Photoinhibition of the Picophytoplankter Synechococcus Is Exacerbated by Ocean Acidification
Abstract
1. Introduction
2. Materials and Methods
2.1. Cultures and Experimental Design
2.2. Carbonate Chemistry System
2.3. Growth Rates
2.4. Chlorophyll a Content and Optical Absorption Cross Section
2.5. Chlorophyll a Fluorescence
2.6. Carbon Fixation Rates
2.7. C and N Analysis
2.8. Data Analysis
3. Results
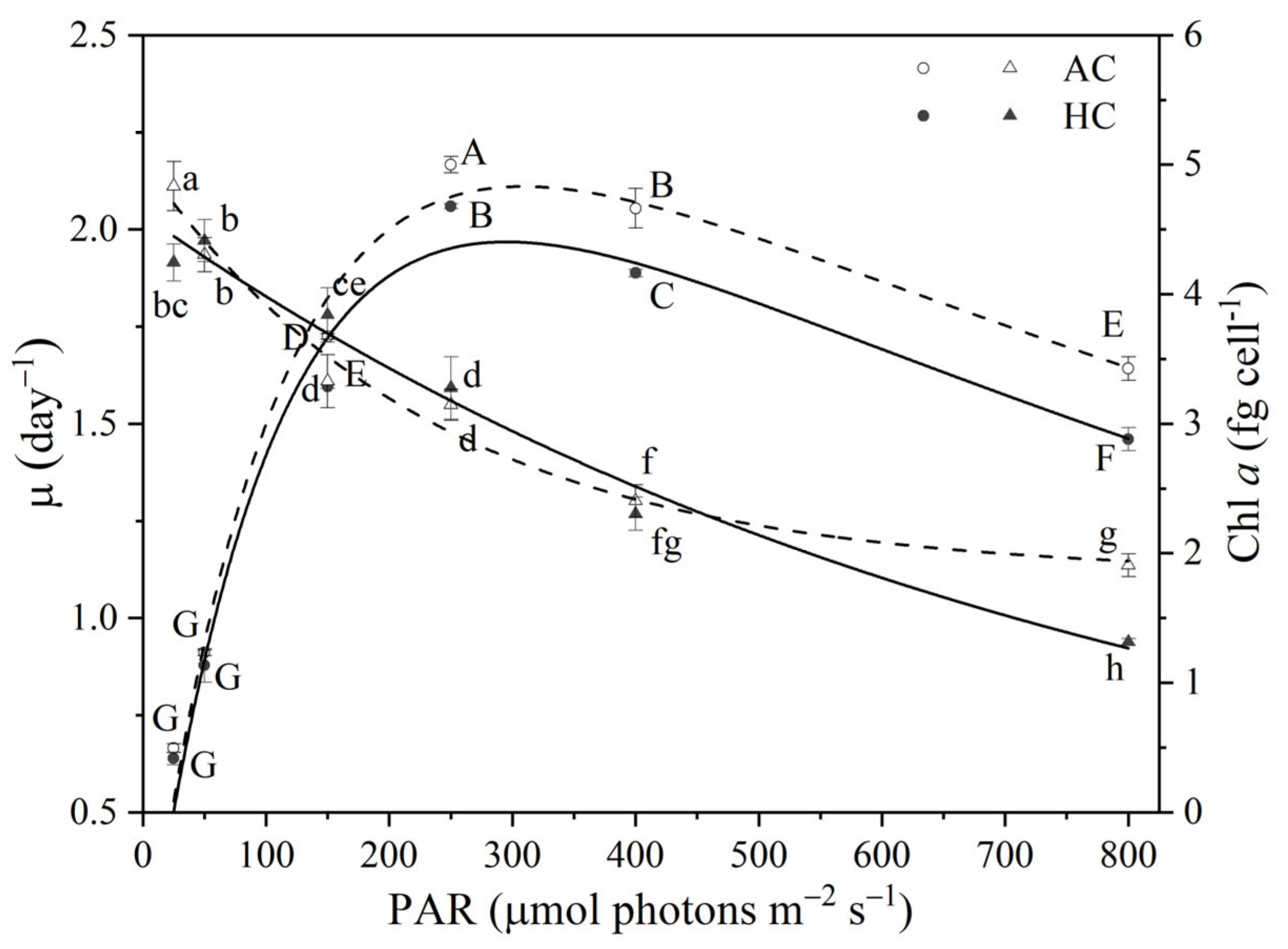
3.1. Growth and Chl a
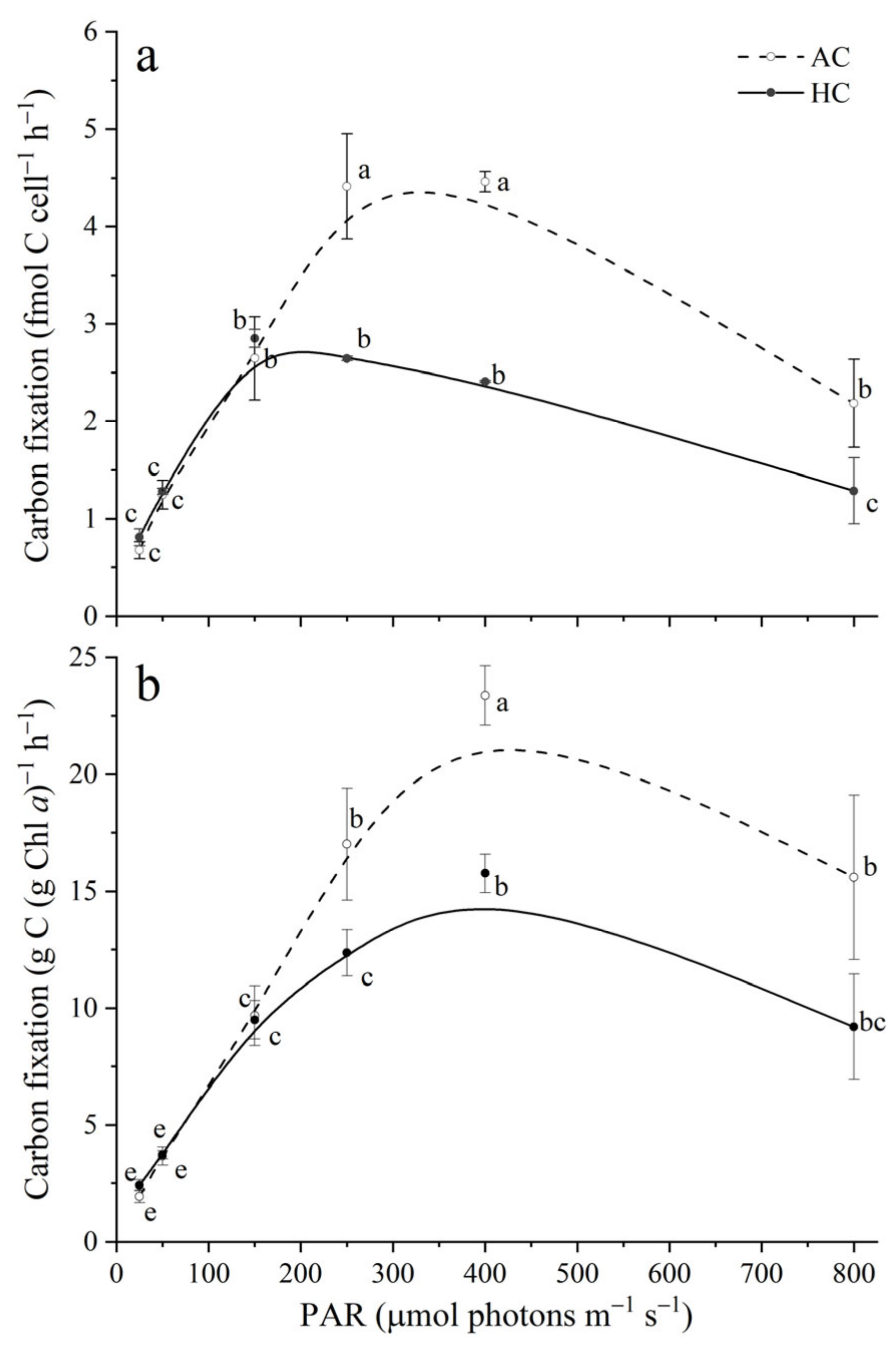
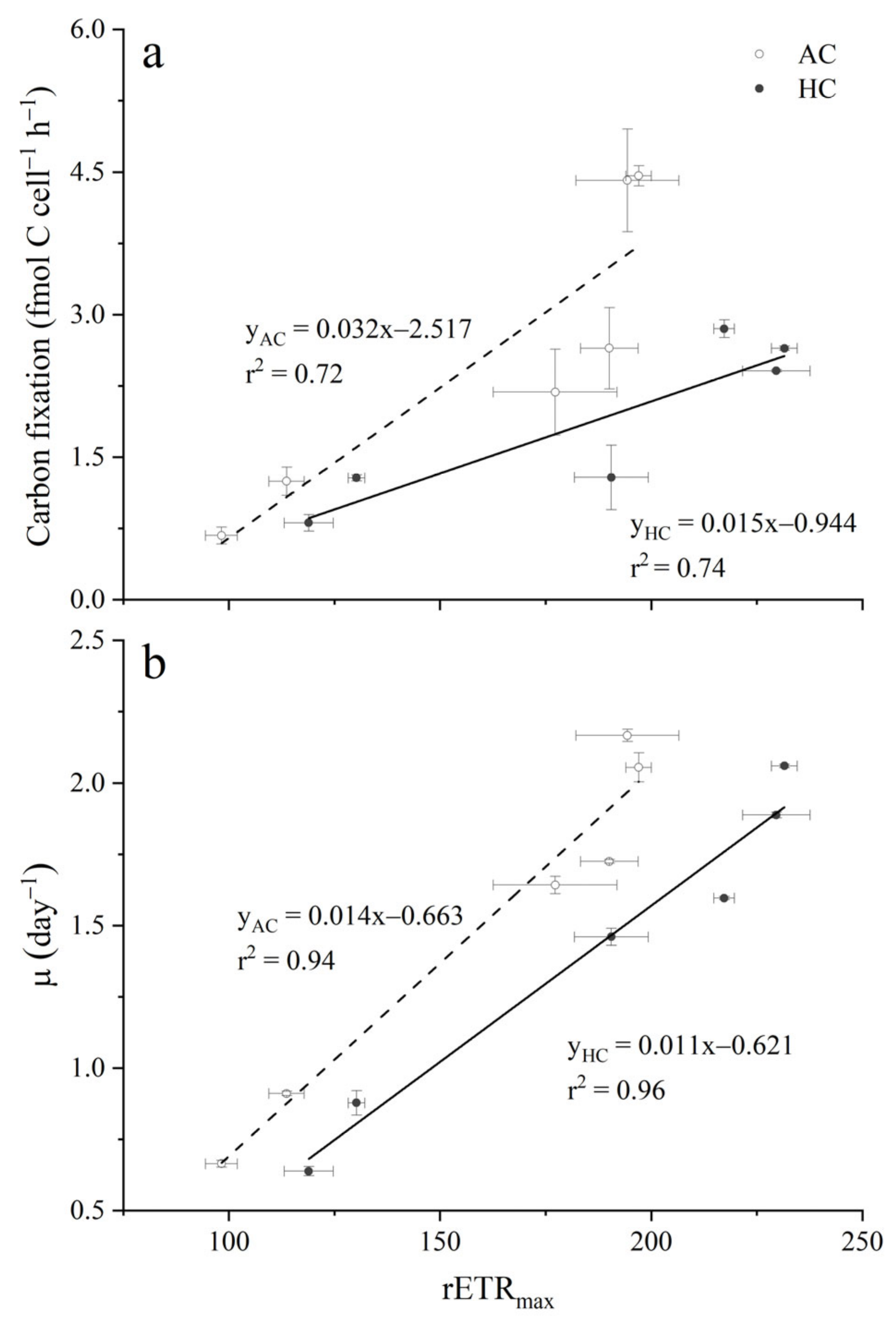
3.2. Carbon Fixation
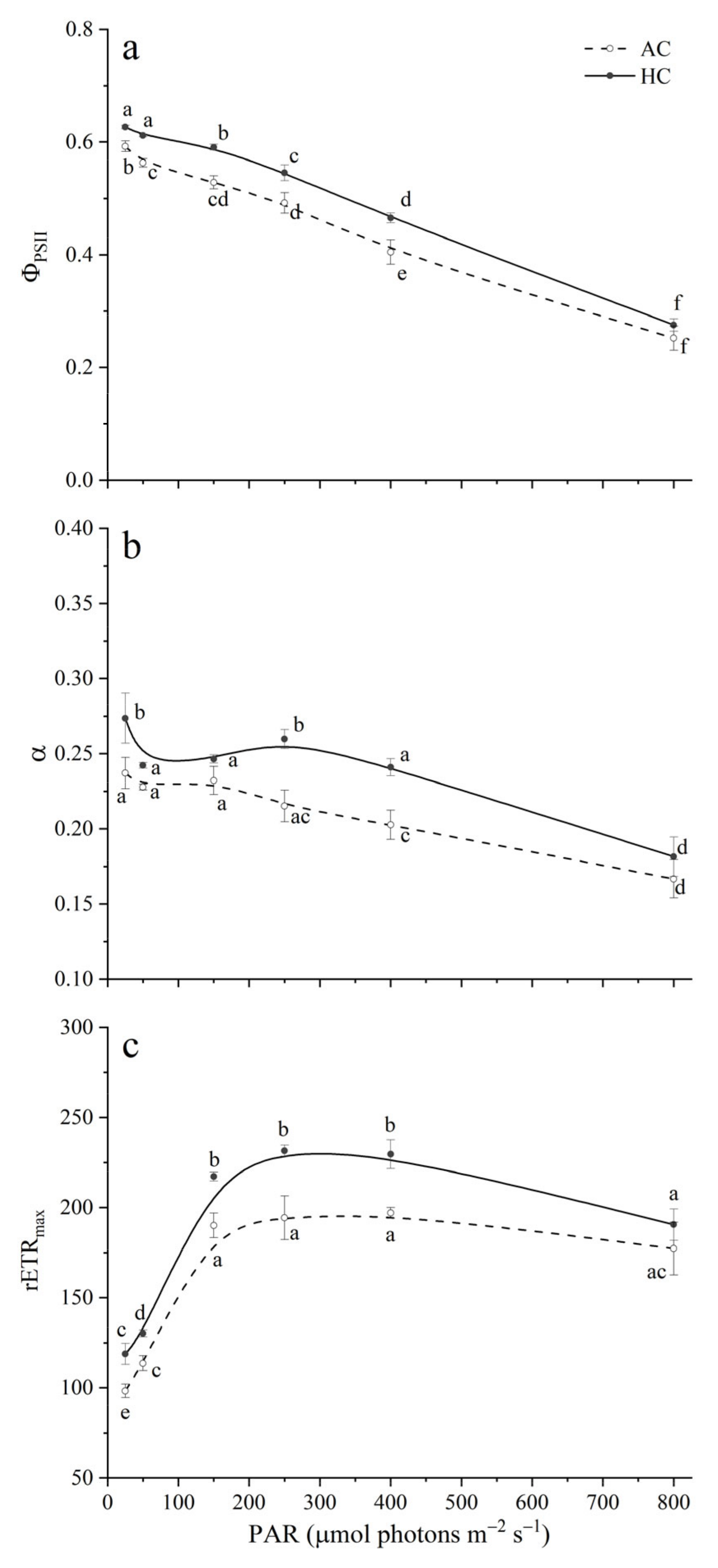
3.3. Chlorophyll a Fluorescence
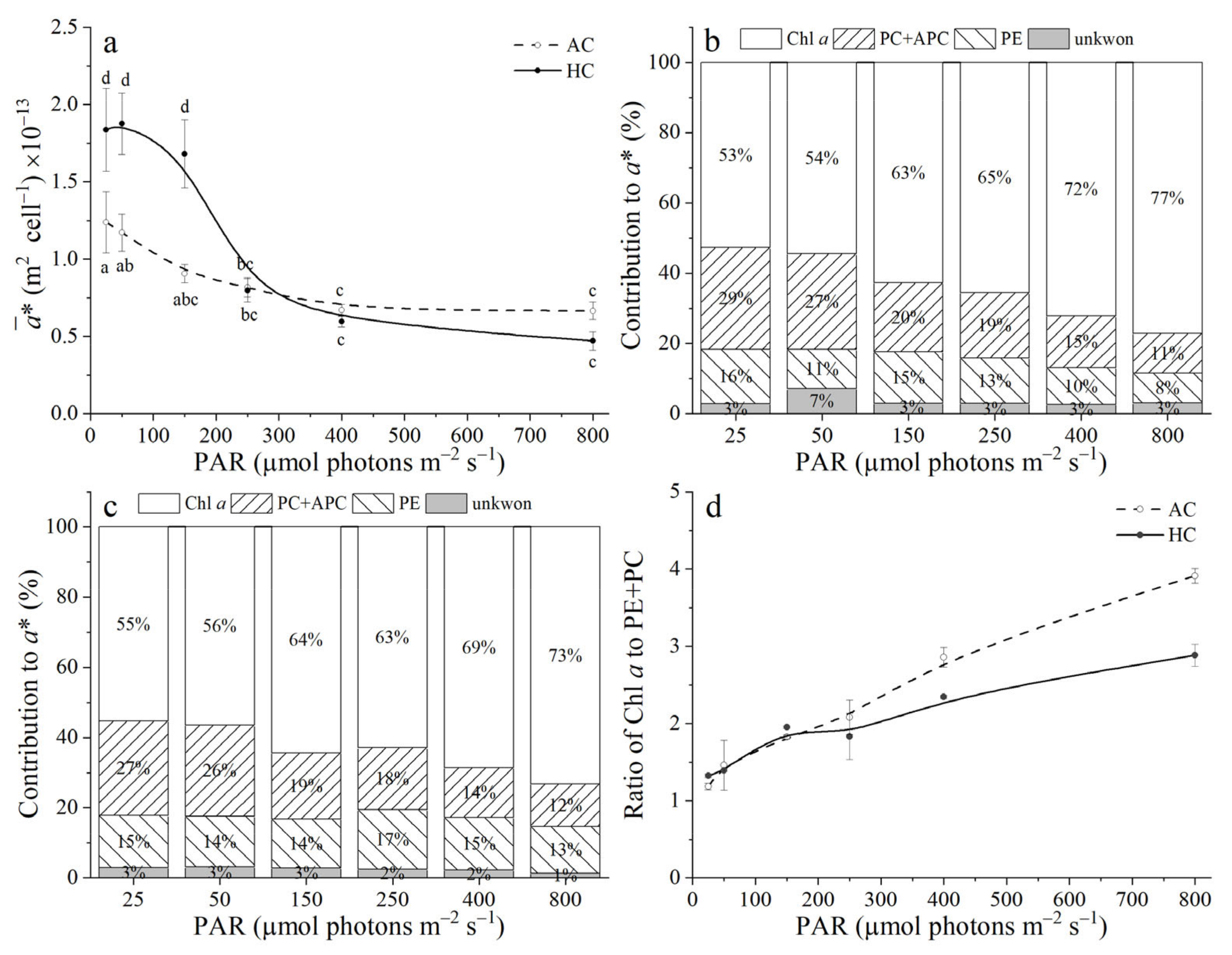
3.4. Optical Absorption Cross Section
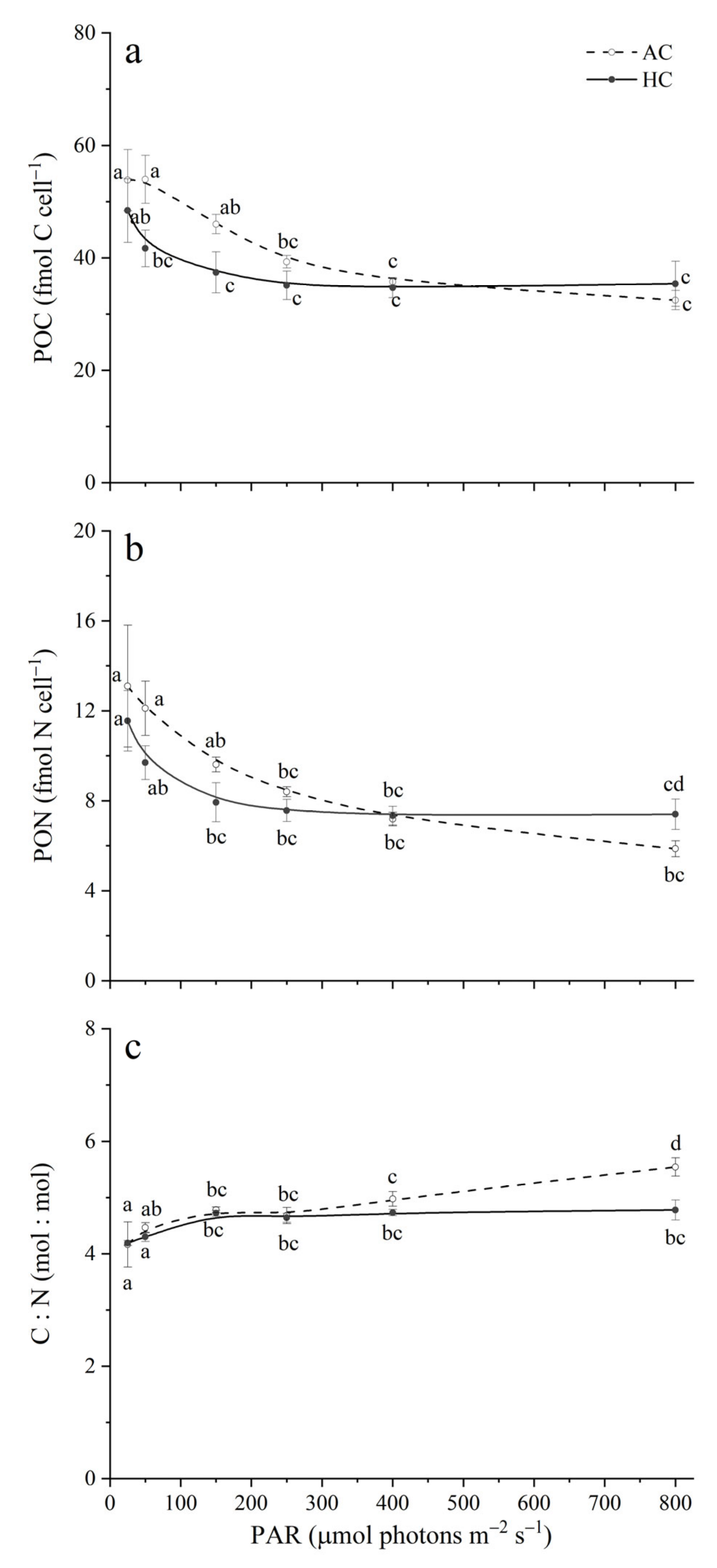
3.5. Cellular POC Content and POC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flombaum, P.; Gallegos, J.L.; Gordillo, R.A.; Rincón, J.; Zabala, L.L.; Jiao, N.; Karl, D.M.; Li, W.K.; Lomas, M.W.; Veneziano, D. Present and future global distributions of the marine Cyanobacteria Prochlorococcus and Synechococcus. Proc. Natl. Acad. Sci. USA 2013, 110, 9824–9829. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Jeon, J.; Kwak, M.S.; Kim, G.H.; Koh, I.; Rho, M. Photosynthetic functions of Synechococcus in the ocean microbiomes of diverse salinity and seasons. PLoS ONE 2018, 13, e0190266. [Google Scholar] [CrossRef] [PubMed]
- Partensky, F.; Blanchot, J.; Vaulot, D. Differential distribution and ecology of Prochlorococcus and Synechococcus in oceanic waters: A review. Bull.-Inst. Oceanogr. Monaco-Numero Spec. 1999, 19, 457–476. [Google Scholar]
- Parli, B.V.; Bhaskar, J.T.; Jawak, S.; Jyothibabu, R.; Mishra, N. Mixotrophic plankton and Synechococcus distribution in waters around Svalbard, Norway during June 2019. Polar Sci. 2021, 30, 100697. [Google Scholar] [CrossRef]
- Fucich, D.; Chen, F. Presence of toxin-antitoxin systems in picocyanobacteria and their ecological implications. ISME J. 2020, 14, 2843–2850. [Google Scholar] [CrossRef]
- Wang, T.; Li, J.; Jing, H.; Qin, S. Picocyanobacterial Synechococcus in marine ecosystem: Insights from genetic diversity, global distribution, and potential function. Mar. Environ. Res. 2022, 177, 105622. [Google Scholar] [CrossRef]
- Guidi, L.; Chaffron, S.; Bittner, L.; Eveillard, D.; Larhlimi, A.; Roux, S.; Darzi, Y.; Audic, S.; Berline, L.; Brum, J.R.; et al. Plankton networks driving carbon export in the oligotrophic ocean. Nature 2016, 532, 465–470. [Google Scholar] [CrossRef]
- Howes, E.L.; Joos, F.; Eakin, C.M.; Gattuso, J.-P. An updated synthesis of the observed and projected impacts of climate change on the chemical, physical and biological processes in the oceans. Front. Mar. Sci. 2015, 2, 36. [Google Scholar] [CrossRef]
- Orr, J.C.; Fabry, V.J.; Aumont, O.; Bopp, L.; Doney, S.C.; Feely, R.A.; Gnanadesikan, A.; Gruber, N.; Ishida, A.; Joos, F. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 2005, 437, 681–686. [Google Scholar] [CrossRef]
- Gattuso, J.-P.; Magnan, A.; Billé, R.; Cheung, W.W.; Howes, E.L.; Joos, F.; Allemand, D.; Bopp, L.; Cooley, S.R.; Eakin, C.M. Contrasting futures for ocean and society from different anthropogenic CO2 emissions scenarios. Science 2015, 349, aac4722. [Google Scholar] [CrossRef]
- Cai, W.-J.; Hu, X.; Huang, W.-J.; Murrell, M.C.; Lehrter, J.C.; Lohrenz, S.E.; Chou, W.-C.; Zhai, W.; Hollibaugh, J.T.; Wang, Y. Acidification of subsurface coastal waters enhanced by eutrophication. Nat. Geosci. 2011, 4, 766–770. [Google Scholar] [CrossRef]
- Feely, R.A.; Alin, S.R.; Newton, J.; Sabine, C.L.; Warner, M.; Devol, A.; Krembs, C.; Maloy, C. The combined effects of ocean acidification, mixing, and respiration on pH and carbonate saturation in an urbanized estuary. Estuar. Coast. Shelf Sci. 2010, 88, 442–449. [Google Scholar] [CrossRef]
- Giordano, M.; Beardall, J.; Raven, J.A. CO2 concentrating mechanisms in algae: Mechanisms, environmental modulation, and evolution. Annu. Rev. Plant Biol. 2005, 56, 99–131. [Google Scholar] [CrossRef]
- Beardall, J.; Giordano, M. Ecological implications of microalgal and cyanobacterial CO2 concentrating mechanisms, and their regulation. Funct. Plant Biol. 2002, 29, 335–347. [Google Scholar] [CrossRef]
- Gao, K.; Gao, G.; Wang, Y.; Dupont, S. Impacts of ocean acidification under multiple stressors on typical organisms and ecological processes. Mar. Life Sci. Technol. 2020, 2, 279–291. [Google Scholar] [CrossRef]
- McNicholl, C.; Koch, M.; Swarzenski, P.; Oberhaensli, F.; Taylor, A.; Batista, M.G.; Metian, M. Ocean acidification effects on calcification and dissolution in tropical reef macroalgae. Coral Reefs 2020, 39, 1635–1647. [Google Scholar] [CrossRef]
- Raven, J.A.; Beardall, J.; Quigg, A. Light-Driven Oxygen Consumption in the Water-Water Cycles and Photorespiration, and Light Stimulated Mitochondrial Respiration. In Photosynthesis in Algae: Biochemical and Physiological Mechanisms; Larkum, A.W.D., Grossman, A.R., Raven, J.A., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 161–178. [Google Scholar]
- Qu, L.; Beardall, J.; Jiang, X.; Gao, K. Elevated pCO2 enhances under light but reduces in darkness the growth rate of a diatom, with implications for the fate of phytoplankton below the photic zone. Limnol. Oceanogr. 2021, 66, 3630–3642. [Google Scholar] [CrossRef]
- Gao, K.; Xu, J.; Gao, G.; Li, Y.; Hutchins, D.A.; Huang, B.; Wang, L.; Zheng, Y.; Jin, P.; Cai, X.; et al. Rising CO2 and increased light exposure synergistically reduce marine primary productivity. Nat. Clim. Chang. 2012, 2, 519–523. [Google Scholar] [CrossRef]
- Jin, P.; Ding, J.; Xing, T.; Riebesell, U.; Gao, K. High levels of solar radiation offset impacts of ocean acidification on calcifying and non-calcifying strains of Emiliania huxleyi. Mar. Ecol. Prog. Ser. 2017, 568, 47–58. [Google Scholar] [CrossRef]
- Ji, Y.; Gao, K. Effects of climate change factors on marine macroalgae: A review. Adv. Mar. Biol. 2021, 88, 91–136. [Google Scholar] [CrossRef]
- Fu, F.X.; Warner, M.E.; Zhang, Y.; Feng, Y.; Hutchins, D.A. Effects of Increased temperature and CO2 on photosynthesis, growth, and elemental ratios in marine Synechococcus and Prochlorococcus (cyanobacteria). J. Phycol. 2007, 43, 485–496. [Google Scholar] [CrossRef]
- Basu, S.; Mackey, K.R. Effect of Rising Temperature and Carbon Dioxide on the Growth, Photophysiology, and Elemental Ratios of Marine Synechococcus: A Multistressor Approach. Sustainability 2022, 14, 9508. [Google Scholar] [CrossRef]
- Mou, S.; Zhang, Y.; Li, G.; Li, H.; Liang, Y.; Tang, L.; Tao, J.; Xu, J.; Li, J.; Zhang, C. Effects of elevated CO2 and nitrogen supply on the growth and photosynthetic physiology of a marine cyanobacterium, Synechococcus sp. PCC7002. J. Appl. Phycol. 2017, 29, 1755–1763. [Google Scholar] [CrossRef]
- Bao, N.; Gao, K. Interactive effects of elevated CO2 concentration and light on the picophytoplankton Synechococcus. Front. Mar. Sci. 2021, 8, 634189. [Google Scholar] [CrossRef]
- Laws, E.A.; McClellan, S.A. Interactive effects of CO2, temperature, irradiance, and nutrient limitation on the growth and physiology of the marine cyanobacterium Synechococcus (Cyanophyceae). J. Phycol. 2022, 58, 703–718. [Google Scholar] [CrossRef]
- Wang, K.; Wommack, K.E.; Chen, F. Abundance and distribution of Synechococcus spp. and cyanophages in the Chesapeake Bay. Appl. Environ. Microb. 2011, 77, 7459–7468. [Google Scholar] [CrossRef]
- Marsan, D.; Wommack, K.E.; Ravel, J.; Chen, F. Draft genome sequence of Synechococcus sp. strain CB0101, isolated from the Chesapeake Bay estuary. Genome Announc. 2014, 2, e01111-13. [Google Scholar] [CrossRef]
- Chen, F.; Wang, K.; Kan, J.; Bachoon, D.S.; Lu, J.; Lau, S.; Campbell, L. Phylogenetic diversity of Synechococcus in the Chesapeake Bay revealed by Ribulose-1, 5-bisphosphate carboxylase-oxygenase (RuBisCO) large subunit gene (rbcL) sequences. Aquat. Microb. Ecol. 2004, 36, 153–164. [Google Scholar] [CrossRef]
- Waterbury, J.B. Biological and ecological characterization of the marine unicellular cyanobacterium Synechococcus. Can Bull. Fish Aquat. Sci. 1986, 214, 71–120. [Google Scholar]
- Lewis, E.; Wallace, D. Program Developed for CO2 System Calculations; Environmental System Science Data Infrastructure for a Virtual Ecosystem. 1998. Available online: https://www.osti.gov/servlets/purl/639712 (accessed on 8 March 2023).
- Platt, T.; Gallegos, C.; Harrison, W.G. Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. J. Mar. Res. 1980, 38, 687–701. [Google Scholar]
- Ritchie, R.J. Consistent sets of spectrophotometric chlorophyll equations for acetone, methanol and ethanol solvents. Photosynth. Res. 2006, 89, 27–41. [Google Scholar] [CrossRef]
- Subramaniam1, A.; Carpenter, E.J.; Karentz, D.; Falkowski, P.G. Bio-optical properties of the marine diazotrophic cyanobacteria Trichodesmium spp. I. Absorption and photosynthetic action spectra. Limnol. Oceanogr. 1999, 44, 608–617. [Google Scholar] [CrossRef]
- Stramski, D.; Reynolds, R.A.; Kaczmarek, S.; Uitz, J.; Zheng, G. Correction of pathlength amplification in the filter-pad technique for measurements of particulate absorption coefficient in the visible spectral region. Appl. Opt. 2015, 54, 6763–6782. [Google Scholar] [CrossRef]
- Küpper, H.; Seibert, S.; Parameswaran, A. Fast, sensitive, and inexpensive alternative to analytical pigment HPLC: Quantification of chlorophylls and carotenoids in crude extracts by fitting with Gauss peak spectra. Anal. Chem. 2007, 79, 7611–7627. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.-M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. BBA-Gen. Subjects 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Eilers, P.; Peeters, J. A model for the relationship between light intensity and the rate of photosynthesis in phytoplankton. Ecol. Model. 1988, 42, 199–215. [Google Scholar] [CrossRef]
- Rodriguez, F.; Chauton, M.; Johnsen, G.; Andresen, K.; Olsen, L.; Zapata, M. Photoacclimation in phytoplankton: Implications for biomass estimates, pigment functionality and chemotaxonomy. Mar. Biol. 2006, 148, 963–971. [Google Scholar] [CrossRef]
- Marie-Rose Vandenhecke, J.; Bastedo, J.; Cockshutt, A.M.; Campbell, D.A.; Huot, Y. Changes in the Rubisco to photosystem ratio dominates photoacclimation across phytoplankton taxa. Photosynth. Res. 2015, 124, 275–291. [Google Scholar] [CrossRef]
- Andresen, E.; Lohscheider, J.; Šetlikova, E.; Adamska, I.; Šimek, M.; Küpper, H. Acclimation of Trichodesmium erythraeum ISM101 to high and low irradiance analysed on the physiological, biophysical and biochemical level. New Phytol. 2010, 185, 173–188. [Google Scholar] [CrossRef]
- Walker, B.J.; Strand, D.D.; Kramer, D.M.; Cousins, A.B. The response of cyclic electron flow around photosystem I to changes in photorespiration and nitrate assimilation. Plant Physiol. 2014, 165, 453–462. [Google Scholar] [CrossRef]
- Fujita, Y. A study on the dynamic features of photosystem stoichiometry: Accomplishments and problems for future studies. Photosynth. Res. 1997, 53, 83–93. [Google Scholar] [CrossRef]
- Xu, J.; Gao, K. Future CO2-induced ocean acidification mediates the physiological performance of a green tide alga. Plant Physiol. 2012, 160, 1762–1769. [Google Scholar] [CrossRef] [PubMed]
- Price, G.D.; Badger, M.R.; Woodger, F.J.; Long, B.M. Advances in understanding the cyanobacterial CO2-concentrating-mechanism (CCM): Functional components, Ci transporters, diversity, genetic regulation and prospects for engineering into plants. J. Exp. Bot. 2008, 59, 1441–1461. [Google Scholar] [CrossRef] [PubMed]
- Hopkinson, B.M.; Dupont, C.L.; Allen, A.E.; Morel, F.M.M. Efficiency of the CO2-concentrating mechanism of diatoms. Proc. Natl. Acad. Sci. USA 2011, 108, 3830–3837. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Gao, K.; Riebesell, U. CO2-induced seawater acidification affects physiological performance of the marine diatom Phaeodactylum tricornutum. Biogeosciences 2010, 7, 2915–2923. [Google Scholar] [CrossRef]
- Suffrian, K.; Schulz, K.G.; Gutowska, M.; Riebesell, U.; Bleich, M. Cellular pH measurements in Emiliania huxleyi reveal pronounced membrane proton permeability. New Phytol. 2011, 190, 595–608. [Google Scholar] [CrossRef]
- Flynn, K.J.; Blackford, J.C.; Baird, M.E.; Raven, J.A.; Clark, D.R.; Beardall, J.; Brownlee, C.; Fabian, H.; Wheeler, G.L. Changes in pH at the exterior surface of plankton with ocean acidification. Nat. Clim. Chang. 2012, 2, 510–513. [Google Scholar] [CrossRef]
- Raven, J.A.; Giordano, M.; Beardall, J.; Maberly, S.C. Algal evolution in relation to atmospheric CO2: Carboxylases, carbon-concentrating mechanisms and carbon oxidation cycles. Philos. Trans. R. Soc. B 2012, 367, 493–507. [Google Scholar] [CrossRef]
- Liu, N.; Beardall, J.; Gao, K. Elevated CO2 and associated seawater chemistry do not benefit a model diatom grown with increased availability of light. Aquat. Microb. Ecol. 2017, 79, 137–147. [Google Scholar] [CrossRef]
- Sun, J.-Z.; Wang, T.; Huang, R.; Yi, X.; Zhang, D.; Beardall, J.; Hutchins, D.A.; Liu, X.; Wang, X.; Deng, Z.; et al. Enhancement of diatom growth and phytoplankton productivity with reduced O2 availability is moderated by rising CO2. Commun. Biol. 2022, 5, 54. [Google Scholar] [CrossRef]
| Growth | Carbon Fixation | |||
|---|---|---|---|---|
| AC | HC | AC | HC | |
| α | 0.0246 ± 0.0006 | 0.0234 ± 0.0017 | 0.0146 ± 0.0028 | 0.0295 ± 0.0013 |
| β | 0.0010 ± 0.0001 | 0.0011 ± 0.0001 | 0.0044 ± 0.0010 | 0.0025 ± 0.0006 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Beardall, J.; Gao, K. Photoinhibition of the Picophytoplankter Synechococcus Is Exacerbated by Ocean Acidification. Water 2023, 15, 1228. https://doi.org/10.3390/w15061228
Li H, Beardall J, Gao K. Photoinhibition of the Picophytoplankter Synechococcus Is Exacerbated by Ocean Acidification. Water. 2023; 15(6):1228. https://doi.org/10.3390/w15061228
Chicago/Turabian StyleLi, He, John Beardall, and Kunshan Gao. 2023. "Photoinhibition of the Picophytoplankter Synechococcus Is Exacerbated by Ocean Acidification" Water 15, no. 6: 1228. https://doi.org/10.3390/w15061228
APA StyleLi, H., Beardall, J., & Gao, K. (2023). Photoinhibition of the Picophytoplankter Synechococcus Is Exacerbated by Ocean Acidification. Water, 15(6), 1228. https://doi.org/10.3390/w15061228







