Spatial Distribution of Vegetation on Stream Bars and the Riparian Zone Reflects Successional Pattern Due to Fluid Dynamics of River
Abstract
:1. Introduction
2. Materials and Methods
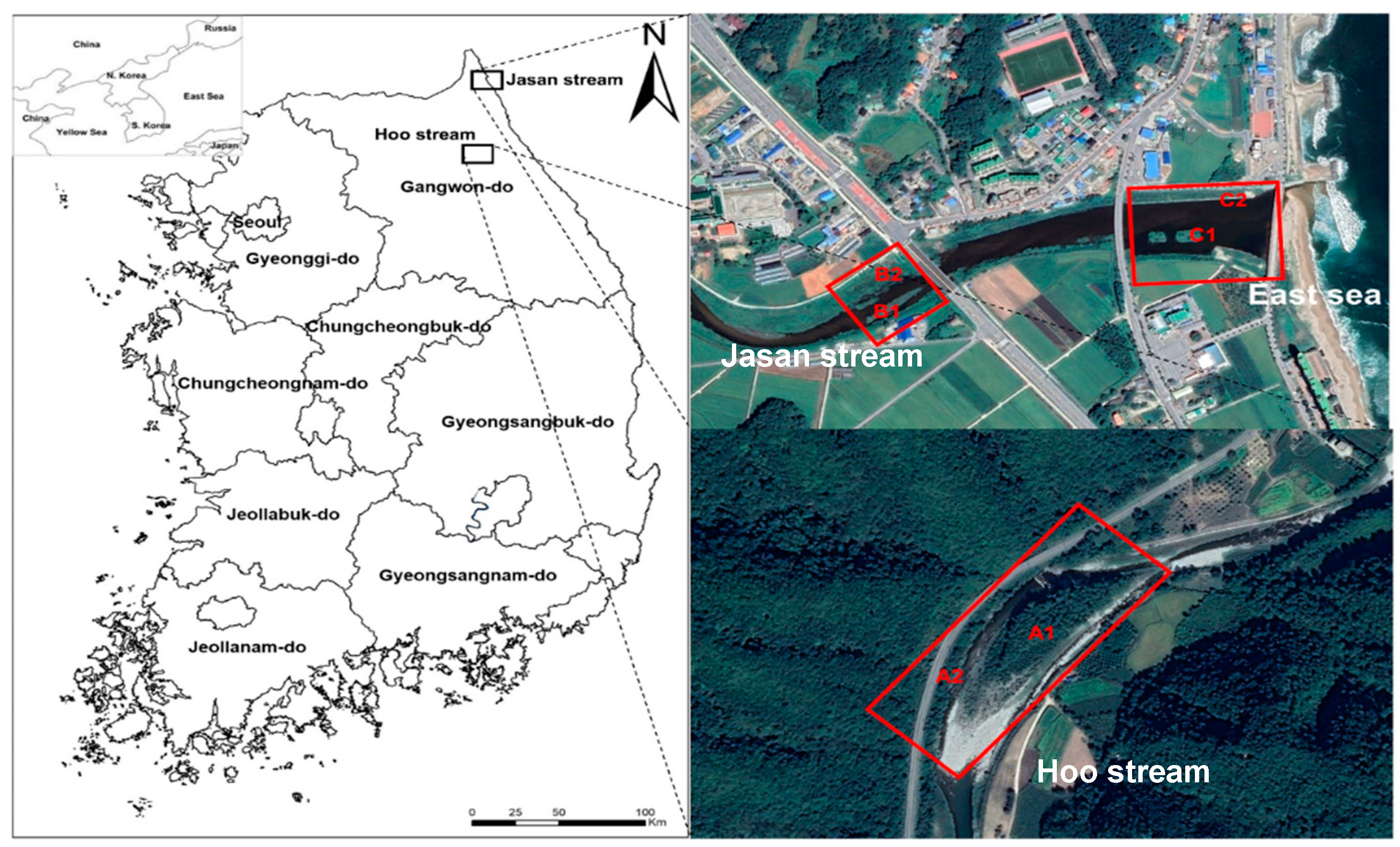
2.1. Study Area
2.2. Methods
3. Results
3.1. Stand Profiles of Vegetation Established on Stream Bars and Riparian Vegetation
3.2. Stand Ordination Based on Vegetation Established on Stream Bars and Riparian Vegetation
3.3. Species Diversity
4. Discussion
4.1. Spatial Distribution of Vegetation and Disturbance Regime
4.2. Formation Process of Stream Bars and Vegetation Succession
4.3. Relationship between the Developmental Stage of Vegetation and Species Diversity
4.4. Ecological Importance of Stream Bars and Riparian Zones
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Prach, K.; Walker, L.R. Four opportunities for studies of ecological succession. Trends Ecol. Evol. 2011, 26, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Egerton, F.N. History of ecological sciences, part 54: Succession, community, and continuum. Bull. Ecol. Soc. Am. 2015, 96, 426–474. [Google Scholar] [CrossRef] [Green Version]
- Meiners, S.J.; Cadotte, M.W.; Fridley, J.D.; Pickett, S.T.; Walker, L.R. Is successional research nearing its climax? New approaches for understanding dynamic communities. Funct. Ecol. 2015, 29, 154–164. [Google Scholar] [CrossRef]
- Walker, L.R.; Wardle, D.A. Plant succession as an integrator of contrasting ecological time scales. Trends Ecol. Evol. 2014, 29, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; HilleRisLambers, J. Integrating succession and community assembly perspectives. F1000Research 2016, 5, F1000. [Google Scholar] [CrossRef]
- HilleRisLambers, J.; Adler, P.B.; Harpole, W.S.; Levine, J.M.; Mayfield, M.M. Rethinking community assembly through the lens of coexistence theory. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 227–248. [Google Scholar] [CrossRef] [Green Version]
- Pulsford, S.A.; Lindenmayer, D.B.; Driscoll, D.A. A succession of theories: Purging redundancy from disturbance theory. Biol. Rev. 2016, 91, 148–167. [Google Scholar] [CrossRef]
- Walker, L.R.; Walker, J.; Hobbs, R.J. Linking Restoration and Ecological Succession; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Connell, J.H.; Slatyer, R.O. Mechanisms of succession in natural communities and their role in community stability and organization. Am. Nat. 1977, 111, 1119–1144. [Google Scholar] [CrossRef]
- van Andel, J.; Bakker, J.; Grootjans, A. Mechanisms of vegetation succession: A review of concepts and perspectives. Acta Bot. Neerl. 1993, 42, 413–433. [Google Scholar] [CrossRef]
- McCook, L. Understanding ecological community succession: Causal models and theories, a review. Vegetatio 1994, 110, 115–147. [Google Scholar] [CrossRef]
- Barbour, M.T. Rapid Bioassessment Protocols for Use in Wadeable Streams and Rivers: Periphyton, Benthic Macroinvertebrates and Fish; US Environmental Protection Agency, Office of Water: Washington, DC, USA, 1999. [Google Scholar]
- Kollmann, J.; Vieli, M.; Edwards, P.; Tockner, K.; Ward, J. Interactions between vegetation development and island formation in the Alpine river Tagliamento. Appl. Veg. Sci. 1999, 2, 25–36. [Google Scholar] [CrossRef]
- Chun, Y.J.; Collyer, M.L.; Moloney, K.A.; Nason, J.D. Phenotypic plasticity of native vs. invasive purple loosestrife: A two-state multivariate approach. Ecology 2007, 88, 1499–1512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.-S.; Cho, Y.-C.; Shin, H.-C.; Park, S. Differences between sand and gravel bars of streams in patterns of vegetation succession. J. Ecol. Environ. 2009, 32, 55–60. [Google Scholar] [CrossRef]
- Pi, J. The Evaluation of Impact of Dam Construction and Different Levels of Restoration on the Naturalness of Riparian Vegetation and Adaptive Management Plans for Improving the Naturalness of the Influenced Rivers of Restoration; Seoul Women’s University: Seoul, Republic of Korea, 2014. [Google Scholar]
- Lee, C.S.; Chun, Y.M.; Lee, H.; Pi, J.H.; Lim, C.H. Establishment, Regeneration, and Succession of Korean Red Pine (Pinus Densiflora S. et Z.) Forest in Korea; IntechOpen: London, UK, 2018. [Google Scholar]
- Gregory, S.V.; Swanson, F.J.; McKee, W.A.; Cummins, K.W. An ecosystem perspective of riparian zones. BioScience 1991, 41, 540–551. [Google Scholar] [CrossRef]
- Lee, C.; Ahn, J.; Pee, J.; Lee, S.; Lee, J. Futuristic direction of river restoration in Korea under changing climate change. J. Restor. Ecol. 2011, 2, 137–143. [Google Scholar]
- Nilsson, C.; Svedmark, M. Basic principles and ecological consequences of changing water regimes: Riparian plant communities. Environ. Manag. 2002, 30, 468–480. [Google Scholar] [CrossRef]
- Naiman, R.J.; Bechtold, J.S.; Drake, D.C.; Latterell, J.J.; O’Keefe, T.C.; Balian, E.V. Origins, patterns, and importance of heterogeneity in riparian systems. In Ecosystem Function in Heterogeneous Landscapes; Springer: New York, NY, USA, 2005; pp. 279–309. [Google Scholar]
- Yarnell, K.; Hall, C.; Royle, C.; Walker, S.L. Domesticated horses differ in their behavioural and physiological responses to isolated and group housing. Physiol. Behav. 2015, 143, 51–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, B.S.; Seol, J.; Kim, A.R.; An, J.H.; Lim, C.H.; Lee, C.S. Succession of the abandoned rice fields restores the riparian forest. Int. J. Environ. Res. Public Health 2022, 19, 10416. [Google Scholar] [CrossRef]
- Auble, G.T.; Scott, M.L.; Friedman, J.M. Use of individualistic streamflow-vegetation relations along the Fremont River, Utah, USA to assess impacts of flow alteration on wetland and riparian areas. Wetlands 2005, 25, 143–154. [Google Scholar] [CrossRef]
- Chen, Y.; Li, W.; Xu, C.; Ye, Z.; Chen, Y. Desert riparian vegetation and groundwater in the lower reaches of the Tarim River basin. Environ. Earth Sci. 2015, 73, 547–558. [Google Scholar] [CrossRef]
- Ferreira, L.V.; Stohlgren, T.J. Effects of river level fluctuation on plant species richness, diversity, and distribution in a floodplain forest in Central Amazonia. Oecologia 1999, 120, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Casanova, M.T.; Brock, M.A. How do depth, duration and frequency of flooding influence the establishment of wetland plant communities? Plant Ecol. 2000, 147, 237–250. [Google Scholar] [CrossRef]
- Shafroth, P.B.; Stromberg, J.C.; Patten, D.T. Riparian vegetation response to altered disturbance and stress regimes. Ecol. Appl. 2002, 12, 107–123. [Google Scholar] [CrossRef]
- Capon, S. Flood variability and spatial variation in plant community composition and structure on a large arid floodplain. J. Arid Environ. 2005, 60, 283–302. [Google Scholar] [CrossRef]
- Arscott, D.B.; Tockner, K.; van der Nat, D.; Ward, J. Aquatic habitat dynamics along a braided alpine river ecosystem (Tagliamento River, Northeast Italy). Ecosystems 2002, 5, 802–814. [Google Scholar] [CrossRef]
- Li, X.; Yang, D.; Zheng, C.; Li, X.; Zhao, W.; Huang, M.; Chen, Y.; Yu, P. Ecohydrology. In The Geographical Sciences During 1986–2015; Springer: Berlin/Heidelberg, Germany, 2017; pp. 407–417. [Google Scholar]
- Blondeaux, P.; Seminara, G. A unified bar–bend theory of river meanders. J. Fluid Mech. 1985, 157, 449–470. [Google Scholar] [CrossRef]
- Fujita, Y.; Muramoto, Y. Studies on the process of development of alternate bars. Bull. Disaster Prev. Res. Inst. 1985, 35, 55–86. [Google Scholar]
- Tsujimoto, T. Development of sand island with vegetation in fluvial fan river under degradation. In Proceedings of the Water Resources Engineering′98, Memphis, TN, USA, 3–7 August 1998; pp. 574–579. [Google Scholar]
- Tsujimoto, T. Fluvial processes in streams with vegetation. J. Hydraul. Res. 1999, 37, 789–803. [Google Scholar] [CrossRef]
- Crosato, A.; Desta, F.B.; Cornelisse, J.; Schuurman, F.; Uijttewaal, W.S. Experimental and numerical findings on the long-term evolution of migrating alternate bars in alluvial channels. Water Resour. Res. 2012, 48, W06524. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, S.; Mosselman, E.; Claude, N.; Wintenberger, C.L.; Juge, P. Alternate bars in a sandy gravel bed river: Generation, migration and interactions with superimposed dunes. Earth Surf. Process. Landf. 2015, 40, 610–628. [Google Scholar] [CrossRef]
- Prach, K. Vegetation succession on river gravel bars across the Northwestern Himalayas, India. Arct. Alp. Res. 1994, 26, 349–353. [Google Scholar] [CrossRef]
- Prandle, D. Generalised theory of estuarine dynamics. Phys. Shallow Estuaries Bays 1986, 16, 41–57. [Google Scholar]
- Verri, G.; Pinardi, N.; Bryan, F.; Tseng, Y.-H.; Coppini, G.; Clementi, E. A box model to represent estuarine dynamics in mesoscale resolution ocean models. Ocean Model. 2020, 148, 101587. [Google Scholar] [CrossRef]
- Jeong, J.-S.; Woo, S.-B.; Lee, H.S.; Gu, B.-H.; Kim, J.W.; Song, J.I. Baroclinic Effect on Inner-Port Circulation in a Macro-Tidal Estuary: A Case Study of Incheon North Port, Korea. J. Mar. Sci. Eng. 2022, 10, 392. [Google Scholar] [CrossRef]
- Zhang, R.; Hong, B.; Zhu, L.; Gong, W.; Zhang, H. Responses of estuarine circulation to the morphological evolution in a convergent, microtidal estuary. Ocean Sci. 2022, 18, 213–231. [Google Scholar] [CrossRef]
- Shin, S.-S.; Park, S.-D.; Lee, S.-K.; Ji, M.-G. Estimating critical stream power by the distribution of gravel-bed materials in the meandering river. J. Korea Water Resour. Assoc. 2012, 45, 151–163. [Google Scholar] [CrossRef] [Green Version]
- Braun-Blanquet, J. Pflanzensoziologie: Grundzüge der Vegetationskunde; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Ellenberg, D.; Mueller-Dombois, D. Aims and Methods of Vegetation Ecology; Wiley: New York, NY, USA, 1974. [Google Scholar]
- Lee, T. Illustrated Flora of Korea; Hyang Moon Sa: Seoul, Republic of Korea, 1985. [Google Scholar]
- Korean Plant Names Index. Available online: http://www.nature.go.kr/kbi/plant/pilbk/selectPlantPilbkGnrlList.do (accessed on 15 February 2023).
- Hill, M. A FORTRAN program for detrended correspondence analysis and reciprocal averaging. In Ecology and Systematics; Cornell University: Ithaca, NY, USA, 1979; 52p. [Google Scholar]
- Magurran, A.E.; Henderson, P.A. Explaining the excess of rare species in natural species abundance distributions. Nature 2003, 422, 714–716. [Google Scholar] [CrossRef]
- Kent, M. Vegetation Description and Data Analysis: A Practical Approach; John and Wiley and Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Technol. J. 1948, 27, 379–423. [Google Scholar] [CrossRef] [Green Version]
- Sousa, W.P. The role of disturbance in natural communities. Annu. Rev. Ecol. Syst. 1984, 15, 353–391. [Google Scholar] [CrossRef]
- Turner, M.G. Disturbance and landscape dynamics in a changing world. Ecology 2010, 91, 2833–2849. [Google Scholar] [CrossRef] [Green Version]
- Herben, T.; Chytrý, M.; Klimešová, J. A quest for species-level indicator values for disturbance. J. Veg. Sci. 2016, 27, 628–636. [Google Scholar] [CrossRef]
- Pielech, R.; Czortek, P. Disentangling effects of disturbance severity and frequency: Does bioindication really work? Ecol. Evol. 2021, 11, 252–262. [Google Scholar] [CrossRef]
- Haghkerdar, J.M.; McLachlan, J.R.; Ireland, A.; Greig, H.S. Repeat disturbances have cumulative impacts on stream communities. Ecol. Evol. 2019, 9, 2898–2906. [Google Scholar] [CrossRef] [Green Version]
- Danehy, R.J.; Bilby, R.E.; Justice, T.E.; Lester, G.T.; Jones, J.E.; Haddadi, S.S.; Merritt, G.D. Aquatic Biological Diversity Responses to Flood Disturbance and Forest Management in Small, Forested Watersheds. Water 2021, 13, 2793. [Google Scholar] [CrossRef]
- Keane, R. Disturbance Regimes and the Historical Range and Variation in Terrestrial Ecosystems; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Shea, K.; Roxburgh, S.H.; Rauschert, E.S. Moving from pattern to process: Coexistence mechanisms under intermediate disturbance regimes. Ecol. Lett. 2004, 7, 491–508. [Google Scholar] [CrossRef]
- White, P.S.; Jentsch, A. The search for generality in studies of disturbance and ecosystem dynamics. In Progress in Botany; Springer: Berlin/Heidelberg, DE, 2001; pp. 399–450. [Google Scholar]
- Burton, P.J.; Jentsch, A.; Walker, L.R. The Ecology of Disturbance Interactions. BioScience 2020, 70, 854–870. [Google Scholar] [CrossRef]
- Lee, C.-S.; You, Y.-H. Creation of an environmental forest as an ecological restoration. Korean J. Ecol. 2001, 24, 101–109. [Google Scholar]
- Bornette, G.; Amoros, C. Disturbance regimes and vegetation dynamics: Role of floods in riverine wetlands. J. Veg. Sci. 1996, 7, 615–622. [Google Scholar] [CrossRef]
- Girel, J. Old distribution procedure of both water and matter fluxes in floodplains of western Europe: Impact on present vegetation. Environ. Manag. 1994, 18, 203–221. [Google Scholar] [CrossRef]
- Ward, J.V.; Tockner, K.; Uehlinger, U.; Malard, F. Understanding natural patterns and processes in river corridors as the basis for effective river restoration. Regul. Rivers Res. Manag. Int. J. Devoted River Res. Manag. 2001, 17, 311–323. [Google Scholar] [CrossRef]
- Tockner, K.; Stanford, J.A. Riverine flood plains: Present state and future trends. Environ. Conserv. 2002, 29, 308–330. [Google Scholar] [CrossRef] [Green Version]
- Rivaes, R.; Pinheiro, A.N.; Egger, G.; Ferreira, T. The Role of river Morphodynamic disturbance and groundwater hydrology as driving factors of riparian landscape Patterns in Mediterranean Rivers. Front. Plant Sci. 2017, 8, 1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naiman, R.J.; Decamps, H. The ecology of interfaces: Riparian zones. Annu. Rev. Ecol. Syst. 1997, 28, 621–658. [Google Scholar] [CrossRef] [Green Version]
- Van Looy, K.; Cavillon, C.; Tormos, T.; Piffady, J.; Landry, P.; Souchon, Y. A scale-sensitive connectivity analysis to identify ecological networks and conservation value in river networks. Landsc. Ecol. 2013, 28, 1239–1249. [Google Scholar] [CrossRef] [Green Version]
- Poff, N.L.; Allan, J.D.; Bain, M.B.; Karr, J.R.; Prestegaard, K.L.; Richter, B.D.; Sparks, R.E.; Stromberg, J.C. The natural flow regime. BioScience 1997, 47, 769–784. [Google Scholar] [CrossRef]
- Bejarano, M.D.; Gonzalez del Tanago, M.; de Jalón, D.G.; Marchamalo, M.; Sordo-Ward, Á.; Solana-Gutiérrez, J. Responses of riparian guilds to flow alterations in a Mediterranean stream. J. Veg. Sci. 2012, 23, 443–458. [Google Scholar] [CrossRef] [Green Version]
- Benda, L.; Poff, N.L.; Miller, D.; Dunne, T.; Reeves, G.; Pess, G.; Pollock, M. The network dynamics hypothesis: How channel networks structure riverine habitats. BioScience 2004, 54, 413–427. [Google Scholar] [CrossRef] [Green Version]
- Foster, A.D.; Claeson, S.M.; Bisson, P.A.; Heimburg, J. Aquatic and riparian ecosystem recovery from debris flows in two western Washington streams, USA. Ecol. Evol. 2020, 10, 2749–2777. [Google Scholar] [CrossRef]
- Johnson, S.L.; Swanson, F.J.; Grant, G.E.; Wondzell, S.M. Riparian forest disturbances by a mountain flood—The influence of floated wood. Hydrol. Process. 2000, 14, 3031–3050. [Google Scholar] [CrossRef]
- James, C.S.; Mackay, S.J.; Arthington, A.H.; Capon, S.J.; Barnes, A.; Pearson, B. Does stream flow structure woody riparian vegetation in subtropical catchments? Ecol. Evol. 2016, 6, 5950–5963. [Google Scholar] [CrossRef]
- Vervuren, P.; Blom, C.; De Kroon, H. Extreme flooding events on the Rhine and the survival and distribution of riparian plant species. J. Ecol. 2003, 91, 135–146. [Google Scholar] [CrossRef]
- You, X.; Liu, J. Modeling the spatial and temporal dynamics of riparian vegetation induced by river flow fluctuation. Ecol. Evol. 2018, 8, 3648–3659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bazzaz, F.A. Characteristics of populations in relation to disturbance in natural and man-modified ecosystems. In Proceedings of the Disturbance and Ecosystems: Components of Response; Springer: Berlin/Heidelberg, Germany, 1983; pp. 259–275. [Google Scholar]
- Schlosser, I.J. Environmental variation, life history attributes, and community structure in stream fishes: Implications for environmental management and assessment. Environ. Manag. 1990, 14, 621–628. [Google Scholar] [CrossRef]
- Gomi, T.; Sidle, R.C.; Richardson, J.S. Understanding processes and downstream linkages of headwater systems: Headwaters differ from downstream reaches by their close coupling to hillslope processes, more temporal and spatial variation, and their need for different means of protection from land use. BioScience 2002, 52, 905–916. [Google Scholar]
- Lite, S.; Bagstad, K.; Stromberg, J. Riparian plant species richness along lateral and longitudinal gradients of water stress and flood disturbance, San Pedro River, Arizona, USA. J. Arid Environ. 2005, 63, 785–813. [Google Scholar] [CrossRef]
- Ito, H.; Ito, S.; Nakao, T.; Kadomoto, K. Effects of fluvial and geomorphic disturbances on forest dynamics of a sedimentation-dominated riparian forest in warm-temperate mountainous region in southern Japan. Jpn. J. For. Environ. Jpn. 2008, 50, 17–24. [Google Scholar]
- Lepori, F.; Hjerdt, N. Disturbance and aquatic biodiversity: Reconciling contrasting views. BioScience 2006, 56, 809–818. [Google Scholar] [CrossRef]
- Bendix, J.; Hupp, C.R. Hydrological and geomorphological impacts on riparian plant communities. Hydrol. Process. 2000, 14, 2977–2990. [Google Scholar] [CrossRef]
- Merritt, D.M.; Scott, M.L.; LeRoy Poff, N.; Auble, G.T.; Lytle, D.A. Theory, methods and tools for determining environmental flows for riparian vegetation: Riparian vegetation-flow response guilds. Freshw. Biol. 2010, 55, 206–225. [Google Scholar] [CrossRef]
- Džubáková, K.; Molnar, P.; Schindler, K.; Trizna, M. Monitoring of riparian vegetation response to flood disturbances using terrestrial photography. Hydrol. Earth Syst. Sci. 2015, 19, 195–208. [Google Scholar] [CrossRef] [Green Version]
- Park, H.; Kim, J.G. Temporal and spatial variations of vegetation in a riparian zone of South Korea. J. Ecol. Environ. 2020, 44, 9. [Google Scholar] [CrossRef] [Green Version]
- Bourgeois, B.; González, E.; Vanasse, A.; Aubin, I.; Poulin, M. Spatial processes structuring riparian plant communities in agroecosystems: Implications for restoration. Ecol. Appl. 2016, 26, 2103–2115. [Google Scholar] [CrossRef] [PubMed]
- Flores-Galicia, N.; Trejo, I.; Ramírez-Marcial, N. Environment-driven changes in diversity of riparian plant communities along a mountain river. Ecol. Evol. 2021, 11, 5690–5701. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Hu, T.; Mao, J.; Montzka, C.; Bol, R.; Wan, S.; Li, J.; Yue, J.; Dai, H. Hydrological Drivers for the Spatial Distribution of Wetland Herbaceous Communities in Poyang Lake. Remote Sens. 2022, 14, 4870. [Google Scholar] [CrossRef]
- Jeon, H.-S.; Obana, M.; Tsujimoto, T. Concept of bed roughness boundary layer and its application to bed load transport in flow with non-submerged vegetation. J. Water Resour. Prot. 2014, 2014, 48190. [Google Scholar] [CrossRef] [Green Version]
- Jeon, H.S.; Obana, M.; Kim, K.H.; Tsujimoto, T. Flow and Sediment Transport with Non-Submerged Riparian Vegetation in 1D Scheme. J. Coast. Res. 2017, 79, 329–333. [Google Scholar] [CrossRef] [Green Version]
- Malanson, G.P.; Butler, D.R. Woody debris, sediment, and riparian vegetation of a subalpine river, Montana, USA. Arct. Alp. Res. 1990, 22, 183–194. [Google Scholar] [CrossRef]
- Fetherston, K.L.; Naiman, R.J.; Bilby, R.E. Large woody debris, physical process, and riparian forest development in montane river networks of the Pacific Northwest. Geomorphology 1995, 13, 133–144. [Google Scholar] [CrossRef]
- Nakamura, F.; Fuke, N.; Kubo, M. Contributions of large wood to the initial establishment and diversity of riparian vegetation in a bar-braided temperate river. Plant Ecol. 2012, 213, 735–747. [Google Scholar] [CrossRef] [Green Version]
- Schwabe, A. A method for the analysis of temporal changes in vegetation pattern at the landscape level. Vegetatio 1991, 95, 1–19. [Google Scholar] [CrossRef]
- Langlade, L.-R.; Decamps, O. Silt accumulation and plant colonization along a gravel bar. Comptes Rendus de l’Academie des Sciences Serie 3 Sciences de la Vie 1995, 318, 1073–1082. [Google Scholar]
- Hughes, F. The flooded forest: Guidance for policy makers and river managers in Europe on the restoration of floodplain forests. FLOBAR2 2003, 2003, 10021977743. [Google Scholar]
- Cendejas-Zarelli, S.J. The Effect of Large Woody Debris, Direct Seeding, and Distance from the Forest Edge on Species Composition on Novel Terraces following Dam Removal on the Elwha River, WA. Master’s Thesis, Western Washington University, Bellingham, WA, USA, 2021. [Google Scholar]
- Li, Z.; Wang, Z.; Pan, B.; Zhu, H.; Li, W. The development mechanism of gravel bars in rivers. Quat. Int. 2014, 336, 73–79. [Google Scholar] [CrossRef]
- Nguyen, N.-T.; Nakajo, S.; Mukunoki, T.; Tsujimoto, G. Estuarine circulation patterns in a complex geometry estuary: Dinh an estuary, Mekong River. Environ. Process. 2018, 5, 503–517. [Google Scholar] [CrossRef]
- Du, Y.; Cheng, Z.; You, Z. Morphological changes in a macro-tidal estuary during extreme flooding events. Front. Mar. Sci. 2023, 9, 1112494. [Google Scholar] [CrossRef]
- Abbe, T.B.; Montgomery, D.R. Large woody debris jams, channel hydraulics and habitat formation in large rivers. Regul. Rivers Res. Manag. 1996, 12, 201–221. [Google Scholar] [CrossRef]
- Picco, L.; Sitzia, T.; Mao, L.; Comiti, F.; Lenzi, M.A. Linking riparian woody communities and fluviomorphological characteristics in a regulated gravel-bed river (Piave River, Northern Italy). Ecohydrology 2016, 9, 101–112. [Google Scholar] [CrossRef]
- Corenblit, D.; Steiger, J.; Gurnell, A.M.; Tabacchi, E.; Roques, L. Control of sediment dynamics by vegetation as a key function driving biogeomorphic succession within fluvial corridors. Earth Surf. Process. Landf. J. Br. Geomorphol. Res. Group 2009, 34, 1790–1810. [Google Scholar] [CrossRef]
- Corenblit, D.; Steiger, J.; Tabacchi, E. Biogeomorphologic succession dynamics in a Mediterranean river system. Ecography 2010, 33, 1136–1148. [Google Scholar] [CrossRef]
- Corenblit, D.; Vautier, F.; González, E.; Steiger, J. Formation and dynamics of vegetated fluvial landforms follow the biogeomorphological succession model in a channelized river. Earth Surf. Process. Landf. 2020, 45, 2020–2035. [Google Scholar] [CrossRef]
- Tilman, D. Niche tradeoffs, neutrality, and community structure: A stochastic theory of resource competition, invasion, and community assembly. Proc. Natl. Acad. Sci. USA 2004, 101, 10854–10861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franklin, J.; Serra-Diaz, J.M.; Syphard, A.D.; Regan, H.M. Global change and terrestrial plant community dynamics. Proc. Natl. Acad. Sci. USA 2016, 113, 3725–3734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, S. Wind as a natural disturbance agent in forests: A synthesis. For. Int. J. For. Res. 2013, 86, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Hupp, C.R.; Osterkamp, W. Bottomland vegetation distribution along Passage Creek, Virginia, in relation to fluvial landforms. Ecology 1985, 66, 670–681. [Google Scholar] [CrossRef]
- Ellenberg, H.H. Vegetation Ecology of Central Europe; Cambridge University Press: Cambridge, UK, 1988. [Google Scholar]
- Schnitzler, A. River dynamics as a forest process: Interaction between fluvial systems and alluvial forests in large European river plains. Bot. Rev. 1997, 63, 40–64. [Google Scholar] [CrossRef]
- Choi, I.-C.; Shin, H.-J.; Nguyen, T.T.; Tenhunen, J. Water Policy Reforms in South Korea: A Historical Review and Ongoing Challenges for Sustainable Water Governance and Management. Water 2017, 9, 717. [Google Scholar] [CrossRef] [Green Version]
- Del Tánago, M.G.; Martínez-Fernández, V.; Aguiar, F.C.; Bertoldi, W.; Dufour, S.; de Jalón, D.G.; Garófano-Gómez, V.; Mandzukovski, D.; Rodríguez-González, P.M. Improving river hydromorphological assessment through better integration of riparian vegetation: Scientific evidence and guidelines. J. Environ. Manag. 2021, 292, 112730. [Google Scholar] [CrossRef]
- Gurnell, A.M.; Petts, G.E. Island-dominated landscapes of large floodplain rivers, a European perspective. Freshw. Biol. 2002, 47, 581–600. [Google Scholar] [CrossRef]
- Décamps, H. The renewal of floodplain forests along rivers: A landscape perspective. Int. Ver. Theor. Angew. Limnol. Verh. 1996, 26, 35–59. [Google Scholar] [CrossRef]
- Odum, E.P. The Strategy of Ecosystem Development: An understanding of ecological succession provides a basis for resolving man’s conflict with nature. Science 1969, 164, 262–270. [Google Scholar] [CrossRef] [Green Version]
- Sousa, W.P. Experimental investigations of disturbance and ecological succession in a rocky intertidal algal community. Ecol. Monogr. 1979, 49, 227–254. [Google Scholar] [CrossRef]
- Whittaker, R.H. Communities Ecosystems, 2nd ed.; Macmillan: New York, USA, 1975. [Google Scholar]
- Baptist, M.J.; Penning, W.E.; Duel, H.; Smits, A.J.; Geerling, G.W.; Van der Lee, G.E.; Van Alphen, J.S. Assessment of the effects of cyclic floodplain rejuvenation on flood levels and biodiversity along the Rhine River. River Res. Appl. 2004, 20, 285–297. [Google Scholar] [CrossRef]
- Vandvik, V.; Heegaard, E.; Måren, I.E.; Aarrestad, P.A. Managing heterogeneity: The importance of grazing and environmental variation on post-fire succession in heathlands. J. Appl. Ecol. 2005, 42, 139–149. [Google Scholar] [CrossRef]
- Sun, C.; Chai, Z.; Liu, G.; Xue, S. Changes in species diversity patterns and spatial heterogeneity during the secondary succession of grassland vegetation on the Loess Plateau, China. Front. Plant Sci. 2017, 8, 1465. [Google Scholar] [CrossRef] [Green Version]
- Tockner, K.; Malard, F.; Ward, J. An extension of the flood pulse concept. Hydrol. Process. 2000, 14, 2861–2883. [Google Scholar] [CrossRef]
- Teurlincx, S.; Verhofstad, M.J.; Bakker, E.S.; Declerck, S.A. Managing successional stage heterogeneity to maximize landscape-wide biodiversity of aquatic vegetation in ditch networks. Front. Plant Sci. 2018, 9, 1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moi, D.A.; García-Ríos, R.; Hong, Z.; Daquila, B.V.; Mormul, R.P. Intermediate disturbance hypothesis in ecology: A literature review. In Proceedings of the Annales Zoologici Fennici; Finnish Zoological and Botanical Publishing Board: Helsinki, Finland, 2020; pp. 67–78. [Google Scholar]
- Zeng, Q.; Shi, L.; Wen, L.; Chen, J.; Duo, H.; Lei, G. Gravel bars can be critical for biodiversity conservation: A case study on Scaly-sided Merganser in South China. PLoS ONE 2015, 10, e0127387. [Google Scholar] [CrossRef]
- Alena, H.; Jan, D.; Jana, D. Threats, biodiversity drivers and restoration in temperate floodplain forests related to spatial scales. Sci. Total Environ. 2022, 854, 158743. [Google Scholar]
- Ock, G.; Kondolf, G.M. Assessment of Ecological Roles of Gravel Bar Features Restored by Gravel Augmentation and Channel Rehabilitation Activities below Lewiston Dam in the Trinity River, California; Trinity River Restoration Program: Weaverville, CA, USA, 2012. [Google Scholar]
- Ock, G.; Gaeuman, D.; McSloy, J.; Kondolf, G.M. Ecological functions of restored gravel bars, the Trinity River, California. Ecol. Eng. 2015, 83, 49–60. [Google Scholar] [CrossRef]
- Lavelle, A.M.; Chadwick, M.A.; Chadwick, D.D.; Pritchard, E.G.; Bury, N.R. Effects of habitat restoration on fish communities in urban streams. Water 2021, 13, 2170. [Google Scholar] [CrossRef]
- Boodoo, K.S.; Fasching, C.; Battin, T.J. Sources, transformation, and fate of dissolved organic matter in the gravel bar of a prealpine stream. J. Geophys. Res. Biogeosci. 2020, 125, e2019JG005604. [Google Scholar] [CrossRef]
- Tsujimoto, T.; Obana, M. Ecosystem function of sand bar segment in sandy river with particular reference to denitrification potential. Procedia Eng. 2012, 28, 835–839. [Google Scholar] [CrossRef] [Green Version]
- Vesipa, R.; Camporeale, C.; Ridolfi, L. Effect of river flow fluctuations on riparian vegetation dynamics: Processes and models. Adv. Water Resour. 2017, 110, 29–50. [Google Scholar] [CrossRef] [Green Version]
- Sabo, J.L.; Sponseller, R.; Dixon, M.; Gade, K.; Harms, T.; Heffernan, J.; Jani, A.; Katz, G.; Soykan, C.; Watts, J. Riparian zones increase regional species richness by harboring different, not more, species. Ecology 2005, 86, 56–62. [Google Scholar] [CrossRef]
- Bennett, A.F.; Nimmo, D.G.; Radford, J.Q. Riparian vegetation has disproportionate benefits for landscape-scale conservation of woodland birds in highly modified environments. J. Appl. Ecol. 2014, 51, 514–523. [Google Scholar] [CrossRef]
- Johnson, R.K.; Almlöf, K. Adapting boreal streams to climate change: Effects of riparian vegetation on water temperature and biological assemblages. Freshw. Sci. 2016, 35, 984–997. [Google Scholar] [CrossRef]
- Piégay, H.; Cuaz, M.; Javelle, E.; Mandier, P. Bank erosion management based on geomorphological, ecological and economic criteria on the Galaure River, France. Regul. Rivers Res. Manag. Int. J. Devoted River Res. Manag. 1997, 13, 433–448. [Google Scholar] [CrossRef]
- Kalníková, V.; Chytrý, K.; Chytrý, M. Early vegetation succession on gravel bars of Czech Carpathian streams. Folia Geobot. 2018, 53, 317–332. [Google Scholar] [CrossRef]
- Lim, B.S.; Kim, D.U.; Kim, A.R.; Seol, J.W.; Lee, C.S. Analysis of Ecodiversity as the Foundation for Conserving Biodiversity and Its Restoration Strategy. Korean J. Ecol. Environ. 2020, 53, 408–426. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seok, J.E.; Lim, B.S.; Moon, J.S.; Kim, G.S.; Lee, C.S. Spatial Distribution of Vegetation on Stream Bars and the Riparian Zone Reflects Successional Pattern Due to Fluid Dynamics of River. Water 2023, 15, 1493. https://doi.org/10.3390/w15081493
Seok JE, Lim BS, Moon JS, Kim GS, Lee CS. Spatial Distribution of Vegetation on Stream Bars and the Riparian Zone Reflects Successional Pattern Due to Fluid Dynamics of River. Water. 2023; 15(8):1493. https://doi.org/10.3390/w15081493
Chicago/Turabian StyleSeok, Ji Eun, Bong Soon Lim, Jeong Sook Moon, Gyung Soon Kim, and Chang Seok Lee. 2023. "Spatial Distribution of Vegetation on Stream Bars and the Riparian Zone Reflects Successional Pattern Due to Fluid Dynamics of River" Water 15, no. 8: 1493. https://doi.org/10.3390/w15081493




