Hydrology as a Determinant of Riparian Habitat Structure in Lowland River Floodplains
Abstract
1. Introduction
2. Materials and Methods
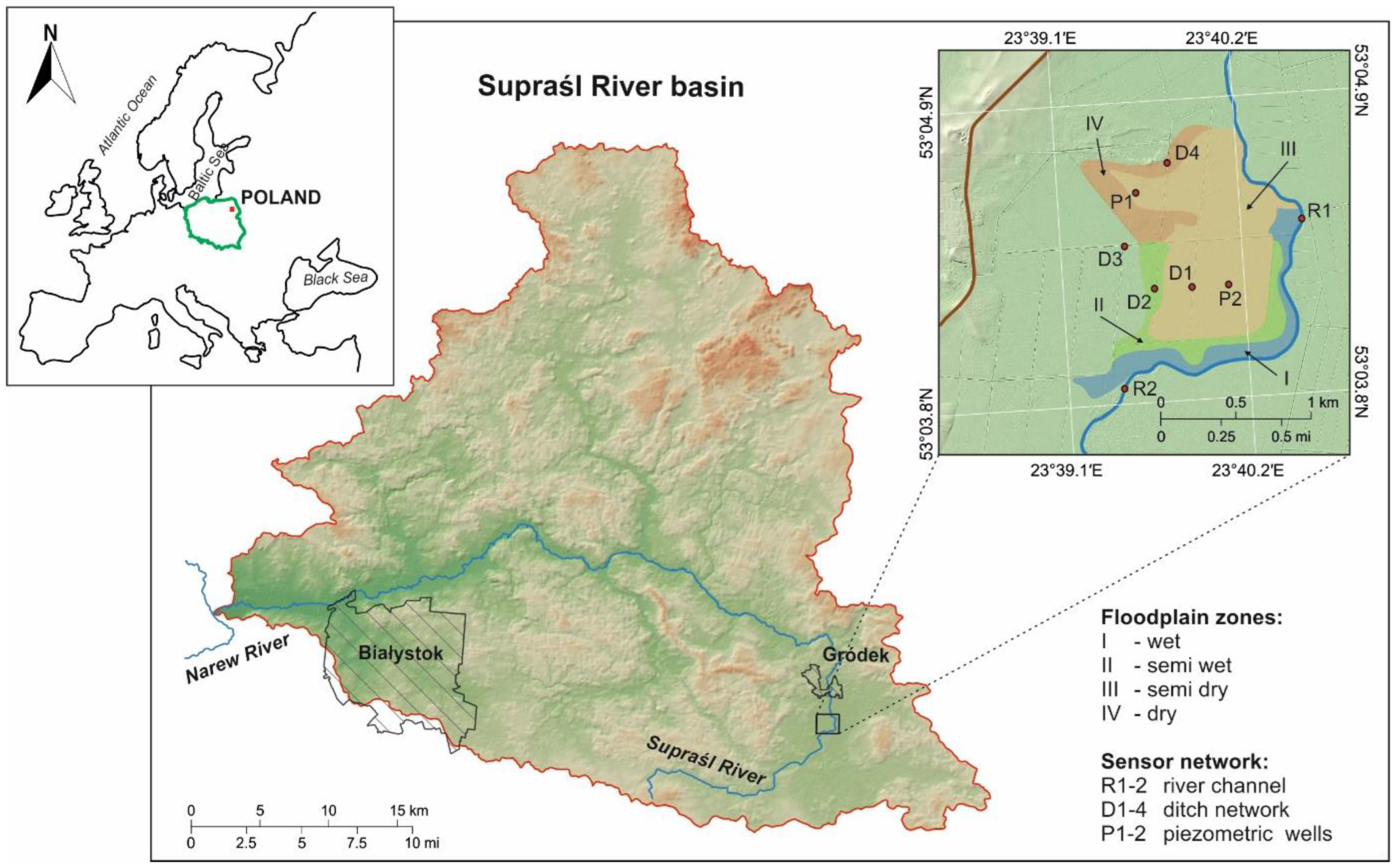
2.1. Study Site
2.2. Hydrological and GIS Data Sources
2.3. Field Observations and Riparian Habitat Classification
2.4. Statistical Analyses
3. Results
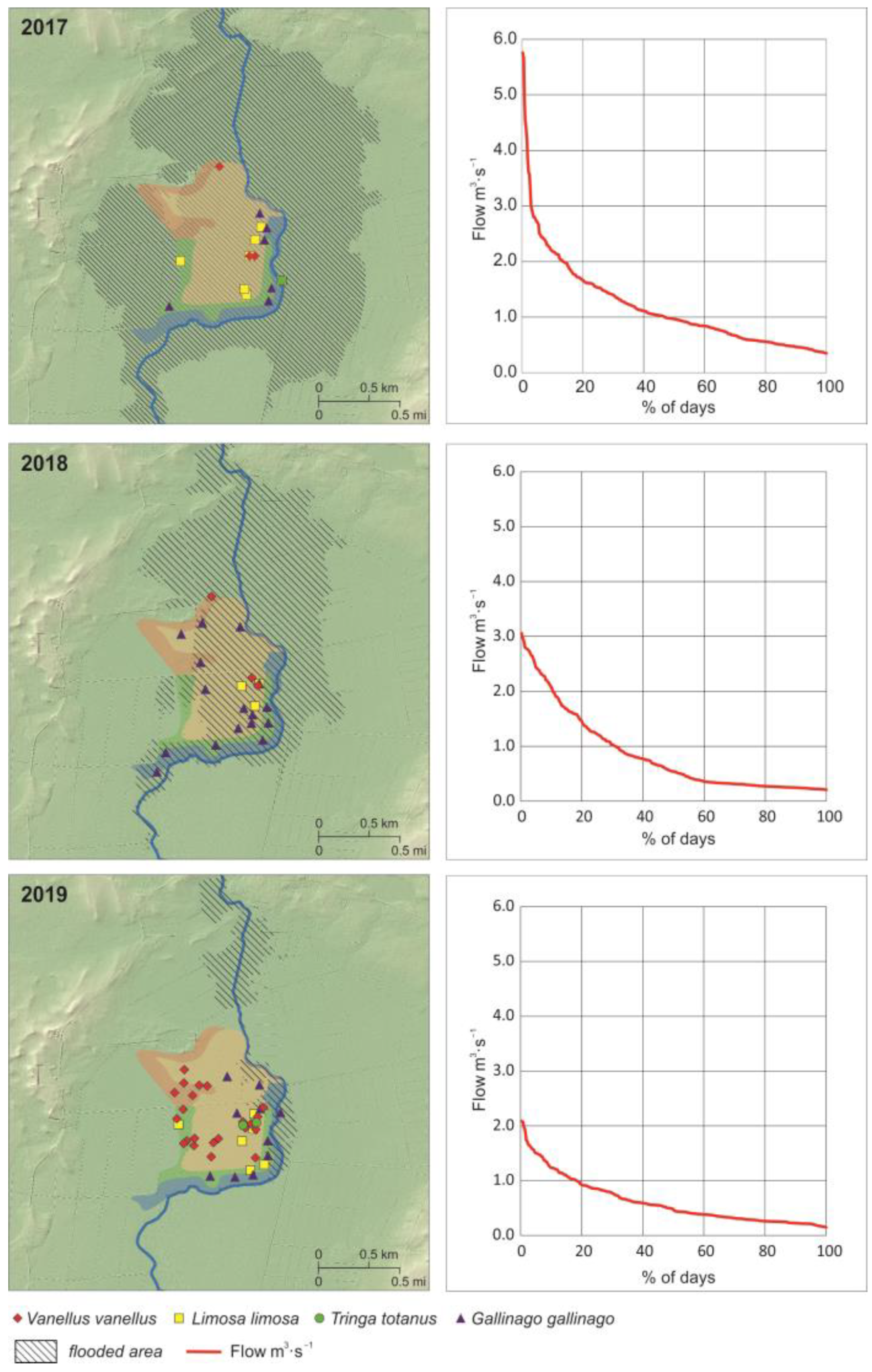
3.1. Hydrological Characteristics
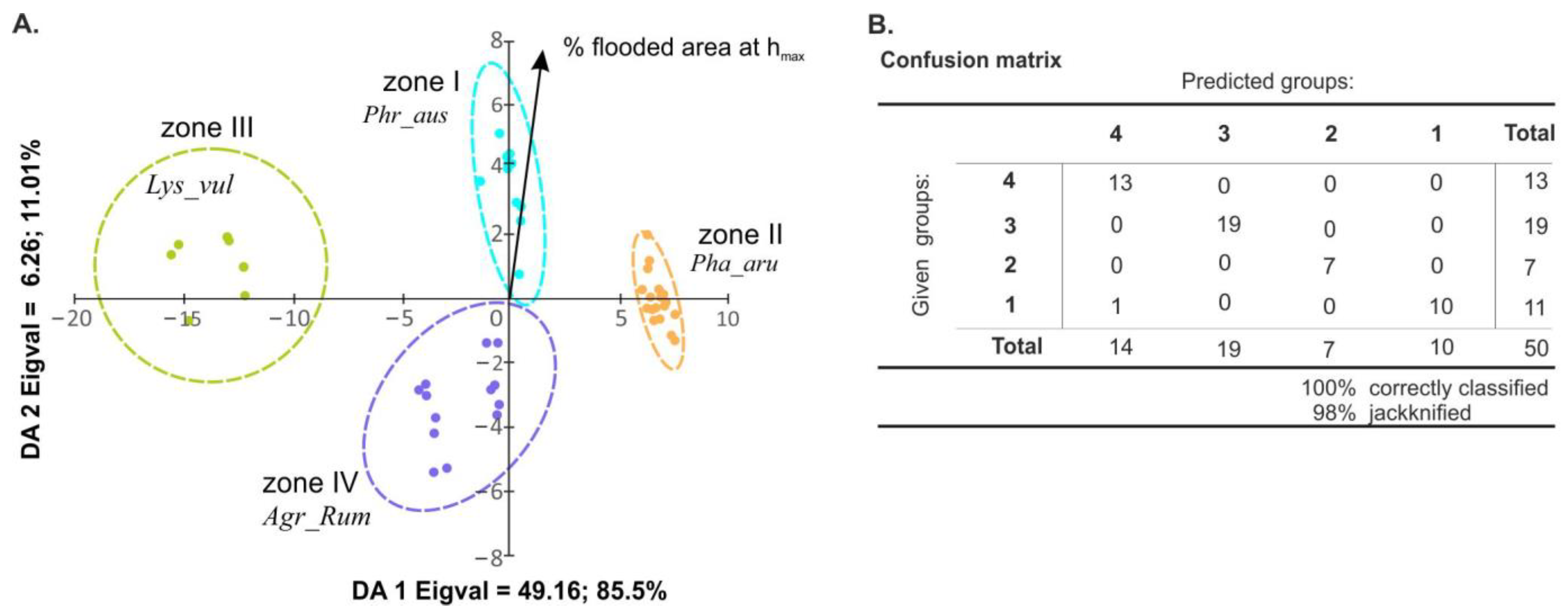
3.2. Riparian Habitats
3.3. Indicator Species
4. Discussion
4.1. Patterns of Vegetation Diversity in Riparian Zones of Rivers
4.2. Changes in Vegetation Cover Due to Hydrological Variations
4.3. Impact of Unstable River Valley Environment on Other Natural Values
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tockner, K.; Pusch, M.; Borchardt, D.; Lorang, M.S. Multiple Stressors in Coupled River-Floodplain Ecosystems. Freshw. Biol. 2010, 55, 135–151. [Google Scholar] [CrossRef]
- Critchley, C.N.R.; Chambers, B.J.; Fowbert, J.A.; Bhogal, A.; Rose, S.C.; Sanderson, R.A. Plant Species Richness, Functional Type and Soil Properties of Grasslands and Allied Vegetation in English Environmentally Sensitive Areas: Species Richness and Type in Relation to Soil Properties of Grassland. Grass Forage Sci. 2002, 57, 82–92. [Google Scholar] [CrossRef]
- Rodríguez-Rojo, M.P.; Font, X.; García-Mijangos, I.; Crespo, G.; Fernández-González, F. An Expert System as an Applied Tool for the Conservation of Semi-Natural Grasslands on the Iberian Peninsula. Biodivers. Conserv. 2020, 29, 1977–1992. [Google Scholar] [CrossRef]
- Petermann, J.S.; Buzhdygan, O.Y. Grassland Biodiversity. Cur. Biol. 2021, 31, R1195–R1201. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, J.; Bullock, J.M.; Egoh, B.; Everson, C.; Everson, T.; O’Connor, T.; O’Farrell, P.J.; Smith, H.G.; Lindborg, R. Grasslands—More important for ecosystem services than you might think. Ecosphere 2019, 10, 02582. [Google Scholar] [CrossRef]
- Grzybowski, M.; Glińska-Lewczuk, K. Principal Threats to the Conservation of Freshwater Habitats in the Continental Biogeographical Region of Central Europe. Biodivers. Conserv. 2019, 28, 4065–4097. [Google Scholar] [CrossRef]
- Grzybowski, M.; Glińska-Lewczuk, K. The Principal Threats to the Peatlands Habitats, in the Continental Bioregion of Central Europe—A Case Study of Peatland Conservation in Poland. J. Nat. Conserv. 2020, 53, 125778. [Google Scholar] [CrossRef]
- Wehn, S.; Burton, R.; Riley, M.; Johansen, L.; Hovstad, K.A.; Rønningen, K. Adaptive Biodiversity Management of Semi-Natural Hay Meadows: The Case of West-Norway. Land Use Policy 2018, 72, 259–269. [Google Scholar] [CrossRef]
- Johansen, L.; Westin, A.; Wehn, S.; Iuga, A.; Ivascu, C.M.; Kallioniemi, E.; Lennartsson, T. Traditional Semi-Natural Grassland Management with Heterogeneous Mowing Times Enhances Flower Resources for Pollinators in Agricultural Landscapes. Glob. Ecol. Conserv. 2019, 18, e00619. [Google Scholar] [CrossRef]
- Bates, A.J.; Sadler, J.P.; Henshall, S.; Hannah, D.M. Ecology and Conservation of Arthropods of Exposed Riverine Sedi-Ments (ERS). Terr. Arthropod Rev. 2009, 2, 77–98. [Google Scholar]
- Kimberley, A.; Hooftman, D.; Bullock, J.M.; Honnay, O.; Krickl, P.; Lindgren, J.; Plue, J.; Poschlod, P.; Traveset, A.; Cousins, S.A.O. Functional Rather than Structural Connectivity Explains Grassland Plant Diversity Patterns Following Landscape Scale Habitat Loss. Landsc. Ecol. 2021, 36, 265–280. [Google Scholar] [CrossRef]
- Gurnell, A.; Surian, N.; Zanoni, L. Multi-Thread River Channels: A Perspective on Changing European Alpine River Systems. Aquat. Sci. 2009, 71, 253–265. [Google Scholar] [CrossRef]
- Zeiringer, B.; Seliger, C.; Greimel, F.; Schmutz, S. River Hydrology, Flow Alteration, and Environmental Flow. Riverine Ecosyst. Manag. 2018, 8, 67–89. [Google Scholar] [CrossRef]
- Ward, J.V.; Tockner, K.; Arscott, D.B.; Claret, C. Riverine Landscape Diversity: Riverine Landscape Diversity. Freshw. Biol. 2002, 47, 517–539. [Google Scholar] [CrossRef]
- Lenssen, J.P.M.; De Kroon, H. Abiotic Constraints at the Upper Boundaries of Two Rumex Species on a Freshwater Flooding Gradient. J. Ecol. 2005, 93, 138–147. [Google Scholar] [CrossRef]
- van Eck, W.H.J.M.; Lenssen, J.P.M.; van de Steeg, H.M.; Blom, C.W.P.M.; de Kroon, H. Seasonal Dependent Effects of Flooding on Plant Species Survival and Zonation: A Comparative Study of 10 Terrestrial Grassland Species. Hydrobiologia 2006, 565, 59–69. [Google Scholar] [CrossRef]
- Arias, M.E.; Wittmann, F.; Parolin, P.; Murray-Hudson, M.; Cochrane, T.A. Interactions between Flooding and Upland Disturbance Drives Species Diversity in Large River Floodplains. Hydrobiologia 2018, 814, 5–17. [Google Scholar] [CrossRef]
- Stanford, J.A.; Lorang, M.S.; Hauer, F.R. The Shifting Habitat Mosaic of River Ecosystems. SIL Proc. 1922–2010 2005, 29, 123–136. [Google Scholar] [CrossRef]
- Mouw, J.E.B.; Stanford, J.A.; Alaback, P.B. Influences of Flooding and Hyporheic Exchange on Floodplain Plant Richness and Productivity. River Res. Applic. 2009, 25, 929–945. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, W.; Woods, R.A.; Shao, Q. Hydrological Effects of Change in Vegetation Components across Global Catchments. J. Hydrol. 2021, 595, 125775. [Google Scholar] [CrossRef]
- Härdtle, W.; Redecker, B.; Assmann, T.; Meyer, H. Vegetation Responses to Environmental Conditions in Floodplain Grasslands: Prerequisites for Preserving Plant Species Diversity. Basic Appl. Ecol. 2006, 7, 280–288. [Google Scholar] [CrossRef]
- Hodapp, D.; Roca, I.T.; Fiorentino, D.; Garilao, C.; Kaschner, K.; Kesner-Reyes, K.; Schneider, B.; Segschneider, J.; Kocsis, Á.T.; Kiessling, W.; et al. Climate Change Disrupts Core Habitats of Marine Species. Glob. Chang. Biol. 2023, 29, 3304–3317. [Google Scholar] [CrossRef] [PubMed]
- Grant, K.; Kreyling, J.; Heilmeier, H.; Beierkuhnlein, C.; Jentsch, A. Extreme Weather Events and Plant–Plant Interactions: Shifts between Competition and Facilitation among Grassland Species in the Face of Drought and Heavy Rainfall. Ecol. Res. 2014, 29, 991–1001. [Google Scholar] [CrossRef]
- Richards, D.R.; Moggridge, H.L.; Warren, P.H.; Maltby, L. Impacts of Hydrological Restoration on Lowland River Floodplain Plant Communities. Wetlands Ecol. Manage. 2020, 28, 403–417. [Google Scholar] [CrossRef]
- Lenssen, J.P.M.; Van De Steeg, H.M.; De Kroon, H. Does Disturbance Favour Weak Competitors? Mechanisms of Chang-Ing Plant Abundance after Flooding. J. Veg. Sci. 2004, 15, 305–314. [Google Scholar] [CrossRef]
- Gattringer, J.P.; Ludewig, K.; Harvolk-Schöning, S.; Donath, T.W.; Otte, A. Interaction between Depth and Duration Matters: Flooding Tolerance of 12 Floodplain Meadow Species. Plant Ecol. 2018, 219, 973–984. [Google Scholar] [CrossRef]
- Lan, Z.; Huang, H.; Chen, Y.; Liu, J.; Chen, J.; Li, L.; Li, L.; Jin, B.; Chen, J. Testing Mechanisms Underlying Responses of Plant Functional Traits to Flooding Duration Gradient in a Lakeshore Meadow. J. Freshwat. Ecol. 2019, 34, 481–495. [Google Scholar] [CrossRef]
- van Eck, W.H.J.M.; van de Steeg, H.M.; Blom, C.W.P.M.; de Kroon, H. Is Tolerance to Summer Flooding Correlated with Distribution Patterns in River Floodplains? A Comparative Study of 20 Terrestrial Grassland Species. Oikos 2004, 107, 393–405. [Google Scholar] [CrossRef]
- Obolewski, K.; Skorbiłowicz, E.; Skorbiłowicz, M.; Glińska-Lewczuk, K.; Astel, A.; Strzelczak, A. The Effect of Metals Accumulated in Reed (Phragmites Australis) on the Structure of Periphyton. Ecotoxicol. Environ. Saf. 2011, 74, 558–568. [Google Scholar] [CrossRef]
- Garssen, A.G.; Baattrup-Pedersen, A.; Voesenek, L.A.C.J.; Verhoeven, J.T.A.; Soons, M.B. Riparian Plant Community Responses to Increased Flooding: A Meta-analysis. Glob. Chang. Biol. 2015, 21, 2881–2890. [Google Scholar] [CrossRef]
- Casanova, M.T.; Brock, M.A. How Do Depth, Duration and Frequency of Flooding Influence the Establishment of Wetland Plant Communities? Plant Ecol. 2000, 147, 237–250. [Google Scholar] [CrossRef]
- Gerard, M.; Kahloun, M.E.; Mertens, W.; Verhagen, B.; Meire, P. Impact of Flooding on Potential and Realised Grassland Species Rich-Ness. Plant Ecol. 2008, 194, 85–98. [Google Scholar] [CrossRef]
- Soomers, H.; Karssenberg, D.; Soons, M.B.; Verweij, P.A.; Verhoeven, J.T.A.; Wassen, M.J. Wind and Water Dispersal of Wetland Plants Across Fragmented Landscapes. Ecosystems 2013, 16, 434–451. [Google Scholar] [CrossRef]
- Moggridge, H.L.; Gurnell, A. Hydrological Controls on the Transport and Deposition of Plant Propagules within Riparian Zones. Riv. Res. Appl. 2010, 26, 512–527. [Google Scholar] [CrossRef]
- Lake, P.S. Drought and Aquatic Ecosystems: Effects and Responses: Lake/Drought and Aquatic Ecosystems: Effects and Responses; John Wiley & Sons, Ltd.: Chichester, UK, 2011. [Google Scholar]
- Junk, W.J.; Bayley, P.B.; Sparks, R.E. The Flood Pulse Concept in River-Floodplain Systems. Can. J. Fish Aquat. Sci. 1989, 106, 110–127. [Google Scholar]
- Davidson, T.A.; Mackay, A.W.; Wolski, P.; Mazebedi, R.; Murray-Hudson, M.; Todd, M. Seasonal and Spatial Hydrological Variability Drives Aquatic Biodiversity in a Flood-Pulsed, Sub-Tropical Wetland: Aquatic Biodiversity in Tropical Flood-Pulsed Wetlands. Freshw. Biol. 2012, 57, 1253–1265. [Google Scholar] [CrossRef]
- Seneviratne, S.I.; Nicholls, N.; Easterling, D.; Goodess, C.M.; Kanae, S.; Kossin, J.; Luo, Y.; Marengo, J.; McInnes, K.; Rahi-Mi, M.; et al. Managing the Risks of Extreme Events and Disasters to Advance Climate Change Adaptation: Special Report of the Intergovernmental Panel on Climate Change; Field, C.B., Barros, V., Stocker, T.F., Dahe, Q., Eds.; Cambridge University Press: Cambridge, NY, USA, 2012; pp. 109–230. [Google Scholar]
- Blom, C.W.P.M.; Bögemann, G.M.; Laan, P.; van der Sman, A.J.M.; van de Steeg, H.M.; Voesenek, L.A.C.J. Adaptations to Flooding in Plants from River Areas. Aquat. Bot. 1990, 38, 29–47. [Google Scholar] [CrossRef]
- Żmihorski, M.; Krupiński, D.; Kotowska, D.; Knape, J.; Pärt, T.; Obłoza, P.; Berg, Å. Habitat Characteristics Associated with Occupancy of Declining Waders in Polish Wet Grasslands. Agric. Ecosyst. Environ. 2018, 251, 236–243. [Google Scholar] [CrossRef]
- Wassen, M.J.; Joosten, J.H.J. In Search of a Hydrological Explanation for Vegetation Changes along a Fen Gradient in the Biebrza Upper Basin (Poland). Vegetatio 1996, 124, 191–209. [Google Scholar] [CrossRef]
- Górniak, A. Ecohydrological Determinants of Seasonality and Export of Total Organic Carbon in Narew River with High Peatland Contribution (North-Eastern Poland). Ecohydrol. Hydrobiol. 2019, 19, 1–13. [Google Scholar] [CrossRef]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World Map of the Köppen-Geiger Climate Classification Updated. Metz 2006, 15, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Giełczewski, M. The Narew River Basin: A Model for the Sustainable Management of Agriculture, Nature and Water Supply; Netherlands Geographical Studies; Utrecht University Repository: Utrecht, The Netherlands, 2003. [Google Scholar]
- Kiryluk, A. Zmiany Siedlisk Pobagiennych i Fitocenoz w Dolinie Supraśli; IMUZ Falenty: Falenty, Poland, 2007; pp. 12–14. [Google Scholar]
- Zolotova, E.; Ivanova, N.; Ivanova, S. Global Overview of Modern Research Based on Ellenberg Indicator Values. Diversity 2022, 15, 14. [Google Scholar] [CrossRef]
- Zarzycki, K.; Trzcińska-Tacik, H.; Różański, W.; Szeląg, Z.; Wołek, J.; Korzeniak, U. Ecological Indicator Values of Vascular Plants of Poland. Ekologiczne Liczby Wskaźnikowe Roślin Naczyniowych Polski; Szafer Institute of Botany, Polish Academy of Sciences: Kraków, Poland, 2002. [Google Scholar]
- Simpson, E.H. Measurement of Diversity. Nature 1949, 163, 668. [Google Scholar] [CrossRef]
- Nkoa, R.; Owen, M.D.K.; Swanton, C.J. Weed Abundance, Distribution, Diversity, and Community Analyses. Weed Sci. 2015, 63, 64–90. [Google Scholar] [CrossRef]
- Głowacka, A.; Flis-Olszewska, E. The Biodiversity of Weed Communities of Dent Maize, Narrow-Leaved Lupin and Oat in Relation to Cropping System and Weed Control. Agron. Sci. 2022, 77, 123–137. [Google Scholar] [CrossRef]
- Szwed, W.; Hennekens, S.M.; Pelsma, T.A.H.M.; Ratyńska, H.; Rusińska, A. A Numerical Data Base and Checklist of Taxa of Polish Flora Applicable in Phytosociology, Particularly for the TURBOVEG. Zesz. Nauk. Wyższej Szkoły Pedagog. W Bydg. 1999, 14, 5–18. [Google Scholar]
- Ratyńskia, H.; Wojterska, M.; Brzeg, A.; Kołacz, M. Multimedialna Encyklopedia Zbiorowisk Roślinnych Polski; Computer File, edition ver. 1.1, OCLC Number 82377940; Instytut Edukacyjnych Technologii Informatycznych: Bydgoszcz, Polish, 2010. [Google Scholar]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeont. Electr. 2001, 4, 9. [Google Scholar]
- Dufrêne, M.; Legendre, P. Species Assemblages and Indicator Species:The Need for a Flexible Asymmetrical Approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- Severns, P.M.; Sykes, E.M. Indicator Species Analysis: A Useful Tool for Plant Disease Studies. Phytopathology 2020, 110, 1860–1862. [Google Scholar] [CrossRef]
- Mccune, B.; Mefford, M.J. PC-ORD. Multivariate Analysis of Ecological Data. Version 5.32; MjM Software: Gleneden Beach, OR, USA, 2006. [Google Scholar]
- Mccabe, D.J. Rivers and Streams: Life in Flowing Water. Nat. Educ. Knowl. 2011, 1, 4. [Google Scholar]
- Schindler, S.; O’Neill, F.H.; Biró, M.; Damm, C.; Gasso, V.; Kanka, R.; van der Sluis, T.; Krug, A.; Lauwaars, S.G.; Sebesvari, Z.; et al. Multifunctional Floodplain Management and Biodiversity Effects: A Knowledge Synthesis for Six European Countries. Biodivers. Conserv. 2016, 25, 1349–1382. [Google Scholar] [CrossRef]
- Kleinhans, M.G.; de Vries, B.; Braat, L.; van Oorschot, M. Living Landscapes: Muddy and Vegetated Floodplain Effects on Fluvial Pattern in an Incised River: Effects of Mud and Vegetation On River Pattern. Earth Surf. Process. Landforms 2018, 43, 2948–2963. [Google Scholar] [CrossRef] [PubMed]
- Tabacchi, E.; Tabacchi, A.-M.P. Functional Significance of Species Composition in Riparian Plant Communities. J. Am Water Resources Assoc. 2001, 37, 1629–1637. [Google Scholar] [CrossRef]
- Brown, R.L.; Peet, R.K. Diversity and Invasibility of Southern Appalachian Plant Communities. Ecology 2003, 84, 32–39. [Google Scholar] [CrossRef]
- Mligo, C. Diversity and Distribution Pattern of Riparian Plant Species in the Wami River System, Tanzania. J. Plant Ecol. 2016, 10, 259–270. [Google Scholar] [CrossRef][Green Version]
- Huston, M. A General Hypothesis of Species Diversity. Am. Nat. 1979, 113, 81–101. [Google Scholar] [CrossRef]
- Bendix, J.; Hupp, C.R. Hydrological and Geomorphological Impacts on Riparian Plant Communities. Hydrol. Process. 2000, 14, 2977–2990. [Google Scholar] [CrossRef]
- Zerbe, S. Rivers and Floodplains. In Restoration of Ecosystems—Bridging Nature and Humans; Springer: Berlin/Heidelberg, Germany, 2023; pp. 209–233. [Google Scholar] [CrossRef]
- Gattringer, J.P.; Donath, T.W.; Eckstein, R.L.; Ludewig, K.; Otte, A.; Harvolk-Schöning, S. Flooding Tolerance of Four Floodplain Meadow Species Depends on Age. PLoS ONE 2017, 12, e0176869. [Google Scholar] [CrossRef]
- Benjankar, R.; Egger, G.; Jorde, K.; Goodwin, P.; Glenn, N.F. Dynamic Floodplain Vegetation Model Development for the Kootenai River, USA. J. Environ. Manage. 2011, 92, 3058–3070. [Google Scholar] [CrossRef]
- Rivaes, R.; Boavida, I.; Santos, J.M.; Pinheiro, A.N.; Ferreira, T. Importance of Considering Riparian Vegetation Requirements for the Long-Term Efficiency of Environmental Flows in Aquatic Microhabitats. Hydrol. Earth Syst. Sci. 2017, 21, 5763–5780. [Google Scholar] [CrossRef]
- Amoros, C.; Bornette, G. Connectivity and Biocomplexity in Waterbodies of Riverine Floodplains: Connectivity and Biocomplexity in Riverine Floodplains. Freshwat. Biol. 2002, 47, 761–776. [Google Scholar] [CrossRef]
- Mackey, R.L.; Currie, D.J. A Re-Examination of the Expected Effects of Disturbance on Diversity. Oikos 2000, 88, 483–493. [Google Scholar] [CrossRef]
- Szoszkiewicz, K.; Jusik, S.; Pietruczuk, K.; Gebler, D. The Macrophyte Index for Rivers (MIR) as an Advantageous Approach to Running Water Assessment in Local Geographical Conditions. Water 2019, 12, 108. [Google Scholar] [CrossRef]
- Kaijser, W.; Birk, S.; Hering, D. Environmental Ranges Discriminating between Macrophytes Groups in European Rivers. PLoS ONE 2022, 17, e0269744. [Google Scholar] [CrossRef] [PubMed]
- Połeć, K.; Grzywna, A.; Tarkowska-Kukuryk, M.; Bronowicka-Mielniczuk, U. Changes in the Ecological Status of Rivers Caused by the Functioning of Natural Barriers. Water 2022, 14, 1522. [Google Scholar] [CrossRef]
- Walters, A.W.; Post, D.M. How Low Can You Go? Impacts of a Low-Flow Disturbance on Aquatic Insect Communities. Ecol. Appl. 2011, 21, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Stromberg, J.C.; Beauchamp, V.B.; Dixon, M.D.; Lite, S.J.; Paradzick, C. Importance of Low-Flow and High-Flow Characteristics to Restoration of Riparian Vegetation along Rivers in Arid South-Western United States. Freshw. Biol. 2007, 52, 651–679. [Google Scholar] [CrossRef]
- Jensch, D.; Poschlod, P. Germination Ecology of Two Closely Related Taxa in the Genus Oenanthe: Fine Tuning for the Habitat? Aquat. Bot. 2008, 89, 345–351. [Google Scholar] [CrossRef]
- Wang, D.; Liu, Y.; Zheng, L.; Li, D. Growing Impacts of Low-Flow Events on Vegetation Dynamics in Hydrologically Connected Wetlands Downstream Yangtze River Basin after the Operation of the Three Gorges Dam. J. Geogr. Sci. 2023, 33, 885–904. [Google Scholar] [CrossRef]
- Finger-Higgens, R.; Bishop, T.B.B.; Belnap, J.; Geiger, E.L.; Grote, E.E.; Hoover, D.L.; Reed, S.C.; Duniway, M.C. Droughting a Megadrought: Ecological Consequences of a Decade of Experimental Drought atop Aridification on the Colorado Plateau. Glob. Chang. Biol. 2023, 29, 3364–3377. [Google Scholar] [CrossRef]
- Ilg, C.; Dziock, F.; Foeckler, F.; Follner, K.; Gerisch, M.; Glaeser, J.; Rink, A.; Schanowski, A.; Scholz, M.; Deichner, O.; et al. Long-Term Reactions of Plants and Macroinvertebrates to Extreme Floods in Floodplain Grassland. Ecology 2008, 89, 2392–2398. [Google Scholar] [CrossRef] [PubMed]
- Larson, D.M.; Carhart, A.M.; Lund, E.M. Aquatic Vegetation Types Identified during Early and Late Phases of Vegetation Recovery in the Upper Mississippi River. Ecosphere 2023, 14, e4468. [Google Scholar] [CrossRef]
- Tockner, K.; Schiemer, F.; Baumgartner, C.; Kum, G.; Weigand, E.; Zweimüller, I.; Ward, J.V. The Danube Restoration Project: Species Diversity Patterns across Connectivity Gradients in the Floodplain System. Regul. Rivers Res. Mgmt. 1999, 15, 245–258. [Google Scholar] [CrossRef]
- Chodkiewicz, A.; Stypiński, P.; Studnicki, M.; Borawska-Jarmułowicz, B. The Influence of Konik Horses Grazing and Meteorological Conditions on Wetland Communities. Agriculture 2023, 13, 325. [Google Scholar] [CrossRef]
- Joyeux, E.; Haie, S.; Le Rest, K.; Quaintenne, G.; Francesiaz, C. Meadow-Breeding Waders in France: Population Sizes, Distribution and Conservation Challenges. Wader Study 2023, 129, 166–176. [Google Scholar] [CrossRef]
- Christen, W. Population Trend and Migration of the Northern Lapwing Vanellus Vanellus in the Aare Plain near Solo-Thurn. Ornithol. Beob. 2007, 104, 173–188. [Google Scholar]
- Meltofte, H.; Amstrup, O.; Petersen, T.L.; Rigét, F.; Tøttrup, A.P. Trends in Breeding Phenology across Ten Decades Show Varying Adjustments to Environmental Changes in Four Wader Species. Bird Study 2018, 65, 44–51. [Google Scholar] [CrossRef]
- Jellesmark, S.; Ausden, M.; Blackburn, T.M.; Hoffmann, M.; McRae, L.; Visconti, P.; Gregory, R.D. The Effect of Conservation Interventions on the Abundance of Breeding Waders within Nature Reserves in the United Kingdom. Ibis 2023, 165, 69–81. [Google Scholar] [CrossRef]
- Osmundson, D.B.; Ryel, R.J.; Lamarra, V.L.; Pitlick, J. Flow–Sediment–Biota Relations: Implications for River Regulation Effects on Native Fish Abundance. Ecol. Appl. 2002, 12, 1719–1739. [Google Scholar] [CrossRef]
- Obolewski, K.; Glińska-Lewczuk, K.; Bąkowska, M. From isolation to connectivity: The effect of floodplain lake restoration on sediments as habitats for macroinvertebrate communities. Aquat. Sci. 2018, 80, 4. [Google Scholar] [CrossRef]



| Moisture Riparian Zone | Species * | Observed Indicator Value (IV) | IV from Randomized Zones | p-Value ** | |
|---|---|---|---|---|---|
| Mean | ±SD | ||||
| I Wet | Phalaris arundinacea | 80.5 | 32 | 8.98 | 0.0002 |
| Phragmites australis | 55.3 | 16.7 | 7.3 | 0.0016 | |
| Symphytum officinale | 45.5 | 12.2 | 6.63 | 0.0008 | |
| Urtica dioica | 34.9 | 13.3 | 6.89 | 0.0132 | |
| Alisma plantago-aquatica | 27.3 | 10 | 5.48 | 0.0304 | |
| Galium palustre | 27.3 | 9.6 | 5.6 | 0.0112 | |
| Carex rostrata | 18.2 | 8.9 | 4.8 | 0.0720 | |
| II Semi-wet | Filipendula ulmaria | 88.6 | 21.7 | 7.33 | 0.0002 |
| Galium palustre | 71.4 | 11.9 | 6.49 | 0.0004 | |
| Cirsium rivulare | 70.4 | 16.4 | 6.88 | 0.0002 | |
| Potentilla erecta | 53.6 | 15.3 | 7.46 | 0.0010 | |
| Veronica scutellata | 52.7 | 13.7 | 6.83 | 0.0006 | |
| Agrostis capillaris | 42.9 | 9.6 | 5.55 | 0.0018 | |
| Galium molugo | 34.7 | 19.8 | 7.4 | 0.0444 | |
| Achillea monticola | 28.6 | 8.5 | 4.6 | 0.0204 | |
| Galium aparine | 28.1 | 9.9 | 5.5 | 0.0106 | |
| III Semi-dry | Deschampsia caespitosa | 70.3 | 39.6 | 11.53 | 0.0134 |
| Festuca arundinacea | 61.5 | 30.3 | 9.82 | 0.0092 | |
| Lythrum salicaria | 51.8 | 29.8 | 10.13 | 0.0296 | |
| Epilobium palustre | 44.5 | 21.1 | 7.25 | 0.0132 | |
| IV Dry | Anthoxanthum odoratum | 76.1 | 22.7 | 9.03 | 0.0002 |
| Achillea millefolium | 72.3 | 26.7 | 9.71 | 0.0010 | |
| Lychnis flos-cuculi | 56.5 | 23.9 | 6.76 | 0.0012 | |
| Juncus compressus | 52.1 | 23.2 | 7.4 | 0.0052 | |
| Festuca pratensis | 50.9 | 29.4 | 7.06 | 0.0114 | |
| Potentilla anserina | 49.2 | 23.9 | 7.28 | 0.0066 | |
| Vicia cracca | 41.6 | 16.1 | 7.55 | 0.0096 | |
| Taraxacum officinale | 30.8 | 10.1 | 5.68 | 0.0132 | |
| Leontodon autumnalis | 68 | 27.9 | 8.04 | 0.0002 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burandt, P.; Grzybowski, M.; Glińska-Lewczuk, K.; Gotkiewicz, W.; Szymańska-Walkiewicz, M.; Obolewski, K. Hydrology as a Determinant of Riparian Habitat Structure in Lowland River Floodplains. Water 2024, 16, 164. https://doi.org/10.3390/w16010164
Burandt P, Grzybowski M, Glińska-Lewczuk K, Gotkiewicz W, Szymańska-Walkiewicz M, Obolewski K. Hydrology as a Determinant of Riparian Habitat Structure in Lowland River Floodplains. Water. 2024; 16(1):164. https://doi.org/10.3390/w16010164
Chicago/Turabian StyleBurandt, Paweł, Mirosław Grzybowski, Katarzyna Glińska-Lewczuk, Wojciech Gotkiewicz, Monika Szymańska-Walkiewicz, and Krystian Obolewski. 2024. "Hydrology as a Determinant of Riparian Habitat Structure in Lowland River Floodplains" Water 16, no. 1: 164. https://doi.org/10.3390/w16010164
APA StyleBurandt, P., Grzybowski, M., Glińska-Lewczuk, K., Gotkiewicz, W., Szymańska-Walkiewicz, M., & Obolewski, K. (2024). Hydrology as a Determinant of Riparian Habitat Structure in Lowland River Floodplains. Water, 16(1), 164. https://doi.org/10.3390/w16010164










