Surface Properties of Supported Pt (Pd) Catalysts. In Infrared Spectroscopy—Materials Science, Engineering and Technology; IntechOpen Limited: London, UK, 2012. [Google Scholar] [CrossRef][Green Version]
- Reddy, N.A.; Lakshmipathy, R.; Sarada, N.C. Application of Citrullus Lanatus Rind as Biosorbent for Removal of Trivalent Chromium from Aqueous Solution. Alexandria Eng. J. 2014, 53, 969–975. [Google Scholar] [CrossRef]
- Zhang, A.J.; Qin, Q.L.; Zhang, H.; Wang, H.T.; Li, X.; Miao, L.; Wu, Y.J. Preparation and Characterisation of Food-Grade Chitosan from Housefly Larvae. Czech J. Food Sci. 2011, 29, 616–623. [Google Scholar] [CrossRef]
- D’Halluin, M.; Rull-Barrull, J.; Bretel, G.; Labrugère, C.; Le Grognec, E.; Felpin, F.X. Chemically Modified Cellulose Filter Paper for Heavy Metal Remediation in Water. ACS Sustain. Chem. Eng. 2017, 5, 1965–1973. [Google Scholar] [CrossRef]
- Md Salim, R.; Asik, J.; Sarjadi, M.S. Chemical Functional Groups of Extractives, Cellulose and Lignin Extracted from Native Leucaena Leucocephala Bark. Wood Sci. Technol. 2021, 55, 295–313. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, B.; Zhang, L.; Huang, J.; Chen, F.; Yang, Z.; Yao, J.; Zhang, Z. Controlled Assembly of Fe3O4 Magnetic Nanoparticles on Graphene Oxide. Nanoscale 2011, 3, 1446–1450. [Google Scholar] [CrossRef]
- Kannan, S.; Gariepy, Y.; Raghavan, V. Optimization of Enzyme Hydrolysis of Seafood Waste for Microwave Hydrothermal Carbonization. Energy Fuels 2015, 29, 8006–8016. [Google Scholar] [CrossRef]
- Abd-Talib, N.; Chuong, C.S.; Mohd-Setapar, S.H.; Asli, U.A.; Pa’ee, K.F.; Len, K.Y.T. Trends in Adsorption Mechanisms of Fruit Peel Adsorbents to Remove Wastewater Pollutants (Cu (II), Cd (II) and Pb (II)). J. Water Environ. Technol. 2020, 18, 290–313. [Google Scholar] [CrossRef]
- Lo, S.F.; Wang, S.Y.; Tsai, M.J.; Lin, L.D. Adsorption Capacity and Removal Efficiency of Heavy Metal Ions by Moso and Ma Bamboo Activated Carbons. Chem. Eng. Res. Des. 2012, 90, 1397–1406. [Google Scholar] [CrossRef]
- Guibal, E. Interactions of Metal Ions with Chitosan-Based Sorbents: A Review. Sep. Purif. Technol. 2004, 38, 43–74. [Google Scholar] [CrossRef]
- Keshvardoostchokami, M.; Babaei, L.; Zamani, A.A.; Parizanganeh, A.H.; Piri, F. Synthesized Chitosan/Iron Oxide Nanocomposite and Shrimp Shell in Removal of Nickel, Cadmium and Lead from Aqueous Solution. Glob. J. Environ. Sci. Manag. 2017, 3, 267–278. [Google Scholar] [CrossRef]
- Ugbe, F.A.; Pam, A.A.; Ikudayisi, A.V. Thermodynamic Properties of Chromium (III) Ion Adsorption by Sweet Orange (Citrus sinensis) Peels. Am. J. Anal. Chem. 2014, 05, 666–673. [Google Scholar] [CrossRef]
- Jisha, T.J.; Lubna, C.H.; Habeeba, V. Removal of Cr (VI) Using Orange Peel as an Adsorbent. Int. J. Adv. Res. Innov. Ideas Educ. 2017, 3, 276–283. [Google Scholar]
- Tejada-Tovar, C.; Gonzalez-Delgado, A.D.; Villabona-Ortiz, A. Removal of Cr (VI) from Aqueous Solution Using Orange Peel-Based Biosorbents. Indian J. Sci. Technol. 2018, 11, 1–13. [Google Scholar] [CrossRef]
- Gönen, F. Adsorption Study on Orange Peel: Removal of Ni(II) Ions from Aqueous Solution. Afr. J. Biotechnol. 2012, 11, 1250–1258. [Google Scholar] [CrossRef]
- Lukum, A.; Paramata, Y.; Botutihe, D.N.; Akume, J.; Sukamto, K.; Paramata, A.R. Development of Bioadsorbent Chitosan from Shrimp Shell Waste to Mercury Absorption Efficiency. IOP Conf. Ser. Earth Environ. Sci. 2020, 589, 012018. [Google Scholar] [CrossRef]
- Jain, G. Removal of Copper and Zinc from Wastewater Using Chitosan. Ph.D. Thesis, National Institute Of Technology, Rourkela Orissa, India, 2013; p. 769008. Available online: https://core.ac.uk/download/pdf/53189819.pdf (accessed on 18 December 2023).
- Pratiwi, R.; Prinajati, P.D. Adsorption for Lead Removal by Chitosan from Shrimp Shells. Indones. J. Urban Environ. Technol. 2018, 2, 35–46. [Google Scholar] [CrossRef]
- Basu, M.; Guha, A.K.; Ray, L. Adsorption of Cadmium on Cucumber Peel: Kinetics, Isotherm and Co-Ion Effect. Indian Chem. Eng. 2018, 60, 179–195. [Google Scholar] [CrossRef]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. J. Chem. 2017, 2017, 3039817. [Google Scholar] [CrossRef]
- Vijayakumar, G.; Tamilarasan, R.; Kumar, M.D. Removal of Cd2+ Ions From Aqueous Solution Using Live and Dead Bacillus Subtilis. Chem. Eng. Res. Bull. 2011, 15, 18–24. Available online: https://www.banglajol.info/index.php/CERB (accessed on 17 December 2023).
- Rahman, M.A.; Hossain, M.M.; Samad, A.; Alam, A.M.S. Removal of Arsenic from Ground Water with Shrimp Shell. Dhaka Univ. J. Sci. 2012, 60, 175–180. [Google Scholar] [CrossRef]
- Ali, M.H.H.; Abdel-Satar, A.M. Removal of Some Heavy Metals from Aqueous Solutions Using Natural Wastes Orange Peel Activated Carbon. IJRDO-J. Appl. Sci. 2017, 3, 13–30. Available online: https://api.semanticscholar.org/CorpusID:219678980 (accessed on 18 December 2023).
- Singh, R.J.; Martin, C.E.; Barr, D.; Rosengren, R.J. Immobilised Apple Peel Bead Biosorbent for the Simultaneous Removal of Heavy Metals from Cocktail Solution. Cogent Environ. Sci. 2019, 5, 2461–2477. [Google Scholar] [CrossRef]
- Akinhanmi, T.F.; Ofudje, E.A.; Adeogun, A.I.; Aina, P.; Joseph, I.M. Orange Peel as Low-Cost Adsorbent in the Elimination of Cd(II) Ion: Kinetics, Isotherm, Thermodynamic and Optimization Evaluations. Bioresour. Bioprocess. 2020, 7, 34. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Zhang, Z.; Cui, W.; Zhang, X.; Wang, S. Removing Copper and Cadmium from Water and Sediment by Magnetic Microspheres—MnFe2O4/Chitosan Prepared by Waste Shrimp Shells. J. Environ. Chem. Eng. 2021, 9, 104647. [Google Scholar] [CrossRef]
- Mo, J.; Yang, Q.; Zhang, N.; Zhang, W.; Zheng, Y.; Zhang, Z. A Review on Agro-Industrial Waste (AIW) Derived Adsorbents for Water and Wastewater Treatment. J. Environ. Manag. 2018, 227, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Iftekhar, S.; Ramasamy, D.L.; Srivastava, V.; Asif, M.B.; Sillanpää, M. Understanding the Factors Affecting the Adsorption of Lanthanum Using Different Adsorbents: A Critical Review. Chemosphere 2018, 204, 413–430. [Google Scholar] [CrossRef]
- Alam, O.; Qiao, X. Influences of Chemically Controlled Ca-Bearing Minerals in Chitosan on Pb2+ Removal Efficiency. J. Environ. Health Sci. Eng. 2020, 18, 993–1005. [Google Scholar] [CrossRef]
- Pinto, P.X.; Al-Abed, S.R.; Reisman, D.J. Biosorption of Heavy Metals from Mining Influenced Water onto Chitin Products. Chem. Eng. J. 2011, 166, 1002–1009. [Google Scholar] [CrossRef]
- Kong, D.; Foley, S.R.; Wilson, L.D. An Overview of Modified Chitosan Adsorbents for the Removal of Precious Metals Species from Aqueous Media. Molecules 2022, 27, 978. [Google Scholar] [CrossRef] [PubMed]
- Pooladi, A.; Bazargan-Lari, R. Simultaneous Removal of Copper and Zinc Ions by Chitosan/Hydroxyapatite/Nano-Magnetite Composite. J. Mater. Res. Technol. 2020, 9, 14841–14852. [Google Scholar] [CrossRef]
- Sargin, I.; Arslan, G. Chitosan/Sporopollenin Microcapsules: Preparation, Characterisation and Application in Heavy Metal Removal. Int. J. Biol. Macromol. 2015, 75, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Chai, Y.; Zeng, L.; Gao, Z.; Zhang, J.; Ji, H. Effcient Removal of Copper Ion from Waste Water Using a Stable Chitosan Gel Material. Molecules 2019, 24, 4205. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhu, Y.; Li, Z.; Tian, D.; Chen, L.; Chen, P. Chitin Nanofibrils for Rapid and Efficient Removal of Metal Ions from Water System. Carbohydr. Polym. 2013, 98, 483–489. [Google Scholar] [CrossRef]
- Cho, D.W.; Jeon, B.H.; Chon, C.M.; Kim, Y.; Schwartz, F.W.; Lee, E.S.; Song, H. A Novel Chitosan/Clay/Magnetite Composite for Adsorption of Cu(II) and As(V). Chem. Eng. J. 2012, 200–202, 654–662. [Google Scholar] [CrossRef]
- Biswas, S.; Rashid, T.U.; Debnath, T.; Haque, P.; Rahman, M.M. Application of Chitosan-Clay Biocomposite Beads for Removal of Heavy Metal and Dye from Industrial Effluent. J. Compos. Sci. 2020, 4, 16. [Google Scholar] [CrossRef]
- Benavente, M.; Moreno, L.; Martinez, J. Sorption of Heavy Metals from Gold Mining Wastewater Using Chitosan. J. Taiwan Inst. Chem. Eng. 2011, 42, 976–988. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).