Physical Separation: Reuse Pollutants and Thermal Energy from Water
Abstract
:1. Introduction
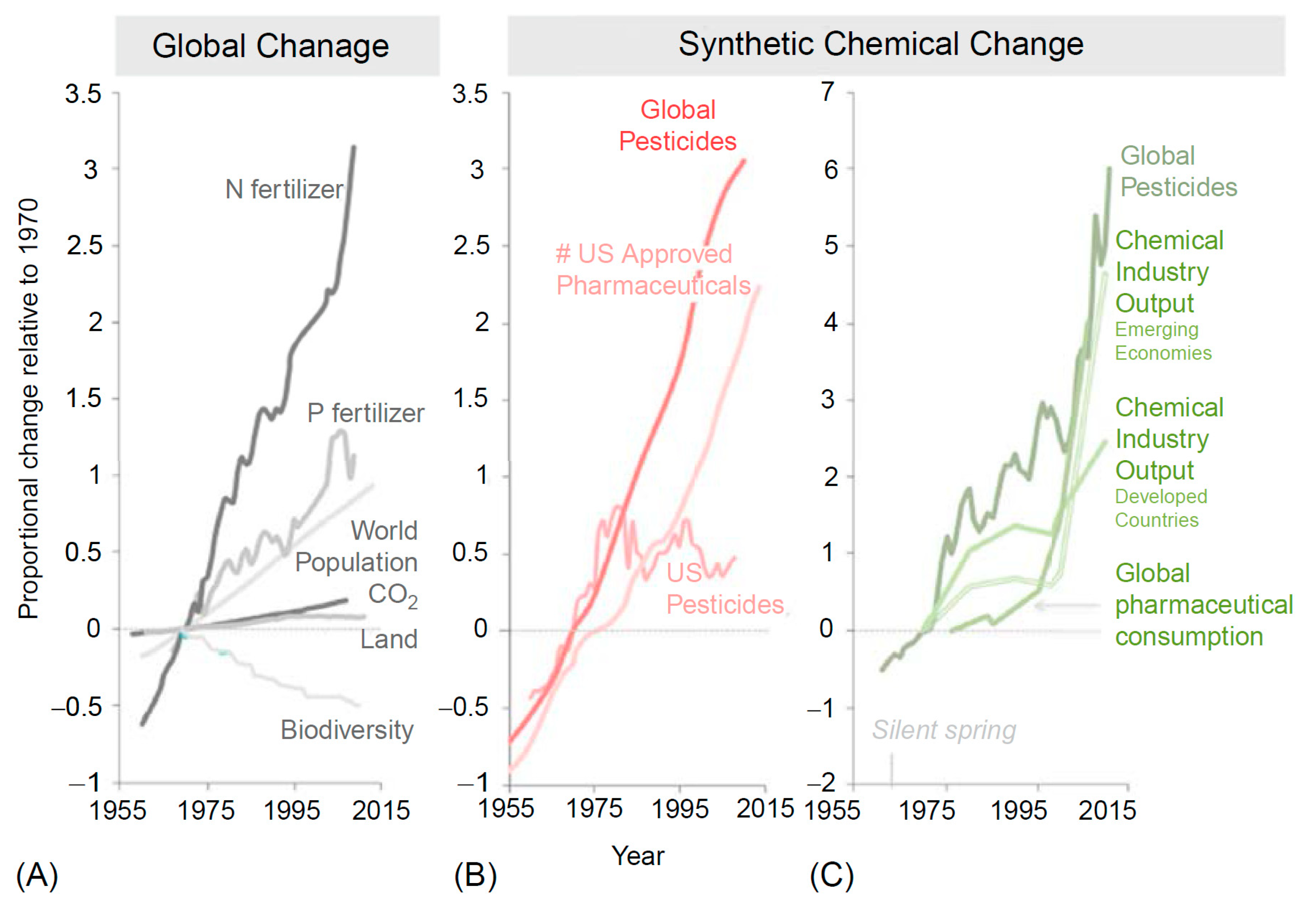
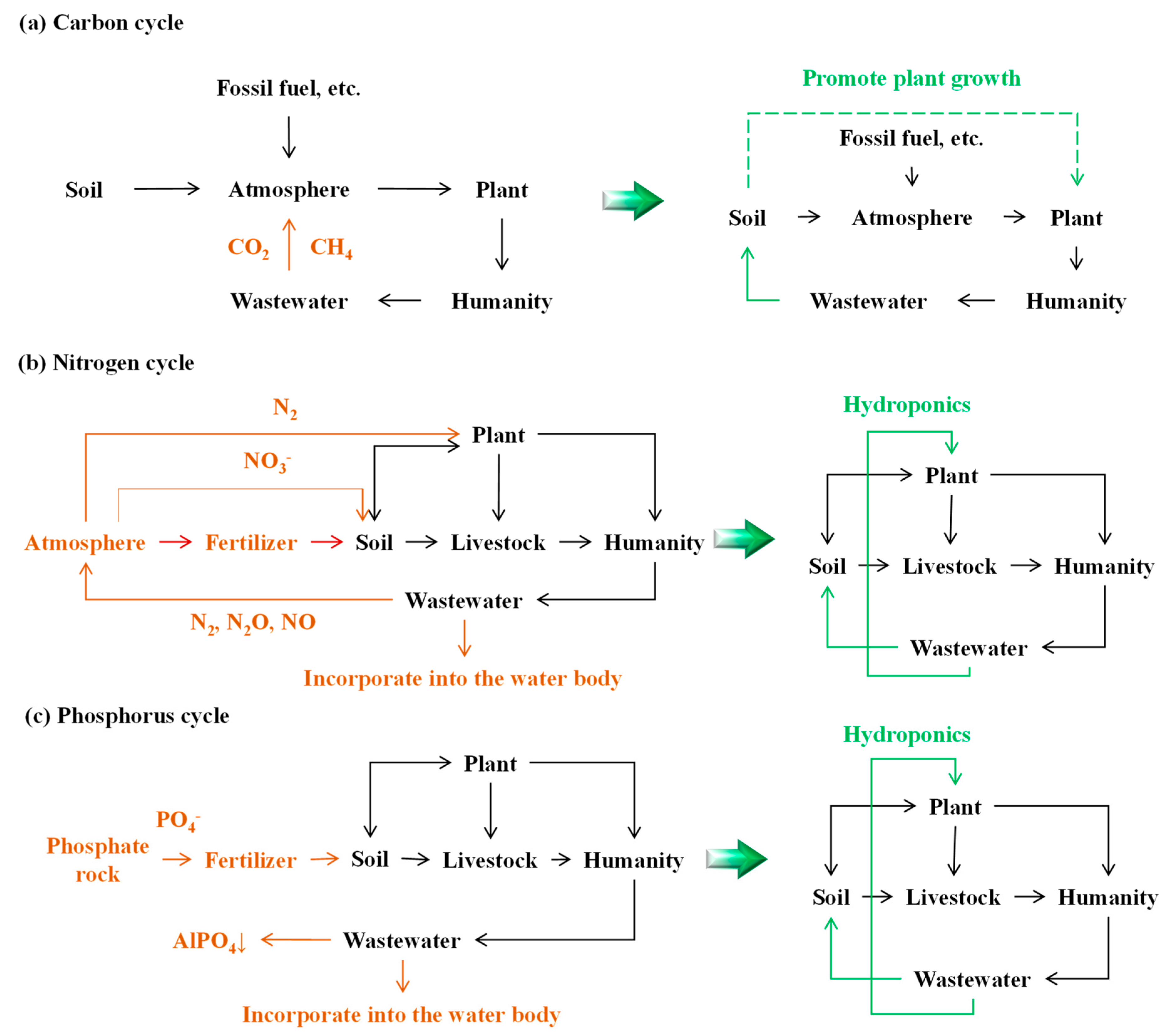
2. Broken Carbon, Nitrogen, and Phosphorus Cycles
2.1. Broken Carbon Cycles
2.2. Broken Nitrogen Cycles
2.3. Broken Phosphorus Cycles
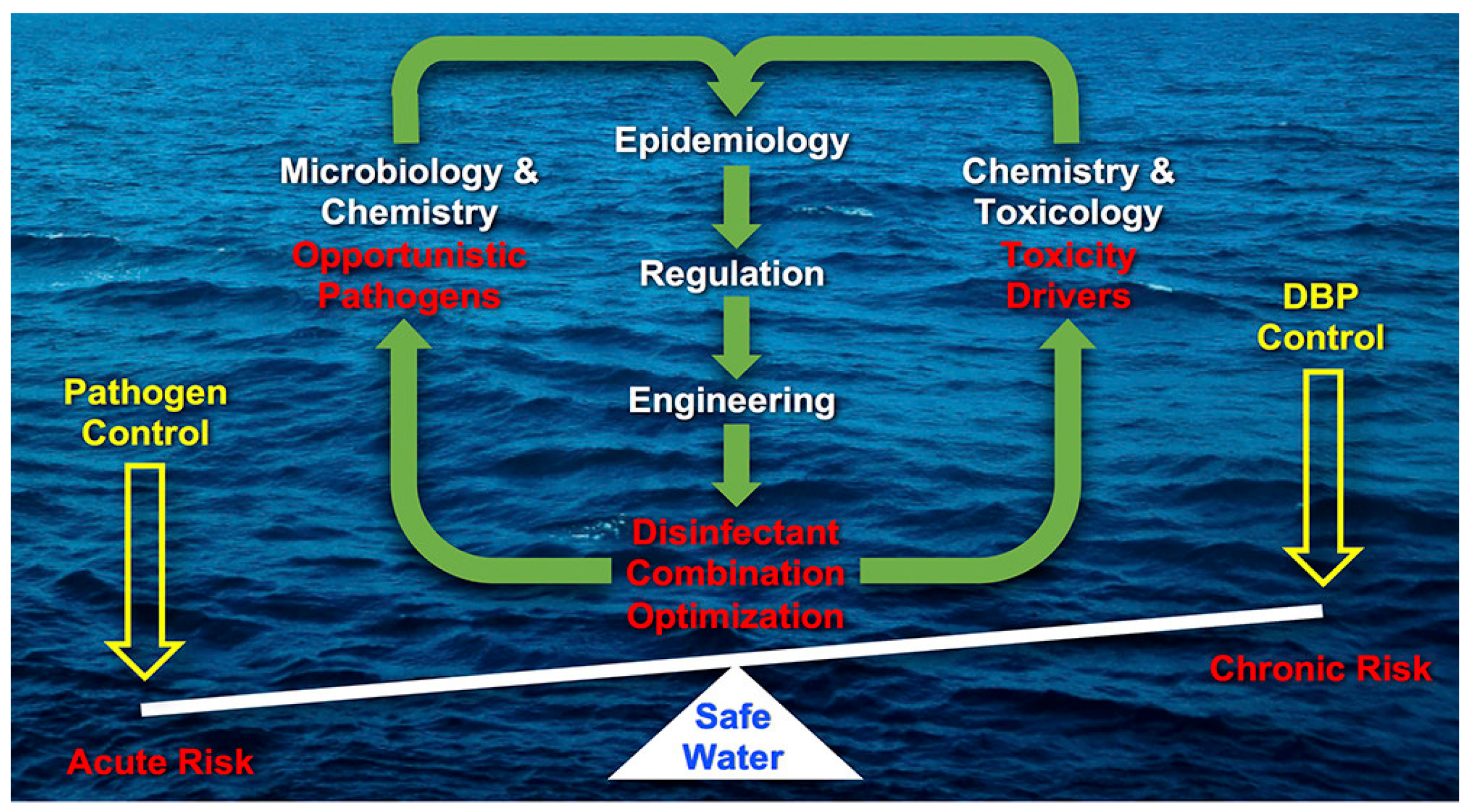
3. Conventional Sewage Treatment Based on Biological and Chemical Methods
4. Promising Sewage Treatment Based on Physical Separation
5. Conclusions
- (1)
- The conventional sewage treatment based on biological and chemical methods breaks the biogeochemical cycles (e.g., the carbon, nitrogen, and phosphorus cycles) and cannot remove hazardous materials including the viruses, microplastics, bacteria, and heavy metals. Accordingly, we should rethink the conceptual revolution of the principles of sewage treatment in 1890s [56], that is, “the replacement of a philosophy that saw sewage purification as the prevention of decomposition with one that tried to facilitate the biological processes that destroy sewage naturally”.
- (2)
- The carbon in the wastewater should be sent back to the soil rather than be used to produce carbon dioxide, methane, or carbonate. The nitrogen and phosphorus in the wastewater should be sent back to the soil or used for hydroponics rather than being mineralized. The thermal energy in the wastewater should be recovered and reused; whereas the chemical energy in the wastewater should be maintained rather than be recovered by producing methane and carbon dioxide. The hazardous materials should be removed.
- (3)
- The proposed promising sewage treatment system based on physical separation mainly consists of the source separators and the insoluble-pollutants separators, soluble-pollutants separators, and the wastewater heat recovery devices in the wastewater treatment plants;
- (4)
- The proposed promising sewage treatment system based on physical separation has the potential to replace conventional sewage treatment based on biological and chemical methods to fix the broken biogeochemical cycles (e.g., the carbon, nitrogen, and phosphorus cycles).
- (5)
- It is urgent to develop more advanced insoluble-pollutants separators and soluble-pollutants separators with high separation efficiency and low energy consumption [58,59,60], especially volume separators. Because the volume separators (e.g., functionalized sand filters) have the potential for replacing the surface separators (e.g., membranes).
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vernadsky, V.I. The Biosphere; Springer Science & Business Media: New York, NY, USA, 1998. [Google Scholar]
- Elser, J.; Bennett, E. A broken biogeochemical cycle. Nature 2011, 478, 29–31. [Google Scholar] [CrossRef]
- Ciais, P.; Sabine, C.; Bala, G.; Bopp, L.; Brovkin, V.; Canadell, J.; Chhabra, A.; DeFries, R.; Galloway, J.; Heimann, M. Carbon and Other Biogeochemical Cycles. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2014; pp. 465–570. [Google Scholar]
- Schlesinger, W.; Bernhardt, E. Biogeochemistry, An Analysis of Global Change, 4th ed.; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Bernhardt, E.S.; Rosi, E.J.; Gessner, M.O. Synthetic chemicals as agents of global change. Front. Ecol. Environ. 2017, 15, 84–90. [Google Scholar] [CrossRef]
- Wolff, E.W. Chemical signals of past climate and environment from polar ice cores and firn air. Chem. Soc. Rev. 2012, 41, 6247–6258. [Google Scholar] [CrossRef]
- Ravishankara, A.R.; Daniel, J.S.; Portmann, R.W. Nitrous Oxide (N2O): The Dominant Ozone-Depleting Substance Emitted in the 21st Century. Science 2009, 326, 123–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stokstad, E. Global efforts to protect biodiversity fall short. Science 2020, 369, 1418. [Google Scholar] [CrossRef]
- Chen, J.H.; Zhang, W.P.; Wan, Z.; Li, S.F.; Huang, T.C.; Fei, Y.J. Oil spills from global tankers: Status review and future governance. J. Clean. Prod. 2019, 227, 20–32. [Google Scholar] [CrossRef]
- Zhao, Z.S.; Cao, Y.L.; Fan, Y.; Yang, H.L.; Feng, X.W.; Li, L.; Zhang, H.L.; Xing, L.; Zhao, M.X. Ladderane records over the last century in the East China sea: Proxies for anammox and eutrophication changes. Water Res. 2019, 156, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Kelly, B.C.; Ikonomou, M.G.; Blair, J.D.; Morin, A.E.; Gobas, F. Food web-specific biomagnification of persistent organic pollutants. Science 2007, 317, 236–239. [Google Scholar] [CrossRef]
- Sokol, J. New mercury compound spotted in mass poisoning. Science 2020, 367, 1415–1416. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Sui, Q.; Lyu, S.; Zhao, W.; Liu, J.; Cai, Z.; Yu, G.; Barcelo, D. Municipal Solid Waste Landfills: An underestimated source of pharmaceutical and personal care products in the water environment. Environ. Sci. Technol. 2020, 54, 9757–9768. [Google Scholar] [CrossRef]
- Li, X.F.; Mitch, W.A. Drinking water disinfection byproducts (DBPs) and human health effects: Multidisciplinary challenges and opportunities. Environ. Sci. Technol. 2018, 52, 1681–1689. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Shi, Y.; Yang, L.; Xiao, L.; Kehoe, D.K.; Gun’ko, Y.K.; Boland, J.J.; Wang, J.J. Microplastic release from the degradation of polypropylene feeding bottles during infant formula preparation. Nat. Food 2020, 1, 746–754. [Google Scholar] [CrossRef]
- Rockstrom, J.; Steffen, W.; Noone, K.; Persson, A.; Chapin, F.S., III; Lambin, E.F.; Lenton, T.M.; Scheffer, M.; Folke, C.; Schellnhuber, H.J.; et al. A safe operating space for humanity. Nature 2009, 461, 472–475. [Google Scholar] [CrossRef] [PubMed]
- Voosen, P. Global Warming New climate models forecast a warming surge. Science 2019, 364, 222–223. [Google Scholar] [CrossRef]
- Cox, P.M.; Betts, R.A.; Jones, C.D.; Spall, S.A.; Totterdell, I.J. Acceleration of global warming due to carbon-cycle feedbacks in a coupled climate model. Nature 2000, 408, 184–187. [Google Scholar] [CrossRef]
- Shepherd, A.; Ivins, E.; Rignot, E.; Smith, B.; Van Den Broeke, M.; Velicogna, I.; Whitehouse, P.; Briggs, K.; Joughin, I.; Krinner, G. Mass balance of the Antarctic Ice Sheet from 1992 to 2017. Nature 2018, 558, 219–222. [Google Scholar]
- Rignot, E.; Mouginot, J.; Scheuchl, B.; van den Broeke, M.; van Wessem, M.J.; Morlighem, M. Four decades of Antarctic Ice Sheet mass balance from 1979–2017. Proc. Natl. Acad. Sci. USA 2019, 116, 1095–1103. [Google Scholar] [CrossRef] [Green Version]
- Garbe, J.; Albrecht, T.; Levermann, A.; Donges, J.F.; Winkelmann, R. The hysteresis of the Antarctic Ice Sheet. Nature 2020, 585, 538–544. [Google Scholar] [CrossRef]
- Pounds, J.A.; Bustamante, M.R.; Coloma, L.A.; Consuegra, J.A.; Fogden, M.P.L.; Foster, P.N.; La Marca, E.; Masters, K.L.; Merino-Viteri, A.; Puschendorf, R.; et al. Widespread amphibian extinctions from epidemic disease driven by global warming. Nature 2006, 439, 161–167. [Google Scholar] [CrossRef]
- Nolan, C.; Overpeck, J.T.; Allen, J.R.M.; Anderson, P.M.; Betancourt, J.L.; Binney, H.A.; Brewer, S.; Bush, M.B.; Chase, B.M.; Cheddadi, R.; et al. Past and future global transformation of terrestrial ecosystems under climate change. Science 2018, 361, 920–923. [Google Scholar] [CrossRef] [Green Version]
- Deutsch, C.A.; Tewksbury, J.J.; Tigchelaar, M.; Battisti, D.S.; Merrill, S.C.; Huey, R.B.; Naylor, R.L. Increase in crop losses to insect pests in a warming climate. Science 2018, 361, 916–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rollo, A.M.; Rollo, A.; Mora, C. The tree-lined path to carbon neutrality. Nat. Rev. Earth Environ. 2020, 1, 332. [Google Scholar] [CrossRef]
- United Nations Environment Programme (UNEP). The Benefits of Soil Carbon: Managing Soils for Multiple Economic, Societal and Environmental Benefits. In UNEP Year Book 2012: Emerging Issues in Our Global Environment; UNEP: Nairobi, Kenya, 2012; pp. 19–33. [Google Scholar]
- United Nations Environment Programme (UNEP). Securing Soil Carbon Benefits: Managing Soils for Multiple Economic, Societal and Environmental Benefits. In UNEP Year Book 2014: Emerging Issues in Our Global Environment; UNEP: Nairobi, Kenya, 2014; pp. 54–59. [Google Scholar]
- Batjes, N.H. Total carbon and nitrogen in the soils of the world. Eur. J. Soil Sci. 2014, 65, 10–21. [Google Scholar] [CrossRef]
- Broadbent, F. The Soil Organic Fraction. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 1953; pp. 153–183. [Google Scholar]
- Crowther, T.W.; Todd-Brown, K.E.O.; Rowe, C.W.; Wieder, W.R.; Carey, J.C.; Machmuller, M.B.; Snoek, B.L.; Fang, S.; Zhou, G.; Allison, S.D.; et al. Quantifying global soil carbon losses in response to warming. Nature 2016, 540, 104–108. [Google Scholar] [CrossRef] [Green Version]
- Pries, C.E.H.; Castanha, C.; Porras, R.C.; Torn, M.S. The whole-soil carbon flux in response to warming. Science 2017, 355, 1420–1423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minasny, B.; Malone, B.P.; McBratney, A.B.; Angers, D.A.; Arrouays, D.; Chambers, A.; Chaplot, V.; Chen, Z.-S.; Cheng, K.; Das, B.S.; et al. Soil carbon 4 per mille. Geoderma 2017, 292, 59–86. [Google Scholar] [CrossRef]
- United Nations Environment Programme (UNEP). The Nitrogen Fix: From Nitrogen Cycle Pollution to Nitrogen Circular Economy. In Frontiers 2018/19: Emerging Issues of Environmental Concern; UNEP: Nairobi, Kenya, 2019; pp. 52–64. [Google Scholar]
- Sutton, M.A.; Howard, C.M.; Erisman, J.W. The European Union Nitrogen Assessment: Sources, Effects and Policy Perspectives; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Gilbert, N. Environment: The disappearing nutrient. Nature 2009, 461, 716–718. [Google Scholar] [CrossRef] [Green Version]
- Kummerer, K.; Dionysiou, D.D.; Olsson, O.; Fatta-Kassinos, D. A path to clean water. Science 2018, 361, 222–224. [Google Scholar] [CrossRef] [Green Version]
- Li, W.W.; Yu, H.Q.; Rittmann, B.E. Chemistry: Reuse water pollutants. Nature 2015, 528, 29–31. [Google Scholar] [CrossRef] [Green Version]
- Ardern, E.; Lockett, W.T. Experiments on the oxidation of sewage without the aid of filters. J. Soc. Chem. Ind. 1914, 33, 523–539. [Google Scholar] [CrossRef] [Green Version]
- Hao, X. Sustainable Treatment Technologies of Wastewater-Wastes; China Architecture Publishing & Media Co., Ltd.: Beijing, China, 2006. [Google Scholar]
- Kartal, B.; Kuenen, J.G.; van Loosdrecht, M.C.M. Sewage treatment with anammox. Science 2010, 328, 702–703. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Guest, J.S.; Peters, C.A.; Zhu, X.; Rau, G.H.; Ren, Z.J. Wastewater treatment for carbon capture and utilization. Nat. Sustain. 2018, 1, 750–758. [Google Scholar] [CrossRef]
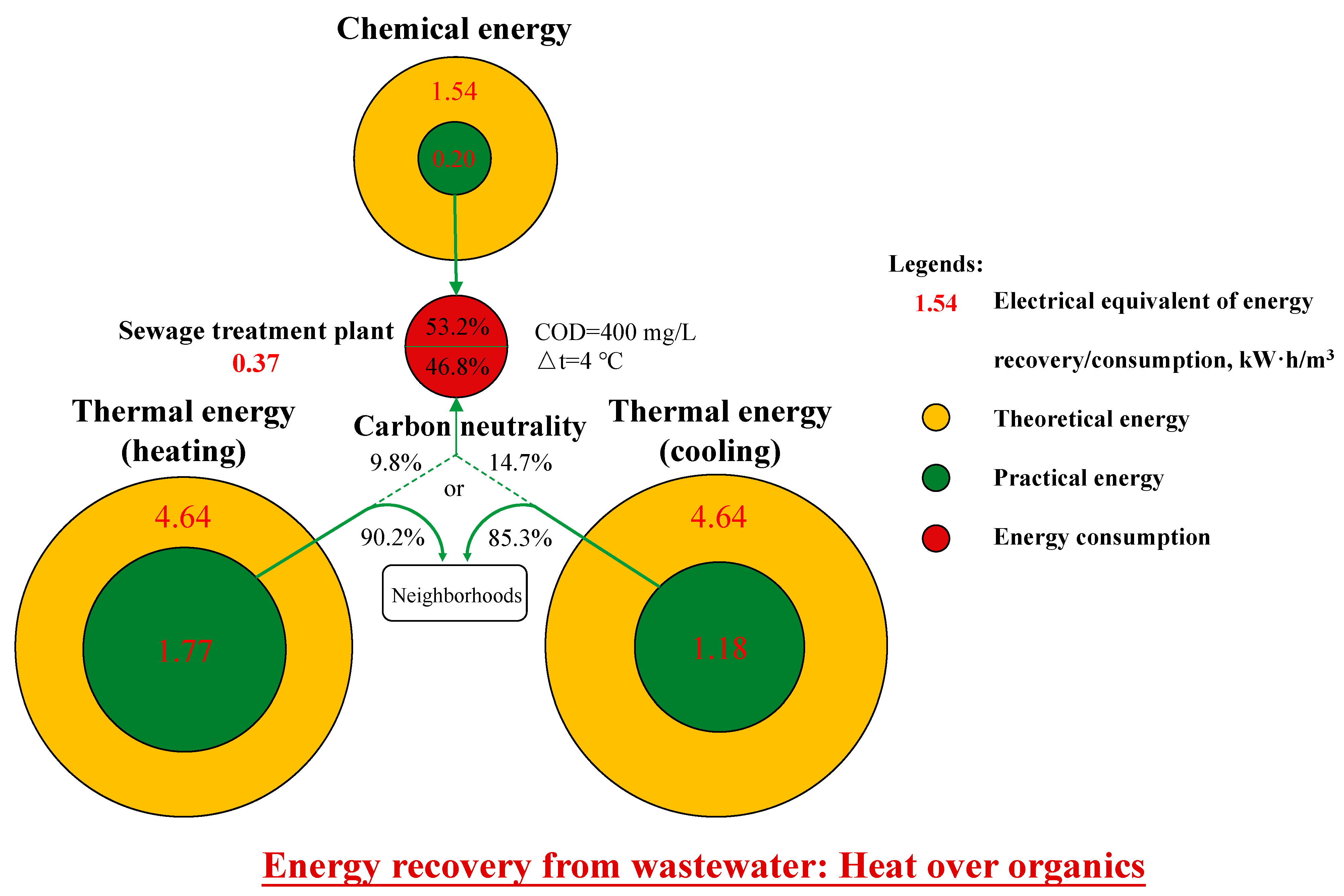
- Hao, X.; Li, J.; van Loosdrecht, M.C.M.; Jiang, H.; Liu, R. Energy recovery from wastewater: Heat over organics. Water Res. 2019, 161, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.D.; Liu, R.B.; Huang, X. Evaluation of the potential for operating carbon neutral WWTPs in China. Water Res. 2015, 87, 424–431. [Google Scholar] [CrossRef]
- Liu, Z.B.; Ma, L.D.; Zhang, J.L. Application of a heat pump system using untreated urban sewage as a heat source. Appl. Therm. Eng. 2014, 62, 747–757. [Google Scholar] [CrossRef]
- Neugebauer, G.; Kretschmer, F.; Kollmann, R.; Narodoslawsky, M.; Ertl, T.; Stoeglehner, G. Mapping thermal energy resource potentials from wastewater treatment plants. Sustainability 2015, 7, 12988–13010. [Google Scholar] [CrossRef] [Green Version]
- Xue, B.X.; Wei, L.; Zhang, C.H.; Li, T.Y.; Xu, H. Study of a double subsurface snow-water utilization system for the melting of snow using the waste heat of urban sewage. Desalin. Water Treat. 2014, 52, 1153–1162. [Google Scholar] [CrossRef]
- Dong, J.K.; Zhang, Z.; Yao, Y.; Jiang, Y.Q.; Lei, B. Experimental performance evaluation of a novel heat pump water heater assisted with shower drain water. Appl. Energy 2015, 154, 842–850. [Google Scholar] [CrossRef]
- Frijns, J.; Hofman, J.; Nederlof, M. The potential of (waste) water as energy carrier, Energy Convers. Manage 2013, 65, 357–363. [Google Scholar]
- Cao, Y.; Van Loosdrecht, M.; Daigger, G. The bottlenecks and causes, and potential solutions for municipal Sewage treatment in China. Water Pract. Technol. 2020, 15, 160–169. [Google Scholar] [CrossRef]
- Qu, J.H.; Wang, H.C.; Wang, K.J.; Yu, G.; Ke, B.; Yu, H.Q.; Ren, H.Q.; Zheng, X.C.; Li, J.; Li, W.W.; et al. Municipal wastewater treatment in China: Development history and future perspectives. Front. Environ. Sci. Eng. 2019, 13, 7. [Google Scholar] [CrossRef]
- Shen, C.; Lei, Z.; Wang, Y.; Zhang, C.; Yao, Y. A review on the current research and application of wastewater source heat pumps in China. Therm. Sci. Eng. Prog. 2018, 6, 140–156. [Google Scholar] [CrossRef]
- Shen, C.; Lei, Z.; Lv, G.; Ni, L.; Deng, S. Experimental performance evaluation of a novel anti-fouling wastewater source heat pump system with a wastewater tower. Appl. Energy 2019, 236, 690–699. [Google Scholar] [CrossRef]
- Smith, A.L.; Stadler, L.B.; Cao, L.; Love, N.G.; Raskin, L.; Skerlos, S.J. Navigating wastewater energy recovery strategies: A life cycle comparison of anaerobic membrane bioreactor and conventional treatment systems with anaerobic digestion. Environ. Sci. Technol. 2014, 48, 5972–5981. [Google Scholar] [CrossRef]
- Rittmann, B.E.; Mayer, B.; Westerhoff, P.; Edwards, M. Capturing the lost phosphorus. Chemosphere 2011, 84, 846–853. [Google Scholar] [CrossRef]
- Diana, M.; Felipe-Sotelo, M.; Bond, T. Disinfection byproducts potentially responsible for the association between chlorinated drinking water and bladder cancer: A review. Water Res. 2019, 162, 492–504. [Google Scholar] [CrossRef]
- Hamlin, C. Edwin Chadwick and the engineers, 1842-1854: Systems and antisystems in the pipe-and-brick sewers war. Technol. Cult. 1992, 33, 680–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, Z.J.; Umble, A.K. Recover wastewater resources locally. Nature 2016, 529, 25. [Google Scholar] [CrossRef] [Green Version]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Marinas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 310. [Google Scholar] [CrossRef]
- Qu, J. Physical technology-Clean water treatment methods worthy of attention. Water Wastewater Eng. 2014, 40, 1. [Google Scholar]
- Zhao, Y.; Tong, T.; Wang, X.; Lin, S.; Reid, E.M.; Chen, Y. Differentiating solutes with precise nanofiltration for next generation environmental separations: A review. Environ. Sci. Technol. 2021, 55, 1359–1376. [Google Scholar] [CrossRef] [PubMed]
- Culp, T.E.; Khara, B.; Brickey, K.P.; Geitner, M.; Zimudzi, T.J.; Wilbur, J.D.; Jons, S.D.; Roy, A.; Paul, M.; Ganapathysubra-manian, B.; et al. Nanoscale control of internal inhomogeneity enhances water transport in desalination membranes. Science 2020, 371, 72–75. [Google Scholar] [CrossRef] [PubMed]
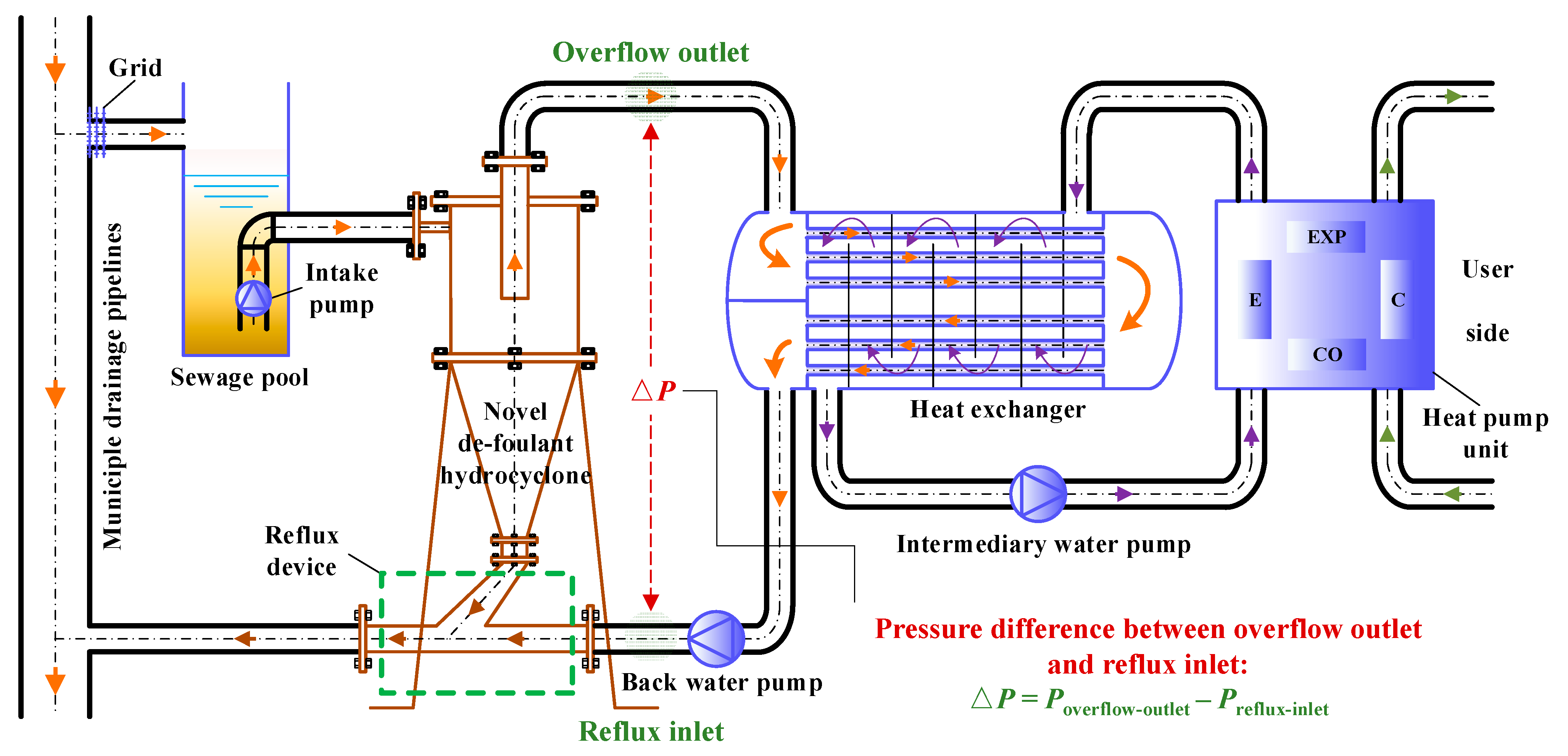
- Tian, J.; Wang, H.; Lv, W.; Huang, Y.; Fu, P.; Li, J.; Liu, Y. An efficient approach to temporarily separate foulants using hydrocyclone with reflux function for thermal energy recovery from sewage. Sep. Purif. Technol. 2021, 259, 118130. [Google Scholar] [CrossRef]
- Ni, L.; Tian, J.; Zhao, J. Experimental study of the effect of underflow pipe diameter on separation performance of a novel de-foulant hydrocyclone with continuous underflow and reflux function. Sep. Purif. Technol. 2016, 171, 270–279. [Google Scholar] [CrossRef]
- Tian, J.; Ni, L.; Song, T.; Olson, J.; Zhao, J. An overview of operating parameters and conditions in hydrocyclones for enhanced separations. Sep. Purif. Technol. 2018, 206, 268–285. [Google Scholar] [CrossRef]
- Ni, L.; Tian, J.Y.; Song, T.; Jong, Y.S.; Zhao, J.N. Optimizing geometric parameters in hydrocyclones for enhanced separations: A review and perspective. Sep. Purif. Rev. 2019, 48, 30–51. [Google Scholar] [CrossRef]
- Xu, Y.X.; Fang, Y.Y.; Wang, Z.H.; Guo, D.; Liu, Y.; Huang, Y.; Fu, P.B.; Jin, J.H.; Wei, C.W.; Wang, H.L.; et al. In-situ sludge reduction and carbon reuse in an anoxic/oxic process coupled with hydrocyclone breakage. Water Res. 2018, 141, 135–144. [Google Scholar] [CrossRef]
- Regmi, P.; Sturm, B.; Hiripitiyage, D.; Keller, N.; Murthy, S.; Jimenez, J. Combining continuous flow aerobic granulation using an external selector and carbon-efficient nutrient removal with AvN control in a full-scale simultaneous nitrification-denitrification process. Water Res. 2022, 210, 117991. [Google Scholar] [CrossRef]
- Gemza, N.; Janiak, K.; Zięba, B.; Przyszlak, J.; Kuśnierz, M. Long-term effects of hydrocyclone operation on activated sludge morphology and full-scale secondary settling tank wet-weather operation in long sludge age WWTP. Sci. Total. Environ. 2022, 845, 157224. [Google Scholar] [CrossRef]
- Liu, W.; Fu, P.; Zhang, Y.; Xu, H.; Wang, H.; Xing, M. Efficient hydrogen production from wastewater remediation by piezoelectricity coupling advanced oxidation processes. Proc. Natl. Acad. Sci. USA 2023, 120, e2218813120. [Google Scholar] [CrossRef]
- Samineni, L.; Xiong, B.; Chowdhury, R.; Pei, A.; Kuehster, L.; Wang, H.; Dickey, R.; Soto, P.E.; Massenburg, L.; Nguyen, T.H.; et al. 7 Log virus removal in a simple functionalized sand filter. Environ. Sci. Technol. 2019, 53, 12706–12714. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Yang, Q.; Liu, S.; Xie, L.-S.; Wang, H.-L. Effect of fibrous coalescer redispersion on W/O emulsion separation. Sep. Purif. Technol. 2016, 159, 50–56. [Google Scholar] [CrossRef]
- Lu, H.; Pan, Z.; Wang, H.; Liu, Y.; Dai, P.; Yang, Q. Fiber coalescence treatment of oily wastewater: A new theory and application. J. Hazard. Mater. 2021, 412, 125188. [Google Scholar] [CrossRef] [PubMed]
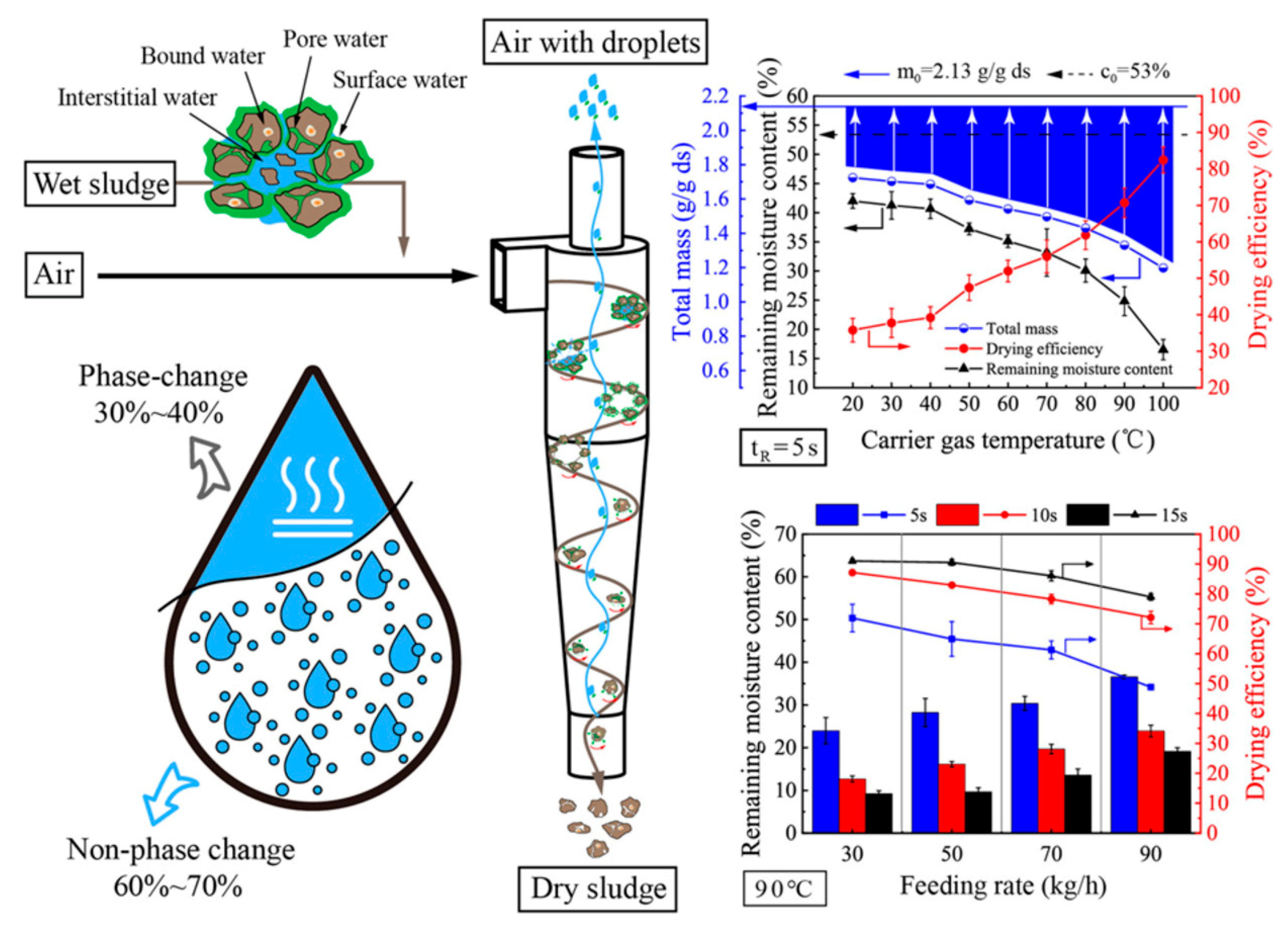
- Li, Q.; Cheng, T.; Lu, Y.; Zhang, B.; Huang, Y.; Yang, Y.; Li, C.; Li, J.; Wang, H.; Fu, P. Sludge low-temperature drying with mainly non-phase change in mere seconds based on particle high-speed self-rotation in cyclone. Water Res. 2022, 224, 119092. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.B.; Jiang, X.; Ma, L.; Yang, Q.; Bai, Z.S.; Yang, X.J.; Chen, J.Q.; Yuan, W.; Wang, H.L.; Lv, W.J. Enhancement of PM2.5 cyclone separation by droplet capture and particle sorting. Environ. Sci. Technol. 2018, 52, 11652–11659. [Google Scholar] [CrossRef] [PubMed]


| Nutrients and Thermal Energy | Flow of Matter and Energy | |
|---|---|---|
| Aerobic activated sludge process [38] | C | COD + O2→CO2 + H2O |
| N | NH4+ + O2→NO3− + H+ + H2O NO3− + COD→N2↑ + CO2 + H2O | |
| P | PO43− + Al3+→AlPO4↓ | |
| Thermal energy | Waste | |
| “Denitrifying phosphorus removal bacteria + Anaerobic treatments” [39] | C | COD→CH4 |
| N, P | NH4+ + O2→NO3− + H+ + H2O NO3− + COD + PO43−→N2↑ + CO2 + H2O + Phosphate | |
| Thermal energy | Waste | |
| “Anaerobic treatment + Anammox process” [40] | C | COD→CH4 |
| N | NH4+ + NO2−→N2↑ + 2H2O NH4+ +1.5O2→NO2− + 2H+ + H2O Together yield: 2NH4+ + 1.5O2→N2↑ + 2H+ + 3H2O | |
| P | PO43− + Al3 + →AlPO4↓ | |
| Thermal energy | Waste | |
| “Anaerobic membrane bioreactor +Microbial electrochemical cells + Ion exchangers” [37] | C | COD→CH4 + Electricity |
| N, P | N, P→Fertilizer | |
| Thermal energy | Waste | |
| “Microbial electrolytic carbon capture + Microalgae cultivation” [41] | C | COD + CO2→Carbonates |
| N, P | N, P→Microalgae cultivation | |
| Thermal energy | Waste |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, J.; Chen, X. Physical Separation: Reuse Pollutants and Thermal Energy from Water. Water 2023, 15, 1196. https://doi.org/10.3390/w15061196
Tian J, Chen X. Physical Separation: Reuse Pollutants and Thermal Energy from Water. Water. 2023; 15(6):1196. https://doi.org/10.3390/w15061196
Chicago/Turabian StyleTian, Jinyi, and Xiurong Chen. 2023. "Physical Separation: Reuse Pollutants and Thermal Energy from Water" Water 15, no. 6: 1196. https://doi.org/10.3390/w15061196
APA StyleTian, J., & Chen, X. (2023). Physical Separation: Reuse Pollutants and Thermal Energy from Water. Water, 15(6), 1196. https://doi.org/10.3390/w15061196






