1. Introduction
The safety of water resources and tap water is directly related to the mortality and health of human beings [
1]. Since the distribution and transportation of living water are highly dependent on water supply networks and distribution systems, their security and stability are of great importance. These pipes and networks can cause serious secondary pollution to the water carried within them due to unavoidable aging [
2]. For example, with continuous water transportation and the use of pipes, a large and complex ecosystem of several kinds of microorganisms and bacteria will gradually accumulate and finally take shape, which is among the main causes of the biofilm attached on the walls of pipes [
3]. Viral pathogens, pathogenic viral hosts, and viral virulence factors (VFs) disseminate from source water to tap water, and the proportion of virus and viral VFs in the biofilm is far less than that in water [
4]. Bacteria accelerate the decay of disinfectants and promote the formation of disinfection by-products, as well as subjecting pipes to more corrosion and polluting water [
5,
6]. Halogenated DBPs such as trihalomethanes (THMs), haloacetic acids (HAAs), and haloacetonitriles (HANs) are cytotoxic and genotoxic, and long-term intake of DBP-containing tap water increases cancer risk as well as having certain adverse effects on the human reproductive system [
7]. Several researchers have reported that among the causes are the growth and dislodging of microorganisms inside the pipes [
8,
9,
10], which will result in cholera, salmonella and other bacteria in the water, causing acute intestinal infectious diseases and diarrhea.
Particulate matter generally refer are composed of macromolecules and multi-molecules with certain solubility and sizes in the range of nm–μm, such as inorganic and organic colloids, mineral particles, living bacteria, and algae. These particulate matter are also a major cause of the limescale within the pipes [
11,
12]. It has been reported that the secondary pollution occuring during water transportation within water supply systems is largely related to the existence and dislodging of biofilm attached on the inner walls of the pipes, bacteria can attach to suspended particulate matter in the pipe network, or attach to sediment on the pipe wall to form biofilm [
13,
14,
15]. However, the effects of particulate matter and some possible synergistic influences caused by particulate matter and biofilm still remain unclear. Furthermore, certain fluctuations in in water quality will occur, especially when hydraulic conditions within the pipes and networks change, as the particulate matter will return to the water body and cause deterioration [
16]. Therefore, it is of great importance to determine the possible impacts of hydraulic conditions on the particulate matter and biofilm within the pipes, and their effect on water quality.
The species, composition, and even structure of bacteria are highly dependent on the hydraulic conditions within the pipes and water supply networks [
17]. Additionally, the composition of bacteria suspended in the water body and within the biofilm attached on the walls is generally different, as the latter usually shows a stronger ability to form EPS, which is beneficial to co-condensation and adhering. In addition, the ability to reduce the matrix, disinfection and washing to remove biofilm is limited because the cohesion of biofilm is highly correlated with the physicochemical properties of EPS [
18]. However, total suspended solids, total bacteria and particle-associated bacterial concentrations also follow different trends as they are affected differently by a complex combination of physicochemical and hydraulic factors [
6,
19]. Based on the above studies, it was concluded that further in-depth studies are needed to determine the key drivers of particle-associated bacterial loading in drinking water distribution systems.
Biofilm are known to exhibit cohesive properties via their EPS matrix, which is critical in both the formation and detachment of the assemblages and is the characteristic difference between planktonic and biofilm communities. The EPS matrix governs the mechanical stability of the biofilm, an attribute which is prioritized in biofilm communities and influenced by drinking water distribution system (DWDS) hydraulics [
2]. Most studies did not relate the impact of particulate matter to EPS, but only described the impact of particulate matter to biofilm abundance in a superficial way. In this study, the influence of particulate matter on the properties of EPS and biofilm within the pipes and the water supply network is investigated thoroughly, which serves as a reference in ensuring the safety of drinking water.
2. Materials and Methods
2.1. Collection of Particulate Matter and the Cultivation of Biofilm
The particulate matter was collected as filtration residues of 60 L tap water left on a 0.2 μm membrane. The trapped particulate matter on the membrane was washed and rinsed into a beaker, and separated through 10 min centrifugation at a speed of 6000 g/min. After that, the solids were dried at 60 °C and sealed for storage and further use, in order to obtain approximately 5 g of particulate matter. The cultivation of biofilm was performed using R2A liquid medium. A certain amount of the above particulates was weighed and added to the medium before 20 min autoclaving at 121 °C. Then, the samples were stored at 4 °C for later use. In order to explore the influence of particulate matter quantity on biofilm, 1 mL tap water was added to 8 mL R2A liquid medium containing different particulate matter concentrations for static culture, with particulate matter concentrations of 0, 100, 200, 400, 800, 1000, 1200 and 1400 mg/L, respectively. In order to explore the influence of particulate matter on biofilm at different rotational speeds, 1 mL tap water was added to 8 mL R2A liquid medium containing particulate matter with a concentration of 400 mg/L, the temperature was set at 37 °C, and the culture was conducted at 0, 50, 80 and 120 rmp, respectively. In a typical cultivation, 1 mL tap water was added to 8 mL R2A liquid medium containing a certain amount of particulate matter with the temperature set at 37 °C. The other three groups were rotated at 50 rpm, 80 rpm and 120 rpm, respectively. After 24 h of cultivation, the liquid medium solution with bacteria was filtered using filtration paper (pore size 20~30 μm) to obtain biofilm microorganisms.
2.2. Determination of Flow Cytometry (FCM)
The count of the microorganisms was obtained using a flow cytometer with a 488 nm argon ion laser at 22 mW (Backman Coulter Quanta SC, USA), as it could be distinguished from abiotic particles through staining. The colorant adopted here was SYBR Green I with high sensitivity, and at least 20 pg DNA could be detected without decolorizing or rinsing, which was significantly higher than that of ethane bromide (EB). Herein, a 2% EDTA-2Na solution was used as the dispersant to disperse the samples for 3 h at 4 °C. After that, 10 μL SYBR Green I was added to the samples diluted at different times, and the samples were settled in dark for 45 min at room temperature. Then, the samples were filtered through a 200 target quasi-sieve, and 300 μL of was taken out and injected into a 96-well plate for determination. The green fluorescence signal collection channel was set as FL1 with a wavelength of 520 ± 20 nm, and the particle signal collection wavelength channel was set as SS.
A volume of 5 mL tap was added water to 200 mL LB liquid medium and incubated at 150 rpm for 24 h at 37 °C. The standard bacterial solution was diluted 40, 50, 60, 80, 200, 300 and 500 fold for FCM determination.
2.3. Extracellular Polymeric Substances (EPS) Analysis Method
EPS extract was detected with a fluorescent photometer (HITACHI F-4600, Japan). The instrument parameters were set as follows: the excitation wavelength (Ex) was scanned from 200 nm to 500 nm in increments of 5 nm; the emission wavelength (Em) was scanned from 250 to 550 nm in increments of 5 nm; the scanning speed was 2400 nm/min. Fourier-transform infrared spectroscopy (Thermo Fisher Scientific Nicolet iS10, USA) was used to analyze the characteristic functional groups in EPS, and the factors affecting EPS could be studied through the change in characteristic peak intensity and the change in characteristic peak wave number. The parameters were a resolution of 0.48 cm−1, a scanning range of 400 cm−1~4000 cm−1, and scanning times 60. We mixed the dried thallus with EPS and potassium bromide and ground them at 1:100, and then the tablets were weighed accurately to reduce the influence of the tablets on the experiment. After the tablet was pressed, we used Fourier-transform infrared spectroscopy (FTIR) for scanning.
3. Results and Discussion
3.1. Impact of Particulate Matter on Biofilm Biomass
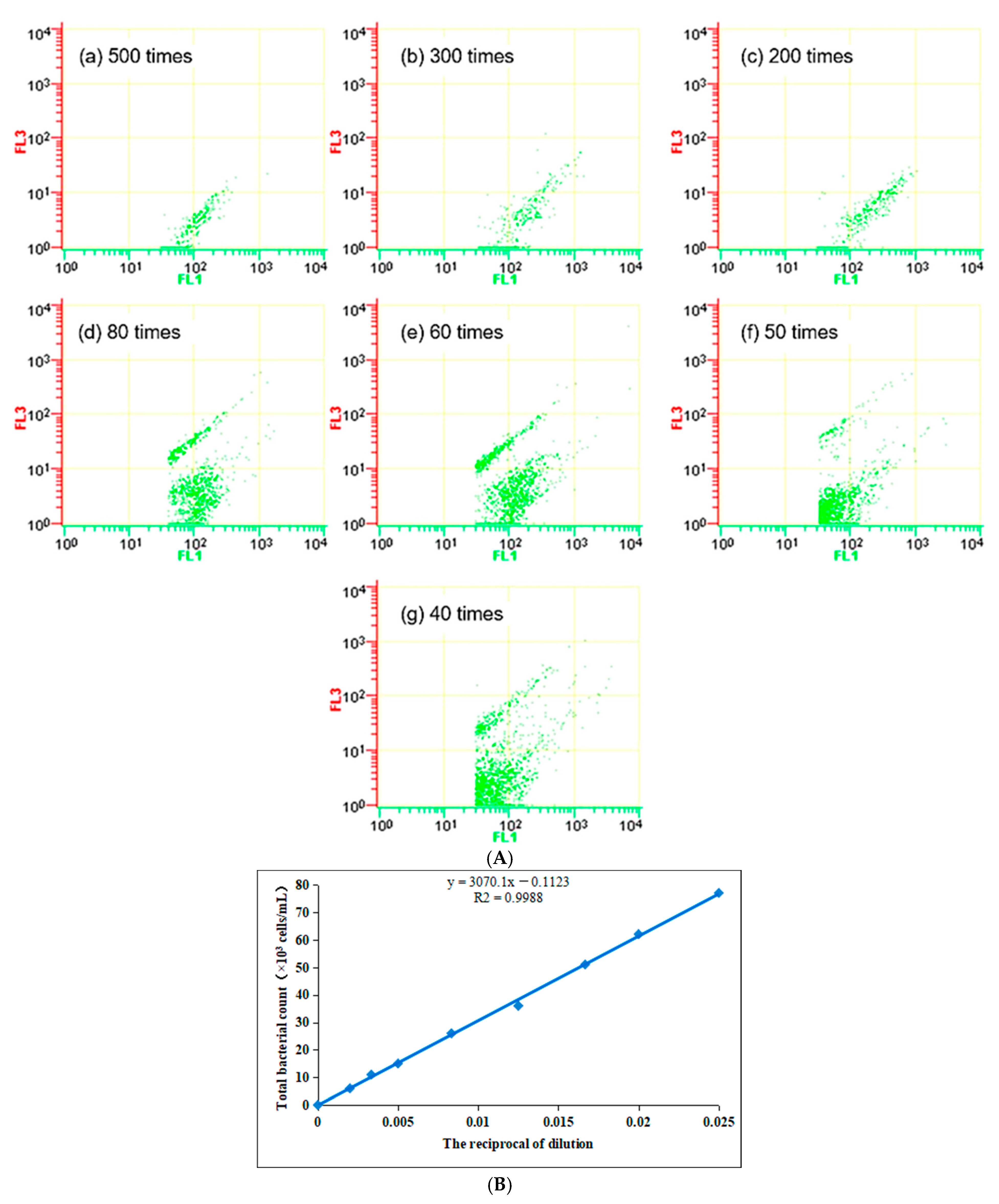
LB liquid culture medium was used as the standard bacterial solution, diluting Standard bacterial solution at different multiples for FCM determination. The results are shown in
Figure 1A, and the standard curve of the FCM measurement is shown in
Figure 1B.
It can be seen from
Figure 1 that with the increase in dilution ratio, the bacterial staining gradually decreases. The initial temperature of the bacterial solution in this experiment was 4 °C, and the correlation coefficient reached 0.9988 under the staining condition at 18 °C, room temperature, indicating the high accuracy of the staining test under this experimental condition. This experiment is the artificial culture of biofilm through drinking water, and the bacteria grow quickly, causing the concentration of bacteria to exceed the linear range of this method, so the sample needs to be diluted properly in the follow-up test.
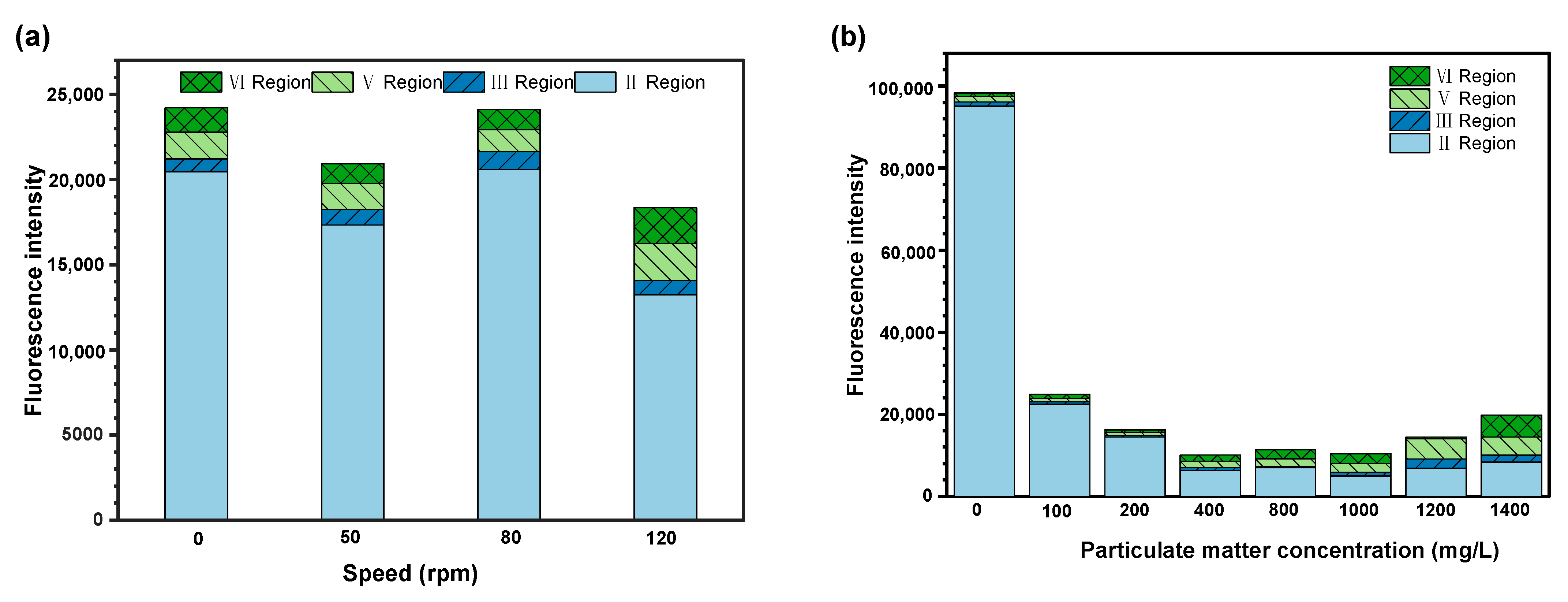
The count of microorganisms within the biofilm cultivated on R2A liquid medium under eight kinds of contents of particulate matter was obtained from FCM, and the results were shown in
Figure 2a. Speed is used to simulate the flow velocity in the actual pipe network, the R2A liquid medium with the same concentration of particulate matter was cultured at different rotational speeds, and the biofilm samples obtained after treatment were diluted at an appropriate multiple and determined by FCM, as shown in
Figure 2b.
The highest biomass in
Figure 2a was obtained in the absence of particulate matter, indicating that bacteria tended to aggregate and form into biofilm under this situation. However, the presence of particulate matter led to significant changes in the quantity of microorganisms within the biofilm—first increased and later decreased with the continuous increase in particulate matter. This could be explained by the fact that the sampled biofilm were generally formed between the bacteria, while the situation for stabled aggregates constituted by particulate matter and bacterial showed opposite behave, increasing at first and later decreasing. This could be attributed to the increased sites due to the presence of particulate matter, which was beneficial for the attachment of microorganisms and therefore gradually formed with stable biofilm.
Since the aggregates of particles and bacteria were difficult to separate, even with the use of an EDTA-2Na dispersant, a 3 h extraction was adopted in the later procedures. The results showed that there still existed some macroscopic aggregates which were relatively stable compared to others and thus not dispersed completely. Further, with the increasing concentration of particulate matter, they tended to make contact with each other more frequently and this made possible the generation of aggregates. Generally, the particles took the major part at the initial stage. However, the unavoidable presence of bacteria within water would produce some secretions which allow its combination with other particles. When the particulate aggregates reached saturation, the bindings between the bacteria were further strengthened, and the dispersion by EDTA-2Na during the extraction resulted in the increase in biofilm biomass. This confirms the important effect of particulate matter on biofilm biomass.
As shown in
Figure 2b, the biofilm microbial biomass first decreased and then increased with the increase in rotation speed. The decreasing trend was caused by the unstable and easily broken nature of the aggregates under fluctuation. A higher flow velocity could improve the microbial biomass of biofilm, and water flow might bring more nutrients to the biofilm [
19]. In this experiment, the media and substrates during the cultivation process were adopted under a eutrophic state. The main reason for this was that the collisions between the bacterial aggregates might increase with the increase in rotation speed and water flow velocity. Then, the increased friction barrier effect on the inner wall accelerated the formation of stable biofilm on it.
3.2. Impact of Particulate Matter on EPS
The three-dimensional fluorescence spectrum (3D-EEM) can reflect the fluorescence intensity changes with the excitation wavelength (Ex) and emission wavelength (Em) at the same time, which provides detailed and comprehensive spectral information rapidly. The three-dimensional fluorescence spectrum of organic matter in natural environment can be roughly divided into 6 different material regions as shown in
Table S1.
The atlas of EPS extract obtained from the 3D-EEM scanning of R2A liquid medium with different concentrations of particulate matter is shown in
Figure 3.
The atlas of EPS extract obtained from 3D-EEM scanning of R2A liquid medium cultured and treated at different rotational speeds is shown in
Figure 4.
Figure 3 and
Figure 4 showed that the fluorescence signals obtained by EPS scanning by 3D-EEM mainly distributed in regions II, III, V and VI (the fluorescence intensity was arranged in descending order), and the intensity of region II was significantly higher than the residues. In other words, the main components of EPS in biofilm cultivated from tap water are generally tyrosine/tryptophan proteins, while the contents of polysaccharides, polyaromatic cyclic humic acid and polycarboxylic acid humic acid are relatively low. In fact, a large number of studies have shown that there are more polysaccharide components measured by a UV spectrophotometer than proteins. For example, studies on sludge floc [
20,
21] showed that the content of polysaccharide in EPS is higher than that of protein, and the same result was found when studying extracellular polymers cultured by pipe network strains [
22]. The reason is that the polysaccharides scanned by 3D fluorescence are different from those in EPS, so that the polysaccharides in EPS cannot be scanned out.
The fluorescence intensity of each region was summed. The relationship between particle concentration and fluorescence intensity is shown in
Figure 5a, and the relationship between rotation speed and fluorescence intensity is shown in
Figure 5b.
As shown in
Figure 5a, EPS fluorescence intensity is the highest when the medium does not contain particulate matter, and EPS fluorescence intensity is relatively small when the medium contains particulate matter. In the process of extraction, EDTA-2Na is more likely to separate EPS from bacteria when there is no particulate matter. The particles adsorb EPS and bind to bacteria to change the chemical properties of EPS, making EPS more stable. In the process of extraction, it is difficult to separate EPS from the particulate matter–bacteria interface.
With the increase in particulate concentration, the fluorescence intensity of EPS first decreased and then increased, indicating that the un-extracted EPS have the same trend. At a low concentration, the adsorption of particles and bacteria plays a major role. Due to the anionic groups in EPS molecules (such as carboxyl group and phosphate group), the ionization of EPS results in negative charge characteristics, and the surface of particulate matter has a strong positive charge. When the concentration increases to a certain level, the aggregation effect between particles through EPS is greater, and relatively stable. The chance of contact between particles through the extracellular polymer increases, and more stable particulate matter aggregates are formed [
23,
24]. EPS was more easily extracted in bacterial and bacterial binding aggregates. In addition, these particulate matter aggregates do not form integral bonds with the biofilm, only physical interaction.
As shown in
Figure 5b, with the increase in the rotation speed, the fluorescence intensity of EPS first decreased and then increased, indicating that the un-extracted EPS have the same tendency. Compared with the static position, the collisions between particles and bacteria with the rotation speed increase, bacteria adheres to the tube wall and forms a stable biofilm. When the rotation speed increased to a certain value, the impact of particles on bacterial aggregates played a dominant role in accelerating biofilm shedding. When they reached dynamic equilibrium, the biofilm showed a large amount of accumulation, so there may be an optimal flow shear force for biofilm formation and succession.
3.3. FTIR Analysis of Particulate Matter
In order to further clarify the influence of particulate matter and hydraulic conditions on EPS, FTIR was used for further analysis. The FTIR spectra of the microorganisms with original enriched particles cultivated under the conditions of (a) different concentrations of particles, and (b) different flow rates, provided in
Figure 6.
All characteristic peaks of the concentration of originally enriched particulate matter are basically the smallest, indicating that during the enrichment of particulate matter, heating has removed most EPS absorbed on the surface, reducing the influence on the experiment. In particular, heating will denature proteins and change functional groups, making the characteristic peaks of proteins the smallest.
According to the corresponding relationship between characteristic peaks and functional groups, the samples contained more polysaccharides (1040~1080 cm−1, 3200~3500 cm−1) and proteins (peptide bond) (1540~1570 cm−1, 1680~1710 cm−1). This also shows that the main components of EPS are polysaccharide and protein. The weak characteristic peak wave number of 2850~2940 cm−1 indicates that there is a certain amount of fat. Compared with three-dimensional fluorescence spectrum, polysaccharides in extracellular polymer cannot be detected and analyzed by three-dimensional fluorescence spectrum scanning. In addition, there is a strong characteristic peak in the spectrum of originally enriched particulate matter, and C-H vibration in methyl group (1380 cm−1). These indicated that the corresponding substances acted with EPS in the process of culture, or were used by microorganisms for transformation.
Figure 6a indicated that sample 1 contained no particulate matter, and the signals of polysaccharides, proteins, aliphatic chains and other components were the weakest compared to the other samples, indicating that particulate matter had a significant effect on EPS. With the increased concentration of particulate matter, the signal of polysaccharides showed a blue shift, e.g., towards the high wave side. This was caused by the reduced bond length of -OH when the particle was combined with the polysaccharide, as more energy was required in this process to form a more stable group. However, a further increase in particulate matter showed little impact on the signals of functional groups such as protein (peptide bond) and fatty chains, revealing that particle concentration does not really affect C=O, C-N, and CH
2 functional groups. Further, since little variation, i.e., deformation and vibration, was detected in the N-H function group, this indicates that there were almost no interactions between the particulate matter, proteins, and fatty chains. These functional groups did not have critical impacts.
Figure 6b shows that the signals of functional groups such as polysaccharides, proteins and fatty chains remained almost unchanged under different hydraulic conditions, indicating that rotational speed posed little interference on the vibration and bending of chemical bonds in EPS. However, with the increase in rotation speed, significant changes were observed in the absorption peaks of polysaccharides and proteins, implying that EPS first increased and then decreased. Briefly, when the rotation speed was low, the particles and bacteria mainly performed Brownian and diffusion motion. With the increase in rotation speed, collisions between particles and bacteria might happen, which would also affect the EPS that adhered within the interface between them. However, if the rotation speed is too high, the chemical bonds between the particulate matter and soluble EPS would be broken and damaged by water flow through shear force, which, in turn, results in the reduction in EPS. The above results revealed that particulate matter mainly adhered to bacteria through EPS. Further, the results also illustrated the critical role of particulate matter in the generation, stabilization, and even deterioration of biofilm, which was consistent with the previous results of 3D-EEM.
4. Conclusions
Particulate matter and hydraulic conditions might affect the biofilm microbial biomass through interfering in the interactions between the particulate matter and EPS. The biofilm which does not contain particulate matter is the largest, as it can also be formed between bacteria. However, biofilm of this kind is commonly unstable, easy to damage, and susceptible to external influences. Compared to biofilm without particulate matter, biofilm integrated by particles and bacteria are usually far more stable, although the stability is largely dependent on working parameters such as rotation speed and water flux. The aggregates formed between bacteria are usually very unstable and tend to be broken up at a lower rotation speed. On the contrary, an appropriate increase in rotation speed contributed to the generation of stable biofilm attached on the inner wall due to the possible collision and adhesion between bacterial polymers.
Particulate matter plays an important role in stabilizing biofilm biomass, and particulate matter–bacteria aggregates are more difficult to destroy than bacteria–bacteria aggregates. The interaction mechanism between particulate matter and EPS still needs to be studied, so biofilm research should pay more attention to the interaction mechanism between particulate matter and EPS, and the function of particulate matter in the biofilm structure. To strengthen the study on the expression of microbial EPS functional genes, the surface theory should be deeply discussed in the subsequent research about relationships between biofilm, extracellular polymers and particulate matter.
EPS is a bridge between particulate matter and microbes. However, the influencing factors of microbial diversity in biofilm are complex, and scholars rarely explore the relationship between particulate matter and biofilm. The mechanism of biofilm formation and succession is still controversial. Therefore, it is of great significance to analyze the influencing factors of biofilm microbial diversity and explore the relationship between them, which will also aid the research in the field of drinking water safety by providing reference values for controlling biofilm growth and avoiding pathogen outbreaks, so as to ensure drinking water safety and reliability.