Integrated Use of Bioaccumulation, Genotoxic, and Haematological Endpoints to Assess the Effect of Water Remediation Strategies on Fish Health: A Complementary Study
Abstract
:1. Introduction
2. Material and Methods
2.1. Chemicals
2.2. Scanning Transmission Electron Microscopy Analysis
2.3. Test Animals
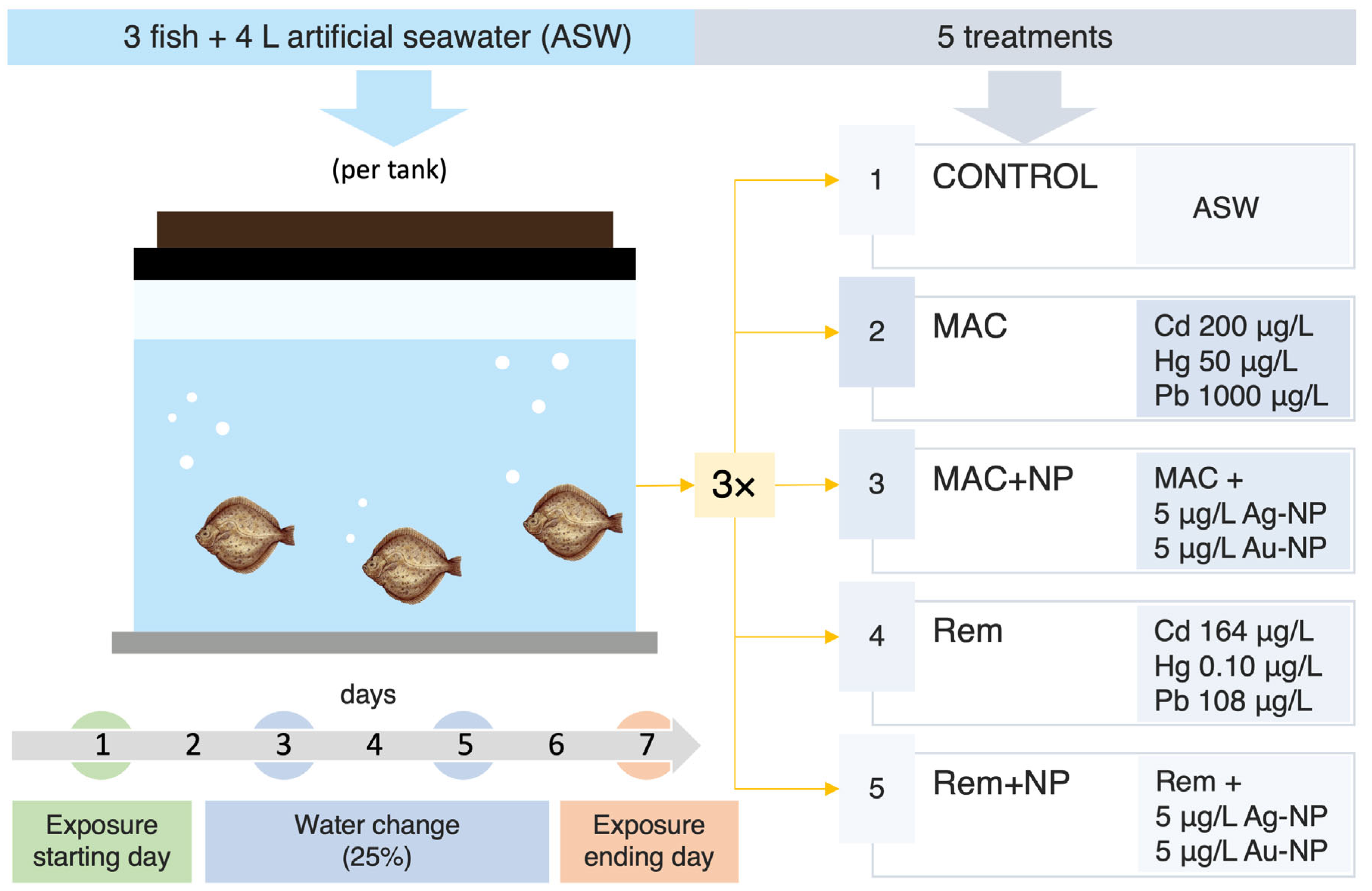
2.4. Experimental Design and Exposure Conditions
2.5. Determination of Cd, Hg, Pb, and Ag in Blood and Kidney
2.6. Genotoxic Potential: Erythrocytic Nuclear Abnormalities (ENA) Assay
2.7. Haematological Dynamics
2.7.1. Erythrocytic Maturity Index (EMI) and Immature Erythrocytes (IE) Frequency
2.7.2. Red Blood Cell (RBC) Count, Haemoglobin (Hb) Concentration, and Mean Cell Haemoglobin (MCH)
2.8. Statistical Analysis and Software
3. Results
3.1. Accumulation of Hg, Cd, Pb, and Ag in the Blood and Kidneys
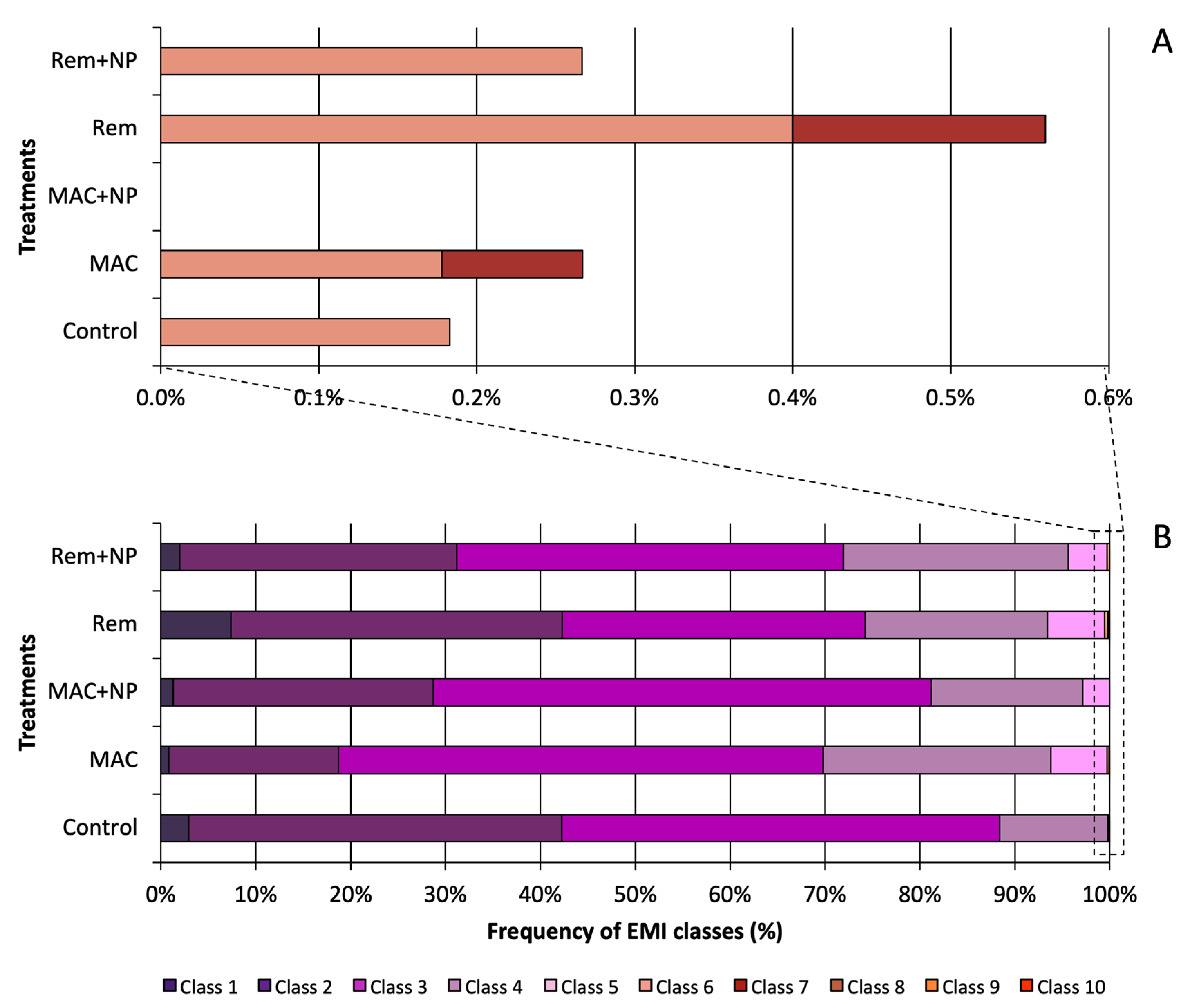
3.2. Genotoxic Potential: Erythrocytic Nuclear Abnormalities (ENA)
3.3. Haematological Dynamics
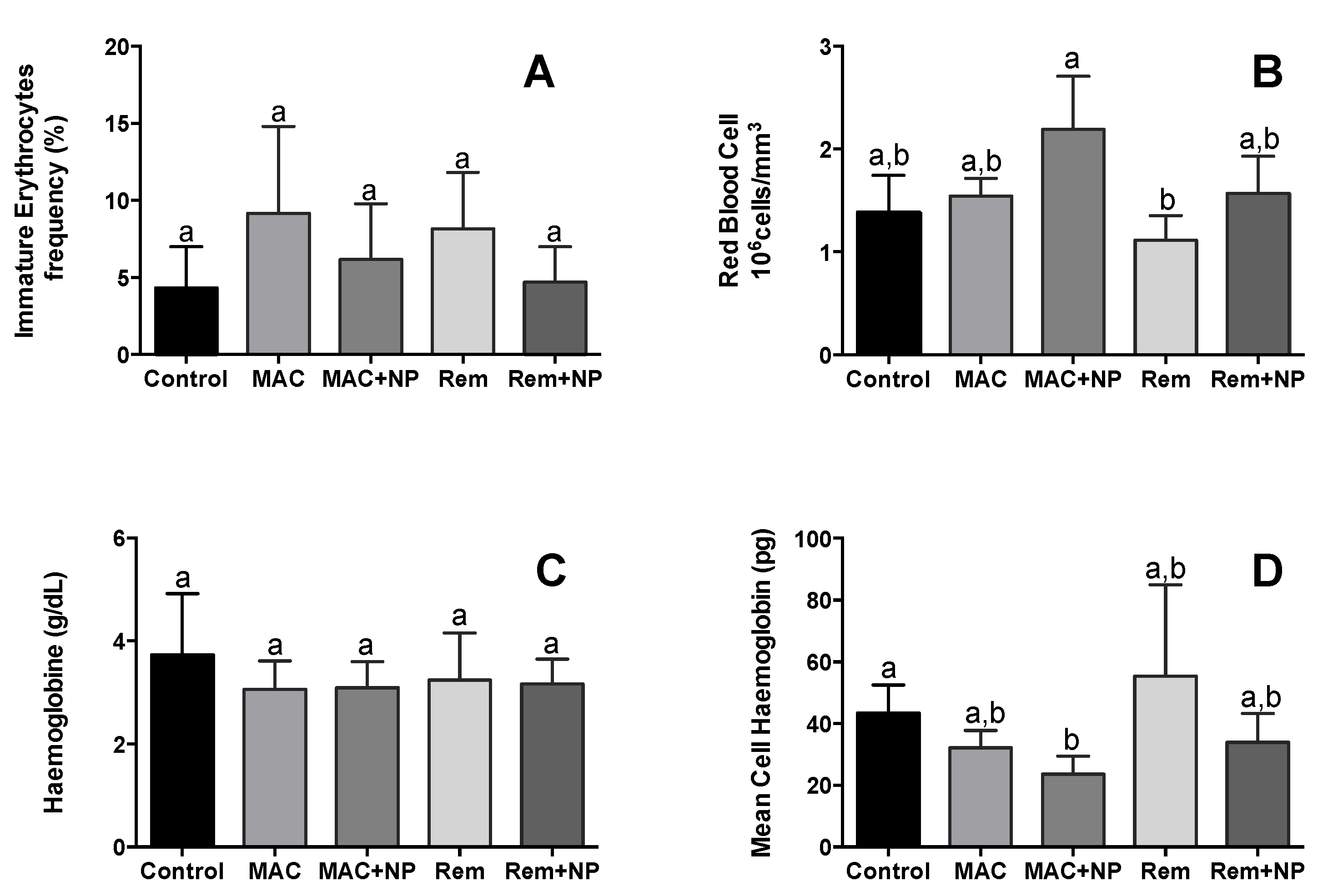
3.3.1. Erythrocytic Maturity Index (EMI) and Immature Erythrocytes Frequency (IE)
3.3.2. Red Blood Cell (RBC) Count, Haemoglobin (Hb) Concentration, and Mean Cell Haemoglobin (MCH)
4. Discussion
4.1. Metals in Fish Blood and Kidneys
4.2. Genotoxicity and Alteration of Haematological Parameters
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The water crisis is worsening. Researchers must tackle it together. Nature 2023, 613, 611–612. [CrossRef] [PubMed]
- Barragán-Peña, P.; Macedo-Miranda, M.G.; Olguin, M.T. Cadmium removal from wastewater in a fixed-bed column system with modified-natural clinoptilolite-rich tuff. Chem. Pap. 2021, 75, 485–491. [Google Scholar] [CrossRef]
- Ozdemir, S.; Dündar, A.; Dizge, N.; Kılınç, E.; Balakrishnan, D.; Prasad, K.S.; Senthilkumar, N. Preconcentrations of Pb(II), Ni(II) and Zn(II) by solid phase bio-extractor using thermophilic Bacillus subtilis loaded multiwalled carbon nanotube biosorbent. Chemosphere 2023, 317, 137840. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Kalamdhad, A.; Sharma, Y.C. Recent advances of nanocellulose as biobased adsorbent for heavy metal ions removal: A sustainable approach integrating with waste management. Environ. Nanotechnol. Monit. Manag. 2023, 20, 100791. [Google Scholar] [CrossRef]
- Pinheiro, P.C.; Tavares, D.S.; Daniel-Da-Silva, A.L.; Lopes, C.B.; Pereira, E.; Araújo, J.P.; Sousa, C.T.; Trindade, T. Ferromagnetic Sorbents Based on Nickel Nanowires for Efficient Uptake of Mercury from Water. ACS Appl. Mater. Interfaces 2014, 6, 8274–8280. [Google Scholar] [CrossRef]
- Fabre, E.; Lopes, C.B.; Vale, C.; Pereira, E.; Silva, C.M. Valuation of banana peels as an effective biosorbent for mercury removal under low environmental concentrations. Sci. Total Environ. 2020, 709, 135883. [Google Scholar] [CrossRef]
- Rocha, L.S.; Lopes, I.; Lopes, C.B.; Henriques, B.; Soares, A.M.V.M.; Duarte, A.C.; Pereira, E. Efficiency of a cleanup technology to remove mercury from natural waters by means of rice husk biowaste: Ecotoxicological and chemical approach. Environ. Sci. Pollut. Res. 2014, 21, 8146–8156. [Google Scholar] [CrossRef]
- Figueira, P.; Henriques, B.; Teixeira, A.; Lopes, C.B.; Reis, A.T.; Monteiro, R.J.R.; Duarte, A.C.; Pardal, M.A.; Pereira, E. Comparative study on metal biosorption by two macroalgae in saline waters: Single and ternary systems. Environ. Sci. Pollut. Res. 2016, 23, 11985–11997. [Google Scholar] [CrossRef]
- Lopes, C.B.; Lopes, I.; Rocha, L.S.; Duarte, A.C.; Soares, A.M.V.M.; Rocha, J.; Pereira, E. A Multidisciplinary Approach to Evaluate the Efficiency of a Clean-Up Technology to Remove Mercury from Water. Bull. Environ. Contam. Toxicol. 2014, 93, 138–143. [Google Scholar] [CrossRef]
- Jonnalagadda, S.; Rao, P. Toxicity, bioavailability and metal speciation. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 1993, 106, 585–595. [Google Scholar] [CrossRef]
- Nurchi, V.M.; Villaescusa, I. Sorption of toxic metal ions by solid sorbents: A predictive speciation approach based on complex formation constants in aqueous solution. Coord. Chem. Rev. 2012, 256, 212–221. [Google Scholar] [CrossRef]
- Liess, M.; Henz, S.; Shahid, N. Modeling the synergistic effects of toxicant mixtures. Environ. Sci. Eur. 2020, 32, 1–10. [Google Scholar] [CrossRef]
- Zorita, I.; Ortiz-Zarragoitia, M.; Apraiz, I.; Cancio, I.; Orbea, A.; Soto, M.; Marigómez, I.; Cajaraville, M.P. Assessment of biological effects of environmental pollution along the NW Mediterranean Sea using red mullets as sentinel organisms. Environ. Pollut. 2008, 153, 157–168. [Google Scholar] [CrossRef]
- Costa, P.M.; Caeiro, S.; Diniz, M.; Lobo-Arteaga, J.; Martins, M.; Ferreira, A.M.; Caetano, M.; Vale, C.; Del Valls, T.; Costa, M.H. Biochemical endpoints on juvenile Solea senegalensis exposed to estuarine sediments: The effect of contaminant mixtures on metallothionein and CYP1A induction. Ecotoxicology 2009, 18, 988–1000. [Google Scholar] [CrossRef]
- EUR-Lex-52012DC0252-EN-EUR-Lex 20. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52012DC0252 (accessed on 20 February 2023).
- Wilk, A.; Romanowski, M.; Wiszniewska, B. Analysis of Cadmium, Mercury, and Lead Concentrations in Erythrocytes of Renal Transplant Recipients from Northwestern Poland. Biology 2021, 10, 62. [Google Scholar] [CrossRef]
- EUR-Lex-32008L0105-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/eli/dir/2008/105/oj (accessed on 20 February 2023).
- Hossain, T.; Khandaker, S.; Bashar, M.M.; Islam, A.; Ahmed, M.; Akter, R.; Alsukaibi, A.K.; Hasan, M.; Alshammari, H.M.; Kuba, T.; et al. Simultaneous toxic Cd(II) and Pb(II) encapsulation from contaminated water using Mg/Al-LDH composite materials. J. Mol. Liq. 2022, 368, 120810. [Google Scholar] [CrossRef]
- Akpor, O.B. Heavy Metal Pollutants in Wastewater Effluents: Sources, Effects and Remediation. Adv. Biosci. Bioeng. 2014, 2, 37. [Google Scholar] [CrossRef]
- Coelho, J.; Mieiro, C.; Pereira, E.; Duarte, A.; Pardal, M. Mercury biomagnification in a contaminated estuary food web: Effects of age and trophic position using stable isotope analyses. Mar. Pollut. Bull. 2013, 69, 110–115. [Google Scholar] [CrossRef]
- Souid, G.; Souayed, N.; Yaktiti, F.; Maaroufi, K. Effect of acute cadmium exposure on metal accumulation and oxidative stress biomarkers of Sparus aurata. Ecotoxicol. Environ. Saf. 2013, 89, 1–7. [Google Scholar] [CrossRef]
- Mieiro, C.L.; Dolbeth, M.; Marques, T.A.; Duarte, A.C.; Pereira, M.E.; Pacheco, M. Mercury accumulation and tissue-specific antioxidant efficiency in the wild European sea bass (Dicentrarchus labrax) with emphasis on seasonality. Environ. Sci. Pollut. Res. 2014, 21, 10638–10651. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, H.; Li, X.; Wang, Z.; Zhu, G.; Jin, T. Effects of lead and cadmium co-exposure on hemoglobin in a Chinese population. Environ. Toxicol. Pharmacol. 2015, 39, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Kang, J.-C. The lead accumulation and hematological findings in juvenile rock fish Sebastes schlegelii exposed to the dietary lead (II) concentrations. Ecotoxicol. Environ. Saf. 2015, 115, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Janicka, M.; Binkowski, J.; Błaszczyk, M.; Paluch, J.; Wojtaś, W.; Massanyi, P.; Stawarz, R. Cadmium, lead and mercury concentrations and their influence on morphological parameters in blood donors from different age groups from southern Poland. J. Trace Elem. Med. Biol. 2015, 29, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.; Kubra, K.T.; Hasan, N.; Awual, E.; Salman, S.; Sheikh, C.; Rehan, A.I.; Rasee, A.I.; Waliullah, R.; Islam, S.; et al. Sustainable ligand-modified based composite material for the selective and effective cadmium(II) capturing from wastewater. J. Mol. Liq. 2023, 371, 121125. [Google Scholar] [CrossRef]
- Organisation for Economic Cooperation and Development (OECD). Nanosafety at the OECD: The First Five Years 2006–2010, Better Policies for Better Lives; OECD: Paris, France, 2011; p. 16. [Google Scholar]
- Farkas, J.; Christian, P.; Urrea, J.G.; Roos, N.; Hassellöv, M.; Tollefsen, K.E.; Thomas, K.V. Effects of silver and gold nanoparticles on rainbow trout (Oncorhynchus mykiss) hepatocytes. Aquat. Toxicol. 2010, 96, 44–52. [Google Scholar] [CrossRef]
- Panda, K.K.; Achary, V.M.M.; Krishnaveni, R.; Padhi, B.K.; Sarangi, S.N.; Sahu, S.N.; Panda, B.B. In vitro biosynthesis and genotoxicity bioassay of silver nanoparticles using plants. Toxicol. In Vitro 2011, 25, 1097–1105. [Google Scholar] [CrossRef]
- Pan, J.-F.; Buffet, P.-E.; Poirier, L.; Amiard-Triquet, C.; Gilliland, D.; Joubert, Y.; Pilet, P.; Guibbolini, M.; de Faverney, C.R.; Roméo, M.; et al. Size dependent bioaccumulation and ecotoxicity of gold nanoparticles in an endobenthic invertebrate: The Tellinid clam Scrobicularia plana. Environ. Pollut. 2012, 168, 37–43. [Google Scholar] [CrossRef]
- Chuang, S.-M.; Lee, Y.-H.; Liang, R.-Y.; Roam, G.-D.; Zeng, Z.-M.; Tu, H.-F.; Wang, S.-K.; Chueh, P.J. Extensive evaluations of the cytotoxic effects of gold nanoparticles. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2013, 1830, 4960–4973. [Google Scholar] [CrossRef]
- Moreno-Garrido, I.; Pérez, S.; Blasco, J. Toxicity of silver and gold nanoparticles on marine microalgae. Mar. Environ. Res. 2015, 111, 60–73. [Google Scholar] [CrossRef]
- Qian, Y.; Zhang, J.; Hu, Q.; Xu, M.; Chen, Y.; Hu, G.; Zhao, M.; Liu, S. Silver nanoparticle-induced hemoglobin decrease involves alteration of histone 3 methylation status. Biomaterials 2015, 70, 12–22. [Google Scholar] [CrossRef]
- Sharma, N.; Rather, M.A.; Ajima, M.N.O.; Gireesh-Babu, P.; Kumar, K.; Sharma, R. Assessment of DNA damage and molecular responses in Labeo rohita (Hamilton, 1822) following short-term exposure to silver nanoparticles. Food Chem. Toxicol. 2016, 96, 122–132. [Google Scholar] [CrossRef]
- Yang, J.; Hou, B.; Wang, J.; Tian, B.; Bi, J.; Wang, N.; Li, X.; Huang, X. Nanomaterials for the Removal of Heavy Metals from Wastewater. Nanomaterials 2019, 9, 424. [Google Scholar] [CrossRef]
- Zahoor, M.; Nazir, N.; Iftikhar, M.; Naz, S.; Zekker, I.; Burlakovs, J.; Uddin, F.; Kamran, A.W.; Kallistova, A.; Pimenov, N.; et al. A Review on Silver Nanoparticles: Classification, Various Methods of Synthesis, and Their Potential Roles in Biomedical Applications and Water Treatment. Water 2021, 13, 2216. [Google Scholar] [CrossRef]
- Karn, B.; Kuiken, T.; Otto, M. Nanotechnology and in Situ Remediation: A Review of the Benefits and Potential Risks. Environ. Health Perspect. 2009, 117, 1813–1831. [Google Scholar] [CrossRef]
- Jha, A.N. Ecotoxicological applications and significance of the comet assay. Mutagenesis 2008, 23, 207–221. [Google Scholar] [CrossRef]
- Patlolla, A.K.; Hackett, D.; Tchounwou, P.B. Genotoxicity study of silver nanoparticles in bone marrow cells of Sprague–Dawley rats. Food Chem. Toxicol. 2015, 85, 52–60. [Google Scholar] [CrossRef]
- Tortella, G.; Rubilar, O.; Durán, N.; Diez, M.; Martínez, M.; Parada, J.; Seabra, A. Silver nanoparticles: Toxicity in model organisms as an overview of its hazard for human health and the environment. J. Hazard. Mater. 2020, 390, 121974. [Google Scholar] [CrossRef]
- Krishnaraj, C.; Harper, S.L.; Yun, S.-I. In Vivo toxicological assessment of biologically synthesized silver nanoparticles in adult Zebrafish (Danio rerio). J. Hazard. Mater. 2016, 301, 480–491. [Google Scholar] [CrossRef]
- Maceda-Veiga, A.; Monroy, M.; Navarro, E.; Viscor, G.; de Sostoa, A. Metal concentrations and pathological responses of wild native fish exposed to sewage discharge in a Mediterranean river. Sci. Total Environ. 2013, 449, 9–19. [Google Scholar] [CrossRef]
- Castro, D.; Mieiro, C.; Coelho, J.; Guilherme, S.; Marques, A.; Santos, M.; Duarte, A.; Pereira, E.; Pacheco, M. Addressing the impact of mercury estuarine contamination in the European eel (Anguilla anguilla L., 1758)—An early diagnosis in glass eel stage based on erythrocytic nuclear morphology. Mar. Pollut. Bull. 2018, 127, 733–742. [Google Scholar] [CrossRef]
- Shahjahan, M.; Islam, J.; Hossain, T.; Alam Mishu, M.; Hasan, J.; Brown, C. Blood biomarkers as diagnostic tools: An overview of climate-driven stress responses in fish. Sci. Total Environ. 2022, 843, 156910. [Google Scholar] [CrossRef] [PubMed]
- EUR-Lex-32009L0090-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32009L0090 (accessed on 20 February 2023).
- Naasz, S.; Altenburger, R.; Kühnel, D. Environmental mixtures of nanomaterials and chemicals: The Trojan-horse phenomenon and its relevance for ecotoxicity. Sci. Total Environ. 2018, 635, 1170–1181. [Google Scholar] [CrossRef] [PubMed]
- EUR-Lex-32010L0063-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32010L0063 (accessed on 20 February 2023).
- Costley, C.T.; Mossop, K.F.; Dean, J.R.; Garden, L.M.; Marshall, J.; Carroll, J. Determination of mercury in environmental and biological samples using pyrolysis atomic absorption spectrometry with gold amalgamation. Anal. Chim. Acta 2000, 405, 179–183. [Google Scholar] [CrossRef]
- Pacheco, M.; Santos, M. Induction of EROD Activity and Genotoxic Effects by Polycyclic Aromatic Hydrocarbons and Resin Acids on the Juvenile Eel (Anguilla anguillaL.). Ecotoxicol. Environ. Saf. 1997, 38, 252–259. [Google Scholar] [CrossRef]
- Marques, A.; Custódio, M.; Guilherme, S.; Gaivão, I.; Santos, M.A.; Pacheco, M. Assessment of chromosomal damage induced by a deltamethrin-based insecticide in fish (Anguilla anguilla L.)—A follow-up study upon exposure and post-exposure periods. Pestic. Biochem. Physiol. 2014, 113, 40–46. [Google Scholar] [CrossRef]
- Guilherme, S.; Válega, M.; Pereira, M.; Santos, M.; Pacheco, M. Erythrocytic nuclear abnormalities in wild and caged fish (Liza aurata) along an environmental mercury contamination gradient. Ecotoxicol. Environ. Saf. 2008, 70, 411–421. [Google Scholar] [CrossRef]
- van Kampen, E.; Zijlstra, W.G. Standardization of hemoglobinometry II. The hemiglobincyanide method. Clin. Chim. Acta 1961, 6, 538–544. [Google Scholar] [CrossRef]
- Muff, S.; Nilsen, E.B.; O’hara, R.B.; Nater, C.R. Rewriting results sections in the language of evidence. Trends Ecol. Evol. 2022, 37, 203–210. [Google Scholar] [CrossRef]
- Heys, K.A.; Shore, R.F.; Pereira, M.G.; Jones, K.C.; Martin, F.L. Risk assessment of environmental mixture effects. RSC Adv. 2016, 6, 47844–47857. [Google Scholar] [CrossRef]
- Ley-Quiñónez, C.; Zavala-Norzagaray, A.; Espinosa-Carreón, T.; Peckham, H.; Marquez-Herrera, C.; Campos-Villegas, L.; Aguirre, A. Baseline heavy metals and metalloid values in blood of loggerhead turtles (Caretta caretta) from Baja California Sur, Mexico. Mar. Pollut. Bull. 2011, 62, 1979–1983. [Google Scholar] [CrossRef]
- Mieiro, C.L.; Pacheco, M.; Pereira, M.E.; Duarte, A.C. Mercury distribution in key tissues of fish (Liza aurata) inhabiting a contaminated estuary—Implications for human and ecosystem health risk assessment. J. Environ. Monit. 2009, 11, 1004–1012. [Google Scholar] [CrossRef]
- Mieiro, C.; Duarte, A.; Pereira, M.; Pacheco, M. Mercury accumulation patterns and biochemical endpoints in wild fish (Liza aurata): A multi-organ approach. Ecotoxicol. Environ. Saf. 2011, 74, 2225–2232. [Google Scholar] [CrossRef]
- Nordberg, G.F.; Fowler, B.A.; Nordberg, M.; Friberg, L.T. Handbook on the Toxicology of Metals; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar] [CrossRef]
- Alissa, E.M.; Ferns, G.A. Heavy Metal Poisoning and Cardiovascular Disease. J. Toxicol. 2011, 2011, 870125. [Google Scholar] [CrossRef]
- De Jonge, M.; Belpaire, C.; Van Thuyne, G.; Breine, J.; Bervoets, L. Temporal distribution of accumulated metal mixtures in two feral fish species and the relation with condition metrics and community structure. Environ. Pollut. 2015, 197, 43–54. [Google Scholar] [CrossRef]
- Lankveld, D.P.K.; Oomen, A.G.; Krystek, P.; Neigh, A.; De Jong, A.T.; Noorlander, C.W.; Van Eijkeren, J.; Geertsma, R.E.; De Jong, W.H. The kinetics of the tissue distribution of silver nanoparticles of different sizes. Biomaterials 2010, 31, 8350–8361. [Google Scholar] [CrossRef]
- Ghedira, J.; Jebali, J.; Bouraoui, Z.; Banni, M.; Guerbej, H.; Boussetta, H. Metallothionein and metal levels in liver, gills and kidney of Sparus aurata exposed to sublethal doses of cadmium and copper. Fish Physiol. Biochem. 2010, 36, 101–107. [Google Scholar] [CrossRef]
- Sonne, C.; Aspholm, O.; Dietz, R.; Andersen, S.; Berntssen, M.H.; Hylland, K. A study of metal concentrations and metallothionein binding capacity in liver, kidney and brain tissues of three Arctic seal species. Sci. Total Environ. 2009, 407, 6166–6172. [Google Scholar] [CrossRef]
- EUR-Lex-32006R1881-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32006R1881 (accessed on 20 February 2023).
- Udroiu, I. The micronucleus test in piscine erythrocytes. Aquat. Toxicol. 2006, 79, 201–204. [Google Scholar] [CrossRef]
- Pacheco, M.; Santos, M.A. Biotransformation, genotoxic, and histopathological effects of environmental contaminants in European eel (Anguilla anguilla L.). Ecotoxicol. Environ. Saf. 2002, 53, 331–347. [Google Scholar] [CrossRef]
- Elahee, K.; Bhagwant, S. Hematological and gill histopathological parameters of three tropical fish species from a polluted lagoon on the west coast of Mauritius. Ecotoxicol. Environ. Saf. 2007, 68, 361–371. [Google Scholar] [CrossRef]
- Adhikari, S.; Sarkar, B.; Chatterjee, A.; Mahapatra, C.; Ayyappan, S. Effects of cypermethrin and carbofuran on certain hematological parameters and prediction of their recovery in a freshwater teleost, Labeo rohita (Hamilton). Ecotoxicol. Environ. Saf. 2004, 58, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.O.; Neto, F.F.; Mela, M.; Silva, P.; Randi, M.; Rabitto, I.; Costa, J.A.; Pelletier, E. Hematological findings in neotropical fish Hoplias malabaricus exposed to subchronic and dietary doses of methylmercury, inorganic lead, and tributyltin chloride. Environ. Res. 2006, 101, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Aceves, M.A.; Lionetti, L.; Faggio, C. Multidisciplinary haematology as prognostic device in environmental and xenobiotic stress-induced response in fish. Sci. Total Environ. 2019, 670, 1170–1183. [Google Scholar] [CrossRef] [PubMed]
- Witeska, M. Erythrocytes in teleost fishes: A review. Zool. Ecol. 2013, 23, 275–281. [Google Scholar] [CrossRef]
- Witeska, M. Stress in fish- hematological and immunological effects of heavy metals. Electron. J. Ichthyol. 2005, 1, 35–41. [Google Scholar]
- Sadauskas-Henrique, H.; Sakuragui, M.M.; Paulino, M.G.; Fernandes, M.N. Using condition factor and blood variable biomarkers in fish to assess water quality. Environ. Monit. Assess. 2011, 181, 29–42. [Google Scholar] [CrossRef]
- Gaber, H.S.; El-Kasheif, M.; Ibrahim, S.; Authman, M.M.N. Effect of water pollution in El-Rahawy drainage canal on hematology and organs of freshwater fish Clarias gariepinus. World Appl. Sci. J. 2013, 21, 329–341. [Google Scholar]
- Fazio, F.; Piccione, G.; Tribulato, K.; Ferrantelli, V.; Giangrosso, G.; Arfuso, F.; Faggio, C. Bioaccumulation of Heavy Metals in Blood and Tissue of Striped Mullet in Two Italian Lakes. J. Aquat. Anim. Health 2014, 26, 278–284. [Google Scholar] [CrossRef]
- Shah, S.L.; Altindag, A. Hematological Parameters of Tench (Tinca tinca L.) after Acute and Chronic Exposure to Lethal and Sublethal Mercury Treatments. Bull. Environ. Contam. Toxicol. 2004, 73, 911–918. [Google Scholar] [CrossRef]
- Azadikhah, D.; Yalsuyi, A.M.; Saha, S.; Saha, N.C.; Faggio, C. Biochemical and Pathophysiological Responses in Capoeta capoeta under Lethal and Sub-Lethal Exposures of Silver Nanoparticles. Water 2023, 15, 585. [Google Scholar] [CrossRef]
- Kondera, E. Haematopoiesis in the head kidney of common carp (Cyprinus carpio L.): A morphological study. Fish Physiol. Biochem. 2011, 37, 355–362. [Google Scholar] [CrossRef]
| Treatments | T-Hg | Cd | Pb | Ag | |
|---|---|---|---|---|---|
| Blood | Control | 0.053 ± 0.020 a | 0.050 | 0.081 | 0.050 |
| MAC | 0.23 ± 0.08 b | 0.20 | 0.27 | 0.010 | |
| MAC + NP | 0.28 ± 0.08 b | 0.25 | 0.16 | 0.004 | |
| Rem | 0.038 ± 0.011 a | 0.097 | 0.44 | <LOD | |
| Rem + NP | 0.041 ± 0.005 a | 0.12 | 0.036 | 0.028 | |
| Kidney | Control | 0.019 ± 0.009 a,c | 0.45 | 0.075 | 0.028 |
| MAC | 0.04 ± 0.01 a,c | 0.73 | 0.52 | 0.033 | |
| MAC + NP | 0.04 ± 0.02 a,c | 0.78 | 0.60 | <LOD | |
| Rem | 0.015 ± 0.002 a | 0.46 | 0.18 | <LOD | |
| Rem + NP | 0.016 ± 0.003 a | 0.66 | 0.16 | 0.007 |
| Treatments | Individual Nuclear Abnormalities | Total ENA | ||||
|---|---|---|---|---|---|---|
| Kidney (k) | Lobed (L) | Segmented (S) | Vacuolated (V) | Micronuclei (MN) | ||
| Control | 3.3 ± 3.1 | 2.7 ± 1.5 | 4.0 ± 5.0 | 0 ± 0 | 0 ± 0 | 10 ± 6 |
| MAC | 4.0 ± 2.0 | 5.3 ± 4.4 | 4.2 ± 4.5 | 0.2 ± 0.4 | 0 ± 0 | 14 ± 9 |
| MAC + NP | 4.3 ± 2.6 | 2.3 ± 3.8 | 3.5 ± 3.7 | 0 ± 0 | 0 ± 0 | 10 ± 9 |
| Rem | 3.8 ± 1.9 | 5.7 ± 3.2 | 3.3 ± 2.5 | 0 ± 0 | 0 ± 0 | 13 ± 6 |
| Rem + NP | 3.0 ± 3.0 | 4.2 ± 3.3 | 2.2 ± 1.6 | 0 ± 0 | 0 ± 0 | 9.3 ± 6.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mieiro, C.L.; Coelho, J.P.; Reis, A.T.; Castro, D.; Figueira, P.; Martinho, F.; Pardal, M.A.; Pereira, E.; Pacheco, M.; Lopes, C.B. Integrated Use of Bioaccumulation, Genotoxic, and Haematological Endpoints to Assess the Effect of Water Remediation Strategies on Fish Health: A Complementary Study. Water 2023, 15, 1564. https://doi.org/10.3390/w15081564
Mieiro CL, Coelho JP, Reis AT, Castro D, Figueira P, Martinho F, Pardal MA, Pereira E, Pacheco M, Lopes CB. Integrated Use of Bioaccumulation, Genotoxic, and Haematological Endpoints to Assess the Effect of Water Remediation Strategies on Fish Health: A Complementary Study. Water. 2023; 15(8):1564. https://doi.org/10.3390/w15081564
Chicago/Turabian StyleMieiro, Cláudia Leopoldina, João Pedro Coelho, Ana Teresa Reis, Diana Castro, Paula Figueira, Filipe Martinho, Miguel A. Pardal, Eduarda Pereira, Mário Pacheco, and Cláudia B. Lopes. 2023. "Integrated Use of Bioaccumulation, Genotoxic, and Haematological Endpoints to Assess the Effect of Water Remediation Strategies on Fish Health: A Complementary Study" Water 15, no. 8: 1564. https://doi.org/10.3390/w15081564






