Individual and Combined Toxic Effects of Nano-ZnO and Polyethylene Microplastics on Mosquito Fish (Gambusia holbrooki)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Microplastics
2.3. Nano-Particles ZnO (ZnO-NPs)
2.4. Fish
2.5. Experimental Design
2.6. Bioaccumulation of MPs
2.7. Bioaccumulation of Zn
2.8. Oxidative Biomarkers
2.9. Evaluation of the Interaction of MPs on the Toxicity of ZnO-NPs
- Predicted effect of the endpoints of fish exposed to MPs and ZnO-NPs, alone
- 2.
- The observed effect of the endpoints of fish exposed to MPs and ZnO-NPs, in combination
- 3.
- The synergistic effect
2.10. Data Analysis
3. Results
3.1. FTIR Assay
3.2. Bioaccumulation of Zn
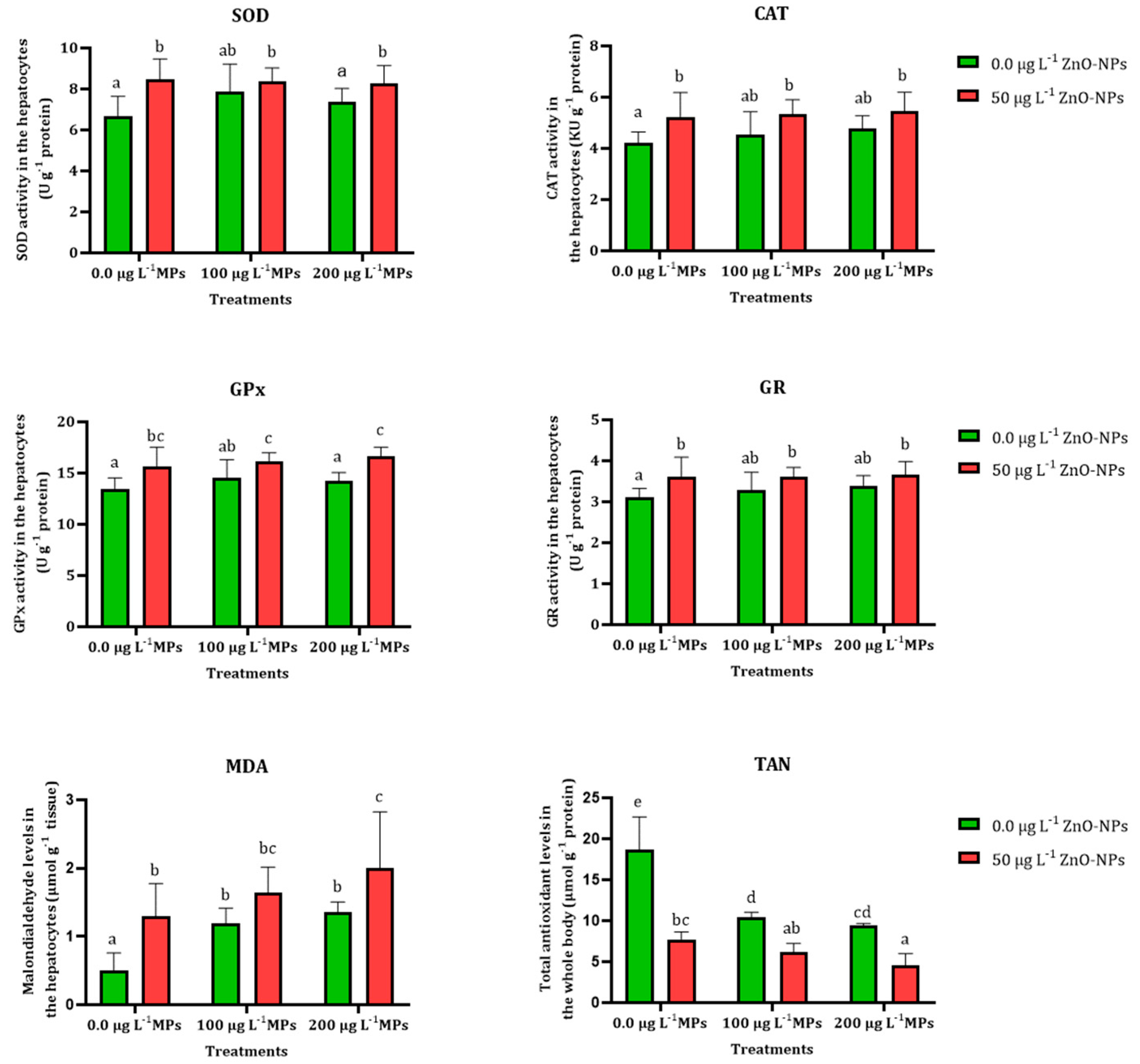
3.3. Antioxidant Enzyme Assay
3.4. Interaction Study between MPs and ZnO-NPs
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- IUCN. Marine Plastic Pollution. Available online: https://www.iucn.org/resources/issues-brief/marine-plastic-pollution (accessed on 25 February 2023).
- Buwono, N.R.; Risjani, Y.; Soegianto, A. Spatio-Temporal Patterns of Occurrence of Microplastics in the Freshwater Fish Gambusia affinis from the Brantas River, Indonesia. Environ. Pollut. 2022, 311, 119958. [Google Scholar] [CrossRef] [PubMed]
- Multisanti, C.R.; Merola, C.; Perugini, M.; Aliko, V.; Faggio, C. Sentinel Species Selection for Monitoring Microplastic Pollution: A Review on One Health Approach. Ecol. Indic. 2022, 145, 109587. [Google Scholar] [CrossRef]
- Prokić, M.D.; Gavrilović, B.R.; Radovanović, T.B.; Gavrić, J.P.; Petrović, T.G.; Despotović, S.G.; Faggio, C. Studying Microplastics: Lessons from Evaluated Literature on Animal Model Organisms and Experimental Approaches. J. Hazard. Mater. 2021, 414, 125476. [Google Scholar] [CrossRef] [PubMed]
- Schirinzi, G.F.; Pedà, C.; Battaglia, P.; Laface, F.; Galli, M.; Baini, M.; Consoli, P.; Scotti, G.; Esposito, V.; Faggio, C. A New Digestion Approach for the Extraction of Microplastics from Gastrointestinal Tracts (GITs) of the Common Dolphinfish (Coryphaena hippurus) from the Western Mediterranean Sea. J. Hazard. Mater. 2020, 397, 122794. [Google Scholar] [CrossRef]
- Manbohi, A.; Mehdinia, A.; Rahnama, R.; Hamzehpour, A.; Dehbandi, R. Sources and Hotspots of Microplastics of the Rivers Ending to the Southern Caspian Sea. Mar. Pollut. Bull. 2023, 188, 114562. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, K.; Xu, S.; Yan, M.; Tao, D.; Chen, L.; Wei, Y.; Wu, C.; Liu, G.; Lam, P.K.S. Heavy Metals in the “Plastisphere” of Marine Microplastics: Adsorption Mechanisms and Composite Risk. Gondwana Res. 2022, 108, 171–180. [Google Scholar] [CrossRef]
- Burgos-Aceves, M.A.; Abo-Al-Ela, H.G.; Faggio, C. Physiological and Metabolic Approach of Plastic Additive Effects: Immune Cells Responses. J. Hazard. Mater. 2021, 404, 124114. [Google Scholar] [CrossRef]
- Pagano, M.; Vazzana, I.; Gentile, A.; Caracappa, G.; Faggio, C. Hematological and Biochemical Parameters in Sea Turtles (Caretta caretta) after Stranding. Reg. Stud. Mar. Sci. 2019, 32, 100832. [Google Scholar] [CrossRef]
- Mallik, A.; Xavier, K.A.M.; Naidu, B.C.; Nayak, B.B. Ecotoxicological and Physiological Risks of Microplastics on Fish and Their Possible Mitigation Measures. Sci. Total Environ. 2021, 779, 146433. [Google Scholar] [CrossRef]
- Tsai, S.C.; Tseng, Y.-J.; Wu, S.M. Reproductive and Developmental Alterations on Zebrafish (Danio rerio) Upon Long-Term Exposure of Di-Ethyl Phthalate (DEP), Di-Isononyl Phthalate (DINP) and Di (2-Ethylhexyl) Phthalate (DEHP). Bull. Environ. Contam. Toxicol. 2023, 110, 49. [Google Scholar] [CrossRef]
- Yu, Y.; Zheng, T.; Li, H.; Hou, Y.; Dong, C.; Chen, H.; Wang, C.; Xiang, M.; Hu, G.; Dang, Y. Growth Inhibition of Offspring Larvae Caused by the Maternal Transfer Effects of Tetrabromobisphenol A in Zebrafish. Environ. Pollut. 2023, 322, 121143. [Google Scholar] [CrossRef] [PubMed]
- Gholamhosseini, A.; Banaee, M.; Sureda, A.; Timar, N.; Zeidi, A.; Faggio, C. Physiological Response of Freshwater Crayfish, Astacus leptodactylus Exposed to Polyethylene Microplastics at Different Temperature. Comp. Biochem. Physiol. Toxicol. Pharmacol. CBP 2023, 267, 109581. [Google Scholar] [CrossRef]
- Possatto, F.E.; Barletta, M.; Costa, M.F.; do Sul, J.A.I.; Dantas, D.V. Plastic Debris Ingestion by Marine Catfish: An Unexpected Fisheries Impact. Mar. Pollut. Bull. 2011, 62, 1098–1102. [Google Scholar] [CrossRef]
- Hoseini, S.M.; Sinha, R.; Fazel, A.; Khosraviani, K.; Hosseinpour Delavar, F.; Arghideh, M.; Sedaghat, M.; Paolucci, M.; Hoseinifar, S.H.; Van Doan, H. Histopathological Damage and Stress- and Immune-related Genes’ Expression in the Intestine of Common Carp, Cyprinus carpio Exposed to Copper and Polyvinyl Chloride Microparticle. J. Exp. Zool. Part Ecol. Integr. Physiol. 2022, 337, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Alimba, C.G.; Faggio, C.; Sivanesan, S.; Ogunkanmi, A.L.; Krishnamurthi, K. Micro(Nano)-Plastics in the Environment and Risk of Carcinogenesis: Insight into Possible Mechanisms. J. Hazard. Mater. 2021, 416, 126143. [Google Scholar] [CrossRef]
- Banaee, M. Alkaline Phosphatase Activity as a Biochemical Biomarker in Aqua-Toxicological Studies. Int. J. Aquat. Biol. 2021, 8, 143–147. [Google Scholar]
- Banihashemi, E.A.; Soltanian, S.; Gholamhosseini, A.; Banaee, M. Effect of Microplastics on Yersinia ruckeri Infection in Rainbow Trout (Oncorhynchus mykiss). Environ. Sci. Pollut. Res. 2022, 29, 11939–11950. [Google Scholar] [CrossRef]
- Lombardo, J.; Solomando, A.; Cohen-Sánchez, A.; Pinya, S.; Tejada, S.; Ferriol, P.; Mateu-Vicens, G.; Box, A.; Faggio, C.; Sureda, A. Effects of Human Activity on Markers of Oxidative Stress in the Intestine of Holothuria tubulosa, with Special Reference to the Presence of Microplastics. Int. J. Mol. Sci. 2022, 23, 9018. [Google Scholar] [CrossRef]
- Banaee, M.; Soltanian, S.; Sureda, A.; Gholamhosseini, A.; Haghi, B.N.; Akhlaghi, M.; Derikvandy, A. Evaluation of Single and Combined Effects of Cadmium and Micro-Plastic Particles on Biochemical and Immunological Parameters of Common Carp (Cyprinus carpio). Chemosphere 2019, 236, 124335. [Google Scholar] [CrossRef]
- Hodkovicova, N.; Hollerova, A.; Svobodova, Z.; Faldyna, M.; Faggio, C. Effects of Plastic Particles on Aquatic Invertebrates and Fish—A Review. Environ. Toxicol. Pharmacol. 2022, 96, 104013. [Google Scholar] [CrossRef]
- Impellitteri, F.; Curpăn, A.-S.; Plăvan, G.; Ciobica, A.; Faggio, C. Hemocytes: A Useful Tool for Assessing the Toxicity of Microplastics, Heavy Metals, and Pesticides on Aquatic Invertebrates. Int. J. Environ. Res. Public. Health 2022, 19, 16830. [Google Scholar] [CrossRef] [PubMed]
- Savuca, A.; Nicoara, M.N.; Faggio, C. Comprehensive Review Regarding the Profile of the Microplastic Pollution in the Coastal Area of the Black Sea. Sustainability 2022, 14, 14376. [Google Scholar] [CrossRef]
- Iswarya, A.; Vaseeharan, B.; Anjugam, M.; Gobi, N.; Divya, M.; Faggio, C. β-1, 3 Glucan Binding Protein Based Selenium Nanowire Enhances the Immune Status of Cyprinus carpio and Protection against Aeromonas hydrophila Infection. Fish Shellfish. Immunol. 2018, 83, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Jeyavani, J.; Sibiya, A.; Sivakamavalli, J.; Divya, M.; Preetham, E.; Vaseeharan, B.; Faggio, C. Phytotherapy and Combined Nanoformulations as a Promising Disease Management in Aquaculture: A Review. Aquac. Int. 2022, 30, 1071–1086. [Google Scholar] [CrossRef]
- Paulpandian, P.; Beevi, I.S.; Somanath, B.; Kamatchi, R.K.; Paulraj, B.; Faggio, C. Impact of Camellia sinensis Iron Oxide Nanoparticle on Growth, Hemato-Biochemical and Antioxidant Capacity of Blue Gourami (Trichogaster trichopterus) Fingerlings. Biol. Trace Elem. Res. 2023, 201, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Rashidian, G.; Lazado, C.C.; Mahboub, H.H.; Mohammadi-Aloucheh, R.; Prokić, M.D.; Nada, H.S.; Faggio, C. Chemically and Green Synthesized ZnO Nanoparticles Alter Key Immunological Molecules in Common Carp (Cyprinus carpio) Skin Mucus. Int. J. Mol. Sci. 2021, 22, 3270. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, S.; Vaseeharan, B.; Sudhakaran, R.; Jeyakandan, J.; Ramasamy, P.; Sonawane, A.; Padhi, A.; Velusamy, P.; Anbu, P.; Faggio, C. Bioinspired Zinc Oxide Nanoparticles Using Lycopersicon esculentum for Antimicrobial and Anticancer Applications. J. Clust. Sci. 2019, 30, 1465–1479. [Google Scholar] [CrossRef]
- Bessemer, R.A.; Butler, K.M.A.; Tunnah, L.; Callaghan, N.I.; Rundle, A.; Currie, S.; Dieni, C.A.; MacCormack, T.J. Cardiorespiratory Toxicity of Environmentally Relevant Zinc Oxide Nanoparticles in the Freshwater Fish Catostomus commersonii. Nanotoxicology 2015, 9, 861–870. [Google Scholar] [CrossRef]
- Khan, G.B.; Akhtar, N.; Khan, M.F.; Ullah, Z.; Tabassum, S.; Tedesse, Z. Toxicological Impact of Zinc Nano Particles on Tilapia Fish (Oreochromis mossambicus). Saudi J. Biol. Sci. 2022, 29, 1221–1226. [Google Scholar] [CrossRef]
- Saddick, S.; Afifi, M.; Abu Zinada, O.A. Effect of Zinc Nanoparticles on Oxidative Stress-Related Genes and Antioxidant Enzymes Activity in the Brain of Oreochromis niloticus and Tilapia zillii. Saudi J. Biol. Sci. 2017, 24, 1672–1678. [Google Scholar] [CrossRef]
- Saha, S.; Chukwuka, A.V.; Mukherjee, D.; Dhara, K.; Saha, N.C.; Faggio, C. Behavioral and Physiological Toxicity Thresholds of a Freshwater Vertebrate (Heteropneustes fossilis) and Invertebrate (Branchiura sowerbyi), Exposed to Zinc Oxide Nanoparticles (NZnO): A General Unified Threshold Model of Survival (GUTS). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 262, 109450. [Google Scholar] [CrossRef]
- Ferreira, P.; Fonte, E.; Soares, M.E.; Carvalho, F.; Guilhermino, L. Effects of Multi-Stressors on Juveniles of the Marine Fish Pomatoschistus Microps: Gold Nanoparticles, Microplastics and Temperature. Aquat. Toxicol. 2016, 170, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Zeidi, A.; Rezaei, M.R.; Sayadi, M.H.; Gholamhosseini, A.; Banaee, M. Evaluation of Polyethylene Microplastic Bio-Accumulation in Hepatopancreas, Intestine and Hemolymph of Freshwater Crayfish, Astacus leptodactylus. Int. J. Aquat. Biol. 2022, 10, 273–279. [Google Scholar]
- Banaee, M.; Akhlaghi, M.; Soltanian, S.; Sureda, A.; Gholamhosseini, A.; Rakhshaninejad, M. Combined Effects of Exposure to Sub-Lethal Concentration of the Insecticide Chlorpyrifos and the Herbicide Glyphosate on the Biochemical Changes in the Freshwater Crayfish Pontastacus leptodactylus. Ecotoxicol. Lond. Engl. 2020, 29, 1500–1515. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Li, X.; Zhou, Y.; Yu, H.; Xie, Y.; Guo, H.; Wang, H.; Li, Y.; Feng, Y.; Wang, Y. Polystyrene Microplastics Induce Hepatotoxicity and Disrupt Lipid Metabolism in the Liver Organoids. Sci. Total Environ. 2022, 806, 150328. [Google Scholar] [CrossRef]
- Shen, R.; Yang, K.; Cheng, X.; Guo, C.; Xing, X.; Sun, H.; Liu, D.; Liu, X.; Wang, D. Accumulation of Polystyrene Microplastics Induces Liver Fibrosis by Activating CGAS/STING Pathway. Environ. Pollut. Barking Essex 2022, 300, 118986. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Y.; Deng, Y.; Jiang, W.; Zhao, Y.; Geng, J.; Ding, L.; Ren, H. Uptake and Accumulation of Polystyrene Microplastics in Zebrafish (Danio rerio) and Toxic Effects in Liver. Environ. Sci. Technol. 2016, 50, 4054–4060. [Google Scholar] [CrossRef]
- Azadikhah, D.; Yalsuyi, A.M.; Saha, S.; Saha, N.C.; Faggio, C. Biochemical and Pathophysiological Responses in Capoeta capoeta under Lethal and Sub-Lethal Exposures of Silver Nanoparticles. Water 2023, 15, 585. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Fileman, E.; Halsband, C.; Goodhead, R.; Moger, J.; Galloway, T.S. Microplastic Ingestion by Zooplankton. Environ. Sci. Technol. 2013, 47, 6646–6655. [Google Scholar] [CrossRef] [PubMed]
- Kannan, M.; Bojan, N.; Swaminathan, J.; Zicarelli, G.; Hemalatha, D.; Zhang, Y.; Ramesh, M.; Faggio, C. Nanopesticides in Agricultural Pest Management and Their Environmental Risks: A Review. Int. J. Environ. Sci. Technol. 2023. [Google Scholar] [CrossRef]
- Abdel-Warith, A.A.; Younis, E.; Al-Asgah, N.A.; Wahbi, M.O. Effect of Zinc Toxicity on Liver Histology of Nile tilapia, Oreochromis niloticus. Sci. Res. Essays 2011, 6, 3760–3769. [Google Scholar] [CrossRef]
- Gomiero, A.; Strafella, P.; Pellini, G.; Salvalaggio, V.; Fabi, G. Comparative Effects of Ingested PVC Micro Particles With and Without Adsorbed Benzo(a)Pyrene vs. Spiked Sediments on the Cellular and Sub Cellular Processes of the Benthic Organism Hediste diversicolor. Front. Mar. Sci. 2018, 5, 99. [Google Scholar] [CrossRef]
- Modesto, K.A.; Martinez, C.B.R. Roundup® Causes Oxidative Stress in Liver and Inhibits Acetylcholinesterase in Muscle and Brain of the Fish Prochilodus lineatus. Chemosphere 2010, 78, 294–299. [Google Scholar] [CrossRef]
- Farkas, J.; Christian, P.; Urrea, J.A.G.; Roos, N.; Hassellöv, M.; Tollefsen, K.E.; Thomas, K.V. Effects of Silver and Gold Nanoparticles on Rainbow Trout (Oncorhynchus mykiss) Hepatocytes. Aquat. Toxicol. Amst. Neth. 2010, 96, 44–52. [Google Scholar] [CrossRef]
- Kim, J.-H.; Yu, Y.-B.; Choi, J.-H. Toxic Effects on Bioaccumulation, Hematological Parameters, Oxidative Stress, Immune Responses and Neurotoxicity in Fish Exposed to Microplastics: A Review. J. Hazard. Mater. 2021, 413, 125423. [Google Scholar] [CrossRef] [PubMed]
- Umamaheswari, S.; Priyadarshinee, S.; Kadirvelu, K.; Ramesh, M. Polystyrene Microplastics Induce Apoptosis via ROS-Mediated P53 Signaling Pathway in Zebrafish. Chem. Biol. Interact. 2021, 345, 109550. [Google Scholar] [CrossRef]
- Martyniuk, V.; Khoma, V.; Matskiv, T.; Yunko, K.; Gnatyshyna, L.; Stoliar, O.; Faggio, C. Combined Effect of Microplastic, Salinomycin and Heating on Unio tumidus. Environ. Toxicol. Pharmacol. 2023, 98, 104068. [Google Scholar] [CrossRef] [PubMed]
- Zicarelli, G.; Romano, C.; Gallo, S.; Valentino, C.; Pepe Bellomo, V.; Leonetti, F.L.; Giglio, G.; Neri, A.; Marsili, L.; Milazzo, C.; et al. Diet and Plastic Ingestion in the Blackmouth Catshark Galeus melastomus, Rafinesque 1810, in Italian Waters. Animals 2023, 13, 1039. [Google Scholar] [CrossRef] [PubMed]
- Canli, E.G.; Canli, M. Effects of Aluminum, Copper and Titanium Nanoparticles on the Liver Antioxidant Enzymes of the Nile Fish (Oreochromis niloticus). Energy Rep. 2020, 6, 62–67. [Google Scholar] [CrossRef]
- Liu, Z.; Malinowski, C.R.; Sepúlveda, M.S. Emerging Trends in Nanoparticle Toxicity and the Significance of Using Daphnia as a Model Organism. Chemosphere 2022, 291, 132941. [Google Scholar] [CrossRef]
- Słowińska, M.; Nynca, J.; Cejko, B.I.; Dietrich, M.A.; Horváth, Á.; Urbányi, B.; Kotrik, L.; Ciereszko, A. Total Antioxidant Capacity of Fish Seminal Plasma. Aquaculture 2013, 400–401, 101–104. [Google Scholar] [CrossRef]
- Banaee, M.; Sagvand, S.; Sureda, A.; Amini, M.; Haghi, B.N.; Sopjani, M.; Faggio, C. Evaluation of Single and Combined Effects of Mancozeb and Metalaxyl on the Transcriptional and Biochemical Response of Zebrafish (Danio rerio). Comp. Biochem. Physiol. Toxicol. Pharmacol. CBP 2023, 268, 109597. [Google Scholar] [CrossRef] [PubMed]
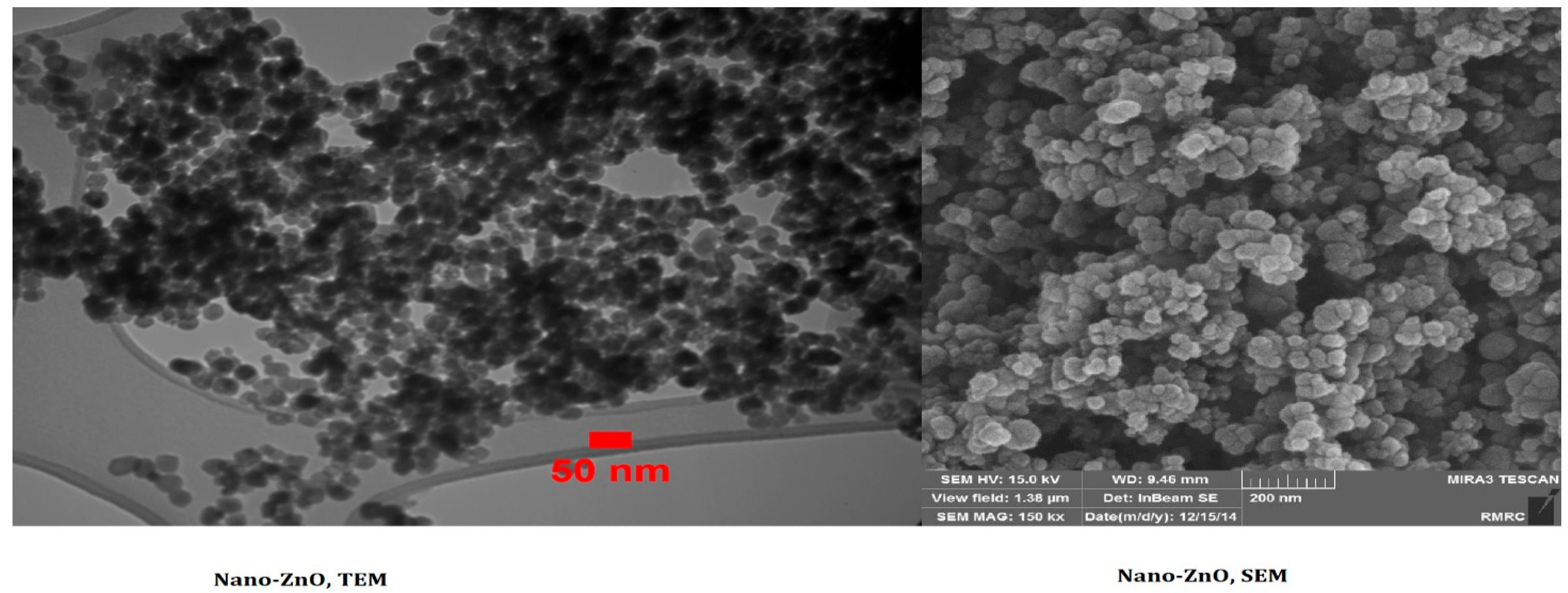
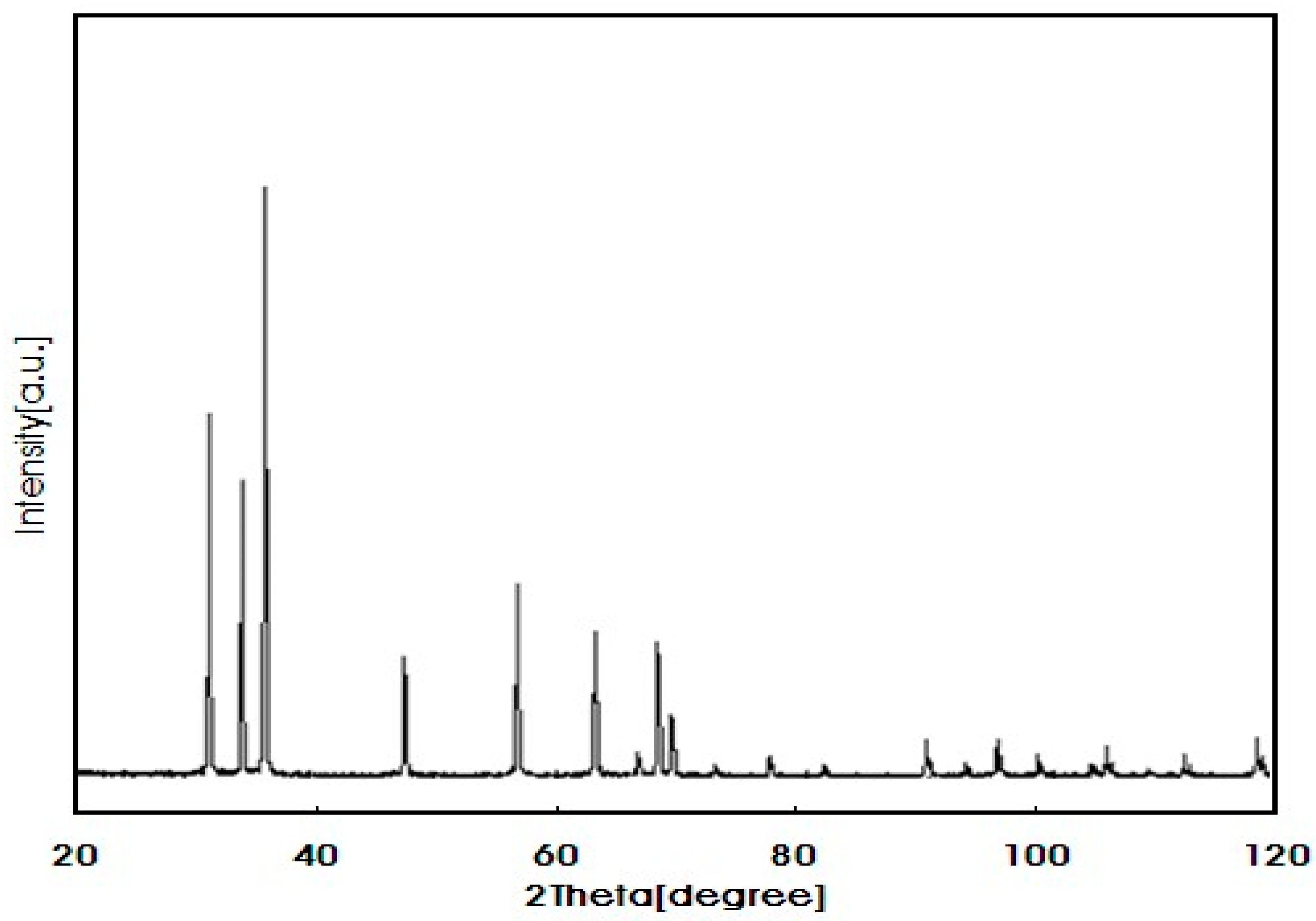
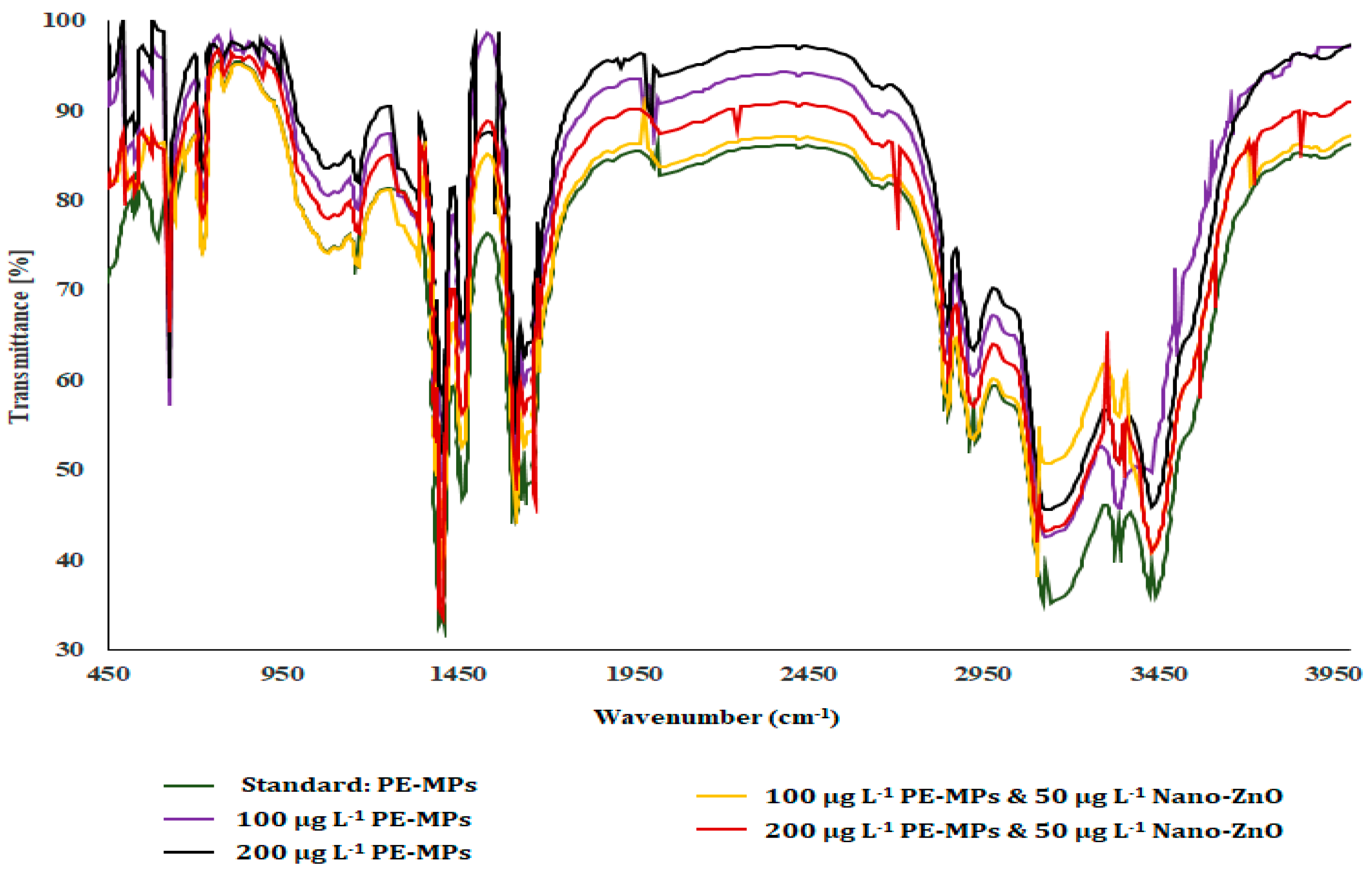
| Physicochemical Properties | Range |
|---|---|
| Purity | +99.9% |
| Average primary particle size (D50) | 10–30 nm |
| Specific surface area (SSA) | 60 m2 g−1 |
| Color | Milky white |
| Bulk density | 5.606 g cm−3 |
| Crystal phase | Single |
| Crystal morphology | Nearly spherical |
| Microplastics (MPs) | ||||||
|---|---|---|---|---|---|---|
| 0.0 µg L−1 | 100 µg L−1 | 200 µg L−1 | ||||
| ZnO Nano-Particles | 0.0 µg L−1 | 50 µg L−1 | 0.0 µg L−1 | 50 µg L−1 | 0.0 µg L−1 | 50 µg L−1 |
| Bioaccumulation (µg g−1 dried tissue) | 0.0 ± 0.0 a | 10.48 ± 0.51 b | 0.0 ± 0.0 a | 13.56 ± 1.12 c | 0.0 ± 0.0 a | 14.17 ± 0.60 c |
| Bioaccumulation factor | 0.0 ± 0.0 a | 0.21 ± 0.01 b | 0.0 ± 0.0 a | 0.27 ± 0.02 c | 0.0 ± 0.0 a | 0.28 ± 0.01 c |
| Oxidative Biomarker | Treatments | Predicated Effect | Observed Effect | Synergy Ratio | Combined Effect |
|---|---|---|---|---|---|
| SOD (U g−1 protein) | 50 µg L−1 ZnO-NPs and 100 µg L−1 PE-MPs | 1.49 | 1.30 | 1.15 | S |
| 50 µg L−1 ZnO-NPs and 200 µg L−1 PE-MPs | 1.40 | 1.24 | 1.13 | S | |
| GPx (U g−1 protein) | 50 µg L−1 ZnO-NPs and 100 µg L−1 PE-MPs | 1.25 | 1.19 | 1.04 | S |
| 50 µg L−1 ZnO-NPs and 200 µg L−1 PE-MPs | 1.22 | 1.23 | 0.99 | A | |
| CAT (KU g−1 protein) | 50 µg L−1 ZnO-NPs and 100 µg L−1 PE-MPs | 1.33 | 1.26 | 1.05 | S |
| 50 µg L−1 ZnO-NPs and 200 µg L−1 PE-MPs | 1.40 | 1.28 | 1.09 | S | |
| GR (U g−1 protein) | 50 µg L−1 ZnO-NPs and 100 µg L−1 PE-MPs | 1.22 | 1.16 | 1.05 | S |
| 50 µg L−1 ZnO-NPs and 200 µg L−1 PE-MPs | 1.26 | 1.15 | 1.09 | S | |
| MDA (µmol g−1 tissue) | 50 µg L−1 ZnO-NPs and 100 µg L−1 PE-MPs | 6.14 | 3.3 | 1.86 | S |
| 50 µg L−1 ZnO-NPs and 200 µg L−1 PE-MPs | 1.74 | 4 | 0.43 | A | |
| TAN (µmol g−1 protein) | 50 µg L−1 ZnO-NPs and 100 µg L−1 PE-MPs | 0.23 | 0.32 | 0.70 | A |
| 50 µg L−1 ZnO-NPs and 200 µg L−1 PE-MPs | 0.20 | 0.24 | 0.85 | A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banaee, M.; Zeidi, A.; Sinha, R.; Faggio, C. Individual and Combined Toxic Effects of Nano-ZnO and Polyethylene Microplastics on Mosquito Fish (Gambusia holbrooki). Water 2023, 15, 1660. https://doi.org/10.3390/w15091660
Banaee M, Zeidi A, Sinha R, Faggio C. Individual and Combined Toxic Effects of Nano-ZnO and Polyethylene Microplastics on Mosquito Fish (Gambusia holbrooki). Water. 2023; 15(9):1660. https://doi.org/10.3390/w15091660
Chicago/Turabian StyleBanaee, Mahdi, Amir Zeidi, Reshma Sinha, and Caterina Faggio. 2023. "Individual and Combined Toxic Effects of Nano-ZnO and Polyethylene Microplastics on Mosquito Fish (Gambusia holbrooki)" Water 15, no. 9: 1660. https://doi.org/10.3390/w15091660







