Determination of Anticancer Drugs in the Aquatic Environment by SPE–LC–MS/MS—A Lebanese Case Study
Abstract
1. Introduction
1.1. Background
1.2. Lebanon Case
2. Materials and Methods
2.1. Materials and Instrumentations
2.2. Health and Safety Considerations
2.3. Stock Solutions Preparation
2.4. LC–MS/MS Method Development
2.5. Solid-Phase Extraction Optimisation
2.6. SPE–LC–MS/MS Method Validation
2.7. Matrix Effect
2.8. Method Application
2.9. Analysis
3. Results and Discussion
3.1. LC–MS/MS Method Development
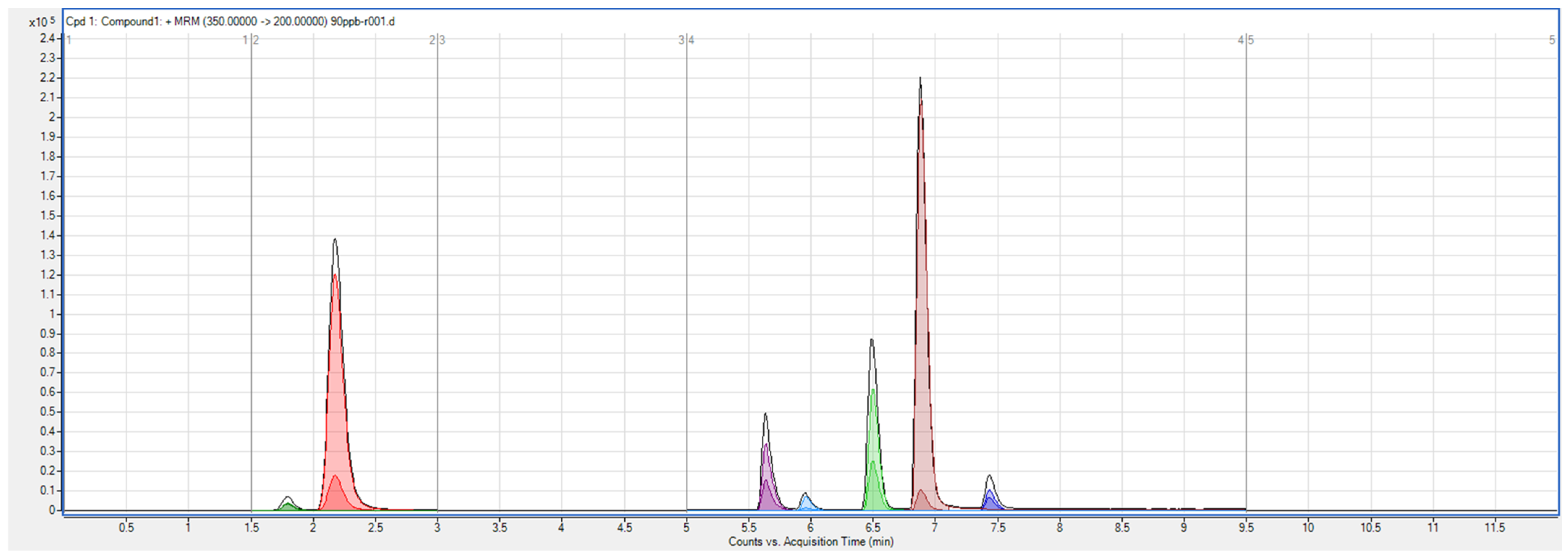
3.2. System Suitability
3.3. Solid-Phase Extraction Optimization
- (1)
- Oasis HLB cartridge for the extraction of methotrexate, cyclophosphamide, tamoxifen, and docetaxel:
- Conditioning: 3 mL methanol
- Equilibrating: 3 mL water (pH 5)
- Sample loading: 50 mL (pH 5)
- Washing: 3 mL water (pH 5)
- Drying: 20 min
- Soaking and eluting: 3 mL methanol (soaking for 5 min before eluting)
- Reconstituting: 495 µL methanol and 5 µL internal standard (600 ng/mL)
- (2)
- Isolute ENV+ cartridge for the extraction of 5-fluorouracil and gemcitabine:
- Conditioning: 3 mL × 2 methanol
- Equilibrating: 3 mL × 2 water (pH 5)
- Sample loading: 50 mL (pH 5)
- Washing: 3 mL × 2 water (pH 5)
- Drying: 2 h
- Soaking and eluting: 3 mL × 2 methanol (soaking for 5 min before eluting)
- Reconstituting: 495 µL water and 5 µL internal standard (600 ng/mL)
3.4. SPE–LC–MS/MS Method Validation
3.5. Matrix Effect
3.6. Method Application
| Compound | Sample Source | Concentration (ng/L) | Ref. |
|---|---|---|---|
| 5-FU | Switzerland—Hospital WW | 27 | [33] |
| France—Hospital WW | 90–4000 | [37] | |
| Slovenia—WWTP influent | 3.1–14 | [35,41] | |
| Spain—WWTP influent | 3.5 | [35] | |
| MET | Italy—WWTP effluent | 12.6 | [68] |
| China—Hospital WW | 4–4689 | [69] | |
| China—WWTP influent | 1.6–18.1 | [70] | |
| Spain—WWTP influent | 2.1–20.1 | [51] | |
| Canada—WWTP influent | 17–60 | [71] | |
| Canada—WWTP effluent | 13–53 | [71] | |
| Spain—WWTP influent | 7.3–55.8 | [48] | |
| Slovenia—WWTP influent | 303 | [35] | |
| Greece—WWTP influent | 433 | [67] | |
| CP | Italy—WWTP effluent | 2.1–9.0 | [68] |
| Switzerland—Surface water | 0.05–0.17 | [72] | |
| China—Hospital WW | 6–2000 | [69] | |
| Canada—WWTP influent | 17–22 | [71] | |
| Canada—WWTP effluent | 18–21 | [71] | |
| Spain—WWTP influent | 13,100 | [61] |
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hernández-Tenorio, R.; González-Juárez, E.; Guzmán-Mar, J.L.; Hinojosa-Reyes, L.; Hernández-Ramírez, A. Review of Occurrence of Pharmaceuticals Worldwide for Estimating Concentration Ranges in Aquatic Environments at the End of the Last Decade. J. Hazard. Mater. Adv. 2022, 8, 100172. [Google Scholar] [CrossRef]
- Courtier, A.; Cadiere, A.; Roig, B. Human Pharmaceuticals: Why and How to Reduce Their Presence in the Environment. Curr. Opin. Green Sustain. Chem. 2019, 15, 77–82. [Google Scholar] [CrossRef]
- Adeleye, A.S.; Xue, J.; Zhao, Y.; Taylor, A.A.; Zenobio, J.E.; Sun, Y.; Han, Z.; Salawu, O.A.; Zhu, Y. Abundance, Fate, and Effects of Pharmaceuticals and Personal Care Products in Aquatic Environments. J. Hazard. Mater. 2022, 424, 127284. [Google Scholar] [CrossRef]
- Li, W.C. Occurrence, Sources, and Fate of Pharmaceuticals in Aquatic Environment and Soil. Environ. Pollut. 2014, 187, 193–201. [Google Scholar] [CrossRef]
- Branchet, P.; Arpin-Pont, L.; Piram, A.; Boissery, P.; Wong-Wah-Chung, P.; Doumenq, P. Pharmaceuticals in the Marine Environment: What Are the Present Challenges in Their Monitoring? Sci. Total Environ. 2021, 766, 142644. [Google Scholar] [CrossRef] [PubMed]
- Dulio, V.; Slobodnik, J. NORMAN-Network of Reference Laboratories, Research Centres and Related Organisations for Monitoring of Emerging Substances. Environ. Sci. Pollut. Res. 2009, 16, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Gomez Cortes, L.; Marinov, D.; Sanseverino, I.; Navarro Cuenca, A.; Niegowska, M.; Porcel Rodriguez, E.; Stefanelli, F.; Lettieri, T. Selection of Substances for the 4th Watch List under the Water Framework Directive; Publications Office of the European Union: Luxembourg, 2022.
- Geara-Matta, D.; Moilleron, R.; El Samarani, A.; Lorgeoux, C.; Chebbo, G. State of Art About Water Uses and Wastewater Management in Lebanon. World Wide Workshop Young Environ. Sci. 2010, 11, 139–152. [Google Scholar]
- MOE/UNDP/ECODIT. State and Trends of the Lebanese Environment. 2011. Available online: https://www.undp.org/lebanon/publications/state-trends-lebanese-environment (accessed on 10 April 2023).
- Houri, A.; El Jeblawi, S.W. Water Quality Assessment of Lebanese Coastal Rivers during Dry Season and Pollution Load into the Mediterranean Sea. J. Water Health 2007, 5, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Merhaby, D.; Ouddane, B.; Net, S.; Halwani, J. Assessment of Persistent Organic Pollutants in Surface Sediments along Lebanese Coastal Zone. Mar. Pollut. Bull. 2020, 153, 110947. [Google Scholar] [CrossRef]
- Abboud-Abi Saab, M.; Hassoun, A.E.R. Effects of Organic Pollution on Environmental Conditions and the Phytoplankton Community in the Central Lebanese Coastal Waters with Special Attention to Toxic Algae. Reg. Stud. Mar. Sci. 2017, 10, 38–51. [Google Scholar] [CrossRef]
- MOE/EU/UNDP. Lebanon Environmental Assessment of the Syrian Conflict & Priority Interventions. 2014. Available online: https://www.undp.org/lebanon/publications/lebanon-environmental-assessment-syrian-conflict (accessed on 10 April 2023).
- World Bank. Lebanon Country Water Sector Assistance Strategy; World Bank: Washington, DC, USA, 2012. [Google Scholar]
- Tokajian, S.; Moghnieh, R.; Salloum, T.; Arabaghian, H.; Alousi, S.; Moussa, J.; Abboud, E.; Youssef, S.; Husni, R. Extended-Spectrum β-Lactamase-Producing Escherichia Coli in Wastewaters and Refugee Camp in Lebanon. Future Microbiol. 2018, 13, 81–95. [Google Scholar] [CrossRef]
- Nehme, N.; Haydar, C.; Koubaissy, B.; Fakih, M.; Awad, S.; Toufaily, J.; Villieras, F.; Hamieh, T. The Distribution of Heavy Metals in the Lower River Basin, Lebanon. Phys. Procedia 2014, 55, 456–463. [Google Scholar] [CrossRef]
- Merhabi, F.; Gomez, E.; Castro, N.A.; Amine, H.; Rosain, D.; Halwani, J.; Fenet, H. Occurrence and Ecological Risk Assessment of Pharmaceutical Products in the Kadicha River in Lebanon. Emerg. Contam. 2021, 7, 196–203. [Google Scholar] [CrossRef]
- Doummar, J.; Geyer, T.; Baierl, M.; Nödler, K.; Licha, T.; Sauter, M. Carbamazepine Breakthrough as Indicator for Specific Vulnerability of Karst Springs: Application on the Jeita Spring, Lebanon. Appl. Geochem. 2014, 47, 150–156. [Google Scholar] [CrossRef]
- Mokh, S.; El Khatib, M.; Koubar, M.; Daher, Z.; Al Iskandarani, M. Innovative SPE-LC-MS/MS Technique for the Assessment of 63 Pharmaceuticals and the Detection of Antibiotic-Resistant-Bacteria: A Case Study Natural Water Sources in Lebanon. Sci. Total Environ. 2017, 609, 830–841. [Google Scholar] [CrossRef] [PubMed]
- Mohty, R.; Tfayli, A. General Oncology Care in Lebanon. In Cancer in the Arab World; Springer: Singapore, 2022; pp. 115–132. ISBN 9789811679445. [Google Scholar]
- Nassour, C.; Barton, S.J.; Nabhani-Gebara, S.; Saab, Y.; Barker, J. Occurrence of Anticancer Drugs in the Aquatic Environment: A Systematic Review. Environ. Sci. Pollut. Res. 2020, 27, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- ICH Harmonised Triopartite Guideline. Validation of Analytical Procedures: Text and Methodology Q2(R1). Available online: https://database.ich.org/sites/default/files/Q2%28R1%29Guideline.pdf (accessed on 13 July 2022).
- Barbarin, N.; Henion, J.D.; Wu, Y. Comparison between Liquid Chromatography-UV Detection and Liquid Chromatography-Mass Spectrometry for the Characterization of Impurities and/or Degradants Present in Trimethoprim Tablets. J. Chromatogr. A 2002, 970, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Kruve, A.; Kaupmees, K. Adduct Formation in ESI/MS by Mobile Phase Additives. J. Am. Soc. Mass Spectrom. 2017, 28, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Mortier, K.A.; Zhang, G.-F.; Van Peteghem, C.H.; Lambert, W.E. Adduct Formation in Quantitative Bioanalysis: Effect of Ionization Conditions on Paclitaxel. J. Am. Soc. Mass Spectrom. 2004, 15, 585–592. [Google Scholar] [CrossRef]
- Blumer, M.R.; Chang, C.H.; Brayfindley, E.; Nunez, J.R.; Colby, S.M.; Renslow, R.S.; Metz, T.O. Mass Spectrometry Adduct Calculator. J. Chem. Inf. Model. 2021, 61, 5721–5725. [Google Scholar] [CrossRef]
- Ravisankar, P.; Naga Navya, C.; Pravallika, D.; Sri, D.N. A Review on Step-by-Step Analytical Method Validation. IOSR J. Pharm. 2015, 5, 2250–3013. [Google Scholar]
- Awasthi, A.; Kumar, A.; Kumar, R.; Vishwas, S.; Khursheed, R.; Kaur, J.; Corrie, L.; Kumar, B.; Gulati, M.; Kumar, D.; et al. RP-HPLC Method Development and Validation for Simultaneous Estimation of Mesalamine and Curcumin in Bulk Form as Well as Nanostructured Lipid Carriers. South African, J. Bot. 2022, 151, 529–537. [Google Scholar] [CrossRef]
- Huang, C.; Liu, C.; Tang, J.; Fang, Y.; Ji, L.; Hu, Q.; Li, Q.; Xie, M.; Chen, Z. Development and Validation of High-Performance Liquid Chromatography Method for Analysis of β-CCT and Its Related Substances. Arab. J. Chem. 2022, 15, 103725. [Google Scholar] [CrossRef]
- Berruex, L.G.; Freitag, R. Separation and Purification of Biochemicals. In Encyclopedia of Physical Science and Technology; Elsevier: Amsterdam, The Netherlands, 2003; pp. 651–673. [Google Scholar]
- Briscoe, C.J.; Stiles, M.R.; Hage, D.S. System Suitability in Bioanalytical LC/MS/MS. J. Pharm. Biomed. Anal. 2007, 44, 484–491. [Google Scholar] [CrossRef]
- Tauxe-Wuersch, A.; De Alencastro, L.F.; Grandjean, D.; Tarradellas, J. Trace Determination of Tamoxifen and 5-Fluorouracil in Hospital and Urban Wastewaters. Int. J. Environ. Anal. Chem. 2006, 86, 473–485. [Google Scholar] [CrossRef]
- Kovalova, L.; McArdell, C.S.; Hollender, J. Challenge of High Polarity and Low Concentrations in Analysis of Cytostatics and Metabolites in Wastewater by Hydrophilic Interaction Chromatography/Tandem Mass Spectrometry. J. Chromatogr. A 2009, 1216, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- Petrie, B.; Youdan, J.; Barden, R.; Kasprzyk-Hordern, B. Multi-Residue Analysis of 90 Emerging Contaminants in Liquid and Solid Environmental Matrices by Ultra-High-Performance Liquid Chromatography Tandem Mass Spectrometry. J. Chromatogr. A 2016, 1431, 64–78. [Google Scholar] [CrossRef] [PubMed]
- Isidori, M.; Lavorgna, M.; Russo, C.; Kundi, M.; Žegura, B.; Novak, M.; Filipič, M.; Mišïk, M.; Knasmueller, S.; de Alda, M.L.; et al. Chemical and Toxicological Characterisation of Anticancer Drugs in Hospital and Municipal Wastewaters from Slovenia and Spain. Environ. Pollut. 2016, 219, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Gros, M.; Petrović, M.; Barceló, D. Tracing Pharmaceutical Residues of Different Therapeutic Classes in Environmental Waters by Using Liquid Chromatography/Quadrupole-Linear Ion Trap Mass Spectrometry and Automated Library Searching. Anal. Chem. 2009, 81, 898–912. [Google Scholar] [CrossRef] [PubMed]
- Mullot, J.U.; Karolak, S.; Fontova, A.; Huart, B.; Levi, Y. Development and Validation of a Sensitive and Selective Method Using GC/MS-MS for Quantification of 5-Fluorouracil in Hospital Wastewater. Anal. Bioanal. Chem. 2009, 394, 2203–2212. [Google Scholar] [CrossRef]
- Ort, C.; Lawrence, M.G.; Rieckermann, J.; Joss, A. Sampling for Pharmaceuticals and Personal Care Products (PPCPs) and Illicit Drugs in Wastewater Systems: Are Sampling for PPCPs in Wastewater Systems: Comparison of Different Sampling Modes and Optimization Strategies. Environ. Sci. Technol. 2010, 44, 6024–6035. [Google Scholar] [CrossRef] [PubMed]
- López-Serna, R.; Petrović, M.; Barceló, D. Development of a Fast Instrumental Method for the Analysis of Pharmaceuticals in Environmental and Wastewaters Based on Ultra High Performance Liquid Chromatography (UHPLC)-Tandem Mass Spectrometry (MS/MS). Chemosphere 2011, 85, 1390–1399. [Google Scholar] [CrossRef]
- Verlicchi, P.; Al Aukidy, M.; Galletti, A.; Petrovic, M.; Barceló, D. Hospital Effluent: Investigation of the Concentrations and Distribution of Pharmaceuticals and Environmental Risk Assessment. Sci. Total Environ. 2012, 430, 109–118. [Google Scholar] [CrossRef]
- Kosjek, T.; Perko, S.; Žigon, D.; Heath, E. Fluorouracil in the Environment: Analysis, Occurrence, Degradation and Transformation. J. Chromatogr. A 2013, 1290, 62–72. [Google Scholar] [CrossRef]
- Česen, M.; Kosjek, T.; Laimou-Geraniou, M.; Kompare, B.; Širok, B.; Lambropolou, D.; Heath, E. Occurrence of Cyclophosphamide and Ifosfamide in Aqueous Environment and Their Removal by Biological and Abiotic Wastewater Treatment Processes. Sci. Total Environ. 2015, 527–528, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Česen, M.; Kosjek, T.; Busetti, F.; Kompare, B.; Heath, E. Human Metabolites and Transformation Products of Cyclophosphamide and Ifosfamide: Analysis, Occurrence and Formation during Abiotic Treatments. Environ. Sci. Pollut. Res. 2016, 23, 11209–11223. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Zhou, J.L. Simultaneous Determination of Various Pharmaceutical Compounds in Water by Solid-Phase Extraction-Liquid Chromatography-Tandem Mass Spectrometry. J. Chromatogr. A 2007, 1154, 205–213. [Google Scholar] [CrossRef]
- Zhou, J.L.; Zhang, Z.L.; Banks, E.; Grover, D.; Jiang, J.Q. Pharmaceutical Residues in Wastewater Treatment Works Effluents and Their Impact on Receiving River Water. J. Hazard. Mater. 2009, 166, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.; Camacho-Muñoz, D.; Santos, J.L.; Aparicio, I.; Alonso, E. Simultaneous Determination of a Selected Group of Cytostatic Drugs in Water Using High-Performance Liquid Chromatography-Triple-Quadrupole Mass Spectrometry. J. Sep. Sci. 2011, 34, 3166–3177. [Google Scholar] [CrossRef]
- Ferrando-Climent, L.; Rodriguez-Mozaz, S.; Barceló, D. Development of a UPLC-MS/MS Method for the Determination of Ten Anticancer Drugs in Hospital and Urban Wastewaters, and Its Application for the Screening of Human Metabolites Assisted by Information-Dependent Acquisition Tool (IDA) in Sewage Samples. Anal. Bioanal. Chem. 2013, 405, 5937–5952. [Google Scholar] [CrossRef]
- Martín, J.; Camacho-Muñoz, D.; Santos, J.L.; Aparicio, I.; Alonso, E. Occurrence and Ecotoxicological Risk Assessment of 14 Cytostatic Drugs in Wastewater. Water. Air. Soil Pollut. 2014, 225, 1896. [Google Scholar] [CrossRef]
- Majors, R.E. Chapter 7—Solid-Phase Extraction. In Handbook of Sample Preparation; Wiley-Blackwell: Hoboken, NJ, USA, 2011; ISBN 9780470099346. [Google Scholar]
- Biel-Maeso, M.; Baena-Nogueras, R.M.; Corada-Fernández, C.; Lara-Martín, P.A. Occurrence, Distribution and Environmental Risk of Pharmaceutically Active Compounds (PhACs) in Coastal and Ocean Waters from the Gulf of Cadiz (SW Spain). Sci. Total Environ. 2018, 612, 649–659. [Google Scholar] [CrossRef]
- Negreira, N.; López de Alda, M.; Barceló, D. On-Line Solid Phase Extraction-Liquid Chromatography-Tandem Mass Spectrometry for the Determination of 17 Cytostatics and Metabolites in Waste, Surface and Ground Water Samples. J. Chromatogr. A 2013, 1280, 64–74. [Google Scholar] [CrossRef]
- Swartz, M.E.; Krull, I.S. Handbook of Analytical Validation; CRC Press: Boca Raton, FL, USA, 2012; ISBN 9781420014488. [Google Scholar]
- Taylor, P.J. Matrix Effects: The Achilles Heel of Quantitative High-Performance Liquid Chromatography-Electrospray-Tandem Mass Spectrometry. Clin. Biochem. 2005, 38, 328–334. [Google Scholar] [CrossRef]
- Kloepfer, A.; Quintana, J.B.; Reemtsma, T. Operational Options to Reduce Matrix Effects in Liquid Chromatography- Electrospray Ionisation-Mass Spectrometry Analysis of Aqueous Environmental Samples. J. Chromatogr. A 2005, 1067, 153–160. [Google Scholar] [CrossRef]
- Matuszewski, B.K.; Constanzer, M.L.; Chavez-Eng, C.M. Strategies for the Assessment of Matrix Effect in Quantitative Bioanalytical Methods Based on HPLC-MS/MS. Anal. Chem. 2003, 75, 3019–3030. [Google Scholar] [CrossRef] [PubMed]
- Ferrer Amate, C.; Unterluggauer, H.; Fischer, R.J.; Fernández-Alba, A.R.; Masselter, S. Development and Validation of a LC-MS/MS Method for the Simultaneous Determination of Aflatoxins, Dyes and Pesticides in Spices. Anal. Bioanal. Chem. 2010, 397, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Raposo, F.; Barceló, D. Challenges and Strategies of Matrix Effects Using Chromatography-Mass Spectrometry: An Overview from Research versus Regulatory Viewpoints. TrAC—Trends Anal. Chem. 2021, 134, 116068. [Google Scholar] [CrossRef]
- Benijts, T.; Dams, R.; Lambert, W.; De Leenheer, A. Countering Matrix Effects in Environmental Liquid Chromatography- Electrospray Ionization Tandem Mass Spectrometry Water Analysis for Endocrine Disrupting Chemicals. J. Chromatogr. A 2004, 1029, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Angeles, L.F.; Aga, D.S. Establishing Analytical Performance Criteria for the Global Reconnaissance of Antibiotics and Other Pharmaceutical Residues in the Aquatic Environment Using Liquid Chromatography-Tandem Mass Spectrometry. J. Anal. Methods Chem. 2018, 2018, 7019204. [Google Scholar] [CrossRef]
- Kovács, R.; Csenki, Z.; Bakos, K.; Urbányi, B.; Horváth, Á.; Garaj-Vrhovac, V.; Gajski, G.; Gerić, M.; Negreira, N.; López de Alda, M.; et al. Assessment of Toxicity and Genotoxicity of Low Doses of 5-Fluorouracil in Zebrafish (Danio Rerio) Two-Generation Study. Water Res. 2015, 77, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Canela, C.; Cortés-Francisco, N.; Oliva, X.; Pujol, C.; Ventura, F.; Lacorte, S.; Caixach, J. Occurrence of Cyclophosphamide and Epirubicin in Wastewaters by Direct Injection Analysis-Liquid Chromatography-High-Resolution Mass Spectrometry. Environ. Sci. Pollut. Res. 2012, 19, 3210–3218. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Canela, C.; Ventura, F.; Caixach, J.; Lacorte, S. Occurrence of Cytostatic Compounds in Hospital Effluents and Wastewaters, Determined by Liquid Chromatography Coupled to High-Resolution Mass Spectrometry. Anal. Bioanal. Chem. 2014, 406, 3801–3814. [Google Scholar] [CrossRef] [PubMed]
- Karnib, A. Assessing Population Coverage of Safely Managed Wastewater Systems: A Case Study of Lebanon. J. Water Sanit. Hyg. Dev. 2016, 6, 313–319. [Google Scholar] [CrossRef]
- Dagher, L.A.; Hassan, J.; Kharroubi, S.; Jaafar, H.; Kassem, I.I. Nationwide Assessment of Water Quality in Rivers across Lebanon by Quantifying Fecal Indicators Densities and Profiling Antibiotic Resistance of Escherichia Coli. Antibiotics 2021, 10, 883. [Google Scholar] [CrossRef] [PubMed]
- Korfali, S.I.; Jurdi, M. Suitability of Surface Water for Domestic Water Use: Awali River Case Study. Eur. Water 2011, 35, 3–12. [Google Scholar]
- Massoud, M.A. Assessment of Water Quality along a Recreational Section of the Damour River in Lebanon Using the Water Quality Index. Environ. Monit. Assess. 2012, 184, 4151–4160. [Google Scholar] [CrossRef] [PubMed]
- Ofrydopoulou, A.; Nannou, C.; Evgenidou, E.; Christodoulou, A.; Lambropoulou, D. Assessment of a Wide Array of Organic Micropollutants of Emerging Concern in Wastewater Treatment Plants in Greece: Occurrence, Removals, Mass Loading and Potential Risks. Sci. Total Environ. 2022, 802, 149860. [Google Scholar] [CrossRef]
- Castiglioni, S.; Bagnati, R.; Calamari, D.; Fanelli, R.; Zuccato, E. A Multiresidue Analytical Method Using Solid-Phase Extraction and High-Pressure Liquid Chromatography Tandem Mass Spectrometry to Measure Pharmaceuticals of Different Therapeutic Classes in Urban Wastewaters. J. Chromatogr. A 2005, 1092, 206–215. [Google Scholar] [CrossRef]
- Yin, J.; Shao, B.; Zhang, J.; Li, K. A Preliminary Study on the Occurrence of Cytostatic Drugs in Hospital Effluents in Beijing, China. Bull. Environ. Contam. Toxicol. 2010, 84, 39–45. [Google Scholar] [CrossRef]
- Yin, J.; Yang, Y.; Li, K.; Zhang, J.; Shao, B. Analysis of Anticancer Drugs in Sewage Water by Selective SPE and UPLC-ESI-MS-MS. J. Chromatogr. Sci. 2010, 48, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Rabii, F.W.; Segura, P.A.; Fayad, P.B.; Sauvé, S. Determination of Six Chemotherapeutic Agents in Municipal Wastewater Using Online Solid-Phase Extraction Coupled to Liquid Chromatography-Tandem Mass Spectrometry. Sci. Total Environ. 2014, 487, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Buerge, I.J.; Buser, H.R.; Poiger, T.; Müller, M.D. Occurrence and Fate of the Cytostatic Drugs Cyclophosphamide and Ifosfamide in Wastewater and Surface Waters. Environ. Sci. Technol. 2006, 40, 7242–7250. [Google Scholar] [CrossRef] [PubMed]
- Nassour, C.; Nabhani-Gebara, S.; Barton, S.J.; Barker, J. Aquatic Ecotoxicology of Anticancer Drugs: A Systematic Review. Sci. Total Environ. 2021, 800, 149598. [Google Scholar] [CrossRef] [PubMed]
- Nassour, C.; Nabhani-Gebara, S.; Barton, S.J.; Barker, J. Anti-Cancer Drug Waste Disposal Practices and Wastewater Management in Hospitals: A Lebanese Survey. J. Oncol. Pharm. Pract. 2023. [Google Scholar] [CrossRef] [PubMed]
| Compound | Concentration Range (ng/L) | Reference | |
|---|---|---|---|
| Analgesic | Acetaminophen | n.d.–242 | [17] |
| Antibiotic | Macrolide | n.d.–2806 | [19] |
| Fluoroquinolone | n.d.–190 | [17,19] | |
| Tetracycline | n.d. | [19] | |
| Rifamycin | n.d.–542 | [19] | |
| Sulfonamide | n.d.–4100 | [17,19] | |
| Trimethoprim | n.d. | [19] | |
| Anticonvulsant | Carbamazepine | n.d.–290 | [17,18] |
| Benzodiazepine | Diazepam | n.d. | [17] |
| Lorazepam | n.d. | [17] | |
| β-Blocker | Atenolol | n.d.–93 | [17] |
| Propranolol | n.d. | [17] | |
| CNS Stimulant | Caffeine | 1–10,234 | [19] |
| Mucolytic | Bromhexine | n.d. | [19] |
| Narcotic | Codeine | n.d. | [17] |
| Norbuprenorphine | n.d. | [17] | |
| NSAID | Diclofenac | n.d.–1055 | [17] |
| Ibuprofen | n.d. | [17] | |
| Ketoprofen | n.d. | [17] | |
| SNRI | Venlafaxine | n.d. | [17] |
| Statin | Pravastatin | n.d. | [17] |
| LC Parameters | |||
|---|---|---|---|
| Column | Kinetex 2.6 µm Phenyl-Hexyl (100 × 3 mm) | ||
| Temperature | 40 °C | ||
| Flow rate | 0.4 mL/min | ||
| Injection volume | 10 µL | ||
| Run time | 12 min | ||
| Mobile phase timetable | Time | % (A) | % (B) |
| 0 | 100 | 0 | |
| 2 | 100 | 0 | |
| 4 | 25 | 75 | |
| 8 | 5 | 95 | |
| 8.5 | 5 | 95 | |
| 8.6 | 100 | 0 | |
| 12 | 100 | 0 | |
| Compound | Precursor Ion | Fragmentor (V) | Product Ions | Collision Energy (eV) | Dwell Time (ms) |
|---|---|---|---|---|---|
| 5-Fluorouracil | 131.01 | 99 | 114 | 13 | 100 |
| 58.1 | 29 | ||||
| Gemcitabine | 264.08 | 99 | 112.1 | 17 | 100 |
| 95.1 | 40 | ||||
| Methotrexate | 455.18 | 118 | 308.3 | 17 | 50 |
| 175 | 40 | ||||
| Cyclophosphamide | 261.03 | 99 | 140 | 21 | 50 |
| 63.1 | 40 | ||||
| Tamoxifen | 372.23 | 137 | 72.2 | 25 | 50 |
| 70.2 | 40 | ||||
| Docetaxel | 830.34 | 137 | 549.3 | 21 | 100 |
| 304.3 | 21 | ||||
| 13C3-Caffeine | 198.1 | 99 | 140 | 17 | 50 |
| 112.1 | 25 |
| Compound | Retention Time (min) | Column Efficiency | Plate Height (cm) | Asymmetry | Resolution |
|---|---|---|---|---|---|
| 5-FU | 1.8 | 1886 | 5.30 × 10−3 | 2 | 1.78 16.22 2.00 3.67 2.67 3.70 |
| GEM | 2.2 | 1672 | 6.00 × 10−3 | 1.5 | |
| MET | 5.6 | 18,496 | 5.41 × 10−4 | 2 | |
| 13C3-CAF | 5.9 | 20,736 | 4.82 × 10−4 | 2 | |
| CP | 6.5 | 33,367 | 2.99 × 10−4 | 2 | |
| TAM | 6.9 | 37,378 | 2.68 × 10−4 | 3 | |
| DOC | 7.4 | 43,820 | 2.28 × 10−4 | 1.3 |
| Compound | 5-FU | GEM | MET | CP | TAM | DOC |
|---|---|---|---|---|---|---|
| Pre-spike response | 3761 | 92,219 | 25,576 | 9046 | 238,716 | 2025 |
| 3450 | 103,297 | 25,608 | 8163 | 222,362 | 2183 | |
| 3299 | 94,225 | 24,214 | 8339 | 221,330 | 1984 | |
| Post-spike response | 4036 | 97,417 | 26,175 | 8324 | 566,720 | 3485 |
| 3277 | 126,620 | 26,419 | 8039 | 584,059 | 3499 | |
| 3438 | 111,605 | 25,288 | 8092 | 557,580 | 3679 | |
| %Recovery | 98.14 | 86.89 | 96.80 | 104.42 | 39.96 | 58.14 |
| ±SD | 6.34 | 6.88 | 0.99 | 3.76 | 2.04 | 4.23 |
| Compound | Range (ng/L) | Regression Coefficient (R2) | Accuracy (%) (±SD) | Interday Precision (%) (±SD) | Intraday Precision (%) (±SD) | LOD (ng/L) | LOQ (ng/L) |
|---|---|---|---|---|---|---|---|
| 5-FU | 5–100 | 0.9975 | 100.3 (±7.74) | 3.7 (±0.94) | 2.7 (±1.09) | 0.9 | 2.8 |
| GEM | 5–100 | 0.9994 | 101.5 (±2.90) | 1.3 (±0.82) | 2.1 (±1.23) | 0.3 | 1.0 |
| MET | 5–100 | 0.9994 | 104.0 (±2.11) | 2.6 (±0.37) | 2.4 (±1.24) | 0.4 | 1.1 |
| CP | 5–100 | 0.9997 | 101.7 (±2.70) | 3.3 (±0.87) | 3.0 (±0.87) | 0.4 | 1.1 |
| TAM | 5–100 | 0.9943 | 100.7 (±8.53) | 2.6 (±0.56) | 2.5 (±1.62) | 0.1 | 0.3 |
| DOC | 5–100 | 0.9983 | 108.6 (±2.08) | 2.9 (±1.65) | 2.8 (±1.36) | 0.3 | 0.8 |
| Compound | Matrix Effect (%) (±SD) | IS Corrected Matrix Effect (%) (±SD) | ||
|---|---|---|---|---|
| 5-FU | L: 32 (±10) | Suppression | L: 62 (±15) | Suppression |
| M: 39 (±4) | Suppression | M: 84 (±12) | Suppression | |
| H: 34 (±18) | Suppression | H: 64 (±37) | Suppression | |
| GEM | L: 81 (±6) | Suppression | L: 161 (±13) | Enhancement |
| M: 81 (±19) | Suppression | M: 177 (±38) | Enhancement | |
| H: 72 (±16) | Suppression | H: 146 (±56) | Enhancement | |
| MET | L: 153 (±24) | Enhancement | L: 344 (±80) | Enhancement |
| M: 95 (±2) | Suppression | M: 189 (±12) | Enhancement | |
| H: 88 (±3) | Suppression | H: 166 (±5) | Enhancement | |
| CP | L: 51 (±28) | Suppression | L: 112 (±68) | Enhancement |
| M: 41 (±4) | Suppression | M: 82 (±9) | Suppression | |
| H: 40 (±2) | Suppression | H: 76 (±8) | Suppression | |
| TAM | L: 51 (±2) | Suppression | L: 114 (±16) | Enhancement |
| M: 47 (±2) | Suppression | M: 93 (±7) | Suppression | |
| H: 49 (±2) | Suppression | H: 92 (±9) | Suppression | |
| DOC | L: 17 (±2) | Suppression | L: 39 (±9) | Suppression |
| M: 16 (±1) | Suppression | M: 32 (±4) | Suppression | |
| H: 16 (±2) | Suppression | H: 30 (±5) | Suppression | |
| IS | Matrix Effect (%) (±SD) | |||
| 13C3-CAF | L: 44 (±5) | Suppression | ||
| M: 51 (±3) | Suppression | |||
| H: 53 (±3) | Suppression | |||
| Sample Location | Concentration (ng/L) (±SD) | |||||
|---|---|---|---|---|---|---|
| 5-FU | GEM | MET | CP | TAM | DOC | |
| Antelias River | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Al Kalb River | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Zahrani River | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Hasbani River | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Litani River | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Wazzani River | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Awali River | 8 ± 3 | <lod | n.d. | n.d. | n.d. | n.d. |
| Damour River | 3 ± 1 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Orontes River | <loq | n.d. | n.d. | n.d. | n.d. | n.d. |
| Al Bared River | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Ibrahim River | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Al Jawz River | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Ostouene River | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Abou Ali River | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| WWTP Joub Jannine Influent | 211 ± 41 * | n.d | n.d. | 3 ± 2 | n.d. | n.d. |
| WWTP Joub Jannine Effluent | 15 ± 2 | <lod | 15 ± 3 | 1.4 ± 1 | n.d. | n.d. |
| WWTP Saida Influent | 118 ± 28 * | n.d. | 20 ± 3 | 1.5 ± 1 | n.d. | n.d. |
| WWTP Saida Effluent | 128 ± 39 * | n.d. | 21 ± 4 | n.d. | n.d. | n.d. |
| WWTP Al Ghadir Influent | 195 ± 59 * | n.d. | 7 ± 2 | 4 ± 2 | n.d. | n.d. |
| WWTP Al Ghadir Effluent | 305 ± 95 * | n.d. | n.d. | 12 ± 1 | n.d. | n.d. |
| Ramlet Al Baida Outfall | 6 ± 3 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Jiyeh Outfall | 130 ± 30 * | n.d. | 31 ± 3 | 2 ± 2 | n.d. | n.d. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nassour, C.; Nabhani-Gebara, S.; Barton, S.J.; Barker, J. Determination of Anticancer Drugs in the Aquatic Environment by SPE–LC–MS/MS—A Lebanese Case Study. Water 2023, 15, 1560. https://doi.org/10.3390/w15081560
Nassour C, Nabhani-Gebara S, Barton SJ, Barker J. Determination of Anticancer Drugs in the Aquatic Environment by SPE–LC–MS/MS—A Lebanese Case Study. Water. 2023; 15(8):1560. https://doi.org/10.3390/w15081560
Chicago/Turabian StyleNassour, Carla, Shereen Nabhani-Gebara, Stephen J. Barton, and James Barker. 2023. "Determination of Anticancer Drugs in the Aquatic Environment by SPE–LC–MS/MS—A Lebanese Case Study" Water 15, no. 8: 1560. https://doi.org/10.3390/w15081560
APA StyleNassour, C., Nabhani-Gebara, S., Barton, S. J., & Barker, J. (2023). Determination of Anticancer Drugs in the Aquatic Environment by SPE–LC–MS/MS—A Lebanese Case Study. Water, 15(8), 1560. https://doi.org/10.3390/w15081560








