Study on Pollution Characteristics of Perfluoroalkyl Substances (PFASs) in Shallow Groundwater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection of the Location of Survey Items
2.1.1. Selection of the Location and Survey Interval of Water Quality Status
2.1.2. Selection of the Location for the In-Depth Survey
2.2. Sample Collections of Groundwater and Analysis on Site Measuring Items
2.3. Analysis on Major Cation and Anion
2.4. Analysis on PFAS Samples
2.5. Risk Assessment of PFASs for Human Body
3. Results and Discussion
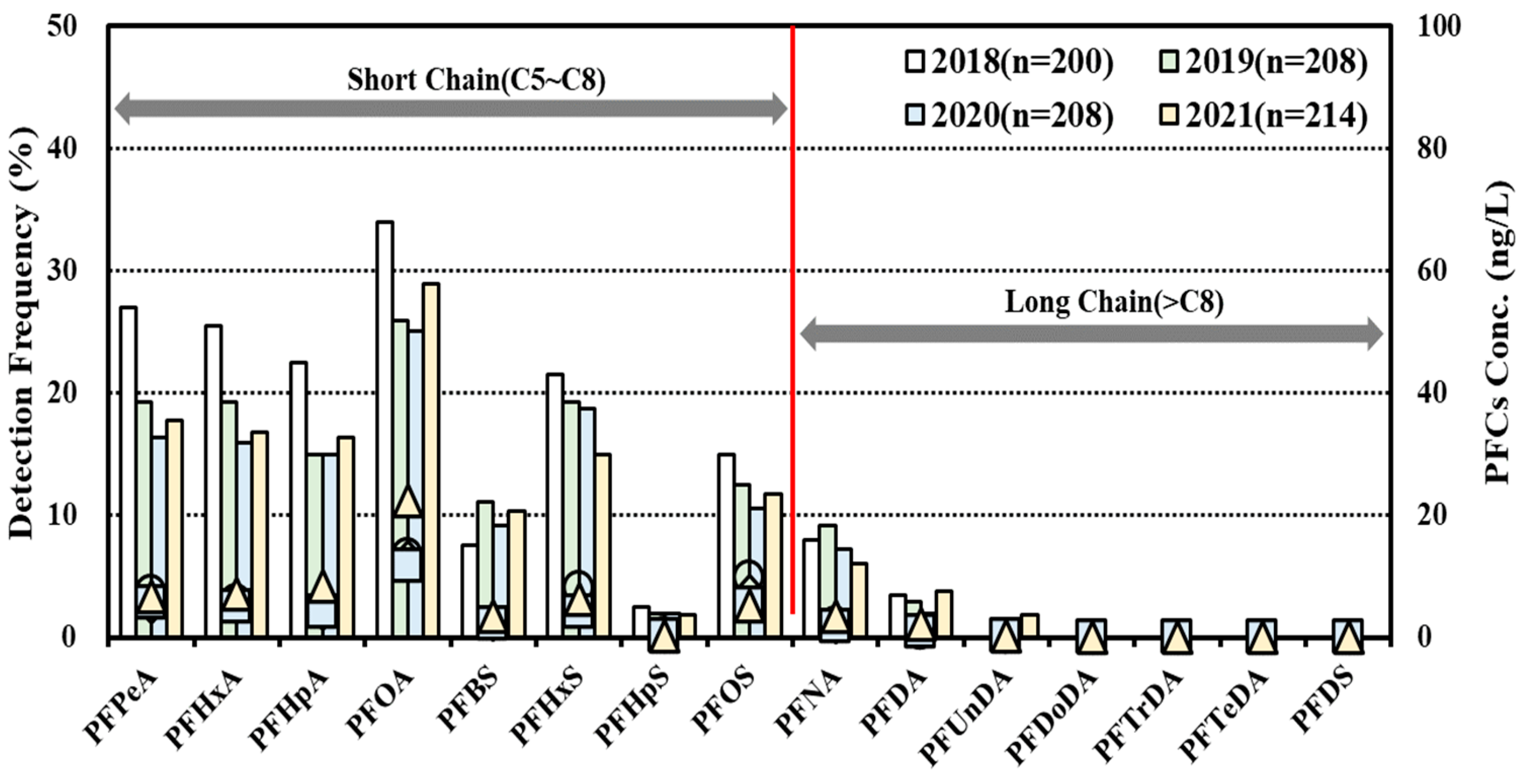
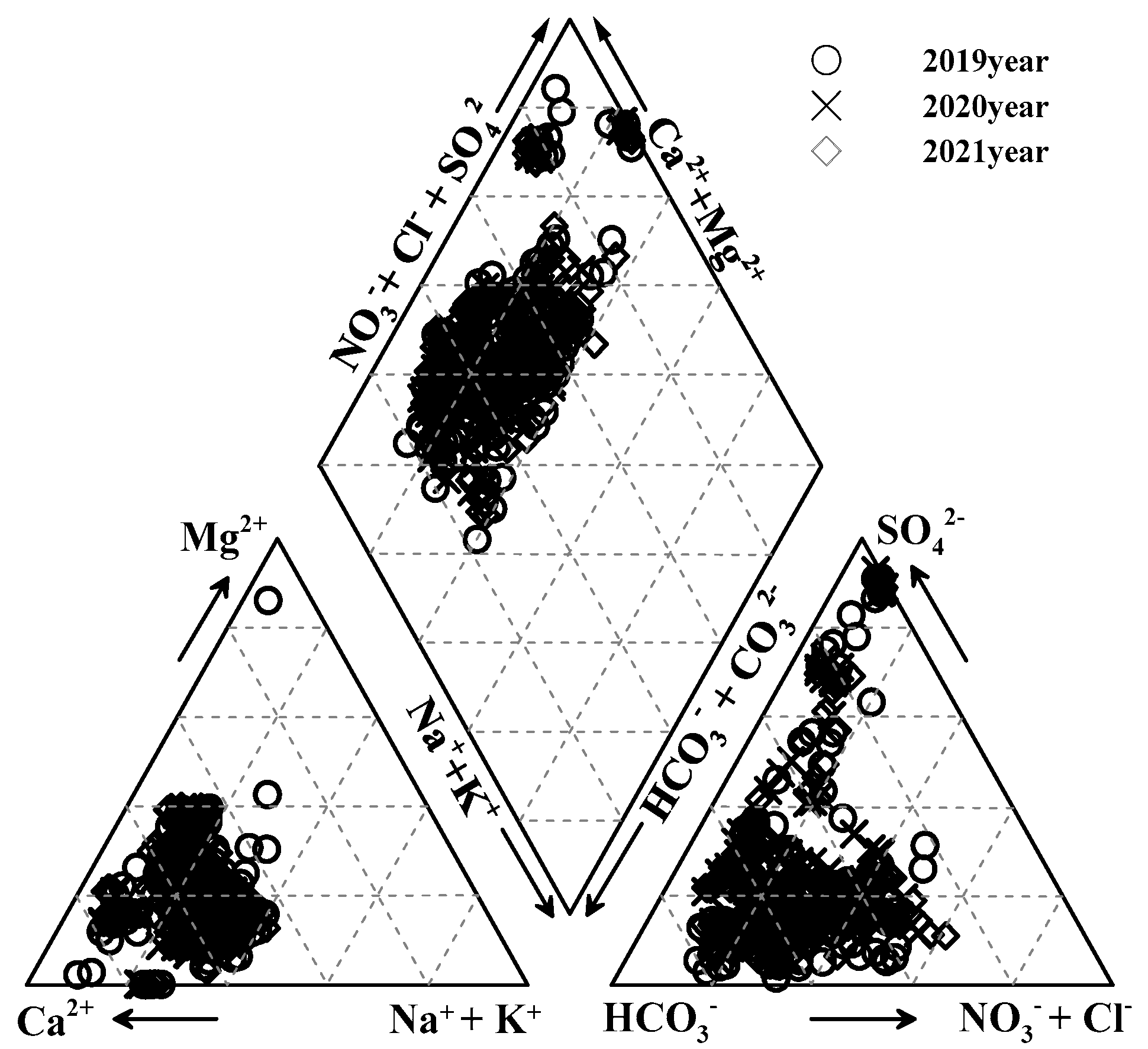
3.1. Survey Results of Water Quality Status
3.2. Results of In-Depth Survey
3.2.1. Analysis Results of Groundwater Quality Classification
3.2.2. Results of In-Depth Monitoring
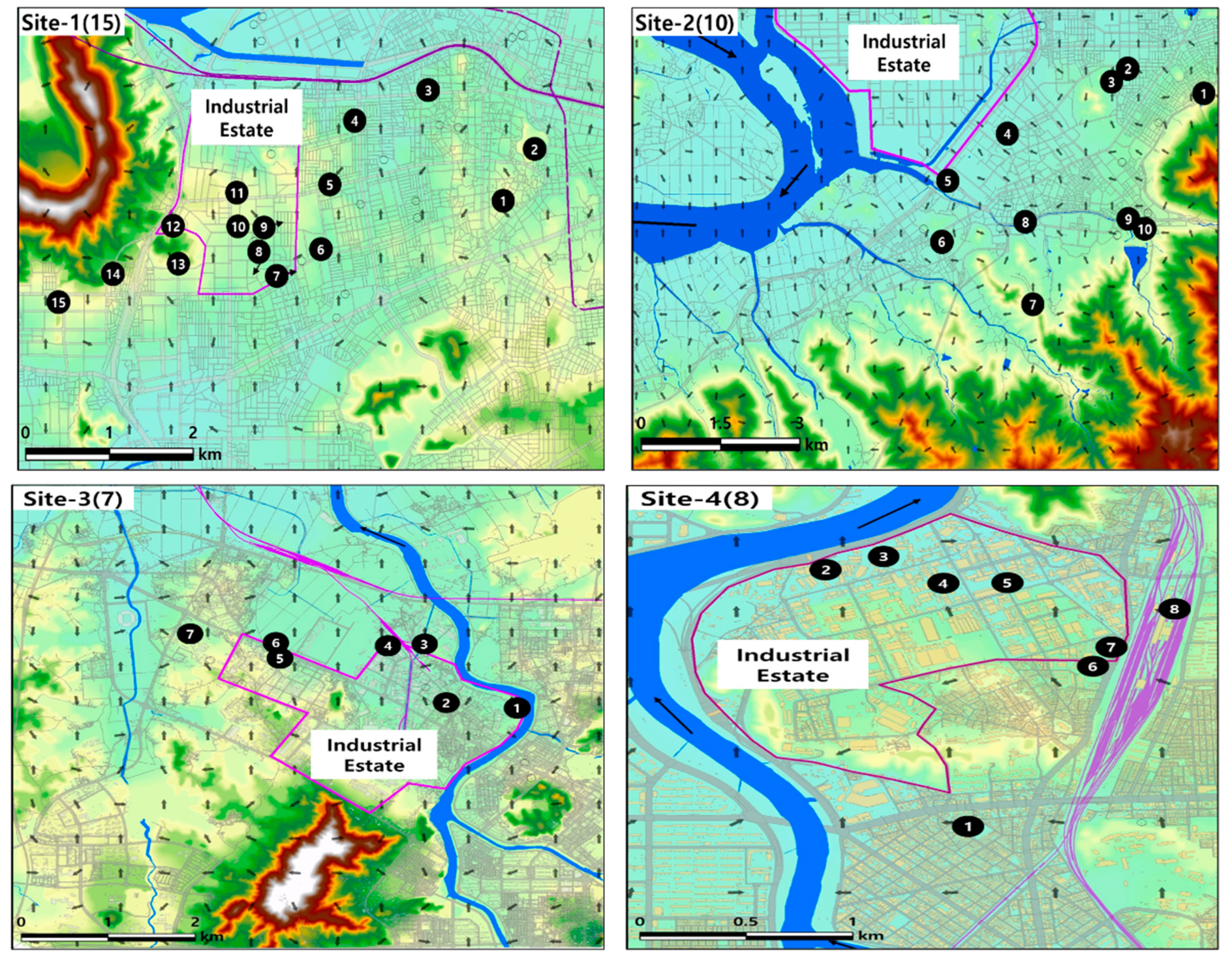
Analysis Results from Site-1
Analysis Results from Site-2
Analysis Results from Site-3
Analysis Results from Site-4
Results of In-Depth Monitoring (4 Sites)
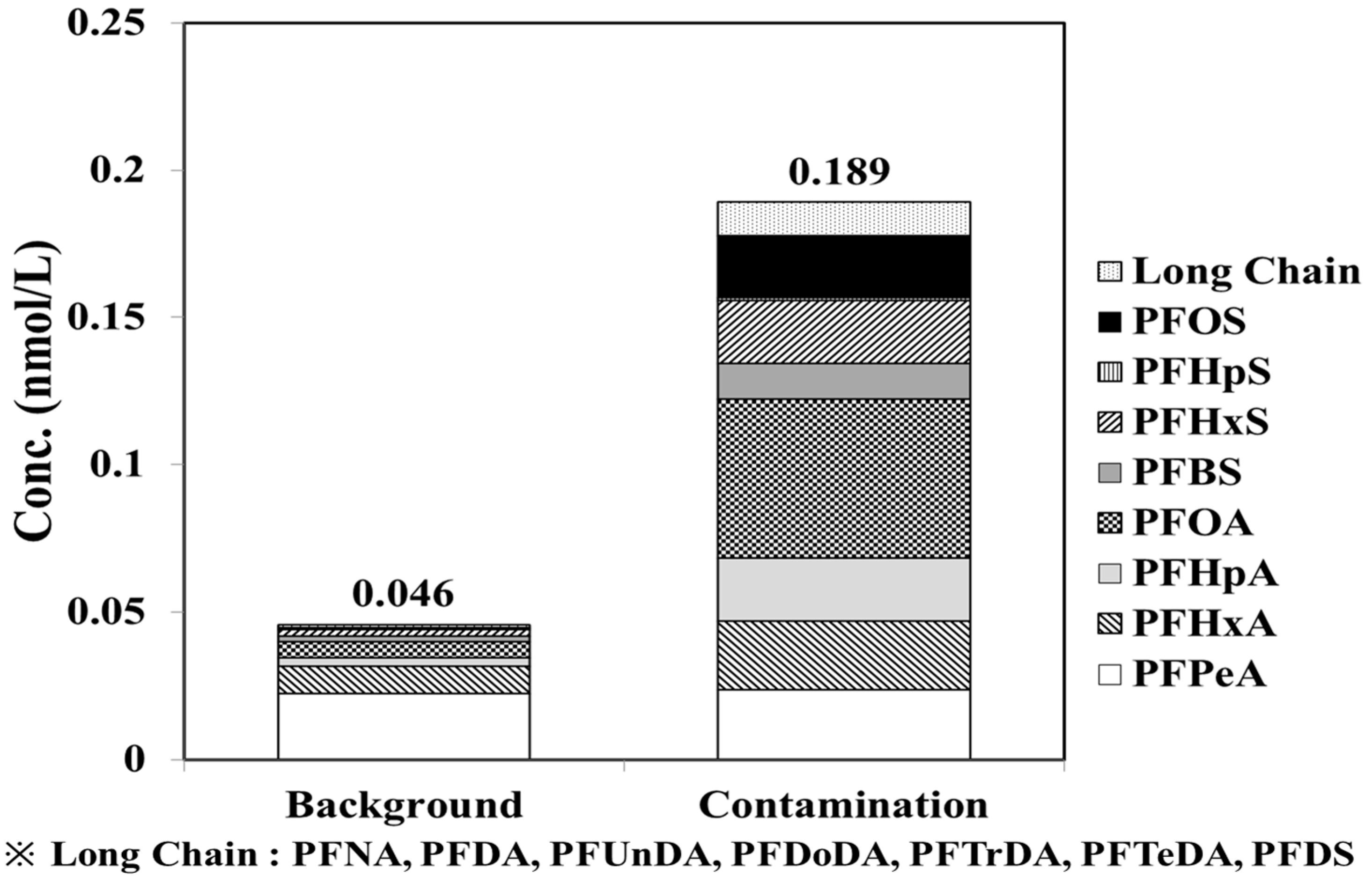
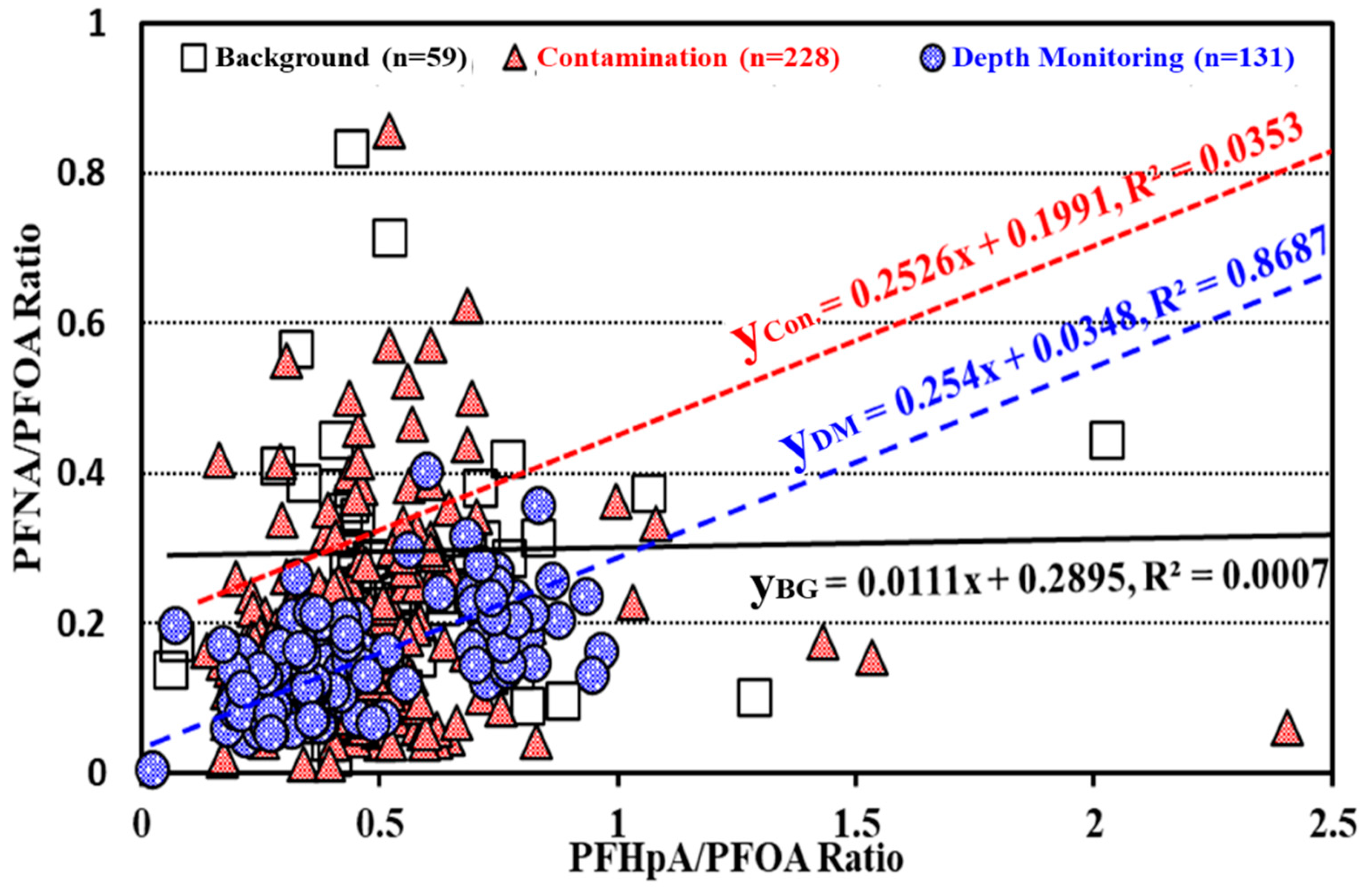
3.2.3. Assessment on Contamination Sources of PFASs in Groundwater
3.3. Results of Assessment of Risk to Human Body (2018–2022)
4. Conclusions
- As a result of the assessment on possibilities of the simultaneous analysis on PFASs in ground water, the accuracy (0.6–11.6%) and precision (90.3–108.0%) of each item meets both Korean and international standards of the analysis methods for PFASs in water. The LOQ by item ranges between 0.3 ng/L and 1.6 ng/L. The reporting limit for all the items were set with 5 ng/L of the LOQ, which is suggested by the Korean Water Pollution Standard Method (ES. 04506.1).
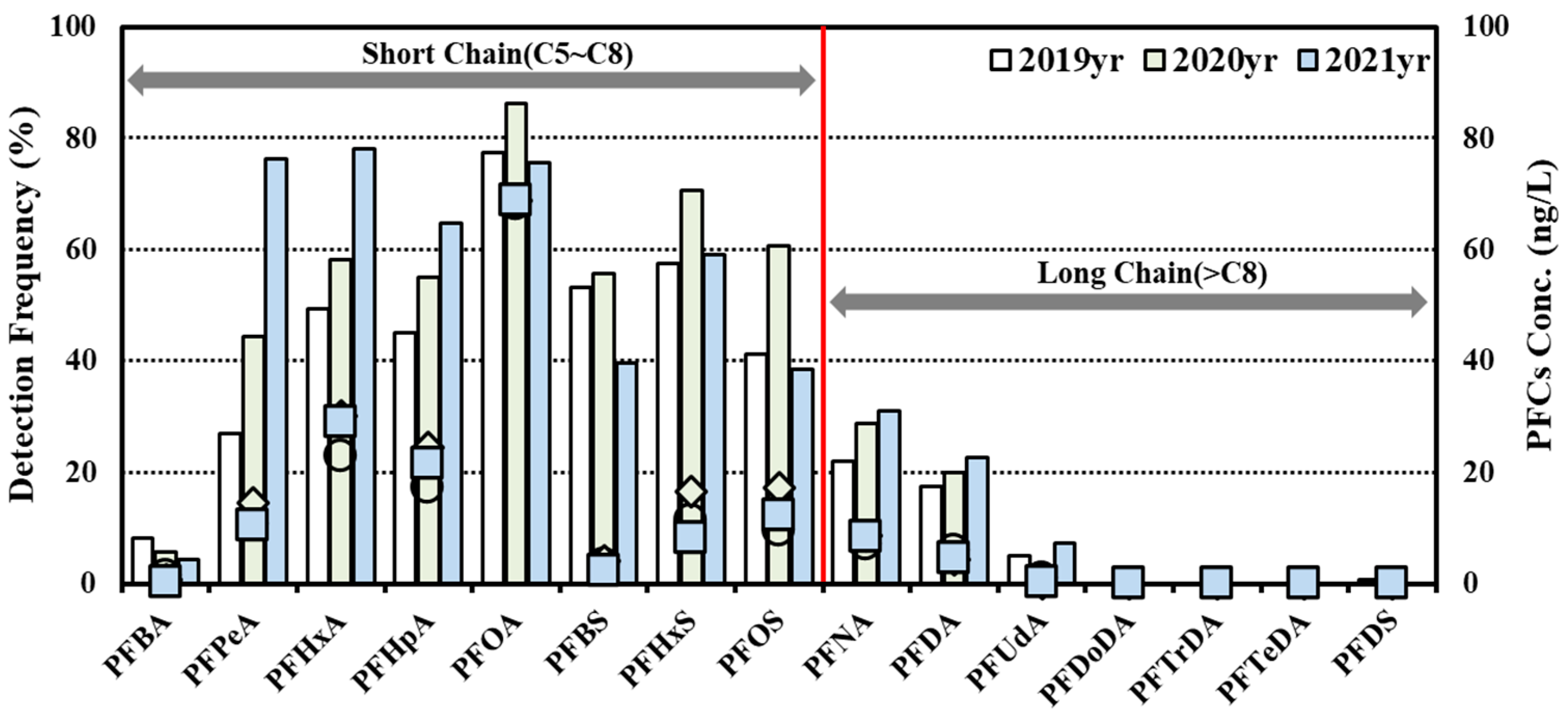
- As a result of the status survey on PFASs in groundwater PFOA (0.025 nmol/L, 28.8%), PFPeA (0.020 nmol/L, 20.1%) and PFHxA (0.013 nmol/L, 19.6%) were detected in the aforementioned order. As a survey result of the distribution rate, depending on different types of PFASs, there were relatively more carboxyl-type PFASs detected than sulfonic PFASs. In addition, PFASs with relatively short chains (8 or less) of carbon showed relatively higher concentrations and frequencies than those with longer chains (9 or more) of carbon.
- As a result of the human risk assessment of PFASs in groundwater, PFOA and PFOS show relatively higher levels of risk (non-carcinogenic risk level: 10−1) compared to other PFASs. When it comes to the assessment of risk contribution to the human body by exposure route, the route of ingestion contributed to risks to the human body among all the items detected from PFASs more than other routes (e.g., inhalation, dermal contact).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cui, D.; Li, X.; Quinete, N. Occurrence, fate, sources and toxicity of PFAS: What we know so far in Florida and major gaps. Trends Anal. Chem. 2020, 130, 115976. [Google Scholar] [CrossRef]
- Wang, Z.; Buser, A.M.; Cousins, I.T.; Demattio, S.; Drost, W.; Johansson, O.; Ohno, K.; Patlewicz, G.; Richard, A.M.; Walker, G.W.; et al. A new OECD definition for per- and polyfluoroalkyl substances. Environ. Sci. Technol. 2021, 55, 15575–15578. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Sa, J.; Kim, J.; Min, S. Study on environmental regulation of aqueous film forming foam and use of C4-based eco-friendly aqueous film forming foam. J. Korean Soc. Hazard Mitig. 2022, 22, 125–131. [Google Scholar] [CrossRef]
- Shahsavari, E.; Rouch, D.; Khudur, L.S.; Thomas, D.; Medina, A.A.; Ball, A.S. Challenges and current status of biological treatment of PFAS-contaminated soils. Front. Bioeng. Biotechnol. 2021, 8, 602040. [Google Scholar] [CrossRef]
- Chen, S.; Jiao, X.C.; Gai, N.; Li, X.J.; Wang, X.C.; Lu, G.H.; Piao, H.T.; Rao, Z.; Yang, Y.L. Perfluorinated compounds in soil, surface water, and groundwater from rural areas in eastern China. Environ. Pollut. 2016, 211, 124–131. [Google Scholar] [CrossRef]
- Patrik, N.B. Toxicological Profile for Perfluoroalkyls; Final Release; ATSDR, Division of Toxicology and Human Health Science: Atlanta, GA, USA, 2021; pp. 1–822.
- Bartell, S.M.; Bolt, H.M.; Cesta, M.F.; Chiu, W.; Cooper, G.; DeWitt, J.; Frisen, M.D.; Fritschi, L.; Fukushima, S.; Hansen, J.; et al. IARC Monographs: Some Chemicals Used as Solvents and in Polymer Manufacture; International Agency for Research on Cancer: Lyon, France, 2017; Volume 110, pp. 37–110.
- Han, X.; Snow, T.A.; Kemper, R.A.; Jepson, G.W. Binding of perfluorooctanoic acid to rat and human plasma proteins. Chem. Res. Toxicol. 2003, 16, 775–781. [Google Scholar] [CrossRef]
- Bartell, S.M.; Vieira, V.M. Critical review on PFOA, kidney cancer, and testicular cancer. J. Air Waste Manag. Assoc. 2017, 71, 663–679. [Google Scholar] [CrossRef]
- IARC Monographs on the Identification of Carcinogenic Hazards to Humans: Report of the Advisory Group to Recommend Priorities for the IARC Monographs During 2020–2024. Available online: https://monographs.iarc.who.int/wp-content-uploads/2019/10/IARCMonographs-AGReport-Priorities_2020-2024.pdf (accessed on 10 March 2023).
- NTP Monograph on Immunotoxicity Associated with Exposure to Perfluorooctanoic Acid (PFOA) or Perfluorooctan Sulfonate (PFOS). Available online: https://ntp.niehs.nih.gov/ntp/ohat/pfoa_pfos/pfoa_pfosmonograph_508.pdf (accessed on 19 March 2023).
- Borg, D.; Lund, B.O.; Lindquist, N.G.; Hakansson, H. Cumulative health risk assessment of 17 perfluoroalkylated and 405 polyfluoroalkylated substances(PFASs) in the Swedish population. Environ. Int. 2013, 59, 112–123. [Google Scholar] [CrossRef]
- Banzhaf, S.; Filipovic, M.; Lewis, J.; Sparrenbom, C.J.; Barthel, R. A review of contamination of surface-, ground-, and drinking water in Sweden by perfluoroalkyl and polyfluoroalkyl substances(PFASs). Ambio 2017, 46, 335–346. [Google Scholar] [CrossRef] [Green Version]
- Arvaniti, O.S.; Stasinakis, A.S. Review on the occurrence, fate and removal of perfluorinated compounds during wastewater treatment. Sci. Total Environ. 2015, 524–525, 81–92. [Google Scholar] [CrossRef]
- Filipovic, M.; Berger, U. Are perfluoroalkyl acids in wastewater treatment plant effluents the result of primary emissions from the technosphere or of environmental recirculation? Chemosphere 2015, 129, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Fact Sheet: PFOA & PFOS Drinking Water Health Advisories. Available online: https://www.epa.gov/sites/default/files/2016-06/documents/drinkingwaterhealthadvisories_pfoa_pfos_updated_5.31.16.pdf (accessed on 14 February 2023).
- Commission Regulation (EU) 2017/1000. Available online: https://www.eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32017R1000&rid=8 (accessed on 14 February 2023).
- Regulation (EU) 2019/1021 of the European Parliament and of the Council. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32019R1021&from=EN (accessed on 14 February 2023).
- Commission Delegated Regulation (EU) 2070/784. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32020R0784&rid=1 (accessed on 14 February 2023).
- Voluntary Groundwater Watch List Concept & Methodology. Available online: https://circabc.europa.eu/sd/a/d3fa0178-0134-4316-a11c-dcfd71efca69/Watch-List_Concept_Final.pdf (accessed on 14 February 2023).
- Sörengård, M.; Bergström, S.; McCleaf, P.; Wiberg, K.; Ahrens, L. Long-distance transport of per- and polyfluoroalkyl substances(PFAS) in a Swedish drinking water aquifer. Environ. Pollut. 2022, 311, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Barreca, S.; Busetto, M.; Vitelli, M.; Colzani, L.; Clerici, L.; Pierluisa, D. Online solid-phase extraction LC-MS/MS: A rapid and valid method for the determination of perfluorinated compounds at sub ng·L−1 level in natural water. J. Chem. 2018, 2018, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Savoca, D.; Melfi, R.; Piccionello, A.P.; Barreca, S.; Buscemi, S.; Arizza, V.; Arculeo, M.; Pace, A. Presence and biodistribution of perfluorooctanoic acid(PFOA) in paracentrotus lividus highlight its potential application for environmental biomonitoring. Nat. Sci. Rep. 2021, 11, 18763. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Park, S.H.; Hwang, J.Y.; Kim, M.S.; Jo, H.J.; Jeon, S.H.; Lee, K.K.; Jeen, S.W. Groundwater qualities of wells around carcass burial areas. Korean J. Geol. Soc. 2017, 53, 433–446. [Google Scholar] [CrossRef]
- National Institute of Environmental Research. Study on Groundwater Standared of Unregulated Contaminants and Remediation Standard for TPH and Chlorinated Compounds (PCE, TCE); National Institute of Environmental Research: Incheon, Republic of Korea, 2018.
- EPA. Guidelines for Exposure Assessment; EPA/600/Z-92/001; United States Environmental Protection Agency: Washington, DC, USA, 1992.
- EPA. Risk Assessment Guidance for Superfund Volume III; Part A, Process for Conducting Probabilistic Risk assessment; United States Environmental Protection Agency: Washington, DC, USA, 2001.
- Kim, S.H. Guidelines for Risk Assessment Reports; Ministry of Food and Drug Safety: Cheongju-si, Republic of Korea, 2015; pp. 1–47.
- EPA. Risk Assessment Guidance for Superfund Volume I-Human Health Evaluation Manual (Part A); EPA/540/1-89/002; United States Environmental Protection Agency: Washington, DC, USA, 1989.
- Brown, L.M.; Pottern, L.M.; Blot, W.J. Lung cancer in relation to environmental pollutants emitted from industrial sources. Environ. Res. 1984, 34, 250–261. [Google Scholar] [CrossRef]
- McKone, T.E. Human exposure to volatile organic compounds in houhold tap water: The indoor inhalation pathway. Environ. Sci. Technol. 1987, 21, 1194–1201. [Google Scholar] [CrossRef]
- Marti, V.; DePablo, J.; Jubany, I.; Rovira, M.; Orejudo, E. Water-air volatilization factors to determine volatile organic compound(VOC) reference levels in water. Toxics 2014, 2, 276–290. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Lu, Y.; Wang, T.; Wang, P.; Li, Q.; Johnson, A.C.; Sarvajayakesavalu, S.; Sweetman, A.J. Risk assessmemnt and source identification of perfluoroalkyl acids in surface and groundwater: Spatial distribution around a mega-fluorochemical industrial park China. Environ. Int. 2016, 91, 69–77. [Google Scholar] [CrossRef] [Green Version]
- Barreca, S.; Busetto, M.; Colzani, L.; Clerici, L.; Marchesi, V.; Tremolada, L.; Daverio, D.; Dellavedova, P. Hyphenated high performance liquid chromatography-tandem mass spectrometry techniques for the determination of perfluorinated alkylated substances in Lombardia region in Italy, profile levels and assessment: One year of monitoring activated during 2018. Seperations 2020, 7, 17. [Google Scholar] [CrossRef] [Green Version]
- Ahrens, L.; Shoeib, M.; DelVento, S.; Codling, G.; Halsall, C. Polyfluoroalkyl compounds in the Canadian arctic atmosphere. Environ. Chem. 2011, 8, 399–406. [Google Scholar] [CrossRef] [Green Version]
- Ruan, T.; Lin, Y.; Wang, T.; Jiang, G.; Wang, N. Methodology for studying biotransformation of polyfluoroalkyl precursors in the environment. Trends Anal. Chem. 2015, 95, 122–131. [Google Scholar] [CrossRef]
- Ateia, M.; Maroli, A.; Tharayil, N.; Karanfil, T. The overlooked short- and ultrashort-chain poly- and perfluorinated substances: A review. Chemosphere 2019, 220, 866–882. [Google Scholar] [CrossRef] [PubMed]
- Yong, Z.Y.; Kim, K.Y.; Oh, J.-E. The occurrence and distributions of per- and polyfluoroalkyl substances (PFAS) in groundwater after a PFAS leakage incident in 2018. Environ. Pollut. 2021, 268, 115395. [Google Scholar] [CrossRef] [PubMed]
- Newell, C.J.; Adamson, D.T.; Kulkarni, P.R.; Nzeribe, B.N.; Conner, J.A.; Prpovic, J.; Stroo, H.F. Monitored natural attenuation to manage PFAS impacts to groundwater: Scientific basis. Groundw. Monit. Remediat. 2021, 41, 76–89. [Google Scholar] [CrossRef]
- Cao, X.; Wang, C.; Lu, Y.; Zhang, M.; Khan, K.; Song, S.; Wang, P.; Wang, C. Occurence, sources and health risk of polyfluoroalkyl substances (PFASs) in soil, water and sediment from a drinking water source area. Ecotoxicol. Environ. Saf. 2019, 174, 208–217. [Google Scholar] [CrossRef]
- Hepburn, E.; Madden, C.; Szabo, D.; Coggan, T.L.; Clarke, B.; Currel, M. Contamination of groundwater with per- and polyfluoroalkyl substances (PFAS) from legacy landfills in an urban re-development precinct. Environ. Pollut. 2019, 248, 101–113. [Google Scholar] [CrossRef]
- Xu, B.; Liu, S.; Zhou, J.L.; Zheng, C.; Jin, W.; Chen, B.; Zhang, T.; Qiu, W. PFAS and their substitutes in groundwater: Occurrence, transformation and remediation. J. Hazard. Mater. 2021, 412, 125159. [Google Scholar] [CrossRef]
- Simcik, M.F.; Dorweiler, K.J. Ratio of perfluorochemical concentrations as a tracer of atmospheric deposition to surface waters. Environ. Sci. Technol. 2005, 39, 8678–8683. [Google Scholar] [CrossRef]
- Yu, X.; Tabake, Y.; Yamamoto, K.; Matsumura, C.; Nishimura, F. Biodegradation property of 8:2 fluorotelomer alcohol (8:2 FTOH) under aerobic/anoxic/anaerobic conditions. J. Water Environ. Technol. 2016, 14, 177–190. [Google Scholar] [CrossRef] [Green Version]
- Lv, J.; Guo, C.; Liang, S.; Zhang, Y.; Xu, J. Partitioning behavior, source identification, and risk assessment of perfluorinated compounds in an industry-influenced river. Environ. Sci. Eur. 2019, 31, 55. [Google Scholar] [CrossRef] [Green Version]
- EPA. Interim Recommendations to Address Groundwater Contaminated with Perfluorooctanoic Acid and Perfluorooctanesulfonate; OLEM Directive No. 9283. 1-47; United States Environmental Protection Agency: Washington, DC, USA, 2019.
- EPA. Questions and Answers: Drinking Water Health Advisories for PFOA, PFOS, GenX Chemicals and PFBS. 2023. Available online: https://epa.gov/sdwa/questions-and-answers-drinking-water-health-advisories-pfoa-pfos-genx-chemicals-and-pfbs (accessed on 29 March 2023).

| Parameter | Condition | |||||
|---|---|---|---|---|---|---|
| Column | ACE 5 C18-PFP 150 × 2.1 mm | |||||
| Mode | Binary gradient | |||||
| Mobile phase | A: 5 mM Ammonium acetate B: Methanol | |||||
| Gradient | Time (min) | 0 | 14.00 | 18.00 | 18.01 | 21.00 |
| Solvent B (%) | 25 | 85 | 100 | 25 | 25 | |
| Column flow rate | 0.2 mL/min | |||||
| Injection volume | 5 μL | |||||
| Column temp | 40 °C | |||||
| Ionization mode | Negative ion electrospray | |||||
| Capillary voltage | 4.00 kV | |||||
| Gas temperature | 280 °C | |||||
| Gas flow | 9 L/min (N2) | |||||
| Compound | Carboxyl /Sulfonyl | Target /Internal | Retention Time (min) | Precursorion, m/z | Production, m/z (CE) | |
|---|---|---|---|---|---|---|
| PFBA | Carboxyl | Target | 5.620 | 213.0 | 169.05 (10) | |
| PFPeA | 8.770 | 262.9 | 219.10 (8) | |||
| PFHxA | 10.868 | 312.9 | 269.05 (9) | 119.00 (21) | ||
| PFHpA | 12.332 | 362.9 | 319.10 (9) | 168.95 (17) | ||
| PFOA | 13.501 | 412.9 | 368.75 (11) | 168.85 (19) | ||
| PFNA | 14.457 | 462.9 | 418.80 (10) | 218.90 (18) | ||
| PFDA | 15.277 | 513.0 | 469.05 (10) | 218.90 (18) | ||
| PFUnDA | 15.228 | 563.0 | 519.00 (13) | 319.05 (19) | ||
| PFDoDA | 16.574 | 612.9 | 568.90 (12) | 169.00 (25) | ||
| PFTrDA | 16.544 | 663.0 | 619.00 (14) | 169.10 (32) | ||
| PFTeDA | 17.074 | 712.9 | 668.95 (13) | 168.90 (35) | ||
| PFBS | Sulfonyl | Target | 9.435 | 298.8 | 98.80 (32) | 79.80 (27) |
| PFHxS | 12.562 | 398.9 | 79.85 (45) | 98.80 (34) | ||
| PFHpS | 13.580 | 449.0 | 79.90 (42) | 98.80 (41) | ||
| PFOS | 14.558 | 499.0 | 79.90 (46) | 98.80 (37) | ||
| PFDS | 15.912 | 598.8 | 98.80 (55) | 80.00 (48) | ||
| M2PFOA | Carboxyl | Internal | 13.704 | 415.0 | 370.40 (13) | 170.40 (36) |
| MPFOS | Sulfonyl | 14.841 | 502.7 | 98.80 (44) | ||
| No. | Compound | MW (g/mol) | Henry’s Constant (atm-m3/mol) | Dwater (m2/s) | Dair (m2/s) | log KOW | Vapor Pressure (Pa, 25 °C) | Saq (mg/L) | SP (cm/hr) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | PFOA | 414.07 | 8.88 × 10−7 | 8.57 × 10−10 | 1.30 × 10−5 | 5.30 | 4.21 | 9500 | 5.52 × 10−2 |
| 2 | PFOS | 500.13 | 2.96 × 10−9 | 8.44 × 10−10 | 1.30 × 10−5 | 6.43 | 0.27 | 570 | - |
| 3 | PFHxS | 400.11 | 1.96 × 10−10 | 8.74 × 10−10 | 1.31 × 10−5 | 5.17 | 5.17 | 2300 | - |
| Drinking (O/X) | Exposure Pathway | Equation | Reference |
|---|---|---|---|
| O | Ingestion | [29] | |
| X | Dermal contact | [i: Water use (Washing, Shower, Farm, etc.)] | [30] |
| Inhalation | [i: Water use (Washing, Shower, Farm, etc.)] | [31] | |
| Total non-cancer risk | [29] | ||
| Total cancer risk | [32] | ||
| Functional Group | Compound | Cancer | 95th Percentile Lifetime Daily Dose (ng/kg-Day) | Hazard Quotients (HQ) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Drinking | Non-Drinking | Total | Drinking | Non-Drinking | Total | |||||
| Ingestion | Dermal Contact | Inhalation | Ingestion | Dermal Contact | Inhalation | |||||
| Carboxyl | PFPeA | X | 0.56 | 0.4 | 5.4 × 10−6 | 0.96 | ◉ | ◉ | ○ | ◉ |
| PFHxA | X | 0.63 | 4.2 × 10−7 | 8.41 × 10−6 | 0.63 | ◉ | ○ | ○ | ◉ | |
| PFHpA | X | 0.63 | 8.7 × 10−3 | 1.14 × 10−5 | 0.64 | ◉ | ○ | ○ | ◉ | |
| PFOA | O | 1.52 | 3.14 × 10−3 | 3.80 × 10−5 | 1.53 | ● | ○ | ○ | ● | |
| PFNA | X | 0.3 | 1.09 × 10−7 | 1.02 × 10−5 | 0.3 | ◉ | ○ | ○ | ◉ | |
| PFDA | X | 0.7 | 5.21 × 10−7 | 3.04 × 10−5 | 0.7 | ◉ | ○ | ○ | ◉ | |
| Sulfonyl | PFBS | X | 0.2 | 7.31 × 10−3 | 2.47 × 10−6 | 0.2 | ◎ | ○ | ○ | ◉ |
| PFHxS | X | 0.38 | 1.77 × 10−7 | 9.35 × 10−6 | 0.38 | ◉ | ○ | ○ | ◉ | |
| PFOS | X | 1.09 | 6.86 × 10−6 | 5.14 × 10−5 | 1.09 | ● | ○ | ○ | ● | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Kim, D.-H.; Yoon, J.-H.; Kwon, J.-B.; Choi, H.; Shin, S.-K.; Kim, M.; Kim, H.-K. Study on Pollution Characteristics of Perfluoroalkyl Substances (PFASs) in Shallow Groundwater. Water 2023, 15, 1480. https://doi.org/10.3390/w15081480
Park S, Kim D-H, Yoon J-H, Kwon J-B, Choi H, Shin S-K, Kim M, Kim H-K. Study on Pollution Characteristics of Perfluoroalkyl Substances (PFASs) in Shallow Groundwater. Water. 2023; 15(8):1480. https://doi.org/10.3390/w15081480
Chicago/Turabian StylePark, Sunhwa, Deok-Hyun Kim, Jong-Hyun Yoon, Jong-Beom Kwon, Hyeonhee Choi, Sun-Kyoung Shin, Moonsu Kim, and Hyun-Koo Kim. 2023. "Study on Pollution Characteristics of Perfluoroalkyl Substances (PFASs) in Shallow Groundwater" Water 15, no. 8: 1480. https://doi.org/10.3390/w15081480
APA StylePark, S., Kim, D.-H., Yoon, J.-H., Kwon, J.-B., Choi, H., Shin, S.-K., Kim, M., & Kim, H.-K. (2023). Study on Pollution Characteristics of Perfluoroalkyl Substances (PFASs) in Shallow Groundwater. Water, 15(8), 1480. https://doi.org/10.3390/w15081480






