Hydrochemical Characteristics and Water Quality of Shallow Groundwater in Desert Area of Kunyu City, Southern Margin of Tarim Basin, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Overview of the Research Area
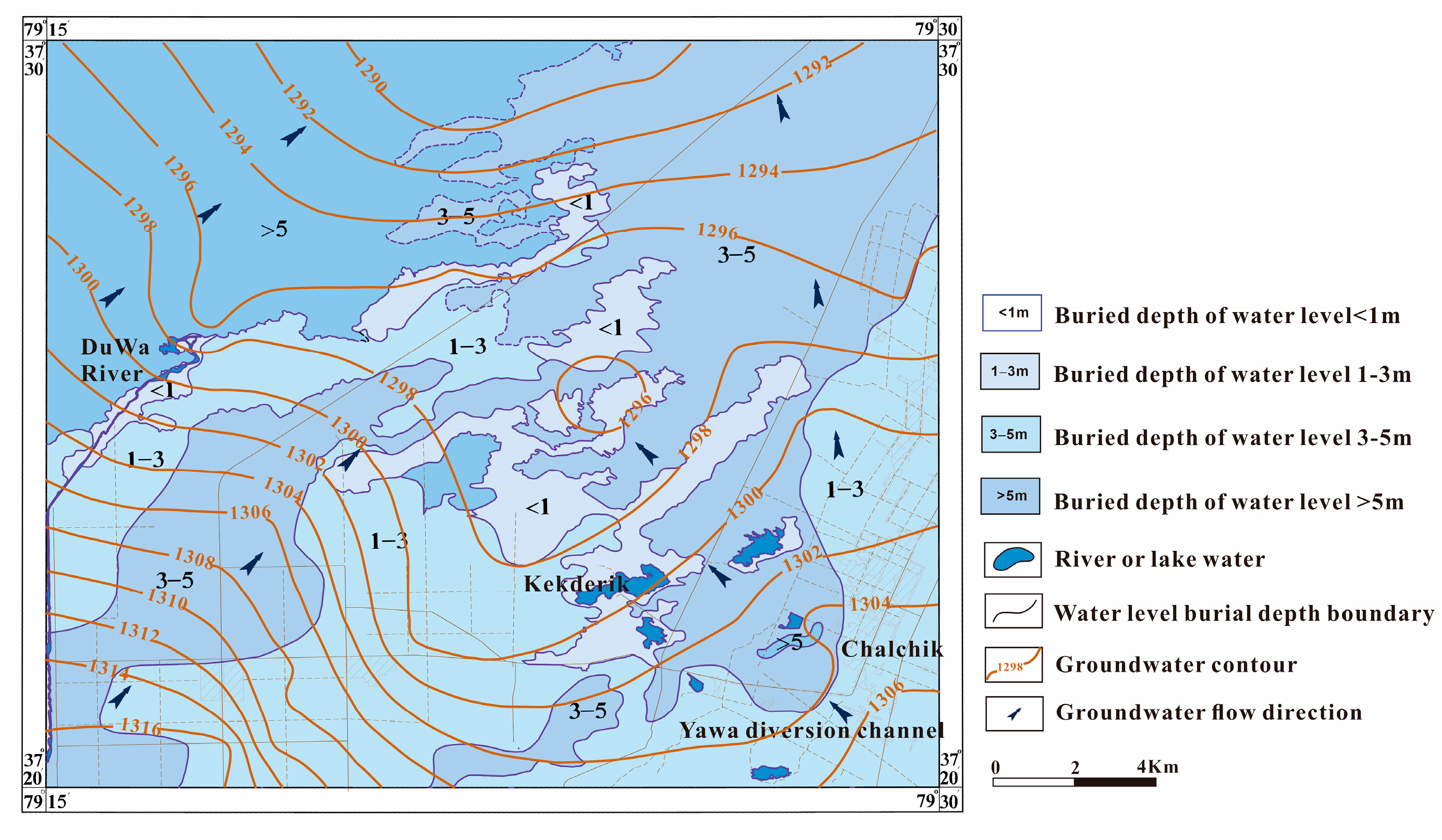
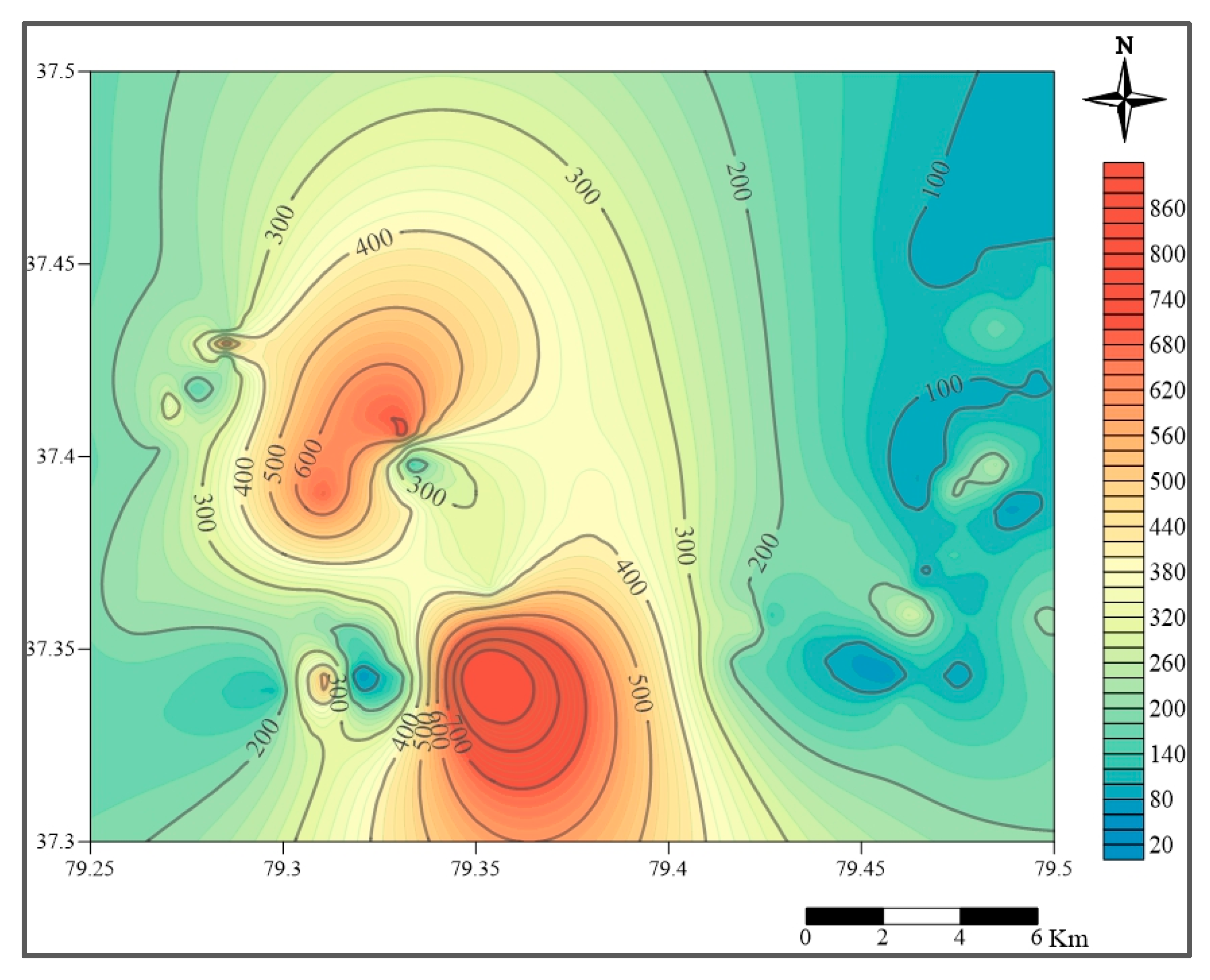
2.2. Hydrogeological Conditions
2.3. Sample Collection and Testing
2.4. Analysis Method
2.4.1. Water Quality Index Method (WQI)
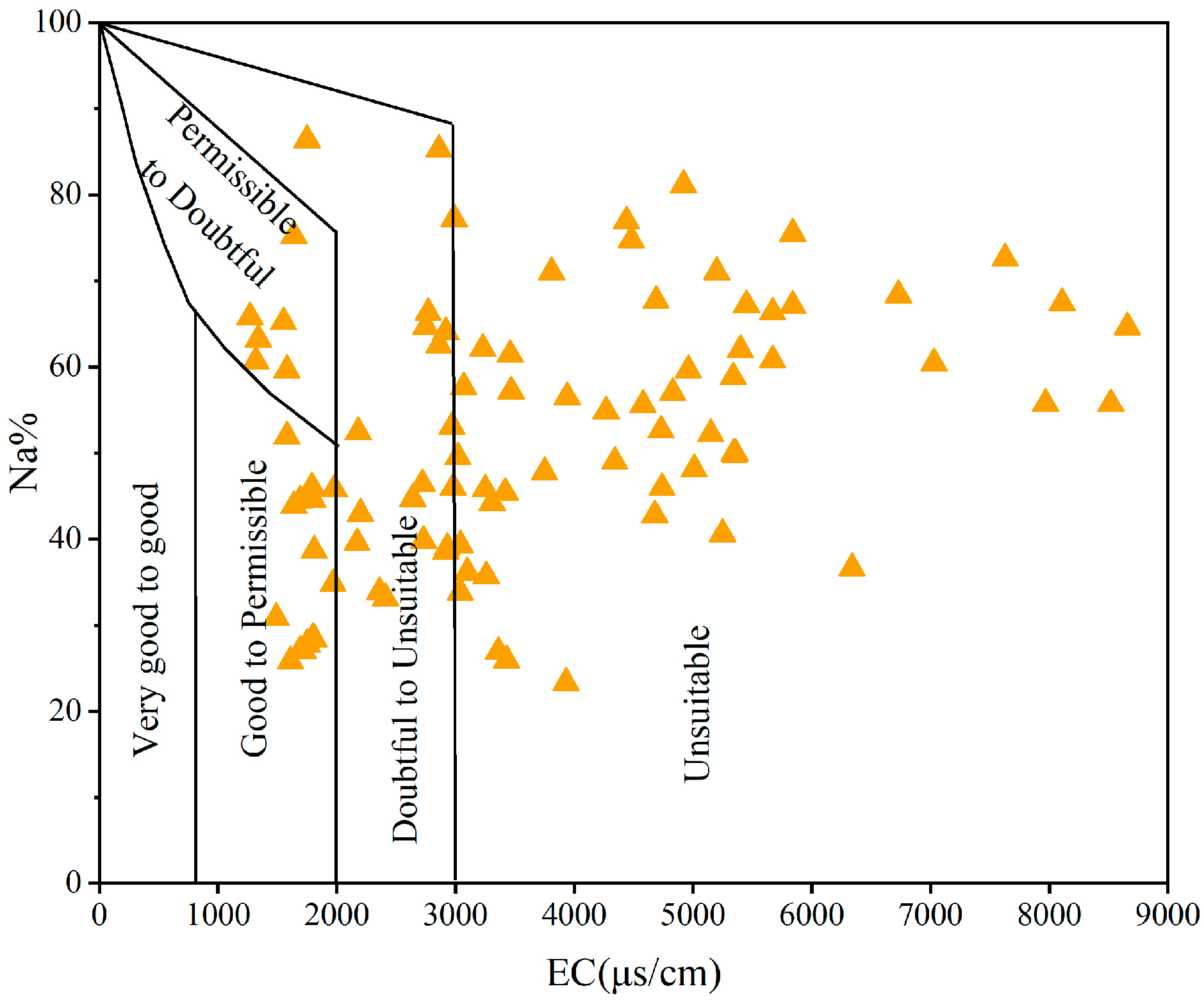
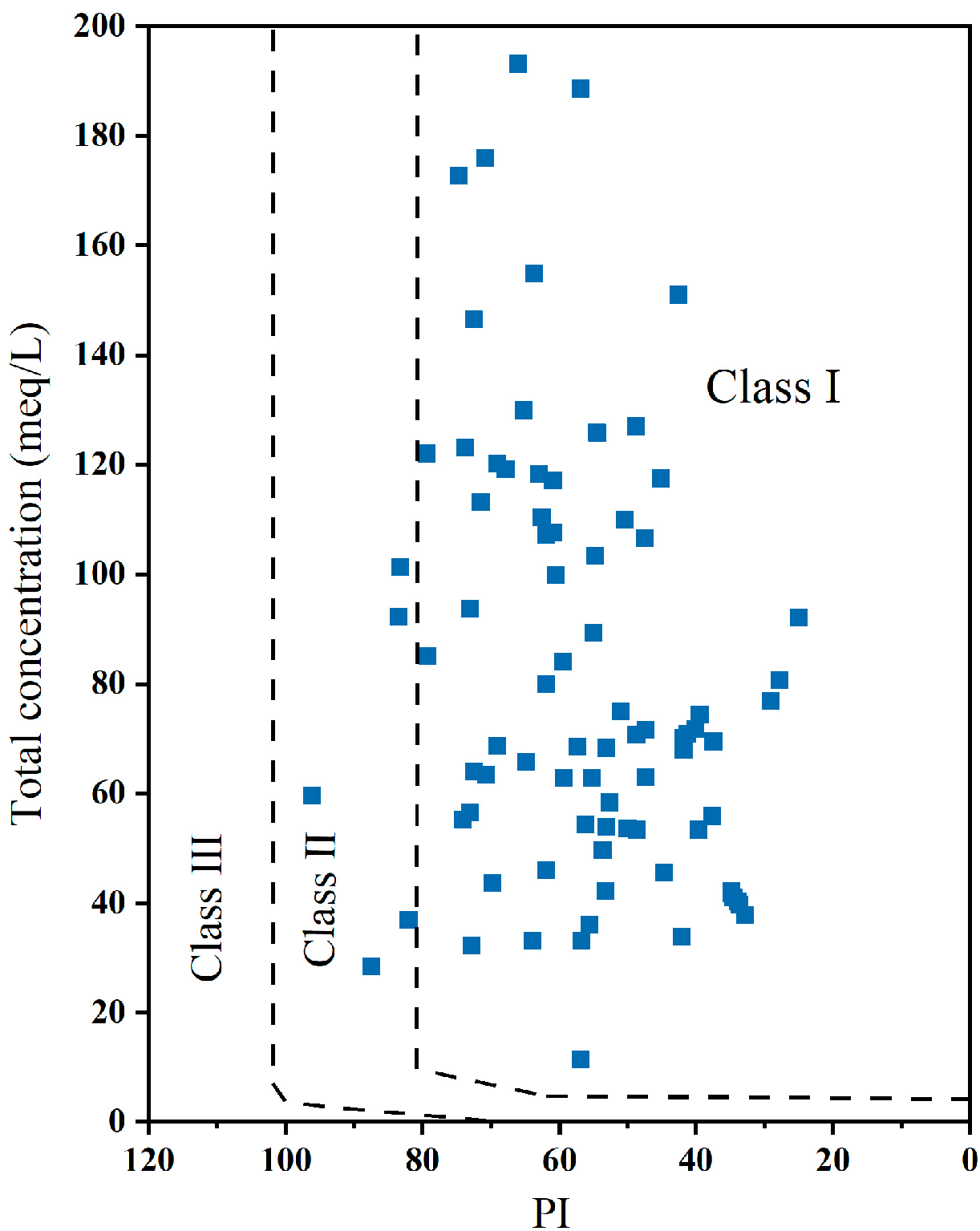
2.4.2. Evaluation Method for Suitability of Irrigation Water
3. Results and Discussion
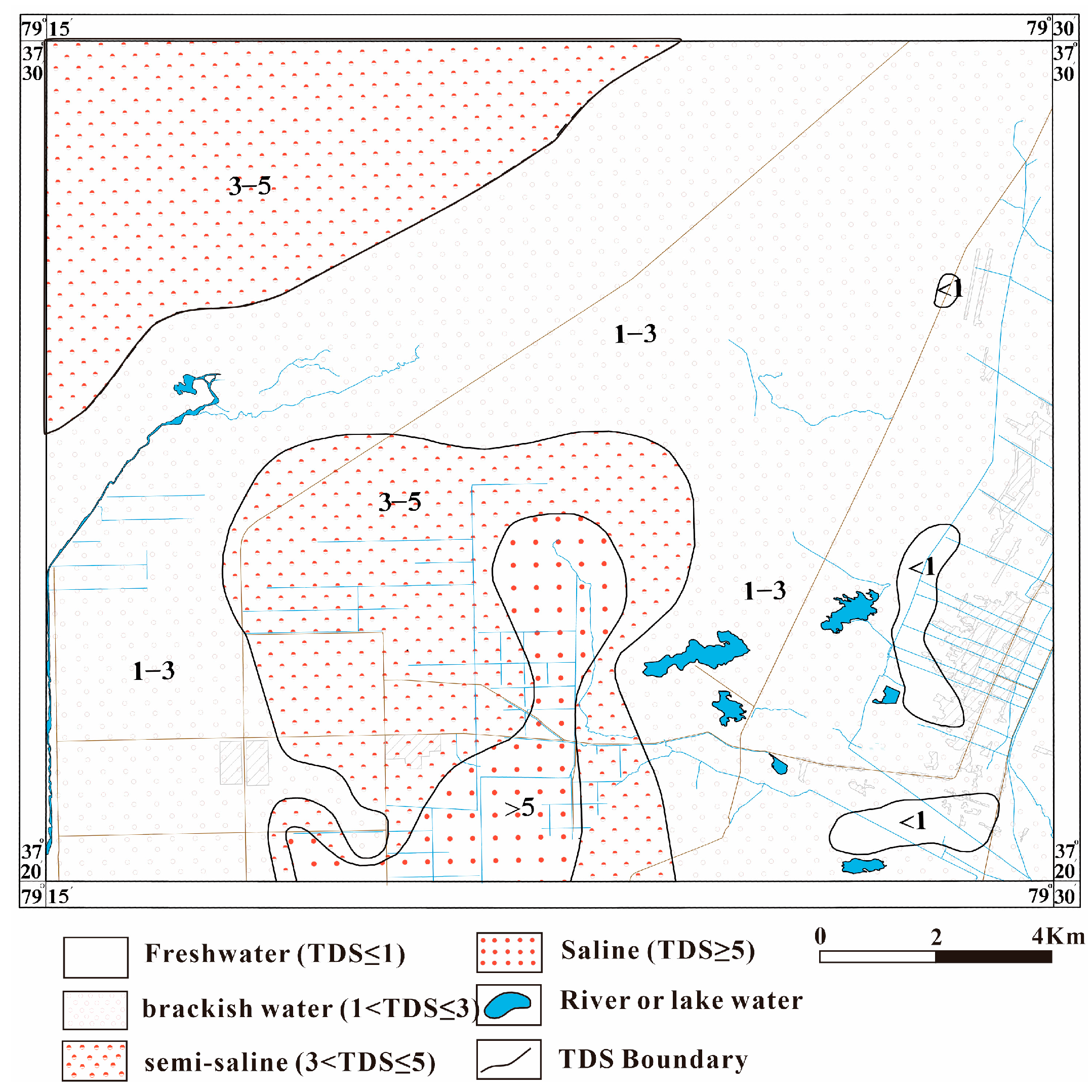
3.1. Basic Characteristics of Hydrochemistry
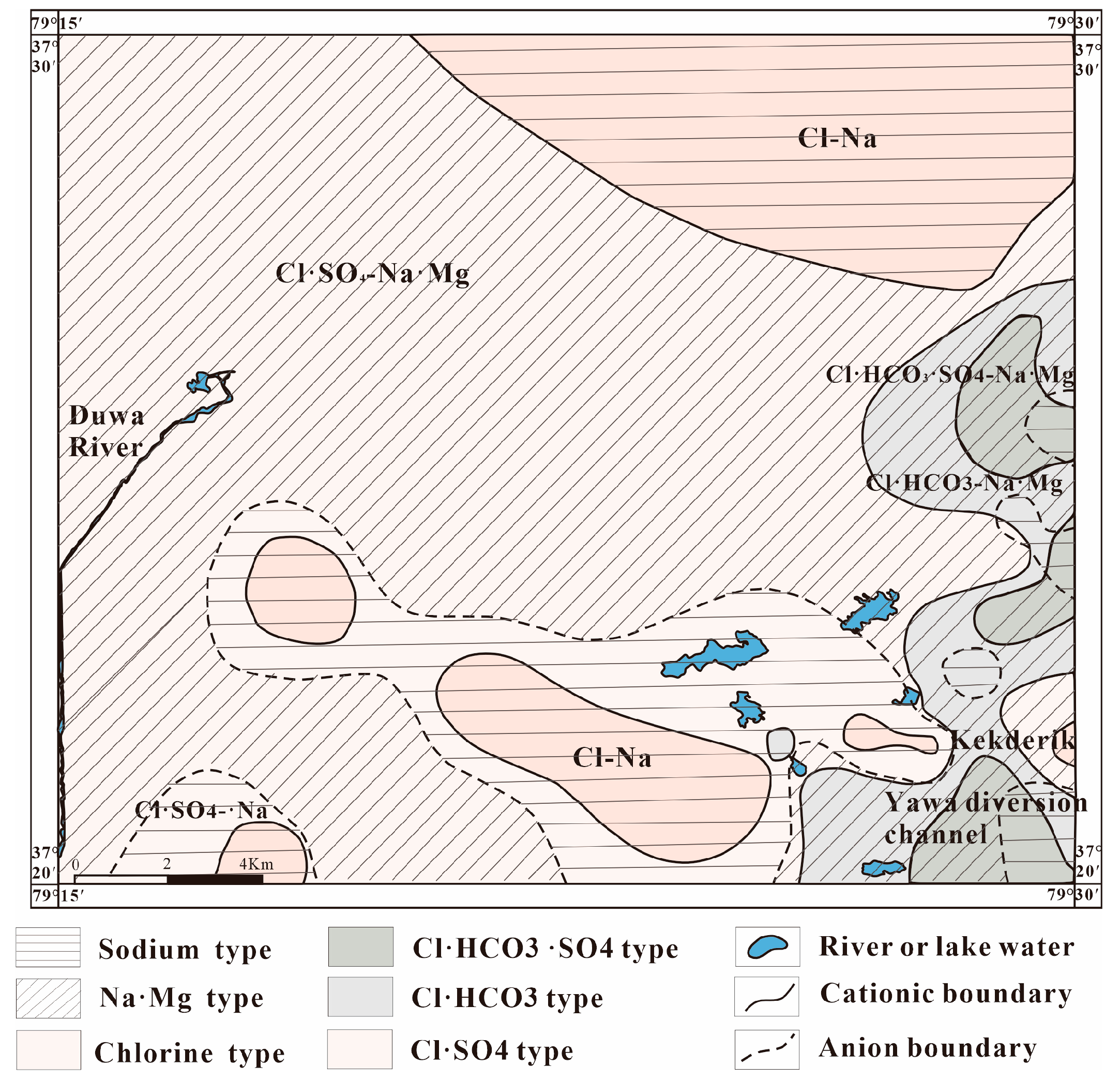
3.2. Hydrochemical Evolution Characteristics
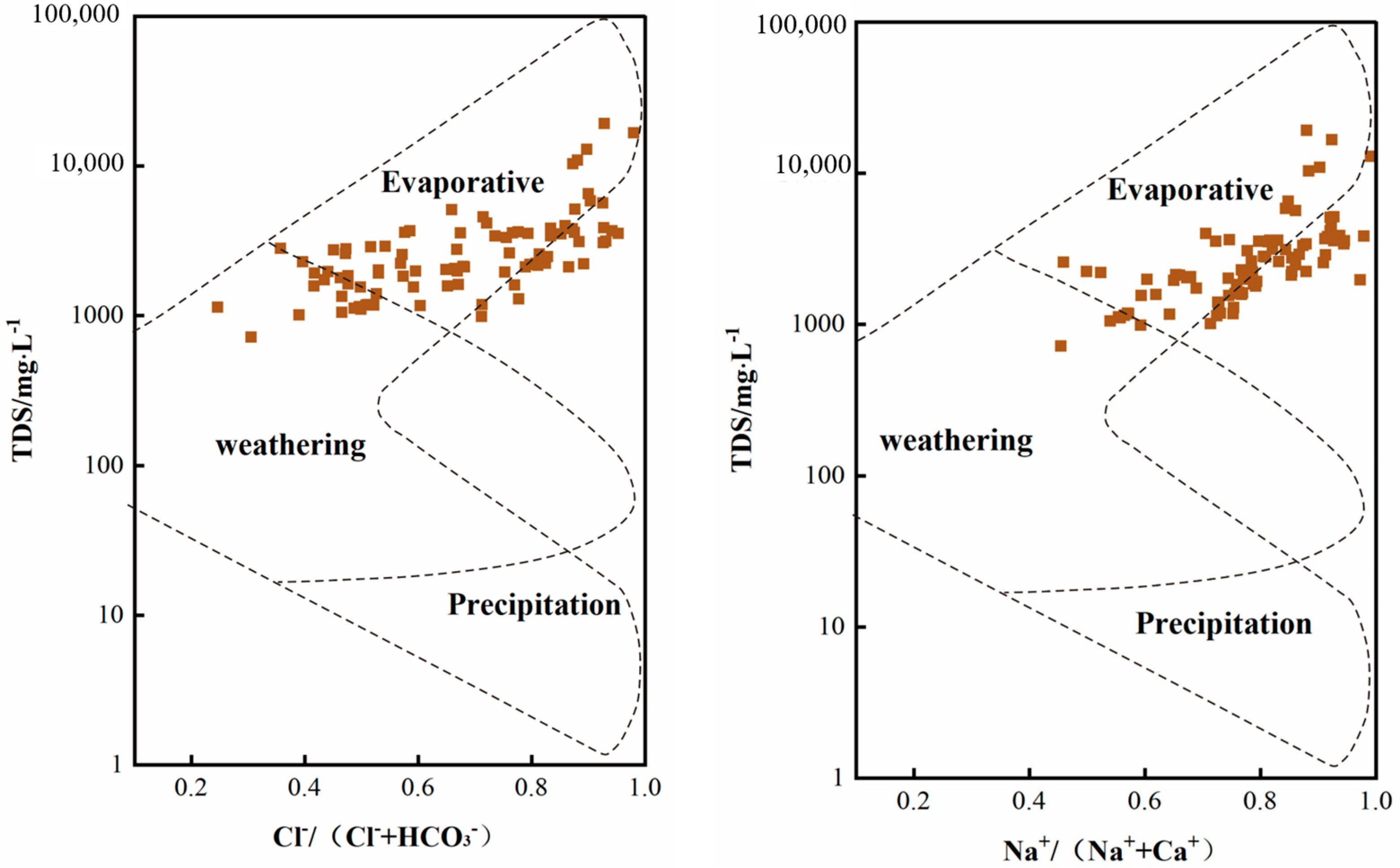
3.3. Analysis of Hydrochemical Origin of Groundwater
3.4. Drinking Water Quality Evaluation
3.5. Irrigation Water Quality Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, P.; Liu, X.; Zhu, W.; Li, C.; Jin, R.; Yan, H.; Gu, C.; Wang, J. Spatio-temporal Changes in Water Conservation Ecosystem Service During 1990–2019 in the Tumen River Basin, Northeast China. Chin. Geogr. Sci. 2023, 33, 102–115. [Google Scholar] [CrossRef]
- Jiang, Y.; Gui, H.; Yu, H.; Wang, M.; Fang, H.; Wang, C.; Chen, C.; Zhang, Y.; Huang, Y. Hydrochemical characteristics and water quality evaluation of rivers in different regions of cities: A case study of Suzhou City in Northern Anhui Province, China. Water 2020, 12, 950. [Google Scholar] [CrossRef]
- Saha, D.; Marwaha, S.; Mukherjee, A. Groundwater resources and sustainable management issues in India. In Clean and Sustainable Groundwater in India; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–11. [Google Scholar]
- Jha, M.K.; Shekhar, A.; Jenifer, M.A. Assessing groundwater quality for drinking water supply using hybrid fuzzy-GIS-based water quality index. Water Res. 2020, 179, 115867. [Google Scholar] [CrossRef] [PubMed]
- Çitakoğlu, H.; Demir, A.; Gemici, B. Regionalization and mapping of dissolved oxygen concentration of sakarya basin by L–moments method. Mühendislik Bilim. Ve Tasarım Derg. 2021, 9, 495–510. [Google Scholar] [CrossRef]
- Li, P.; Qian, H. Water resources research to support a sustainable China. Int. J. Water Resour. Dev. 2018, 34, 327–336. [Google Scholar] [CrossRef]
- Wu, J.; Wang, L.; Wang, S.; Tian, R.; Xue, C.; Feng, W.; Li, Y. Spatiotemporal variation of groundwater quality in an arid area experiencing long-term paper wastewater irrigation, northwest China. Environ. Earth Sci. 2017, 76, 1–14. [Google Scholar] [CrossRef]
- Wu, J.; Sun, Z. Evaluation of shallow groundwater contamination and associated human health risk in an alluvial plain impacted by agricultural and industrial activities, mid-west China. Expo. Health 2016, 8, 311–329. [Google Scholar] [CrossRef]
- Li, X.; Wu, H.; Qian, H.; Gao, Y. Groundwater chemistry regulated by hydrochemical processes and geological structures: A case study in Tongchuan, China. Water 2018, 10, 338. [Google Scholar] [CrossRef]
- Singh, C.K.; Shashtri, S.; Mukherjee, S.; Kumari, R.; Avatar, R.; Singh, A.; Singh, R.P. Application of GWQI to assess effect of land use change on groundwater quality in lower Shiwaliks of Punjab: Remote sensing and GIS based approach. Water Resour. Manag. 2011, 25, 1881–1898. [Google Scholar] [CrossRef]
- Abbasnia, A.; Yousefi, N.; Mahvi, A.H.; Nabizadeh, R.; Radfard, M.; Yousefi, M.; Alimohammadi, M. Evaluation of groundwater quality using water quality index and its suitability for assessing water for drinking and irrigation purposes: Case study of Sistan and Baluchistan province (Iran). Hum. Ecol. Risk Assess. Int. J. 2019, 25, 988–1005. [Google Scholar] [CrossRef]
- Chitsazan, M.; Aghazadeh, N.; Mirzaee, Y.; Golestan, Y. Hydrochemical characteristics and the impact of anthropogenic activity on groundwater quality in suburban area of Urmia city, Iran. Environ. Dev. Sustain. 2019, 21, 331–351. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Singh, P.K.; Mahato, M.K. Environmental geochemistry and a quality assessment of mine water of the west Bokaro Coalfield, India. Mine Water Environ. 2016, 35, 1–11. [Google Scholar] [CrossRef]
- Yan, J.; Chen, J.; Zhang, W. Study on the groundwater quality and its influencing factor in Songyuan City, Northeast China, using integrated hydrogeochemical method. Sci. Total Environ. 2021, 773, 144958. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, A.K.; Sachdeva, P. GIS-based groundwater quality assessment using GQIs and fuzzy-logic approach. Water Environ. J. 2022, 36, 172–182. [Google Scholar] [CrossRef]
- Gharibi, H.; Mahvi, A.H.; Nabizadeh, R.; Arabalibeik, H.; Yunesian, M.; Sowlat, M.H. A novel approach in water quality assessment based on fuzzy logic. J. Environ. Manag. 2012, 112, 87–95. [Google Scholar] [CrossRef]
- Islam, A.T.; Shen, S.; Haque, M.A.; Bodrud-Doza, M.; Maw, K.W.; Habib, M.A. Assessing groundwater quality and its sustainability in Joypurhat district of Bangladesh using GIS and multivariate statistical approaches. Environ. Dev. Sustain. 2018, 20, 1935–1959. [Google Scholar] [CrossRef]
- Maurya, P.K.; Ali, S.A.; Zaidi, S.K.; Wasi, S.; Tabrez, S.; Malav, L.C.; Ditthakit, P.; Son, C.T.; Cabral-Pinto, M.M.S. Assessment of groundwater geochemistry for drinking and irrigation suitability in Jaunpur district of Uttar Pradesh using GIS-based statistical inference. Environ. Sci. Pollut. Res. 2023, 30, 29407–29431. [Google Scholar] [CrossRef] [PubMed]
- ÇITAKOĞLU, H.; Çetin, M.; Çobaner, M.; HAKTANIR, T. Mevsimsel yağışların jeoistatistiksel yöntemle modellenmesi ve gözlemi olmayan noktalarda tahmin edilmesi. Tek. Dergi 2017, 28, 7725–7745. [Google Scholar] [CrossRef]
- Li, P.; Tian, R.; Xue, C.; Wu, J. Progress, opportunities, and key fields for groundwater quality research under the impacts of human activities in China with a special focus on western China. Environ. Sci. Pollut. Res. 2017, 24, 13224–13234. [Google Scholar] [CrossRef]
- Chen, J.; Wu, H.; Qian, H.; Gao, Y. Assessing nitrate and fluoride contaminants in drinking water and their health risk of rural residents living in a semiarid region of Northwest China. Expo. Health 2017, 9, 183–195. [Google Scholar] [CrossRef]
- Chen, J.; Wu, H.; Qian, H.; Li, X. Challenges and prospects of sustainable groundwater management in an agricultural plain along the Silk Road Economic Belt, north-west China. Int. J. Water Resour. Dev. 2018, 34, 354–368. [Google Scholar] [CrossRef]
- Lu, Q.; Liu, F.; Li, Y.; Wang, D. Study on the Relationship between Water Resources Utilization and Economic Growth in Tarim River basin from the Perspective of Water Footprint. Water 2022, 14, 1655. [Google Scholar] [CrossRef]
- Huang, L.; Sun, Z.; Zhou, A.; Bi, J.; Liu, Y. Source and enrichment mechanism of fluoride in groundwater of the Hotan Oasis within the Tarim Basin, Northwestern China. Environ. Pollut. 2022, 300, 118962. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, Y.; Wang, W.; Jiang, J.; Cai, M.; Xu, Y. Evolution characteristics of groundwater and its response to climate and land-cover changes in the oasis of dried-up river in Tarim Basin. J. Hydrol. 2021, 594, 125644. [Google Scholar] [CrossRef]
- Wang, W.; Chen, Y.; Wang, W. Groundwater recharge in the oasis-desert areas of northern Tarim Basin, Northwest China. Hydrol. Res. 2020, 51, 1506–1520. [Google Scholar] [CrossRef]
- Kopáček, J.; Hejzlar, J.; Mosello, R. Estimation of organic acid anion concentrations and evaluation of charge balance in atmospherically acidified colored waters. Water Res. 2000, 34, 3598–3606. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, X.; Chen, Y.; Cai, Y.; Deng, J. Assessing river water quality using water quality index in Lake Taihu Basin, China. Sci. Total Environ. 2018, 612, 914–922. [Google Scholar] [CrossRef]
- Ener, E.; Davraz, A. Evaluation of water quality using water quality index (WQI) method and GIS in Aksu River (SW-Turkey). Sci. Total Environ. 2017, s584–s585, 131–144. [Google Scholar]
- Abbasnia, A.; Alimohammadi, M.; Mahvi, A.H.; Nabizadeh, R.; Yousefi, M.; Mohammadi, A.A.; Pasalari, H.; Mirzabeigi, M. Assessment of groundwater quality and evaluation of scaling and corrosiveness potential of drinking water samples in villages of Chabahr city, Sistan and Baluchistan province in Iran. Data Brief 2018, 16, 182–192. [Google Scholar] [CrossRef]
- Sahoo, S.; Khaoash, S. Impact assessment of coal mining on groundwater chemistry and its quality from Brajrajnagar coal mining area using indexing models. J. Geochem. Explor. 2020, 215, 106559. [Google Scholar] [CrossRef]
- Elsayed, S.; Hussein, H.; Moghanm, F.S.; Khedher, K.M.; Eid, E.M.; Gad, M. Application of irrigation water quality indices and multivariate statistical techniques for surface water quality assessments in the Northern Nile Delta, Egypt. Water 2020, 12, 3300. [Google Scholar] [CrossRef]
- Khosravi, R.; Eslami, H.; Almodaresi, S.A.; Heidari, M.; Fallahzadeh, R.A.; Taghavi, M.; Khodadadie, M.; Peirovi, R. Use of geographic information system and water quality index to assess groundwater quality for drinking purpose in Birjand City, Iran. Desalin Water Treat 2017, 67, 74–83. [Google Scholar] [CrossRef]
- Ministry of Health, PRC. Sanitary Standard for Drinking Water: GB 5749-2006; China Standards Press: Beijing, China, 2007. [Google Scholar]
- Wu, J.; Li, P.; Qian, H. Hydrochemical characterization of drinking groundwater with special reference to fluoride in an arid area of China and the control of aquifer leakage on its concentrations. Environ. Earth Sci. 2015, 73, 8575–8588. [Google Scholar] [CrossRef]
- Miao, Q.; Li, X.; Xu, Y.; Liu, C.; Xie, R.; Lv, Z. Chemical characteristics of groundwater and source identification in a coastal city. PLoS ONE 2021, 16, e0256360. [Google Scholar] [CrossRef]
- Li, P.; He, X.; Li, Y.; Xiang, G. Occurrence and Health Implication of fluoride in groundwater of loess aquifer in the Chinese Loess Plateau: A Case Study of Tongchuan, Northwest China. Expo. Health 2019, 11, 95–107. [Google Scholar] [CrossRef]
- Akshitha, V.; Balakrishna, K.; Udayashankar, H.N. Assessment of hydrogeochemical characteristics and saltwater intrusion in selected coastal aquifers of southwestern India. Mar. Pollut. Bull. 2021, 173, 112989. [Google Scholar] [CrossRef]
- Ju, Q.; Liu, Y.; Hu, Y.; Wang, Y.; Liu, Q.; Wang, Z. Hydrogeochemical evolution and control mechanism of underground multiaquifer system in coal mine area. Geofluids 2020, 2020, 8820650. [Google Scholar] [CrossRef]
- Li, P.; Wu, J.; Qian, H. Hydrochemical appraisal of groundwater quality for drinking and irrigation purposes and the major influencing factors: A case study in and around Hua County, China. Arab. J. Geosci. 2016, 9, 15. [Google Scholar] [CrossRef]
- Li, P.; Tian, R.; Liu, R. Solute geochemistry and multivariate analysis of water quality in the Guohua phosphorite mine, Guizhou Province, China. Expo. Health 2019, 11, 81–94. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, Z.; Sun, Y. Mechanism of Changes in goaf water hydrogeochemistry: A case study of the menkeqing coal mine. Int. J. Environ. Res. Public Health 2022, 20, 536. [Google Scholar] [CrossRef]
- Gountô, A.; Kouamé, I.K.; Mangoua, J.M.O.; Kouassi, A.K.; Savané, I. Using water quality index for assessing of physicochemical quality of quaternary groundwater in the southern part of Abidjan district (Cte d′Ivoire). J. Water Resour. Prot. 2019, 11, 14. [Google Scholar]
- Esmaeili-Vardanjani, M.; Rasa, I.; Amiri, V.; Yazdi, M.; Pazand, K. Evaluation of groundwater quality and assessment of scaling potential and corrosiveness of water samples in Kadkan aquifer, Khorasan-e-Razavi Province, Iran. Environ. Monit. Assess. 2015, 187, 53. [Google Scholar] [CrossRef] [PubMed]
- Ketata, M.; Gueddari, M.; Bouhlila, R. Suitability assessment of shallow and deep groundwaters for drinking and irrigation use in the £1 Khairat aquifer (Enfidha, Tunisian Sahel). Environ. Geol. 2012, 65, 313–330. [Google Scholar] [CrossRef]
- Doneen, L.D. Water Quality for Agriculture; Department of Irrigation: Lalitpur, Nepal, 1964. [Google Scholar]






| Evaluation Parameters | Calculation Formulas |
|---|---|
| SAR | SAR = Na/[(Ca + Mg)/2]0.5 |
| Na% | Na% = (Na + K)/(Ca + Mg + Na + K) × 100% |
| MH | MH = Mg/(Ca + Mg) × 100% |
| PI | PI = (Na + )/(Ca + Mg + Na) × 100% |
| Project | Na+ | K+ | Ca2+ | Mg2+ | Cl− | SO42− | HCO3− | NO3− | CODMn | F− | TDS | TH | pH | EC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | 54.5 | 1.8 | 10.9 | 10.9 | 49.4 | 71.3 | 51.7 | 0.1 | 0.8 | 0.3 | 714 | 126.4 | 6.9 | 1320.0 |
| Max | 4830.0 | 171.0 | 592.0 | 750 | 7575.0 | 6416.0 | 913.0 | 354.0 | 7.0 | 2.9 | 19,100.0 | 35,350.0 | 9.3 | 8660.0 |
| Mean | 696.1 | 36.8 | 118.3 | 135.0 | 950.4 | 824.1 | 341.9 | 15.2 | 2.3 | 1.2 | 3028.7 | 1232.8 | 7.9 | 3953.4 |
| SD | 821.4 | 30.7 | 93.6 | 103.0 | 1139.6 | 872.4 | 212.3 | 45.3 | 1.6 | 0.9 | 3030.3 | 3665.5 | 0.4 | 1842.9 |
| CV% | 118.0 | 83.5 | 79.1 | 76.3 | 119.9 | 105.9 | 62.1 | 297.3 | 68.1 | 74.5 | 100.0 | 297.3 | 4.9 | 46.6 |
| Indicators | TDS | NO3− | SO42− | COD | PH | F− | Cl− | Na+ | TH |
|---|---|---|---|---|---|---|---|---|---|
| Limit value | 1000 mg/L | 10 mg/L | 250 mg/L | 3 mg/L | 6.5–8.5 | 1 mg/L | 250 mg/L | 200 mg/L | 450 mg/L |
| Weight | 5 | 5 | 4 | 4 | 4 | 4 | 3 | 2 | 1 |
| Relative weights | 0.156 | 0.156 | 0.125 | 0.125 | 0.125 | 0.125 | 0.094 | 0.063 | 0.031 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, R.; Dong, S.; Zhang, M.; Zhou, Z.; Zhang, C.; Li, P.; Bai, M. Hydrochemical Characteristics and Water Quality of Shallow Groundwater in Desert Area of Kunyu City, Southern Margin of Tarim Basin, China. Water 2023, 15, 1563. https://doi.org/10.3390/w15081563
Tang R, Dong S, Zhang M, Zhou Z, Zhang C, Li P, Bai M. Hydrochemical Characteristics and Water Quality of Shallow Groundwater in Desert Area of Kunyu City, Southern Margin of Tarim Basin, China. Water. 2023; 15(8):1563. https://doi.org/10.3390/w15081563
Chicago/Turabian StyleTang, Runchi, Shuning Dong, Mengfei Zhang, Zhenfang Zhou, Chenghang Zhang, Pei Li, and Mengtong Bai. 2023. "Hydrochemical Characteristics and Water Quality of Shallow Groundwater in Desert Area of Kunyu City, Southern Margin of Tarim Basin, China" Water 15, no. 8: 1563. https://doi.org/10.3390/w15081563
APA StyleTang, R., Dong, S., Zhang, M., Zhou, Z., Zhang, C., Li, P., & Bai, M. (2023). Hydrochemical Characteristics and Water Quality of Shallow Groundwater in Desert Area of Kunyu City, Southern Margin of Tarim Basin, China. Water, 15(8), 1563. https://doi.org/10.3390/w15081563







