Screening for Microplastic Uptake in an Urbanized Freshwater Ecosystem: Chondrostoma nasus (Linnaeus, 1758) Case Study
Abstract
:1. Introduction
2. Materials and Methods
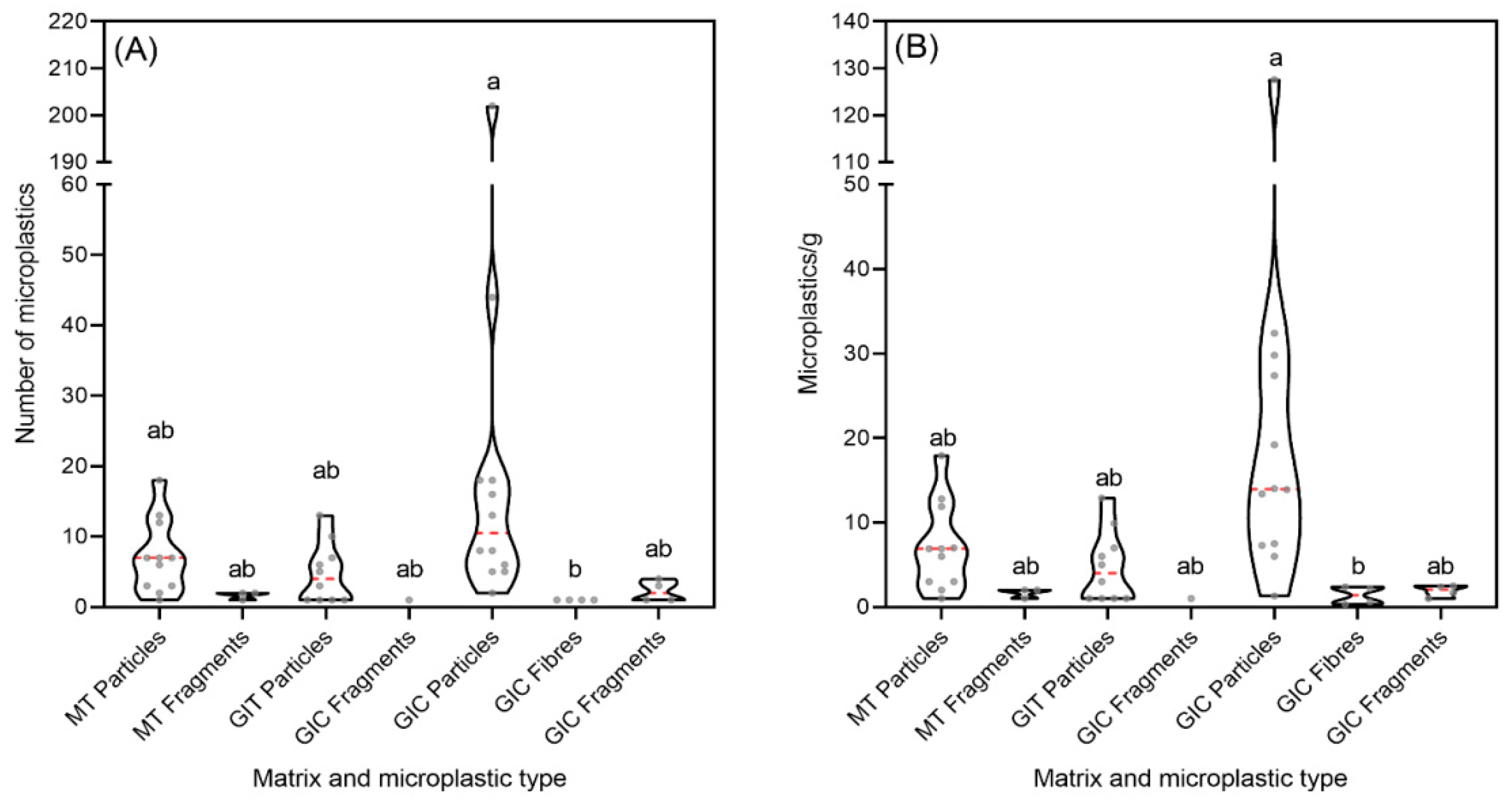
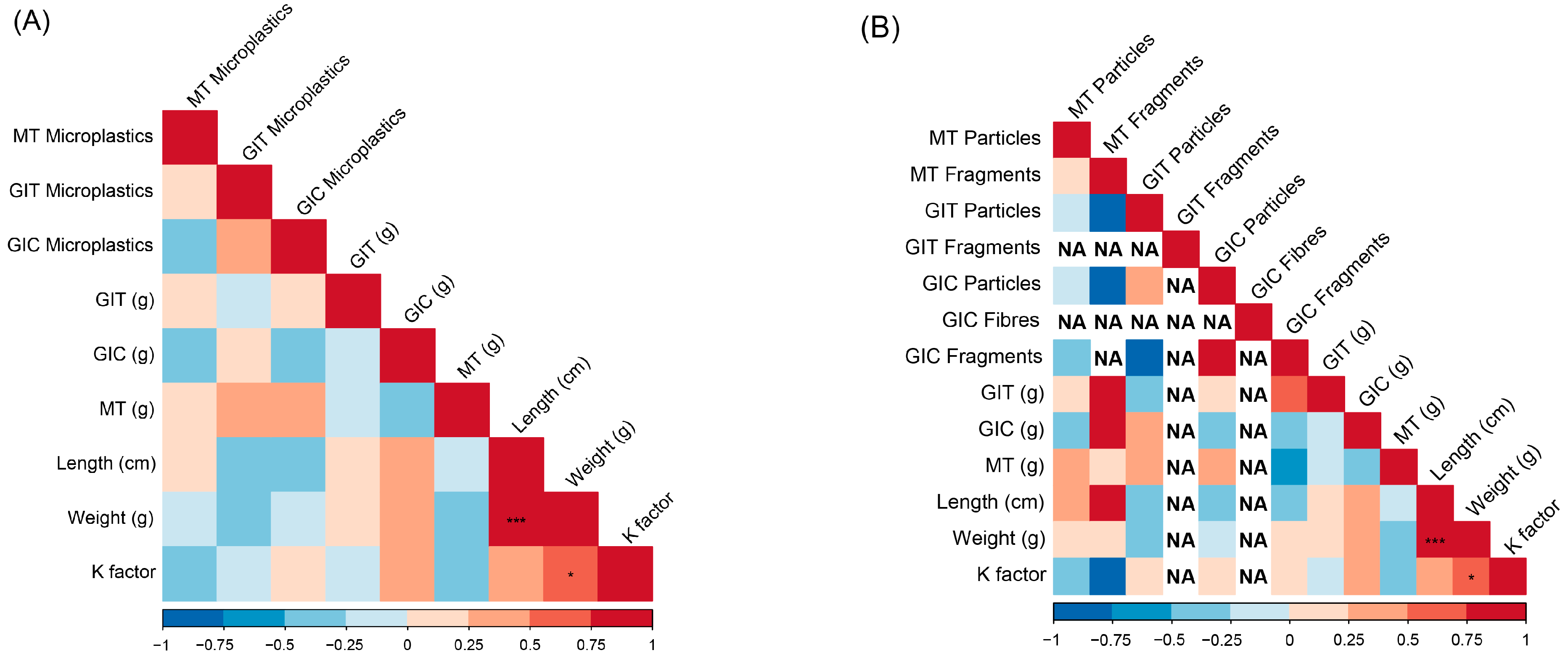
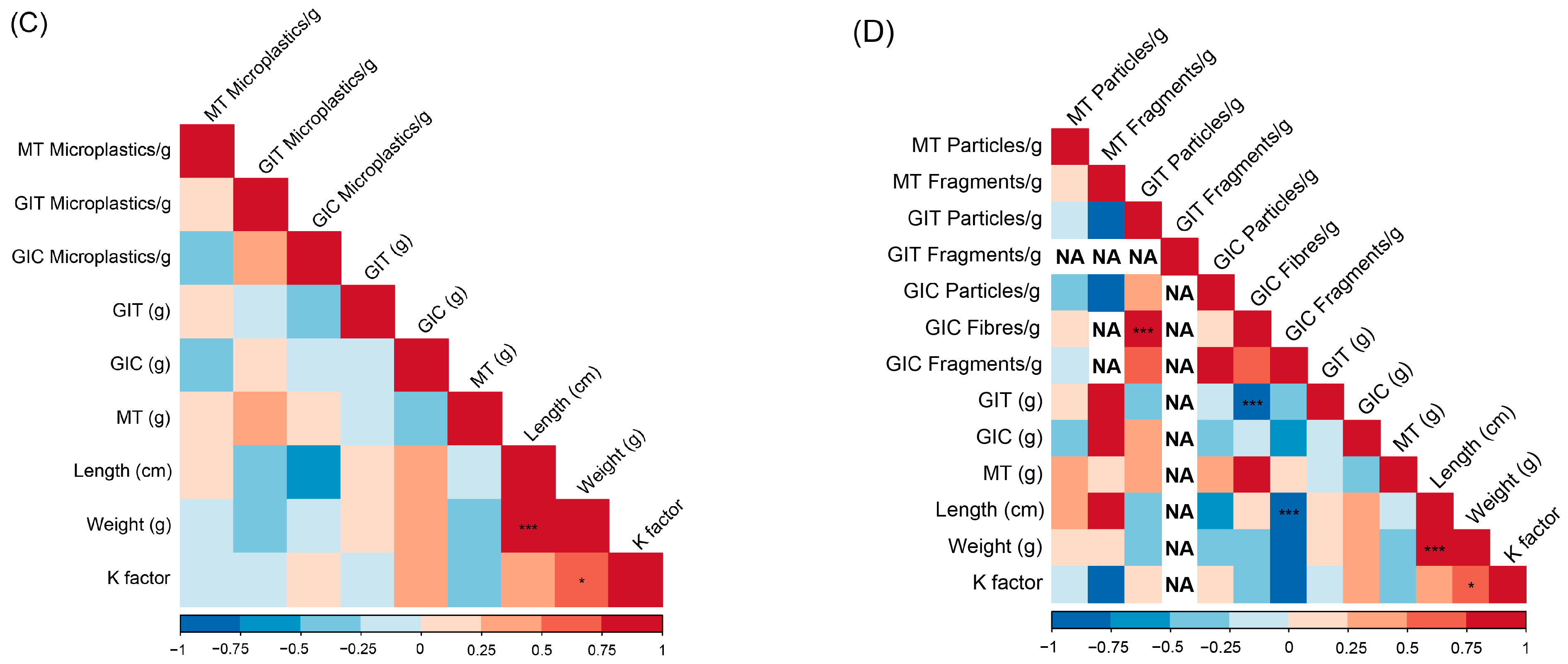
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bănăduc, D.; Simić, V.; Cianfaglione, K.; Barinova, S.; Afanasyev, S.; Öktener, A.; McCall, G.; Simić, S.; Curtean-Bănăduc, A. Freshwater as a Sustainable Resource and Generator of Secondary Resources in the 21st Century: Stressors, Threats, Risks, Management and Protection Strategies, and Conservation Approaches. Int. J. Environ. Res. Public Health 2022, 19, 16570. [Google Scholar] [CrossRef] [PubMed]
- Bănăduc, D.; Barinova, S.; Cianfaglione, K.; Curtean-Bănăduc, A. Editorial: Multiple Freshwater Stressors—Key Drivers For The Future of Freshwater Environments. Front. Environ. Sci. 2023, 11, 1143706. [Google Scholar] [CrossRef]
- Wittmann, F.; Householder, E.; de Olivera Wittmann, A.; Junk, W.J.; Piedade, M.T. Implementation of the Ramsar Convention on South American wetlands: An update. Res. Rep. Biodiv. Stud. 2015, 4, 47–58. [Google Scholar] [CrossRef]
- Woodward, G.; Gessner, O.M.; Giller, S.P.; Gulis, V.; Hladyz, S.; Lecerf, A.; Malmqvist, B.; McKie, G.B.; Tiegs, D.S.; Cariss, H.; et al. Continental-scale effects of nutrient pollution on stream ecosystem functioning. Science 2012, 336, 1438–1440. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, S.; Soltani, N.; Keshavarzi, B.; Moore, F.; Turner, A.; Hassanaghaei, M. Microplastics In Different Tissues of Fish And Prawn From The Musa Estuary, Persian Gulf. Chemosphere 2018, 205, 80–87. [Google Scholar] [CrossRef]
- Akoueson, F.; Sheldon, L.M.; Danapoulos, E.; Morris, S.; Hoften, J.; Chapman, E.; Li, J.; Rotchell, J.M. A Preliminary Analysis of Microplastics in Edible Versus Non-edible Tissues from Seafood Samples. Environ. Pollut. 2020, 263, 114452. [Google Scholar] [CrossRef]
- Almeida, C.M.R.; Saez-Zamacona, I.; Silva, D.M.; Rodrigues, S.M.; Pereira, R.; Ramos, S.D. The Role of Estuarine Wetlands (Saltmarshes) in Sediment Microplastics Retention. Water 2023, 15, 1382. [Google Scholar] [CrossRef]
- Batel, A.; Linti, F.; Scherer, M.; Erdinger, L.; Braunbeck, T. Transfer of benzo[a]pyrene from microplastics to Artemia nauplii and further to zebrafish via a trophic food Web experiment: CYP1A induction and visual tracking of persistent organic pollutants. Environ. Toxicol. Chem. 2016, 35, 1656–1666. [Google Scholar] [CrossRef] [PubMed]
- Bessa, F.; Frias, J.; Kogel, T.; Lusher, A.; Andrade, J.M.; Antunes, J.; Sobral, P.; Pagter, E.; Nash, R.; O’Connor, I.; et al. Harmonized Protocol for Monitoring Microplastics in Biota; Techical Report; JPI-Oceans BASEMAN Project: Oostende, Belgium, 2019. [Google Scholar]
- Bianchi, J.; Valente, T.; Scacco, U.; Cimmaruta, R.; Sbrana, A.; Silvestri, C.; Matiddi, M. Food preference determines the best suitable digestion protocol for analysing microplastic ingestion by fish. Mar. Pollut. Bull. 2020, 154, 111050. [Google Scholar] [CrossRef]
- Chang, X.; Xue, Y.; Li, J.; Zou, L.; Tang, M. Potential health impact of environmental microand nanoplastics pollution. J. Appl. Toxicol. 2019, 40, 4–15. [Google Scholar] [CrossRef]
- Sturm, M.T.; Myers, E.; Schober, D.; Korzin, A.; Thege, C.; Schuhen, K. Comparison of AOP, GAC, and Novel Organosilane-Based Process for the Removal of Microplastict at a Municipal Wastewater Treatment Plant. Water 2023, 15, 1164. [Google Scholar] [CrossRef]
- Galloway, T.S. Micro and Nano-plastics and Human Health. In Marine Anthropogenic Litter; Bergmann, M., Gutaw, L., Klages, M., Eds.; Springer International Publishing: Berlin, Germany, 2015; p. 13. [Google Scholar]
- Barboza, L.G.A.; Lopes, C.; Oliveira, P.; Bessa, F.; Otero, V.; Henriques, B.; Raimundo, J.; Caetano, M.; Vale, C.; Guilhermino, L. Microplastics in wild fish from North East Atlantic Ocean and its potential for causing neurotoxic effects, lipid oxidative damage, and human health risks associated with ingestion exposure. Sci. Total Environ. 2020, 717, 134625. [Google Scholar] [CrossRef] [PubMed]
- PlasticsEurope. Available online: https://www.plasticseurope.org/de/resources/publications/4312-plastics-facts-2020 (accessed on 3 March 2022).
- Ballent, A.M. Anthropogenic Particles in Natural Sediment Sinks: Microplastics Accumulation in Tributary, Beach and Lake Bottom Sediments of Lake Ontario, North America. Ph.D. Thesis, The University of Western Ontario, London, ON, Canada, 2016. [Google Scholar]
- Faure, F.; Corbaz, M.; Baecher, H.; de Alencastro, L. Pollution due to plastics and microplastics in Lake Geneva and in the Mediterranean Sea. Arch. Sci. 2012, 65, 157–164. [Google Scholar]
- Mattsson, K.; Jocic, S.; Doverbratt, I.; Hansson, L.A. Nanoplastics in the Aquatic Environment: Microplastic Contamination in Aquatic Environments; Zheng, E.Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 379–399. [Google Scholar]
- Chen, J.; Deng, Y.; Chen, Y.; Peng, X.; Qin, H.; Wang, T.; Zhao, C. Distribution Patterns of Microplastics Pollution in Urban Fresh Waters: A Case Study of Rivers in Chengdu, China. Int. J. Environ. Res. Public Health 2022, 19, 8972. [Google Scholar] [CrossRef]
- Lusher, A.L.; Tirelli, V.; O’Connor, I.; Officer, R. Microplastics in Arctic polar waters: The first reported values of particles in surface and sub-surface samples. Sci. Rep. 2015, 5, 14947. [Google Scholar] [CrossRef] [PubMed]
- Cózar, A.; Echevarría, F.; González-Gordillo, J.I.; Irigoien, X.; Úbeda, B.; Hernández-León, S.; Palma, Á.T.; Navarro, S.; García-de-Lomas, J.; Ruiz, A.; et al. Plastic debris in the open ocean. Proc. Natl. Acad. Sci. USA 2014, 111, 10239–10244. [Google Scholar] [CrossRef]
- Eriksen, M.; Lebreton, L.C.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic pollution in the world’s oceans: More than 5 trillion plastic pieces weighing over 250,000 tons afloat at sea. PLoS ONE 2014, 9, 1–15. [Google Scholar] [CrossRef]
- Ni’am, A.C.; Hassan, F.; Shiu, R.-F.; Jiang, J.-J. Microplastics in Sediments of East Surabaya, Indonesia: Regional Characteristics and Potential Risks. Int. J. Environ. Res. Public Health 2022, 19, 12348. [Google Scholar] [CrossRef]
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1985–1998. [Google Scholar] [CrossRef]
- Han, X.; Wang, S.; Yu, X.; Vogt, R.D.; Feng, J.; Zhai, L.; Zhu, L.; Lu, X. Kinetics and Size Effects on adsorption of CU(II), Cr(III), and Pb(II) Onto Polyethylene, Polypropylene, and Polyethylene Terephthalate Microplastic Particles. Front. Mar. Sci. 2021, 8, 785146. [Google Scholar] [CrossRef]
- Gasperi, J.; Wright, S.L.; Dris, R.; Collard, F.; Mandin, C.; Guerrouache, M.; Langlois, V.; Kelly, F.J.; Tassin, B. Microplastics in air: Are we breathing it in? Curr. Opin. Environ. Sci. Health 2018, 1, 1–5. [Google Scholar] [CrossRef]
- Batel, A.; Baumann, L.; Carteny, C.C.; Cormier, B.; Keiter, S.H.; Braunbeck, T. Histological, enzymatic and chemical analyses of the potential effects of differently sized microplastic particles upon long-term ingestion in zebrafish (Danio rerio). Mar. Pollut. Bull. 2020, 153, 111022. [Google Scholar] [CrossRef]
- Klavins, M.; Klavins, L.; Stabnikova, O.; Stabnikov, V.; Marynin, A.; Ansone-Bertina, L.; Mezulis, M.; Vaseashta, A. Interaction between Microplastics and Pharmaceuticals Depending on the Composition of Aquatic Environment. Microplastics 2022, 1, 520–535. [Google Scholar] [CrossRef]
- Lechner, A. “Down by the River”: (Micro-) Plastic Pollution of Running Freshwaters with Special Emphasis on the Austrian Danube. Mare Plast.-Plast. Sea Combat. Plast. Pollut. Sci. Art 2020, 1, 141–185. [Google Scholar]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.; McGonigle, D.; Russell, A.E. Lost at sea: Where is all the plastic? Science 2022, 304, 838. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, E.M.; Ehlers, S.M.; Kiriakoulakis, K.; Schuchert, P.; Jones, N.H.; Kregting, L.; Woodall, L.C.; Dick, J.T. The accumulation of microplastic pollution in a commercially important fishing ground. Sci. Rep. 2022, 12, 4217. [Google Scholar] [CrossRef]
- Corcoran, P.L. Degradation of Microplastics in the Environment. In Handbook of Microplastics in the Environment; Rocha-Santos, T., Costa, M.F., Mouneyrac, C., Eds.; Springer: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Nikolić, M.D.; Milošković, A.M.; Jakovljević, M.M.; Radenković, N.M.; Veličković, T.Z.; Đuretanović, S.R.; Kojadinović, N.M.; Nikolić, M.N.; Simić, V.M. The first observation of the presence of microplastics in wild common bleak (Alburnus alburnus L.) and standardization of extraction protocols. Kragujevac J. Sci. 2022, 44, 267–282. [Google Scholar] [CrossRef]
- Kvale, K.; Prowe, A.E.F.; Chien, C.T.; Landolfi, A.; Oschlies, A. The global biological microplastic particle sink. Sci. Rep. 2020, 10, 16670. [Google Scholar] [CrossRef]
- Bertora, A.; Grosman, F.; Sanzano, P.; Rosso, J.J. Combined effects of urbanization and longitudinal disruptions in riparian and in-stream habitat on water quality of a praire stream. Knowl. Manag. Aquat. Ecosyst. 2022, 423, 15. [Google Scholar] [CrossRef]
- Hadwen, W.L.; Arthington, A.H.; Stuart, E.B.; Mosisch, T. Effects of Tourism on Fraser Island’s Dune Lakes; CRC for Sustainable Tourism: Gold Coast, Australia, 2004; ISBN 1 876685 84. [Google Scholar]
- Pojar, I.; Stănică, A.; Stock, F.; Kochleus, C.; Schultz, M.; Bradley, C. Sedimentary microplastic concentrations from the Romanian Danube River to the Black Sea. Sci. Rep. 2021, 11, 1–9. [Google Scholar] [CrossRef]
- Rodrigues, M.O.; Abrantes, N.; Gonçalves, F.J.M.; Nogueira, H.; Marques, J.C.; Gonçalves, A.M.M. Spatial and temporal distribution of microplastics in water and sediments of a freshwater system (Antuã River, Portugal). Sci. Total Environ. 2018, 633, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, R.; Turner, S.D.; Rose, N.L. Microplastics in the sediments of a UK urban lake. Environ. Pollut. 2017, 229, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.; Worch, E.; Knepper, T.P. Occurrence and spatial distribution of microplastics in river shore sediments of the Rhine-main area in Germany. Environ. Sci. Technol. 2015, 49, 6070–6076. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, W.; Bender, C.; Porcher, J.M. Wild gudgeons (Gobio gobio) from French rivers are contaminated by microplastics: Preliminary study and first evidence. Environ. Res. 2014, 128, 98–100. [Google Scholar] [CrossRef]
- Faure, F.; Demars, C.; Wieser, O.; Kunz, M.; De Alencastro, L.F. Plastic pollution in Swiss surface waters: Nature and concentrations, interaction with pollutants. Environ. Chem. 2015, 12, 582–591. [Google Scholar] [CrossRef]
- Roch, S.; Brinker, A. Rapid and efficient method for the detection of microplastic in the gastrointestinal tract of fishes. Environ. Sci. Technol. 2017, 51, 4522–4530. [Google Scholar] [CrossRef]
- McGoran, A.R.; Clark, P.F.; Morritt, D.J.E.P. Presence of microplastic in the digestive tracts of European flounder, Platichthys flesus, and European smelt, Osmerus eperlanus, from the River Thames. Environ. Pollut. 2017, 220, 744–751. [Google Scholar] [CrossRef]
- Horton, A.A.; Jürgens, M.D.; Lahive, E.; van Bodegom, P.M.; Vijver, M.G. The influence of exposure and physiology on microplastic ingestion by the freshwater fish Rutilus rutilus (roach) in the River Thames, UK. Environ. Pollut. 2018, 236, 188–194. [Google Scholar] [CrossRef]
- Slootmaekers, B.; Carteny, C.C.; Belpaire, C.; Saverwyns, S.; Fremout, W.; Blust, R.; Bervoets, L. Microplastic contamination in gudgeons (Gobio gobio) from Flemish rivers (Belgium). Environ. Pollut. 2019, 244, 675–684. [Google Scholar] [CrossRef]
- Roch, S.; Walter, T.; Ittner, L.D.; Friedrich, C.; Brinker, A. A systematic study of the microplastic burden in freshwater fishes of south-western Germany-Are we searching at the right scale? Sci. Total Environ. 2019, 689, 1001–1011. [Google Scholar] [CrossRef]
- Kuśmierek, N.; Popiołek, M. Microplastics in freshwater fish from Central European lowland river (Widawa R., SW Poland). Environ. Sci. Pollut. Res. 2020, 27, 11438–11442. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.icpdr.org/main/danube-basin/countries-danube-river-basin (accessed on 3 March 2022).
- Popa, G.O.; Khalaf, M.; Dudu, A.; Curtean-Bănăduc, A.; Bănăduc, D.; Georgescu, S.E.; Costache, M. Brown trout’s populations genetic diversity using mitochondrial markers in relative similar geographical and ecological conditions—A Carpathian case study. Transylv. Rev. Ecol. Res. 2013, 15.2, 125–132. [Google Scholar]
- Bănăduc, D.; Maric, S.; Cianfaglione, K.; Afanasyev, S.; Somogy, D.; Nyeste, K.; Antal, L.; Kosko, J.; Caleta, M.; Wanzenbock, J.; et al. Stepping Stone Wetlands, Last Sanctuaries for European Mudminnow: How Can the Human Impact, Climate Change, and Non-Native Species Drive a Fish to the Edge of Extinction? Sustainability 2022, 14, 13493. [Google Scholar] [CrossRef]
- Bănăduc, D.; Curtean-Bănăduc, A.; Lenhardt, M.; Guti, G. “Porţile de Fier/Iron Gates” Gorges area (Danube) fish fauna. Transylv. Rev. Syst. Ecol. Res. 2014, 16, 171–196. [Google Scholar] [CrossRef]
- Antipa, G. Fauna Ihtiologică a României; Inst. De Arte Grafice “Carol Göbl” Publishing House: Bucharest, Romania; Publicaţiile Fondului: Adamachi, Romania, 1909; Volume XVI, p. 294. [Google Scholar]
- Bănărescu, P.M.; Fauna, R.P.R. Pisces-Osteichthyes (Peşti Ganoizi şi Osoşi); Editura Academiei Republicii Populare Române: Bucharest, Romania, 1964; Volume XIII, p. 959. [Google Scholar]
- Schneider-Binder, E. The habitats along the upper Danube in Germany and changes to them induced by human impacts. In Human Impact on Danube Watershed Biodiversity in the XXI Century; Springer International Publishing: Berlin, Germany, 2020; pp. 27–49. ISBN 978-3-030-37241. [Google Scholar]
- Burga, C.A.; Landolt, E. The Upper Engadine—Headwater region of the river Inn. A Swiss hot spot of plant diversity and premium tourism region. In Human Impact on Danube Watershed Biodiversity in the XXI Century; Springer International Publishing: Berlin, Germany, 2020; pp. 49–64. ISBN 978-3-030-37241. [Google Scholar]
- Cianfaglione, K.; Pedroti, F. Italy in the Danube: Territory, landscape, environment, vegetation, fauna, culture, human management and outlooks for the future. In Human Impact on Danube Watershed Biodiversity in the XXI Century; Springer International Publishing: Berlin, Germany, 2020; pp. 87–118. ISBN 978-3-030-37241. [Google Scholar]
- Čarni, A.; Juvan, N. Forest vegetation along the Mura River in Slovenia. In Human Impact on Danube Watershed Biodiversity in the XXI Century; Springer International Publishing: Berlin, Germany, 2020; pp. 119–134. [Google Scholar]
- Adámek, Z.; Jurajdová, Z.; Janáč, M.; Zahrádkova, S.; Nĕmejcová, D.; Jurajda, P. The response of fish assemblages to human impacts along the lower stretch of the rivers Morava and Dyje (Danube river basin, Czech Republic). In Human Impact on Danube Watershed Biodiversity in the XXI Century; Springer International Publishing: Berlin, Germany, 2020; pp. 135–150. ISBN 978-3-030-37241. [Google Scholar]
- Ćaleta, M.; Mustafić, P.; Zanella, D.; Buj, I.; Marčić, Z.; Mrakovčić, M. Human impacts of the Dobra River (Croatia). In Human Impact on Danube Watershed Biodiversity in the XXI Century; Springer International Publishing: Berlin, Germany, 2020; pp. 151–168. ISBN 978-3-030-37241. [Google Scholar]
- Dekić, R.; Ivanc, A.; Ćetković, D.; Lolić, S. Anthropogenic impact and environmental quality of different tributaries of the river Vrbas (Bosnia and Herzegovina). In Human Impact on Danube Watershed Biodiversity in the XXI Century; Springer International Publishing: Berlin, Germany, 2020; pp. 169–214. ISBN 978-3-030-37241. [Google Scholar]
- Đikanović, V.; Nikčević, M.; Mićković, B.; Hegediš, A.; Mrdak, D.; Pešić, V. Anthropogenic pressures on watercourses of the Danube River basin in Montenegro. In Human Impact on Danube Watershed Biodiversity in the XXI Century; Springer International Publishing: Berlin, Germany, 2020; pp. 241–256. ISBN 978-3-030-37241. [Google Scholar]
- Lenhardt, M.; Smederevac-Lalić, M.; Hegediš, A.; Skrić, S.; Cvijanović, G.; Višnjić-Jeftić, Ž.; Djikanović, V.; Jovičić, K.; Jaćimović, M.; Jarić, I. Human impacts on fish fauna in the Danube River in Serbia: Current status and ecological implications. In Human Impact on Danube Watershed Biodiversity in the XXI Century; Springer International Publishing: Berlin, Germany, 2020; pp. 257–280. ISBN 978-3-030-37241. [Google Scholar]
- Mišiková, E.; Makovinská, J. Assessment of the aquatic ecosystem in the Slovak stretch of the Danube River. In Human Impact on Danube Watershed Biodiversity in the XXI Century; Springer International Publishing: Berlin, Germany, 2020; pp. 281–300. ISBN 978-3-030-37241. [Google Scholar]
- Maślanko, W.; Ferencz, B.; Dawidek, J. State and changes of natural environment in Polish part of the Danube River basin Poland. In Human Impact on Danube Watershed Biodiversity in the XXI Century; Springer International Publishing: Berlin, Germany, 2020; pp. 301–326. ISBN 978-3-030-37241. [Google Scholar]
- Afanasyev, S.; Lyashenko, A.; Iarochevitch, A.; Lietytska, O.; Zorina-Sakharova, E.; Marushevska, O. Pressures and impacts on ecological status of surface water bodies in Ukrainian part of the Danube River basin. In Human Impact on Danube Watershed Biodiversity in the XXI Century; Springer International Publishing: Berlin, Germany, 2020; pp. 327–358. ISBN 978-3-030-37241. [Google Scholar]
- Bakiu, R. Drina River (Sava’s tributary of Danube River) and human impact in Albania. In Human Impact on Danube Watershed Biodiversity in the XXI Century; Springer International Publishing: Berlin, Germany, 2020; pp. 359–380. ISBN 978-3-030-37241. [Google Scholar]
- Kostov, V.; Slavevska-Stamenkovic, V.; Ristovska, M.; Stojov, V.; Marić, S. Characteristics of the Danube drainage area in the Republic of Macedonia. In Human Impact on Danube Watershed Biodiversity in the XXI Century; Springer International Publishing: Berlin, Germany, 2020; pp. 381–392. ISBN 978-3-030-37241. [Google Scholar]
- Kenderv, L.; Trichkova, T. Long-term changes in the ecological conditions of the Iskar River (Danube River basin, Bulgaria). In Human Impact on Danube Watershed Biodiversity in the XXI Century; Springer International Publishing: Berlin, Germany, 2020; pp. 393–424. ISBN 978-3-030-37241. [Google Scholar]
- Simić, V.; Bănăduc, D.; Curtean-Bănăduc, A.; Petrovic, A.; Velickovic, T.; Stojkovic-Piperac, M.; Simić, S. Assessment of the ecological sustainability of river basins based on the modified the ESHIPPOfish model on the example of the Velika Morava basin (Serbia, Central Balkans). Front. Environ. Sci. 2022, 10, 952692. [Google Scholar] [CrossRef]
- Sasa, M.; Bănăduc, D.; Gajic, D.; Sanda, R.; Velickovic, T.Z. Sabanejewia romanica (Băcescu, 1943) (Actinopterygii: Cobitidae), a new species for the ichthyofaunal of Serbia. Acta Zool. Bulg. 2022, 74, 369–377. [Google Scholar]
- Bănăduc, D.; Joy, M.; Olosutean, H.; Afanasyev, S.; Curtean-Bănăduc, A. Natural and anthropogenic driving forces as key elements in the Lower Danube Basin-South-Eastern Carpathians-North-Western Black Sea coast area lakes: A broken stepping stones for fish in a climatic change scenario? Environ. Sci. Eur. 2020, 32, 73. [Google Scholar] [CrossRef]
- Bănăduc, D.; Rey, S.; Trichkova, T.; Lenhardt, M.; Curtean-Bănăduc, A. The Lower Danube River-Danube Delta-North West Black Sea: A pivotal area of major interest for the past, present and future of its fish fauna—A short review. Sci. Total Environ. 2016, 545, 137–151. [Google Scholar] [CrossRef]
- Boeraş, I.; Curtean-Bănăduc, A.; Bănăduc, D.; Cioca, G. Anthropogenic sewage water circuit as vector for SARS-CoV-2 viral ARN transport and public health assessment, monitoring and forecasting—Sibiu metropolitan area (Transylvania/Romania) study case. Int. J. Environ. Res. Public Health 2022, 19, 11725. [Google Scholar] [CrossRef]
- Boeraş, I.; Burcea, A.; Coman, C.; Bănăduc, D.; Curtean-Bănăduc, A. Bacterial microbiomes in the sediments of lotic systems ecologic drivers and role: A case study from the Mureş River, Transylvania, Romania. Water 2021, 13, 3518. [Google Scholar] [CrossRef]
- Bănăduc, D.; Sas, A.; Cianfaglione, K.; Barinova, S.; Curtean-Bănăduc, A. The role of aquatic refuge habitats for fish, and threats in the context of climate change and human impact, during seasonal hydrological drought in the Saxon Villages area (Transylvania, Romania). Atmosphere 2021, 12, 9–1209. [Google Scholar] [CrossRef]
- Curtean-Bănăduc, A.; Burcea, A.; Mihuţ, C.-M.; Bănăduc, D. The benthic trophic corner stone compartment in POPs transfer from abiotic environment to higher trophic levels—Trichoptera and Ephemeroptera pre-alert indicator role. Water 2021, 13, 1778. [Google Scholar] [CrossRef]
- Bănăduc, D.; Curtean-Bănăduc, A.; Cianfaglione, K.; Akeroyd, J.R.; Cioca, L.-I. Proposed environmental risk management elements in a Carpathian valley basin, within the Roşia Montană European historical mining area. Int. J. Environ. Res. Public Health 2021, 18, 4565. [Google Scholar] [CrossRef] [PubMed]
- Costea, G.; Push, M.T.; Bănăduc, D.; Cosmoiu, D.; Curtean-Bănăduc, A. A review of hydropower plants in Romania: Distribution, current knowledge, and their effects on fish in headwater streams. Renew. Sust. Energy Rev. 2021, 54, 111003. [Google Scholar] [CrossRef]
- Burcea, A.; Boeraş, I.; Mihuţ, C.-M.; Bănăduc, D.; Matei, C.; Curtean-Bănăduc, A. Adding the Mureş River Basin (Transylvania, Romania) to the list of hotspots with high contamination with pharmaceuticals. Sustainability 2020, 12, 10197. [Google Scholar] [CrossRef]
- Curtean-Bănăduc, A.; Burcea, A.; Mihuţ, C.-M.; Berg, V.; Lyche, J.L.; Bănăduc, D. Bioaccumulation of persistent organic pollutants in the gonads of Barbus barbus (Linnaeus, 1758). Ecotoxicol. Environ. Saf. 2020, 201, 110852. [Google Scholar] [CrossRef]
- Curtean-Bănăduc, A.; Bănăduc, D.; Bucşa, C. Watersheds Management (Transylvania/Romania): Implications, risks, solutions. In Strategies to Enhance Environmental Security in Transition Countries, NATO Science for Peace and Security Series C-Environmental Security; Springer: Berlin/Heidelberg, Germany, 2007; pp. 225–238. ISSN 1971-4668. ISBN 978-1-4020-5994-0. [Google Scholar]
- Curtean-Bănăduc, A.; Marić, S.; Gabor, G.; Didenko, A.; Rey Planellas, S.; Bănăduc, D. Hucho hucho (Linnaeus, 1758): Last natural viable population in the Eastern Carpathians—Conservation elements. Turk. J. Zool 2019, 43, 215–223. [Google Scholar] [CrossRef]
- Curtean-Bănăduc, A.; Didenko, A.; Guti, G.; Bănăduc, D. Telestes souffia (Risso, 1827) species conservation at the eastern limit of range—Vişeu River basin, Romania. Appl. Ecol. Environ. Res. 2018, 16, 291–303. [Google Scholar] [CrossRef]
- Andrade, M.C.; Winemiller, K.O.; Barbosa, P.S.; Fortunati, A.; Chelazzi, D.; Cincinelli, A.; Giarrizzo, T. First account of plastic pollution impacting freshwater fishes in the Amazon: Ingestion of plastic debris by piranhas and other serrasalmids with diverse feeding habits. Environ. Pollut. 2019, 244, 766–773. [Google Scholar] [CrossRef]
- Campbell, S.H.; Williamson, P.R.; Hall, B.D. Microplastics in the gastrointestinal tracts of fish and the water from an urban prairie creek. Facets 2017, 2, 395–409. [Google Scholar] [CrossRef]
- Carson, H.S. The incidence of plastic ingestion by fishes: From the prey’s perspective. Mar. Pollut. Bull. 2013, 74, 170–174. [Google Scholar] [CrossRef]
- McNeish, R.E.; Kim, L.H.; Barrett, H.A.; Mason, S.A.; Kelly, J.J.; Hoellein, T.J. Microplastic in riverine fish is connected to species traits. Sci. Rep. 2018, 8, 11639. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Ribeiro, A.; Hylland, K.; Guilhermino, L. Single and combined effects of microplastics and pyrene on juveniles (0+ group) of the common goby Pomatoschistus microps (Teleostei, Gobiidae). Ecol. Indic. 2013, 34, 641–647. [Google Scholar] [CrossRef]
- Rochman, C.M.; Hoh, E.; Kurobe, T.; Teh, S.J. Ingested plastic transfers hazardous chemicals to fish and induces hepatic stress. Sci. Rep. 2013, 3, 3263. [Google Scholar] [CrossRef] [PubMed]
- Silva-Cavalcanti, J.S.; Silva, J.D.B.; de França, E.J.; de Araújo, M.C.B.; Gusmao, F. Microplastics ingestion by a common tropical freshwater fishing resource. Environ. Pollut. 2017, 221, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Del Monte-Luna, P.; Lluch-Belda, D.; Arreguín-Sanchez, F.; Lluch-Cota, S.; Villalobos-Ortiz, H. Approaching the potential of world marine fisheries. Transylv. Rev. Syst. Ecol. Res. 2016, 18, 45–56. [Google Scholar] [CrossRef]
- Thiele, C.J.; Hudson, M.D.; Russell, A.E.; Saluveer, M.; Sidaoui-Haddad, G. Microplastics in fish and fishmeal: An emerging environmental challenge? Sci. Rep. 2021, 11, 2045. [Google Scholar] [CrossRef]
- Karami, A.; Golieskardi, A.; Ho, Y.B.; Larat, V.; Salamatinia, B. Microplastics in eviscerated flesh and excised organs of dried fish. Sci. Rep. 2017, 7, 5473. [Google Scholar] [CrossRef]
- De Sales-Ribeiro, C.; Brito-Casillas, Y.; Fernandez, A.; Caballero, M.J. An end to the controversy over the microscopic detection and effects of pristine microplastics in fish organs. Sci. Rep. 2020, 10, 12434. [Google Scholar] [CrossRef]
- Roch, S.; Friedrich, C.; Brinker, A. Uptake routes of microplastics in fishes: Practical and theoretical approaches to test existing theories. Sci. Rep. 2020, 10, 3896. [Google Scholar] [CrossRef]
- Billard, R. Les Poissons d’Eau Douce des Rivières de France. In Identification, Inventaire et Répartition des 83 Espèces; Delachaux & Niestlé: Lausanne, Switzerland, 1997; p. 192. [Google Scholar]
- Kottelat, M.; Freyhof, J. Handbook of European Freshwater Fishes; Publications Kottelat, Cornol and Freyhof: Berlin, Germany, 2007; p. 646. [Google Scholar]
- Prata, J.C.; Reis, V.; Matos, J.T.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T. A new approach for routine quantification of microplastics using Nile Red and automated software (MP-VAT). Sci. Total Environ. 2019, 690, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- McCall, G.S. Strategies for Quantitative Research: Archaeology by Numbers; Routledge: New York, NY, USA, 2018. [Google Scholar]
- Prata, J.C.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T. Methods for sampling and detection of microplastics in water and sediment: A critical review. TrAC Trends Anal. Chem. 2019, 110, 150–159. [Google Scholar] [CrossRef]

| Chondrostoma nasus | MT (g ww) | GIT (g ww) | GIC (g ww) | Length (cm) | Weight (g) | K |
|---|---|---|---|---|---|---|
| Minimum | 1.00 | 1.00 | 0.42 | 24 | 230 | 264.37 |
| Maximum | 1.01 | 1.01 | 3.15 | 36 | 550 | 583.33 |
| Mean | 1.01 | 1.00 | 1.04 | 31.83 | 437.5 | 457.90 |
| Median | 1.01 | 1.00 | 0.75 | 33.5 | 450 | 465.86 |
| Standard deviation | 0.01 | 0.00 | 0.75 | 3.91 | 86.04 | 72.22 |
| Microplastics | Total Microplastics | Total Particles | Total Fibers | Total Fragments | |
|---|---|---|---|---|---|
| MT | Minimum | 2 | 1 | 0 | 1 |
| Maximum | 20 | 18 | 0 | 2 | |
| Mean | 7.64 | 7.18 | 0 | 1.67 | |
| Median | 7 | 7 | 0 | 2 | |
| Standard deviation | 5.21 | 5.01 | 0 | 0.47 | |
| GIT | Minimum | 1 | 1 | 0 | 1 |
| Maximum | 13 | 13 | 0 | 1 | |
| Mean | 4.45 | 4.8 | 0 | 1 | |
| Median | 3 | 4 | 0 | 1 | |
| Standard deviation | 3.99 | 4.02 | 0 | 0 | |
| GIC | Minimum | 2 | 2 | 1 | 1 |
| Maximum | 207 | 202 | 1 | 4 | |
| Mean | 29.83 | 28.75 | 1 | 2.25 | |
| Median | 11.5 | 10.5 | 1 | 2 | |
| Standard deviation | 54.66 | 53.32 | 0 | 1.3 | |
| SED | Minimum | 11 | 10 | 4 | 1 |
| Maximum | 93 | 75 | 4 | 14 | |
| Mean | 52 | 42.5 | 2 | 7.5 | |
| Median | 52 | 42.5 | 2 | 7.5 | |
| Standard deviation | 57.98 | 45.96 | 2.83 | 9.19 |
| Microplastics | Microplastics/g ww | Particles/g ww | Fibers/g ww | Fragments/g ww | |
|---|---|---|---|---|---|
| MT | Minimum | 1.99 | 1.00 | 0 | 1.00 |
| Maximum | 19.84 | 17.86 | 0 | 2.00 | |
| Mean | 7.58 | 7.12 | 0 | 1.66 | |
| Median | 6.93 | 6.90 | 0 | 1.98 | |
| Standard deviation | 5.16 | 4.96 | 0 | 0.47 | |
| GIT | Minimum | 0.99 | 0.99 | 0 | 0.99 |
| Maximum | 12.94 | 12.94 | 0 | 0.99 | |
| Mean | 4.44 | 4.78 | 0 | 0.99 | |
| Median | 2.99 | 3.97 | 0 | 0.99 | |
| Standard deviation | 3.97 | 4.00 | 0 | 0 | |
| GIC | Minimum | 1.30 | 1.30 | 0.32 | 0.95 |
| Maximum | 130.72 | 127.57 | 2.40 | 2.53 | |
| Mean | 26.06 | 24.97 | 1.40 | 1.88 | |
| Median | 15.45 | 13.91 | 1.44 | 2.03 | |
| Standard deviation | 33.06 | 32.36 | 0.93 | 0.63 |
| Microplastics | Microplastics (µm) | Particles (µm) | Fibers (µm) | Fragments (µm) | |
|---|---|---|---|---|---|
| MT | Minimum | 80.75 | 80.75 | 0 | 198.84 |
| Maximum | 740.52 | 719.28 | 0 | 740.52 | |
| Mean | 227.91 | 214.62 | 0 | 437.78 | |
| Median | 196.47 | 188.46 | 0 | 427.71 | |
| Standard deviation | 135.40 | 120.46 | 0 | 197.26 | |
| GIT | Minimum | 69.12 | 69.12 | 0 | 550.38 |
| Maximum | 557.86 | 557.86 | 0 | 550.38 | |
| Mean | 251.04 | 244.81 | 0 | 550.38 | |
| Median | 196.54 | 192.57 | 0 | 550.38 | |
| Standard deviation | 142.26 | 136.83 | 0 | 0 | |
| GIC | Minimum | 67.77 | 67.77 | 433.96 | 167.33 |
| Maximum | 1236.38 | 701.05 | 1236.38 | 875.66 | |
| Mean | 261.45 | 250.80 | 906.41 | 382.91 | |
| Median | 226.64 | 223.08 | 977.64 | 364.44 | |
| Standard deviation | 150.64 | 125.68 | 385.80 | 219.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Curtean-Bănăduc, A.; Mihuţ, C.; Burcea, A.; McCall, G.S.; Matei, C.; Bănăduc, D. Screening for Microplastic Uptake in an Urbanized Freshwater Ecosystem: Chondrostoma nasus (Linnaeus, 1758) Case Study. Water 2023, 15, 1578. https://doi.org/10.3390/w15081578
Curtean-Bănăduc A, Mihuţ C, Burcea A, McCall GS, Matei C, Bănăduc D. Screening for Microplastic Uptake in an Urbanized Freshwater Ecosystem: Chondrostoma nasus (Linnaeus, 1758) Case Study. Water. 2023; 15(8):1578. https://doi.org/10.3390/w15081578
Chicago/Turabian StyleCurtean-Bănăduc, Angela, Claudia Mihuţ, Alexandru Burcea, Grant S. McCall, Claudiu Matei, and Doru Bănăduc. 2023. "Screening for Microplastic Uptake in an Urbanized Freshwater Ecosystem: Chondrostoma nasus (Linnaeus, 1758) Case Study" Water 15, no. 8: 1578. https://doi.org/10.3390/w15081578







