Co-Transport of Aniline and TNT with Loess Colloid Particles in Saturated Loess Columns: Mechanism and Processes
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of Loess Colloid Particles
2.2. Adsorption Experiments
2.3. Column Experiments
2.4. Methods for Determining the Concentration of Aniline and TNT
2.5. Methods for the Analysis of Leachate
3. Results and Discussion
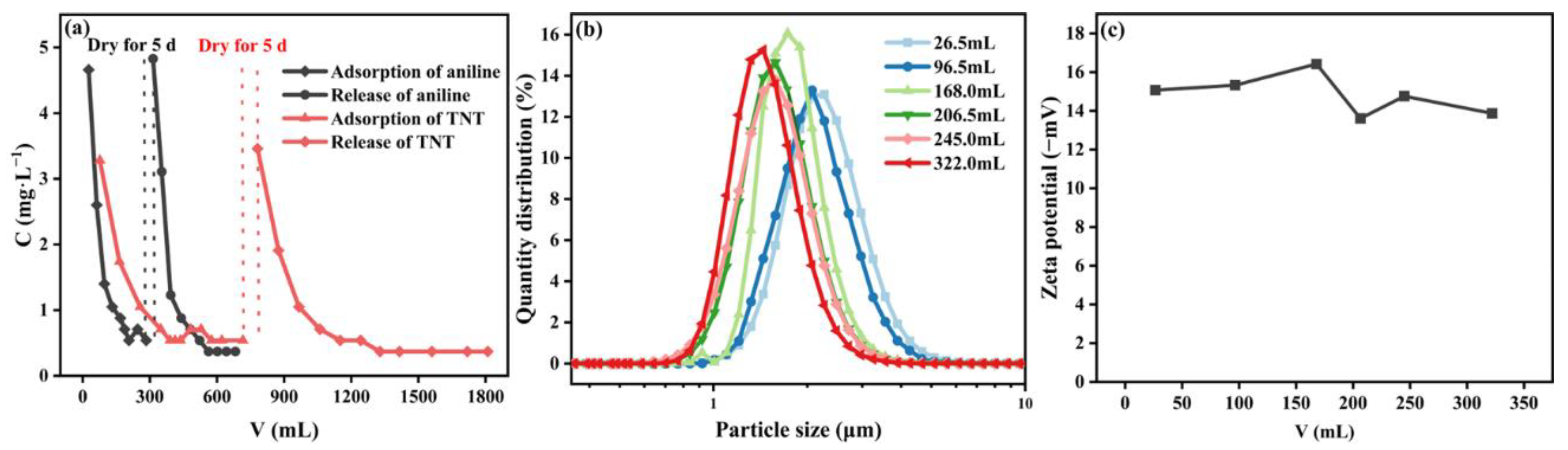
3.1. Basic Properties of Loess Colloid Particles
3.2. Adsorption Characteristics of Aniline and TNT on Loess Colloid Particles
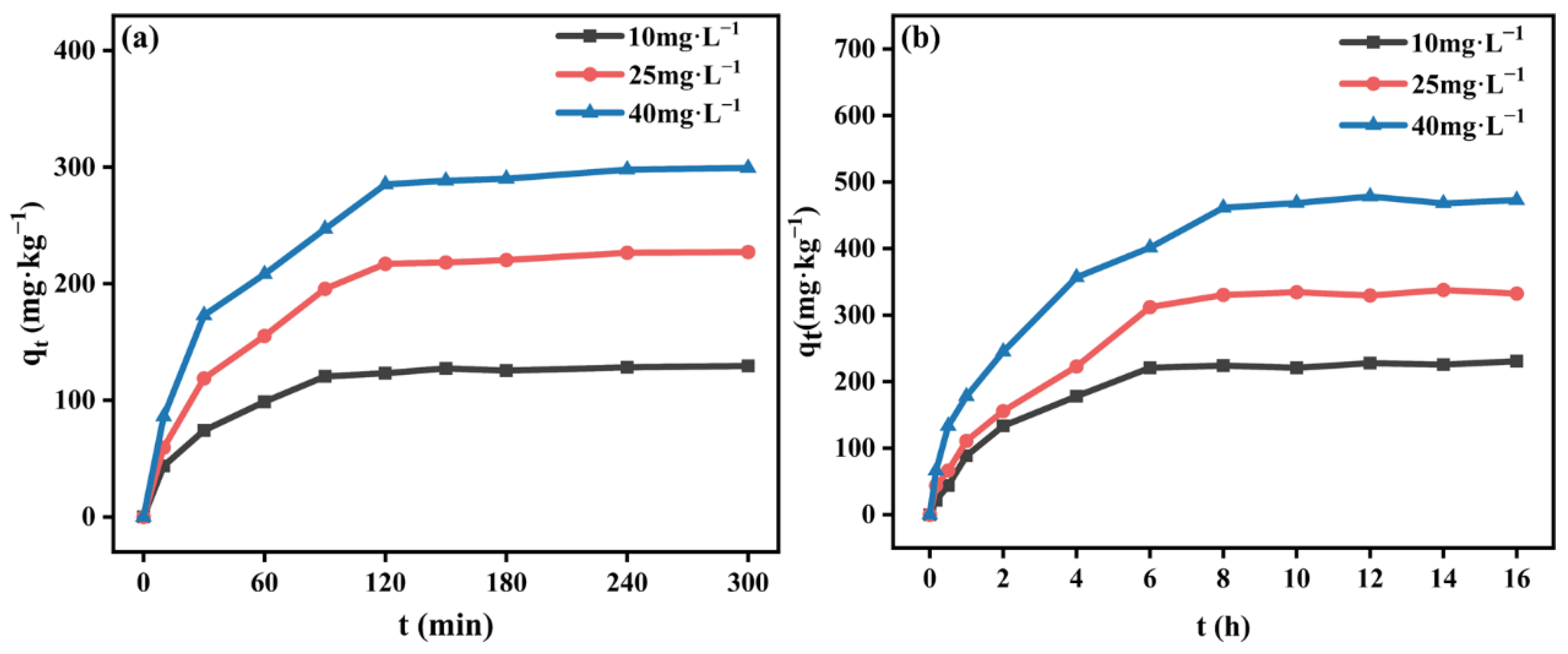
3.2.1. Adsorption Kinetics
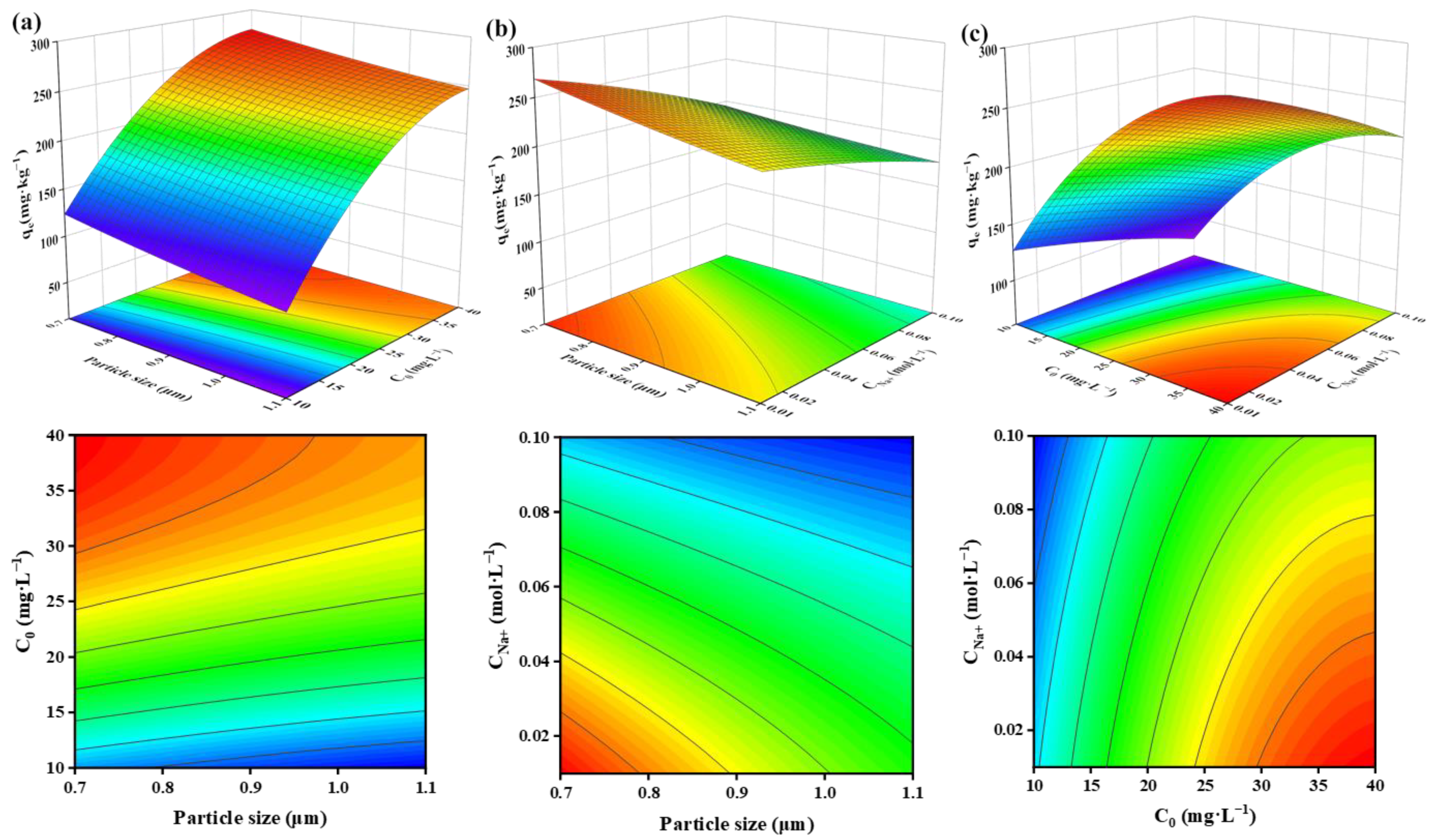
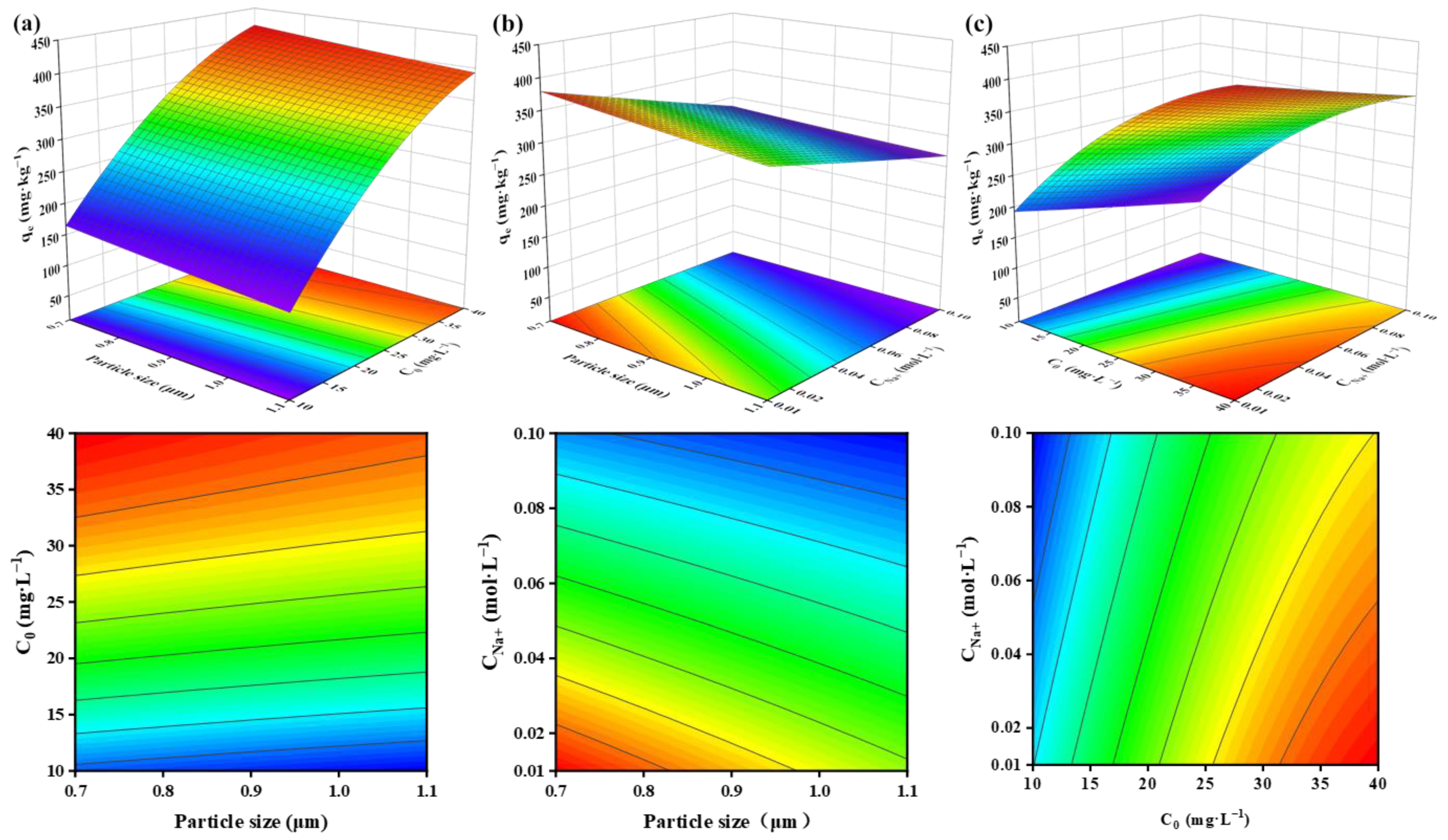
3.2.2. Effect of Factors on Adsorption Capacity
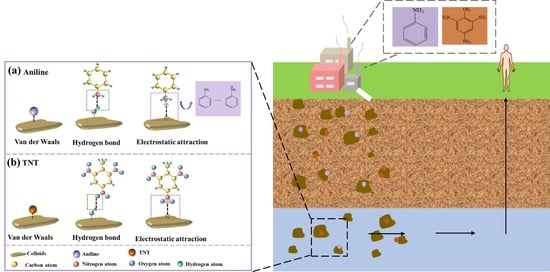
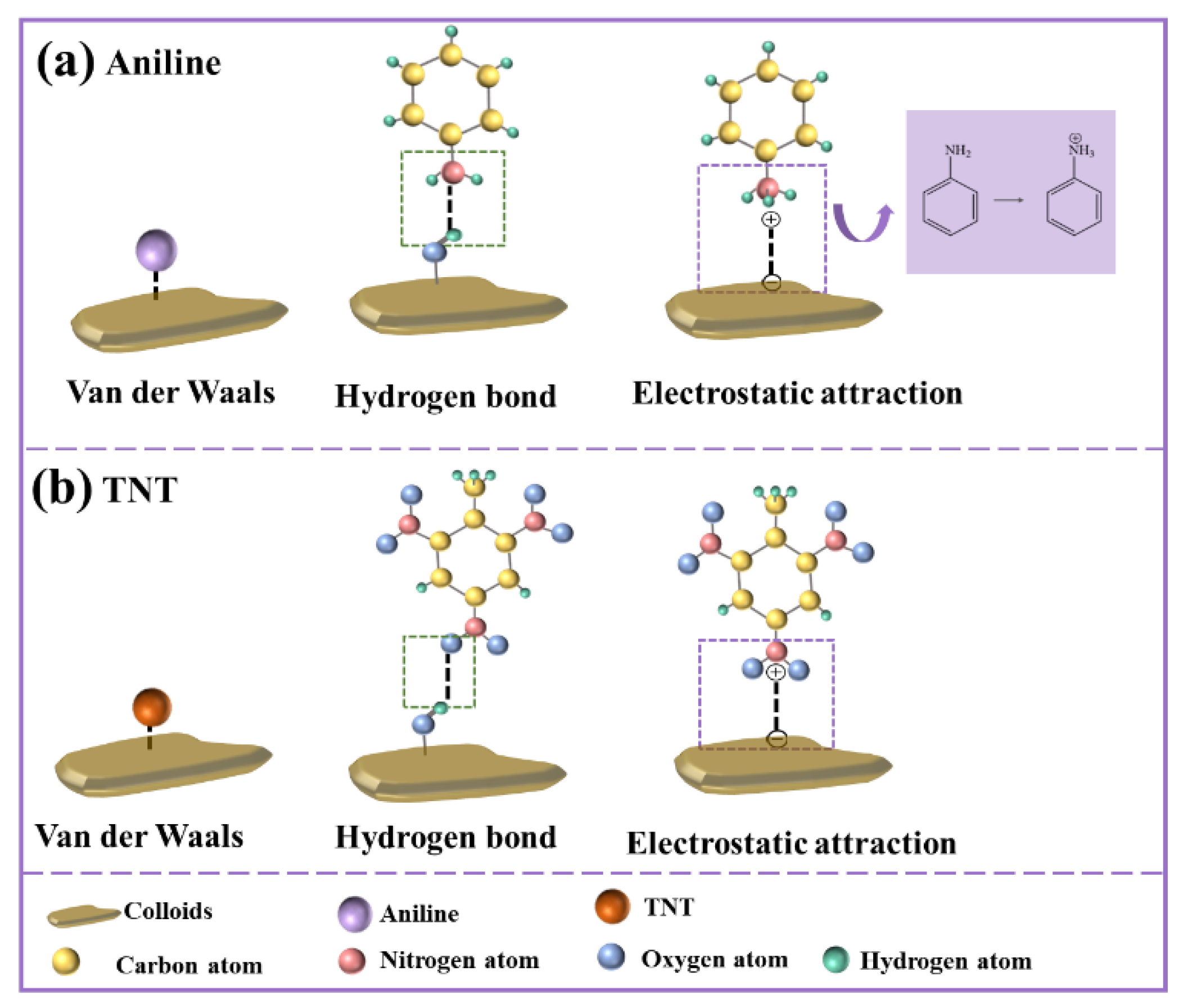
3.2.3. The Proposed Adsorption Mechanism
3.3. Co-Transport of Loess Colloid Particles with Aniline and TNT
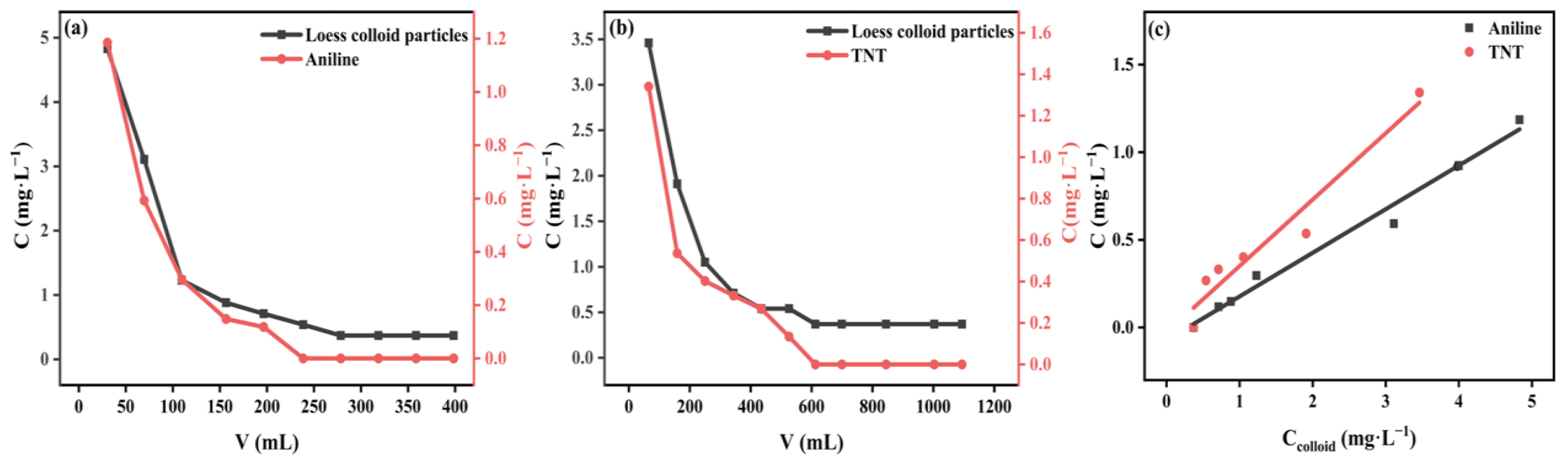
3.3.1. Dynamic Adsorption of Aniline and TNT on Loess
3.3.2. Mechanism of Loess Colloid Particles Release
3.3.3. Mechanism of Co-Transport of Loess Colloid Particles with Aniline and TNT
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fawcett-Hirst, W.; Temple, T.J.; Ladyman, M.K.; Coulon, F. A review of treatment methods for insensitive high explosive contaminated wastewater. Heliyon 2021, 7, e07438. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.M.; Zheng, W.W.; Wang, X.J.; Shen, F. Nitrification progress of nitrogen-rich heterocyclic energetic compounds: A review. Molecules 2022, 27, 1465. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; An, Y.; Row, K.H. Recoverable deep eutectic solvent-based aniline organic pollutant separation technology using choline salt as adsorbent. J. Mol. Liq. 2020, 306, 112910. [Google Scholar] [CrossRef]
- Gao, D.; Hu, Q.; Pan, H.; Jiang, J.; Wang, P. High-capacity adsorption of aniline using surface modification of lignocellulose-biomass jute fibers. Bioresour. Technol. 2015, 193, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Lingamdinne, L.P.; Roh, H.; Choi, Y.-L.; Koduru, J.R.; Yang, J.-K.; Chang, Y.-Y. Influencing factors on sorption of TNT and RDX using rice husk biochar. J. Ind. Eng. Chem. 2015, 32, 178–186. [Google Scholar] [CrossRef]
- Mohanta, V.L.; Mishra, B.K. Integration of cancer and non-cancer human health risk assessment for Aniline enriched groundwater: A fuzzy inference system-based approach. Environ. Geochem. Health 2020, 42, 3623–3639. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yin, W.; Li, P.; Li, X.; Zhang, C.; Stagnitti, F.; Xiong, X. Distribution and migration of nitrobenzene in water following a simulated spill. J. Hazard. Mater. 2010, 182, 787–791. [Google Scholar] [CrossRef]
- Cheng, H.; Song, Y.; Bian, Y.; Ji, R.; Wang, F.; Gu, C.; Yang, X.; Ye, M.; Ouyang, G.; Jiang, X. Meso-/microporous carbon as an adsorbent for enhanced performance in solid-phase microextraction of chlorobenzenes. Sci. Total Environ. 2019, 681, 392–399. [Google Scholar] [CrossRef]
- Silva, L.F.O.; Santosh, M.; Schindler, M.; Gasparotto, J.; Dotto, G.L.; Oliveira, M.L.S.; Hochella, M.F., Jr. Nanoparticles in fossil and mineral fuel sectors and their impact on environment and human health: A review and perspective. Gondwana Res. 2021, 92, 184–201. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, J.; Guo, G.; Li, H.; Jones, K. A comprehensive comparison and analysis of soil screening values derived and used in China and the UK. Environ. Pollut. 2020, 256, 113404. [Google Scholar] [CrossRef]
- Xu, C.; Lin, X.; Yin, S.; Liu, K.; Liu, W. Spatio-vertical characterization of the BTEXS group of VOCs in Chinese agricultural soils. Sci. Total Environ. 2019, 694, 133631. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, S.; He, Z.L.; Harris, W.G. Natural Nanoparticles: Implications for Environment and Human Health. Crit. Rev. Environ. Sci. Technol. 2015, 45, 861–904. [Google Scholar] [CrossRef]
- Ngueleu, S.K.; Grathwohl, P.; Cirpka, O.A. Effect of natural particles on the transport of lindane in saturated porous media: Laboratory experiments and model-based analysis. J. Contam. Hydrol. 2013, 149, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Mendes, P.A.D.; Thomsen, L.; Garcia, R.; Gust, G. Transport of persistent organic pollutants by organo-mineral aggregates (OMAs) in the Lisboa-Setubal Canyon system. Deep.-Sea Res. Part Ii-Top. Stud. Oceanogr. 2011, 58, 2345–2353. [Google Scholar] [CrossRef]
- Mishra, S.B.; Marutheeswaran, S.; Roy, S.C.; Natarajan, V.; Rai, P.K.; Nanda, B.R.K. Adsorption and degradation mechanism of 2,4,6-trinitrotoluene on TiO2 (110) surface. Surf. Sci. 2021, 713, 121902. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, R.; Zheng, Y.; Bai, H.; Shi, J.; Zhang, J.; Zhou, X.; Cai, M.; Fan, S.; Li, C. Graphene oxide-Fe3O4 nanocomposite used as aniline adsorbent with a wide pH range. Colloid Polym. Sci. 2022, 300, 83–93. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, W.; Zhao, B.; Wang, H.; Qin, L.; Han, J. Preparation of high-performance toluene adsorbents by sugarcane bagasse carbonization combined with surface modification. Rsc Adv. 2020, 10, 23749–23758. [Google Scholar] [CrossRef]
- Kasel, D.; Bradford, S.A.; Simunek, J.; Heggen, M.; Vereecken, H.; Klumpp, E. Transport and retention of multi-walled carbon nanotubes in saturated porous media: Effects of input concentration and grain size. Water Res. 2013, 47, 933–944. [Google Scholar] [CrossRef]
- Ma, J.; Guo, H.; Lei, M.; Li, Y.; Weng, L.; Chen, Y.; Ma, Y.; Deng, Y.; Feng, X.; Xiu, W. Enhanced transport of ferrihydrite colloid by chain-shaped humic acid colloid in saturated porous media. Sci. Total Environ. 2018, 621, 1581–1590. [Google Scholar] [CrossRef]
- Wang, D.; Jin, Y.; Jaisi, D.P. Effect of Size-Selective Retention on the Cotransport of Hydroxyapatite and Goethite Nanoparticles in Saturated Porous Media. Environ. Sci. Technol. 2015, 49, 8461–8470. [Google Scholar] [CrossRef]
- Wang, C.; Bobba, A.D.; Attinti, R.; Shen, C.; Lazouskaya, V.; Wang, L.-P.; Jin, Y. Retention and Transport of Silica Nanoparticles in Saturated Porous Media: Effect of Concentration and Particle Size. Environ. Sci. Technol. 2012, 46, 7151–7158. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wang, Y.; Sharma, P.; Li, B.; Liu, K.; Liu, J.; Flury, M.; Shang, J. Effect of naphthalene on transport and retention of biochar colloids through saturated porous media. Colloids Surf. A-Physicochem. Eng. Asp. 2017, 530, 146–154. [Google Scholar] [CrossRef]
- Chen, J.; Chen, W.; Lu, T.; Song, Y.; Zhang, H.; Wang, M.; Wang, X.; Qi, Z.; Lu, M. Effects of phosphate on the transport of graphene oxide nanoparticles in saturated clean and iron oxide-coated sand columns. J. Environ. Sci. 2021, 103, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.; Yan, Z.; Liu, Y.; Lu, G. Transport of nanoparticles in porous media and its effects on the co-existing pollutants. Environ. Pollut. 2021, 283, 117098. [Google Scholar] [CrossRef] [PubMed]
- Hameed, R.; Lei, C.; Fang, J.; Lin, D. Co-transport of biochar colloids with organic contaminants in soil column. Environ. Sci. Pollut. Res. 2021, 28, 1574–1586. [Google Scholar] [CrossRef]
- Lu, L.; Chen, B. Biochar-amendment-reduced cotransport of graphene oxide nanoparticles and dimethyl phthalate in saturated porous media. Sci. Total Environ. 2020, 705, 135094. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, L.; Song, X.; Adeel, M.; Yang, Y. Natural colloids facilitated transport of steroidal estrogens in saturated porous media: Mechanism and processes. Environ. Pollut. 2022, 315, 120315. [Google Scholar] [CrossRef]
- Liu, F.; Xu, B.; He, Y.; Brookes, P.C.; Tang, C.; Xu, J. Differences in transport behavior of natural soil colloids of contrasting sizes from nanometer to micron and the environmental implications. Sci. Total Environ. 2018, 634, 802–810. [Google Scholar] [CrossRef]
- Ma, Y.-L.; Ma, J.; Chen, Y.-L.; Lei, M.; Guo, H.-M.; Weng, L.-P.; Li, Y.-T. Arsenic Adsorption and Its Species on Ferrihydrite and Ferrihydrite Colloid. Huan Jing Ke Xue 2018, 39, 179–186. [Google Scholar] [CrossRef]
- Du, W.; Li, R.; Liu, X.-M.; Tian, R.; Li, H. Specific ion effects on ion exchange kinetics in charged clay. Colloids Surf. A-Physicochem. Eng. Asp. 2016, 509, 427–432. [Google Scholar] [CrossRef]
- Zhu, Y.; Ma, L.Q.; Dong, X.; Harris, W.G.; Bonzongo, J.C.; Han, F. Ionic strength reduction and flow interruption enhanced colloid-facilitated Hg transport in contaminated soils. J. Hazard. Mater. 2014, 264, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Ma, J.; Wu, X.J.; Weng, L.P.; Li, Y.T. Sedimentation and Transport of Different Soil Colloids: Effects of Goethite and Humic Acid. Water 2020, 12, 980. [Google Scholar] [CrossRef]
- Ma, J.; Guo, H.; Lei, M.; Wan, X.; Zhang, H.; Feng, X.; Wei, R.; Tian, L.; Han, X. Blocking effect of colloids on arsenate adsorption during co-transport through saturated sand columns. Environ. Pollut. 2016, 213, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Wang, D.; Cang, L.; Hao, X.; Chu, L. Transport and re-entrainment of soil colloids in saturated packed column: Effects of pH and ionic strength. J. Soils Sediments 2011, 11, 491–503. [Google Scholar] [CrossRef]
- Garcia-Delgado, C.; Marin-Benito, J.M.; Sanchez-Martin, M.J.; Rodriguez-Cruz, M.S. Organic carbon nature determines the capacity of organic amendments to adsorb pesticides in soil. J. Hazard. Mater. 2020, 390, 122162. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Lu, T.; Chen, W.; Zhang, H.; Qi, W.; Song, Y.; Qi, Z. Enhanced role of humic acid on the transport of iron oxide colloids in saturated porous media under various solution chemistry conditions. Colloids Surf. A-Physicochem. Eng. Asp. 2020, 607, 125486. [Google Scholar] [CrossRef]
- Hu, W.; Shao, M.A.; Wan, L.; Si, B.C. Spatial variability of soil electrical conductivity in a small watershed on the Loess Plateau of China. Geoderma 2014, 230, 212–220. [Google Scholar] [CrossRef]
- Mohanty, S.K.; Bulicek, M.C.D.; Metge, D.W.; Harvey, R.W.; Ryan, J.N.; Boehm, A.B. Mobilization of Microspheres from a Fractured Soil during Intermittent Infiltration Events. Vadose Zone J. 2015, 14, 1–10. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, F.; Sun, W. Investigating colloid-associated transport of cadmium and lead in a clayey soil under preferential flow conditions. Water Sci. Technol. 2021, 84, 2486–2498. [Google Scholar] [CrossRef]
- Zhou, B.; Zhang, Z.; Wang, S.; Wu, Y.; Hu, S.; Sun, R. Batch Adsorption and Column Leaching Studies of Aniline in Chinese Loess Under Different Hydrochemical Conditions. Bull. Environ. Contam. Toxicol. 2020, 104, 511–519. [Google Scholar] [CrossRef]
- Pochapski, D.J.; dos Santos, C.C.; Leite, G.W.; Pulcinelli, S.H.; Santilli, C.V. Zeta Potential and Colloidal Stability Predictions for Inorganic Nanoparticle Dispersions: Effects of Experimental Conditions and Electrokinetic Models on the Interpretation of Results. Langmuir 2021, 37, 13379–13389. [Google Scholar] [CrossRef] [PubMed]
- Sharaf, O.Z.; Taylor, R.A.; Abu-Nada, E. On the colloidal and chemical stability of solar nanofluids: From nanoscale interactions to recent advances. Phys. Rep.-Rev. Sect. Phys. Lett. 2020, 867, 1–84. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, D.; Wang, L.; Jiao, G.; Gou, H.; Li, Z.; Zhang, G. Role of tailing colloid from vanadium-titanium magnetite in the adsorption and cotransport with vanadium. Environ. Sci. Pollut. Res. 2023, 30, 34069–34084. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Luo, L.; Chang, F.; Chen, Z.; Yuan, J.; Qiu, H. Adsorption property and mechanism of aniline on coal activated carbon. Sci. Technol. Rev. 2021, 39, 55–62. [Google Scholar]
- Baytar, O.; Sahin, O.; Horoz, S.; Kutluay, S. High-performance gas-phase adsorption of benzene and toluene on activated carbon: Response surface optimization, reusability, equilibrium, kinetic, and competitive adsorption studies. Environ. Sci. Pollut. Res. 2020, 27, 26191–26210. [Google Scholar] [CrossRef]
- Tian, H.; Zhang, Q.; Tian, X.; Xie, Z.-F.; Pu, F.; Wang, Q.-J. Analysis of influencing factors of phenanthrene adsorption by different soils in Guanzhong basin based on response surface method. Sci. Rep. 2022, 12, 20906. [Google Scholar] [CrossRef]
- Guler, U.A.; Tuncel, E.; Ersan, M. Evaluation of factors affecting tetracycline and diclofenac adsorption by agricultural soils using response surface methodology. Environ. Prog. Sustain. Energy 2023, 42, 13939. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, X.; Wang, Y.; Lin, H.; Zhang, H. Evaluation of phenanthrene removal from soil washing effluent by activated carbon adsorption using response surface methodology. Chin. J. Chem. Eng. 2022, 42, 399–405. [Google Scholar] [CrossRef]
- Sharma, A.; Kawamoto, K.; Moldrup, P.; de Jonge, L.W.; Komatsu, T. Transport and Deposition of Variably Charged Soil Colloids in Saturated Porous Media. Vadose Zone J. 2011, 10, 1228–1241. [Google Scholar] [CrossRef]
- Liu, G.; Xue, W.; Wang, J.; Liu, X. Transport behavior of variable charge soil particle size fractions and their influence on cadmium transport in saturated porous media. Geoderma 2019, 337, 945–955. [Google Scholar] [CrossRef]
- Xu, Z.; Sun, Y.; Niu, Z.; Xu, Y.; Wei, X.; Chen, X.; Pan, D.; Wu, W. Kinetic determination of sedimentation for GMZ bentonite colloids in aqueous solution: Effect of pH, temperature and electrolyte concentration. Appl. Clay Sci. 2020, 184, 105393. [Google Scholar] [CrossRef]
- Xu, Z.; Niu, Z.W.; Pan, D.Q.; Zhao, X.D.; Wei, X.Y.; Li, X.L.; Tan, Z.Y.; Chen, X.M.; Liu, C.L.; Wu, W.S. Mechanisms of bentonite colloid aggregation, retention, and release in saturated porous media: Role of counter ions and humic acid. Sci. Total Environ. 2021, 793, 148545. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Liu, D.; Zheng, Y.; Liang, S.; Liu, Z. Sorption isotherm and kinetic modeling of aniline on Cr-bentonite. J. Hazard. Mater. 2009, 167, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.Y.; Song, M.; Wei, Y.X.; Wang, Y.L. The contribution of oxygen-containing functional groups to the gas-phase adsorption of volatile organic compounds with different polarities onto lignin-derived activated carbon fibers. Environ. Sci. Pollut. Res. 2019, 26, 7195–7204. [Google Scholar] [CrossRef]
- Wu, W.H.; Lan, Y.; Zeng, Y.X.; Lin, D.H.; Yang, K. Nonlinear sorption of phenols and anilines by organobentonites: Nonlinear partition and space limitation for partitioning. Sci. Total Environ. 2020, 736, 139609. [Google Scholar] [CrossRef]
- Ren, L.F.; Lin, D.H.; Yang, K. Nonlinear partition of nonionic organic compounds into humus-like substance humificated from lignin. Sci. Total Environ. 2021, 764, 142887. [Google Scholar] [CrossRef] [PubMed]
- Chai, R.; Liu, Y.; Yang, L.; Zhang, Y.; Xin, J.; Ma, J. Adsorption Mechanism of Two Organic Molecules with Different Polarities on Calcite(104)Surface: Density Functional Theory Study. Chin. J. Comput. Phys. 2020, 37, 221–230. [Google Scholar]
- Zhou, Q.; Zhang, S.; Peng, Y.; Fang, X.; Zhao, X.; Yu, G.; Xie, Y.; Li, J.; Feng, Y. Ca2+-Triggered Interaction of Amphiphilic Alginate and Soil to Facilitate Agrochemical Adsorption. J. Polym. Environ. 2023, 31, 1628–1641. [Google Scholar] [CrossRef]
- Hao, Q.-L.; Qiao, H.; Zhou, C.-Z.; Peng, W.; Zhang, K.; Lu, S. Effects of Fulvic Acid on TNT Adsorption in Soil. Soil Sediment Contam. 2018, 27, 186–199. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, L.; Xiao, S.; Chen, J. Preparation of a novel manganese oxide-modified diatomite and its aniline removal mechanism from solution. Chem. Eng. J. 2016, 284, 609–619. [Google Scholar] [CrossRef]
- Sharma, P.; Mayes, M.A.; Tang, G. Role of soil organic carbon and colloids in sorption and transport of TNT, RDX and HMX in training range soils. Chemosphere 2013, 92, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, F.P.; Nyman, M.C. Short-term interactions of aniline and benzidine with three soils in both natural and artificial matrices. Chemosphere 2006, 65, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Li, T.; Wei, C. Competitive Adsorption Between Phenol, Aniline and n-Heptane in Tailrace Coking Wastewater. Water Air Soil Pollut. 2013, 224, 1365. [Google Scholar] [CrossRef]
- Zhou, B.; Wu, Y.G.; Chan, J.W.; Wang, S.C.; Qiao, Z.X.; Hu, S.H. Wetting-drying cycles enhance the release and transport of autochthonous colloidal particles in Chinese loess. Hum. Ecol. Risk Assess. 2019, 25, 335–353. [Google Scholar] [CrossRef]
- Albarran, N.; Degueldre, C.; Missana, T.; Alonso, U.; Garcia-Gutierrez, M.; Lopez, T. Size distribution analysis of colloid generated from compacted bentonite in low ionic strength aqueous solutions. Appl. Clay Sci. 2014, 95, 284–293. [Google Scholar] [CrossRef]
- Bessho, K.; Degueldre, C. Generation and sedimentation of colloidal bentonite particles in water. Appl. Clay Sci. 2009, 43, 253–259. [Google Scholar] [CrossRef]
- Liu, X.; Sun, R.; Hu, S.; Zhong, Y.; Wu, Y. Aromatic compounds releases aroused by sediment resuspension alter nitrate transformation rates and pathways during aerobic-anoxic transition. J. Hazard. Mater. 2022, 424, 127365. [Google Scholar] [CrossRef]
- Zhang, H.J.; Lu, T.T.; Zhang, R.Y.; Wang, M.J.; Krishnan, S.; Liu, S.H.; Zhou, Y.M.; Li, D.L.; Qi, Z.C. Effects of clay colloids on ciprofloxacin transport in saturated quartz sand porous media under different solution chemistry conditions. Ecotoxicol. Environ. Saf. 2020, 199, 110754. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, Z.; Hu, S.; Sun, R.; Meng, C.; Wu, Y.; Sun, X. Co-Transport of Aniline and TNT with Loess Colloid Particles in Saturated Loess Columns: Mechanism and Processes. Water 2024, 16, 180. https://doi.org/10.3390/w16010180
Meng Z, Hu S, Sun R, Meng C, Wu Y, Sun X. Co-Transport of Aniline and TNT with Loess Colloid Particles in Saturated Loess Columns: Mechanism and Processes. Water. 2024; 16(1):180. https://doi.org/10.3390/w16010180
Chicago/Turabian StyleMeng, Zhaohui, Sihai Hu, Ran Sun, Chengzhen Meng, Yaoguo Wu, and Xiaofeng Sun. 2024. "Co-Transport of Aniline and TNT with Loess Colloid Particles in Saturated Loess Columns: Mechanism and Processes" Water 16, no. 1: 180. https://doi.org/10.3390/w16010180
APA StyleMeng, Z., Hu, S., Sun, R., Meng, C., Wu, Y., & Sun, X. (2024). Co-Transport of Aniline and TNT with Loess Colloid Particles in Saturated Loess Columns: Mechanism and Processes. Water, 16(1), 180. https://doi.org/10.3390/w16010180