Effect of Environmental Factors on Nitrite Nitrogen Absorption in Microalgae–Bacteria Consortia of Oocystis borgei and Rhodopseudomonas palustris
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microalgae and Bacteria
2.2. Single-Factor Test Method
2.3. Orthogonal Experimental Design
2.4. Sample 15N Measurement and Data Analysis
3. Results
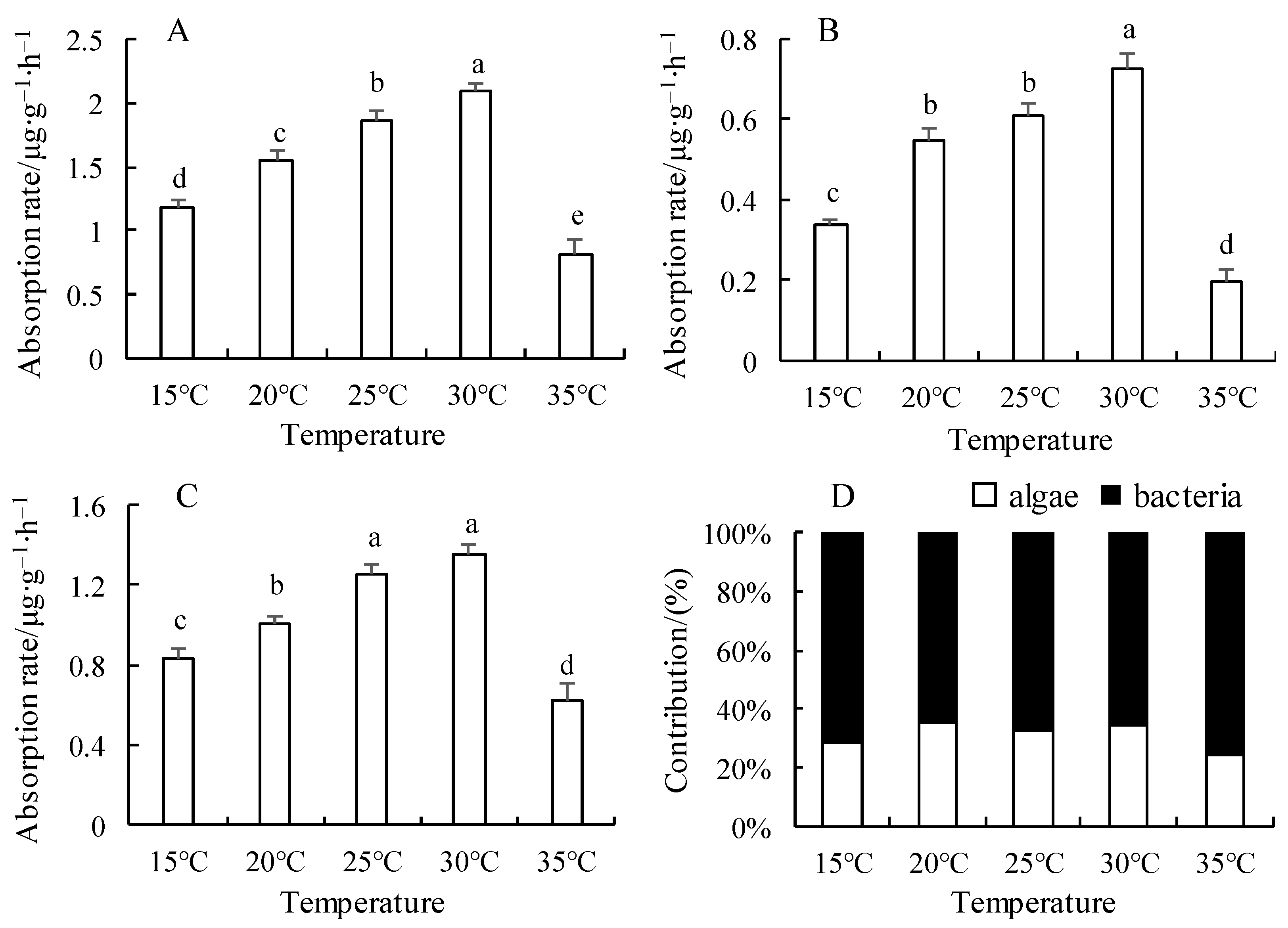
3.1. Effect of Temperature on Nitrite Nitrogen Uptake Rate and Its Contribution Rate in the Microalgae–Bacteria Consortia
3.2. Effect of Salinity on Nitrite Nitrogen Uptake Rate and Its Contribution Rate in the Microalgae–Bacteria Consortia
3.3. Effect of Illumination on Nitrite Nitrogen Uptake Rate and Its Contribution Rate in the Microalgae–Bacteria Consortia
3.4. Optimal Culture Conditions for Nitrite Nitrogen Uptake by the Microalgae–Bacteria Consortia
4. Discussion
4.1. Temperature on Nitrite Nitrogen Uptake by the Microalgae–Bacteria Consortia
4.2. Salinity on Nitrite Nitrogen Uptake by the Microalgae–Bacteria Consortia
4.3. Illumination on Nitrite Nitrogen Uptake by the Microalgae–Bacteria Consortia
4.4. Advantages of the Algae–Bacteria Consortia over Separate Algae and Bacteria
4.5. Suggestions for the Application of the Microalgae–Bacteria Consortia in Shrimp Ponds to Control Nitrite Nitrogen
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xiao, J.; Liu, Q.Y.; Du, J.H.; Zhu, W.L.; Li, Q.Y.; Chen, X.L.; Chen, X.H.; Liu, H.; Zhou, X.Y.; Zhao, Y.Z.; et al. Integrated analysis of physiological, transcriptomic and metabolomic responses and tolerance mechanism of nitrite exposure in Litopenaeus vannamei. Sci. Total Environ. 2020, 711, 134416. [Google Scholar] [CrossRef] [PubMed]
- Valencia-Castañeda, G.; Frías-Espericueta, M.G.; Vanegas-Pérez, R.C.; Chávez-Sánchez, M.C.; Páez-Osuna, F. Toxicity of ammonia, nitrite and nitrate to Litopenaeus vannamei juveniles in low-salinity water in single and ternary exposure experiments and their environmental implications. Environ. Toxicol. Pharmacol. 2019, 70, 103193. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.S.; Ma, S.; Shan, H.W.; Wang, T.; Xiao, W. Responses of hemocyanin and energy metabolism to acute nitrite stress in juveniles of the shrimp Litopenaeus vannamei. Ecotoxicol. Environ. Saf. 2019, 186, 109753. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Liu, Q.; Wang, Y.; Zhang, J.; Xiong, D. Impairment of the intestine barrier function in Litopenaeus vannamei exposed to ammonia and nitrite stress. Fish Shellfish Immunol. 2018, 78, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Ciji, A.; Akhtar, M.S. Nitrite implications and its management strategies in aquaculture: A review. Rev. Aquac. 2020, 12, 878–908. [Google Scholar] [CrossRef]
- Liu, M.; Huang, X.; Zhang, R.; Li, C.; Gu, B. Uptake of urea nitrogen by Oocystis borgei in prawn (Litopenaeus vannamei) aquaculture ponds. Bull. Environ. Contam. Toxicol. 2018, 101, 586–591. [Google Scholar] [CrossRef]
- Pandey, L.K.; Han, T.; Gaur, J.P. Response of a phytoplanktonic assemblage to copper and zinc enrichment in microcosm. Ecotoxicology 2015, 24, 573–582. [Google Scholar] [CrossRef]
- Liu, M.; Huang, X.H.; Li, C.L.; Li, C.L.; Gu, B. Study on the uptake of dissolved nitrogen by Oocystis borgei in prawn (Litopenaeus vannamei) aquaculture ponds and establishment of uptake model. Aquac. Int. 2020, 28, 1445–1458. [Google Scholar] [CrossRef]
- Huang, X.; Li, X.; Wang, Y.; Zhou, M. Effects of environmental factors on the uptake rates of dissolved nitrogen by a salt-water green alga (Oocystis borgei Snow). Bull. Environ. Contam. Toxicol. 2012, 89, 905–909. [Google Scholar] [CrossRef]
- Bent, S.J.; Gucker, C.L.; Oda, Y.; Forney, L.J. Spatial distribution of Rhodopseudomonas palustris ecotypes on a local scale. Appl. Environ. Microbiol. 2003, 69, 5192–5197. [Google Scholar] [CrossRef]
- Larimer, F.W.; Chain, P.; Hauser, L.; Lamerdin, J.; Malfatti, S.; Do, L.; Land, M.L.; Pelletier, D.A.; Beatty, J.T.; Lang, A.S.; et al. Complete genome sequence of the metabolically versatile photosynthetic bacterium Rhodopseudomonas palustris. Nat. Biotechnol. 2004, 22, 55–61. [Google Scholar] [CrossRef]
- Zhang, X.; Shu, M.; Wang, Y.; Fu, L.; Li, W.; Deng, B.; Liang, Q.; Shen, W. Effect of photosynthetic bacteria on water quality and microbiota in grass carp culture. World J. Microbiol. Biotechnol. 2014, 30, 2523–2531. [Google Scholar] [CrossRef]
- Sakpirom, J.; Kantachote, D.; Siripattanakul-Ratpukdi, S.; McEvoy, J.; Khan, E. Simultaneous bioprecipitation of cadmium to cadmium sulfide nanoparticles and nitrogen fixation by Rhodopseudomonas palustris TN110. Chemosphere 2019, 223, 455–464. [Google Scholar] [CrossRef]
- Lee, D.Y.; Ramos, A.; Macomber, L.; Shapleigh, J.P. Taxis response of various denitrifying bacteria to nitrate and nitrite. Appl. Environ. Microbiol. 2002, 68, 2140–2147. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, G.; Li, J.; Zhang, J. Effects of metal ions on biomass and 5-aminolevulinic acid production in Rhodopseudomonas palustris wastewater treatment. Water Sci. Technol. 2016, 73, 382–388. [Google Scholar] [CrossRef]
- Cai, T.; Park, S.Y.; Li, Y. Nutrient recovery from wastewater streams by microalgae: Status and prospects. Renew. Sustain. Energy Rev. 2013, 19, 360–369. [Google Scholar] [CrossRef]
- Alcantara, C.; Munoz, R.; Norvill, Z.; Plouviez, M.; Guieysse, B. Nitrous oxide emissions from high rate algal ponds treating domestic wastewater. Bioresour. Technol. 2015, 177, 110–117. [Google Scholar] [CrossRef]
- Liang, Q.; Zhang, X.; Lee, K.H.; Wang, Y.; Yu, K.; Shen, W.; Fu, L.; Shu, M.; Li, W. Nitrogen removal and water microbiota in grass carp culture following supplementation with Bacillus licheniformis BSK-4. World J. Microbiol. Biotechnol. 2015, 31, 1711–1718. [Google Scholar] [CrossRef]
- Van Rijn, J.; Tal, Y.; Schreier, H.J. Denitrification in recirculating systems: Theory and applications. Aquac. Eng. 2006, 34, 364–376. [Google Scholar] [CrossRef]
- Xu, C.; Wang, L.; Liu, Z.; Cai, G.; Zhan, J. Nitrogen and phosphorus removal efficiency and algae viability in an immobilized algae and bacteria symbiosis system with pink luminescent filler. Water Sci. Technol. 2022, 85, 104–115. [Google Scholar] [CrossRef]
- Karya, N.G.A.I.; Van der Steen, N.P.; Lens, P.N.L. Photooxygenation to support nitrification in an algal-bacterial consortium treating artificial wastewater. Bioresour. Technol. 2013, 134, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Yuan, Q. Removal of nitrogen from wastewater using microalgae and microalgae-bacteria consortia. Cogent Environ. Sci. 2016, 2, 1275089. [Google Scholar] [CrossRef]
- Fan, S.; Ji, B.; Abu Hasan, H.; Fan, J.; Guo, S.; Wang, J.; Yuan, J. Microalgal–bacterial granular sludge process for non-aerated aquaculture wastewater treatment. Bioprocess Biosyst. Eng. 2021, 44, 1733–1739. [Google Scholar] [CrossRef] [PubMed]
- Bankston, E.; Wang, Q.; Higgins, B.T. Algae support populations of heterotrophic, nitrifying, and phosphate-accumulating bacteria in the treatment of poultry litter anaerobic digestate. Chem. Eng. J. 2020, 398, 125550. [Google Scholar] [CrossRef]
- Cao, F.; Zhao, C.; You, J.; Hu, J.; Zheng, D.; Song, G.L. The inhibitive effect of artificial seawater on magnesium corrosion. Adv. Eng. Mater. 2019, 21, 1900363. [Google Scholar] [CrossRef]
- Lewicka-Szczebak, D.; Jansen-Willems, A.; Müller, C.; Dyckmans, J.; Well, R. Nitrite isotope characteristics and associated soil N transformations. Sci. Rep. 2021, 11, 5008. [Google Scholar] [CrossRef]
- Duan, P.; Zhang, Q.; Xiong, Z. Temperature decouples ammonia and nitrite oxidation in greenhouse vegetable soils. Sci. Total Environ. 2020, 733, 139391. [Google Scholar] [CrossRef]
- Hou, X.W.; Niu, Y.J.; Li, W.W.; Wang, G.J.; Sun, H.W. Analysis of the Effect of Temperature on the Microbial Flora Structure During the Nitrite Oxidation Process Using 16S rRNA High-throughput Sequencing. Environ. Sci. 2020, 41, 3773–3780. [Google Scholar]
- Xia, Z.; Wang, Q.; She, Z.; Gao, M.; Zhao, Y.; Guo, L.; Jin, C. Nitrogen removal pathway and dynamics of microbial community with the increase of salinity in simultaneous nitrification and denitrification process. Sci. Total Environ. 2019, 697, 134047. [Google Scholar] [CrossRef]
- Ji, J.; Peng, Y.; Wang, B.; Mai, W.; Li, X.; Zhang, Q.; Wang, S. Effects of salinity build-up on the performance and microbial community of partial-denitrification granular sludge with high nitrite accumulation. Chemosphere 2018, 209, 53–60. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, P. Effect of temperature and light on the growth of algae species: A review. Renew. Sustain. Energy Rev. 2015, 50, 431–444. [Google Scholar] [CrossRef]
- Amiri, R.; Ahmadi, M. Treatment of wastewater in sewer by Spirogyra sp. green algae: Effects of light and carbon sources. Water Environ. J. 2020, 34, 311–321. [Google Scholar] [CrossRef]
- Li, X.; Cai, F.; Luan, T.; Lin, L.; Chen, B. Pyrene metabolites by bacterium enhancing cell division of green algae Selenastrum capricornutum. Sci. Total Environ. 2019, 689, 287–294. [Google Scholar] [CrossRef]
- Arcila, J.S.; Buitrón, G. Influence of solar irradiance levels on the formation of microalgae-bacteria aggregates for municipal wastewater treatment. Algal Res. 2017, 27, 190–197. [Google Scholar] [CrossRef]
- Sepehri, A.; Sarrafzadeh, M.H.; Avateffazeli, M. Interaction between Chlorella vulgaris and nitrifying-enriched activated sludge in the treatment of wastewater with low C/N ratio. J. Clean. Prod. 2019, 247, 119164. [Google Scholar] [CrossRef]
- Su, Y.; Mennerich, A.; Urban, B. Synergistic cooperation between wastewater-born algae and activated sludge for wastewater treatment: Influence of algae and sludge inoculation ratios. Bioresour. Technol. 2012, 105, 67–73. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Peng, S.; Zhao, D.; Miao, L. Autotrophic biological nitrogen removal in a bacterial-algal symbiosis system: Formation of integrated algae/partial-nitrification/anammox biofilm and metagenomic analysis. Chem. Eng. J. 2022, 439, 135689. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Varjani, S.; Jeevanantham, S.; Yaashikaa, P.R.; Thamarai, P.; Abirami, B.; George, C.S. A review on algal-bacterial symbiotic system for effective treatment of wastewater. Chemosphere 2021, 271, 129540. [Google Scholar] [CrossRef]
- Li, Y.; Cao, P.; Wang, S.; Xu, X. Research on the treatment mechanism of anthraquinone dye wastewater by algal-bacterial symbiotic system. Bioresour. Technol. 2022, 347, 126691. [Google Scholar] [CrossRef]
- Huang, X.H.; Liu, M.; Zhou, M.H.; Gu, B.H. Effect of water environmental factors on the uptake rate of nitrite nitrogen by Oocystis borgei. Fish. Mod. 2012, 39, 40–45. [Google Scholar]
- Brown, B.; Wilkins, M.; Saha, R. Rhodopseudomonas palustris: A biotechnology chassis. Biotechnol. Adv. 2022, 60, 108001. [Google Scholar] [CrossRef] [PubMed]
- Nowruzi, B.; Shishir, M.A.; Porzani, S.J.; Ferdous, U.T. Exploring the Interactions between Algae and Bacteria. Mini Rev. Med. Chem. 2022, 22, 2596–2607. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, P.Y.; Zhao, M.; Li, J. Photosynthetic bacteria VOTO1-G isolated from river sediment and its removal capacity of nitrogen and phosphorus. Asian J. Chem. 2014, 26, 6647–6649. [Google Scholar] [CrossRef]
- Delgadillo-Mirquez, L.; Lopes, F.; Taidi, B.; Pareau, D. Nitrogen and phosphate removal from wastewater with a mixed microalgae and bacteria culture. Biotechnol. Rep. 2016, 11, 18–26. [Google Scholar] [CrossRef]
- Korth, F.; Deutsch, B.; Liskow, I.; Voss, M. Uptake of dissolved organic nitrogen by size-fractionated plankton along a salinity gradient from the North Sea to the Baltic Sea. Biogeochemistry 2012, 111, 347–360. [Google Scholar] [CrossRef]
- Kumar, S.D.; Santhanam, P.; Park, M.S.; Kim, M.K. Development and application of a novel immobilized marine microalgae biofilter system for the treatment of shrimp culture effluent. J. Water Process Eng. 2016, 13, 137–142. [Google Scholar] [CrossRef]
- Perković, L.; Djedović, E.; Vujović, T.; Baković, M.; Paradžik, T.; Čož-Rakovac, R. Biotechnological Enhancement of Probiotics through Co-Cultivation with Algae: Future or a Trend? Mar. Drugs 2022, 20, 142. [Google Scholar] [CrossRef]


| Level | Temperature/(°C) | Salinity/(‰) | Illumination/(μmol·m−2·s−1) |
|---|---|---|---|
| 1 | 25 | 5 | 25 |
| 2 | 30 | 15 | 35 |
| 3 | 35 | 25 | 45 |
| Level | Temperature (°C) | Salinity (‰) | Illumination (μmol·m−2·s−1) | Absorption (μg·g−1·h−1) |
|---|---|---|---|---|
| 1 | 25 (1) | 0 (1) | 35 (2) | 2.446 |
| 2 | 25 (1) | 5 (2) | 45 (3) | 2.936 |
| 3 | 25 (1) | 15 (3) | 25 (1) | 1.119 |
| 4 | 30 (2) | 0 (1) | 25 (1) | 2.439 |
| 5 | 30 (2) | 5 (2) | 35 (2) | 3.204 |
| 6 | 30 (2) | 15 (3) | 45 (3) | 2.086 |
| 7 | 35 (3) | 0 (1) | 45 (3) | 1.383 |
| 8 | 35 (3) | 5 (2) | 25 (1) | 1.625 |
| 9 | 35 (3) | 15 (3) | 35 (2) | 1.104 |
| Resource of Gap | Quadratic Sum | Degree of Freedom | Mean Square | F | P |
|---|---|---|---|---|---|
| calibration model | 42.091 | 7 | 6.013 | 119.582 | 0.008 |
| temperature | 2.257 | 2 | 1.128 | 22.442 | 0.043 |
| salinity | 2.003 | 2 | 1.001 | 19.915 | 0.048 |
| illumination | 0.453 | 2 | 0.227 | 4.508 | 0.182 |
| error | 0.101 | 2 | 0.05 | ||
| grand total | 42.192 | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Luo, Z.; Zhong, J.; Zhang, H.; Huang, X.; Li, C.; Zhang, Y. Effect of Environmental Factors on Nitrite Nitrogen Absorption in Microalgae–Bacteria Consortia of Oocystis borgei and Rhodopseudomonas palustris. Water 2023, 15, 1722. https://doi.org/10.3390/w15091722
Ma Y, Luo Z, Zhong J, Zhang H, Huang X, Li C, Zhang Y. Effect of Environmental Factors on Nitrite Nitrogen Absorption in Microalgae–Bacteria Consortia of Oocystis borgei and Rhodopseudomonas palustris. Water. 2023; 15(9):1722. https://doi.org/10.3390/w15091722
Chicago/Turabian StyleMa, Yukun, Zhishen Luo, Jiazhan Zhong, Hanyi Zhang, Xianghu Huang, Changling Li, and Yulei Zhang. 2023. "Effect of Environmental Factors on Nitrite Nitrogen Absorption in Microalgae–Bacteria Consortia of Oocystis borgei and Rhodopseudomonas palustris" Water 15, no. 9: 1722. https://doi.org/10.3390/w15091722
APA StyleMa, Y., Luo, Z., Zhong, J., Zhang, H., Huang, X., Li, C., & Zhang, Y. (2023). Effect of Environmental Factors on Nitrite Nitrogen Absorption in Microalgae–Bacteria Consortia of Oocystis borgei and Rhodopseudomonas palustris. Water, 15(9), 1722. https://doi.org/10.3390/w15091722





