Y-Type Zeolite Synthesized from an Illite Applied for Removal of Pb(II) and Cu(II) Ions from Aqueous Solution: Box-Behnken Design and Kinetics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
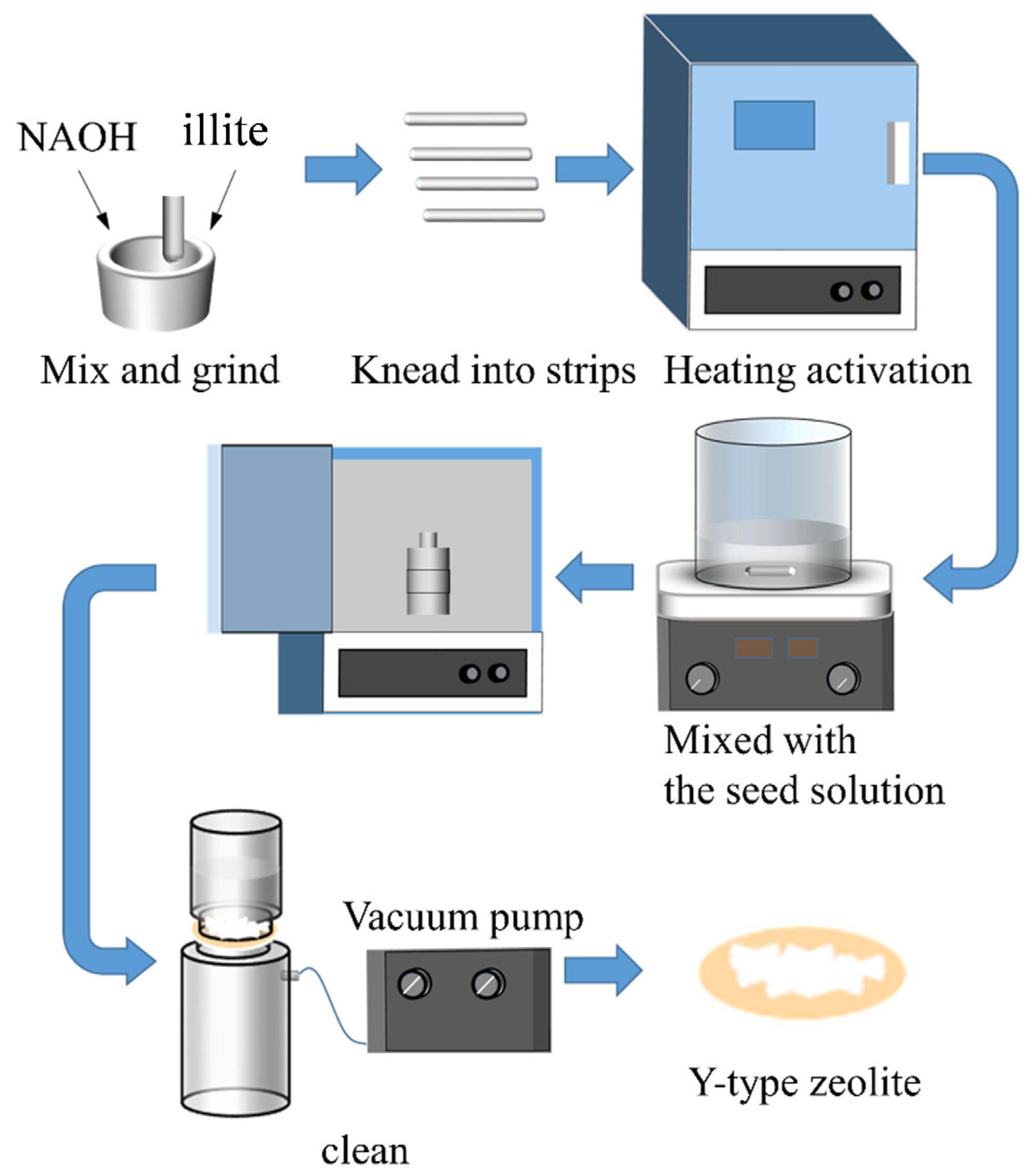
2.2. Preparation of Synthetic Y-Type Zeolite from Illite
2.2.1. Pretreatment of Illite Minerals
2.2.2. Synthesis of ZMA-T-R-t from AAP-T-R-t
2.3. Instrumentation and Techniques
2.4. BBD Optimizes the Synthesis Conditions of Y-Type Zeolite
2.5. Adsorption Kinetics and Isotherms
2.6. Desorption and Reusability of ZMA
3. Results and Discussions
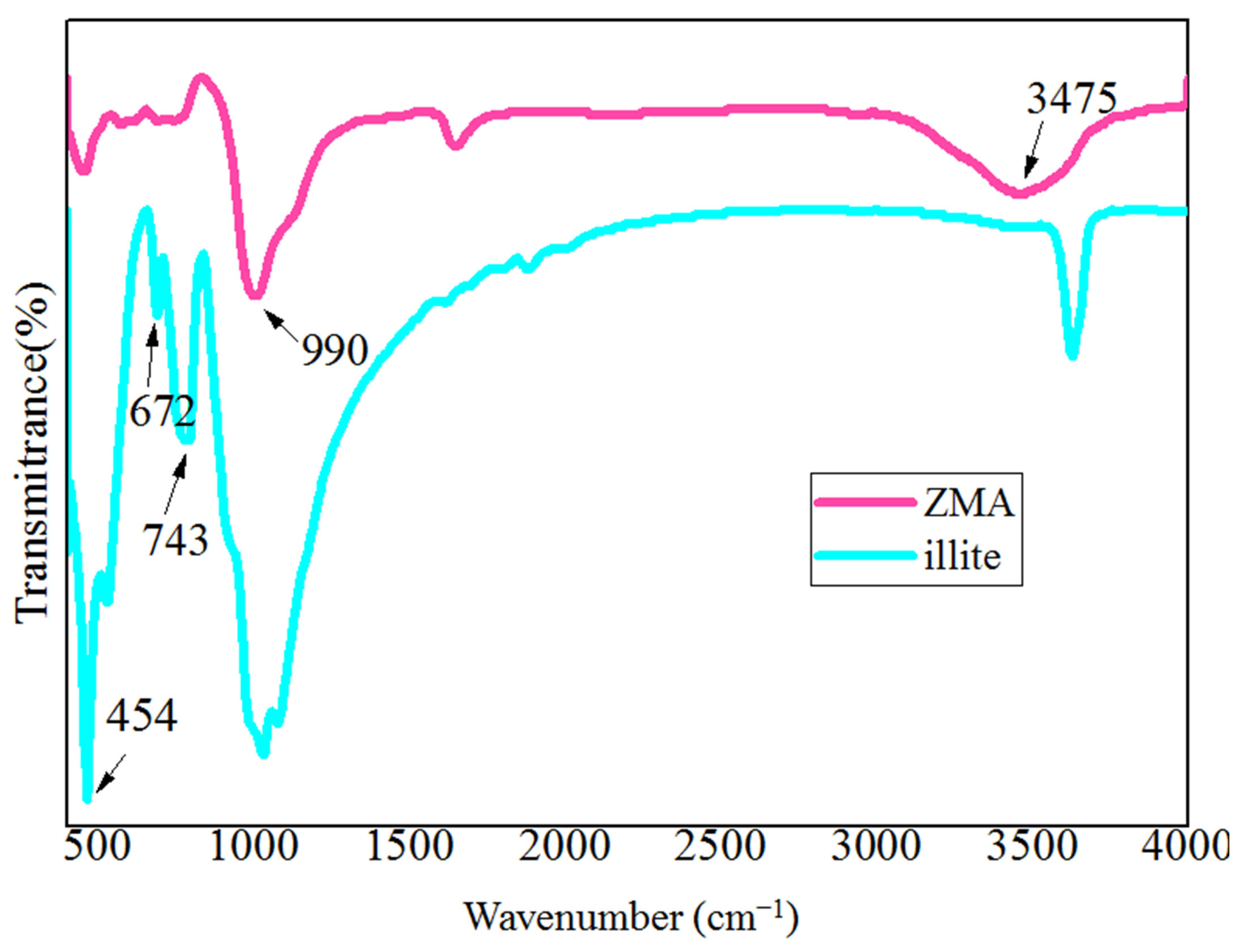
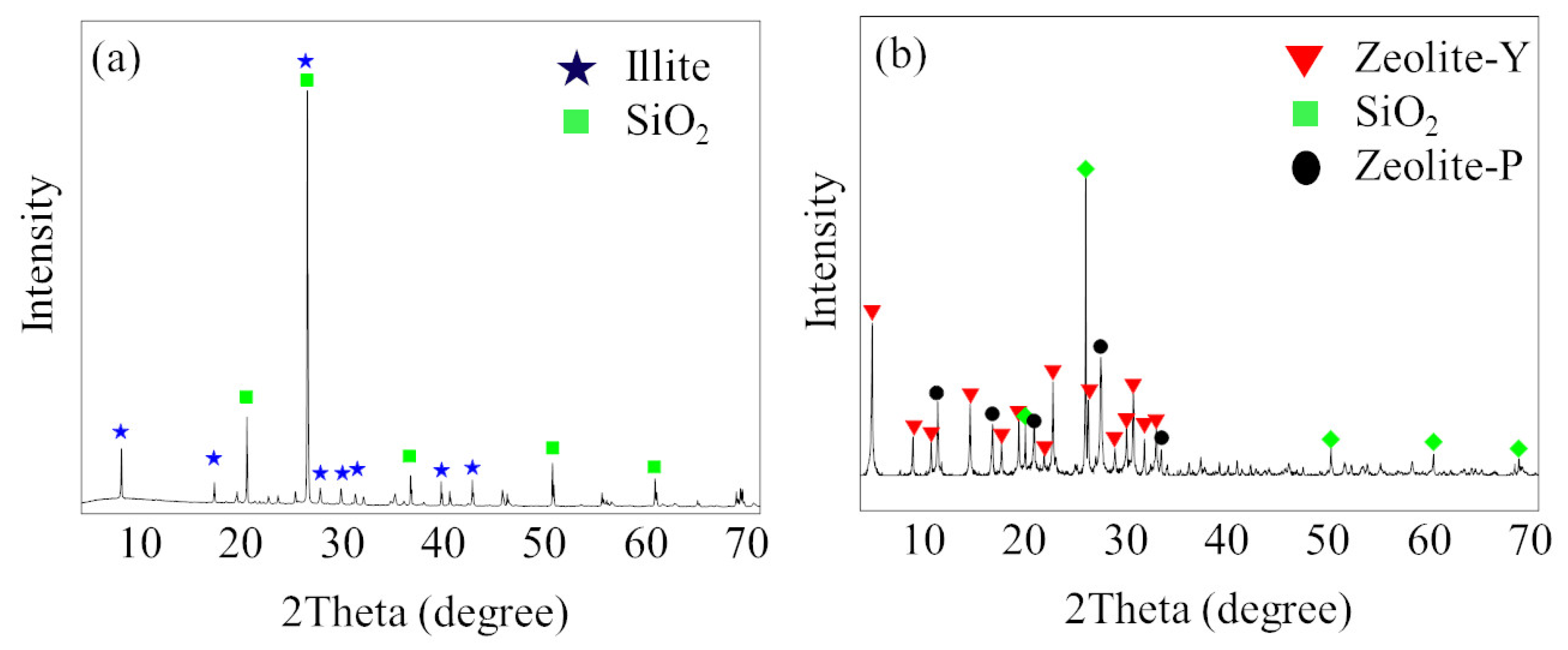
3.1. Characterization of the Synthesized Y-Type Zeolite
3.2. BBD Experimental Analysis
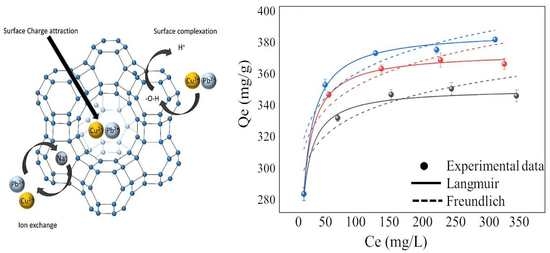
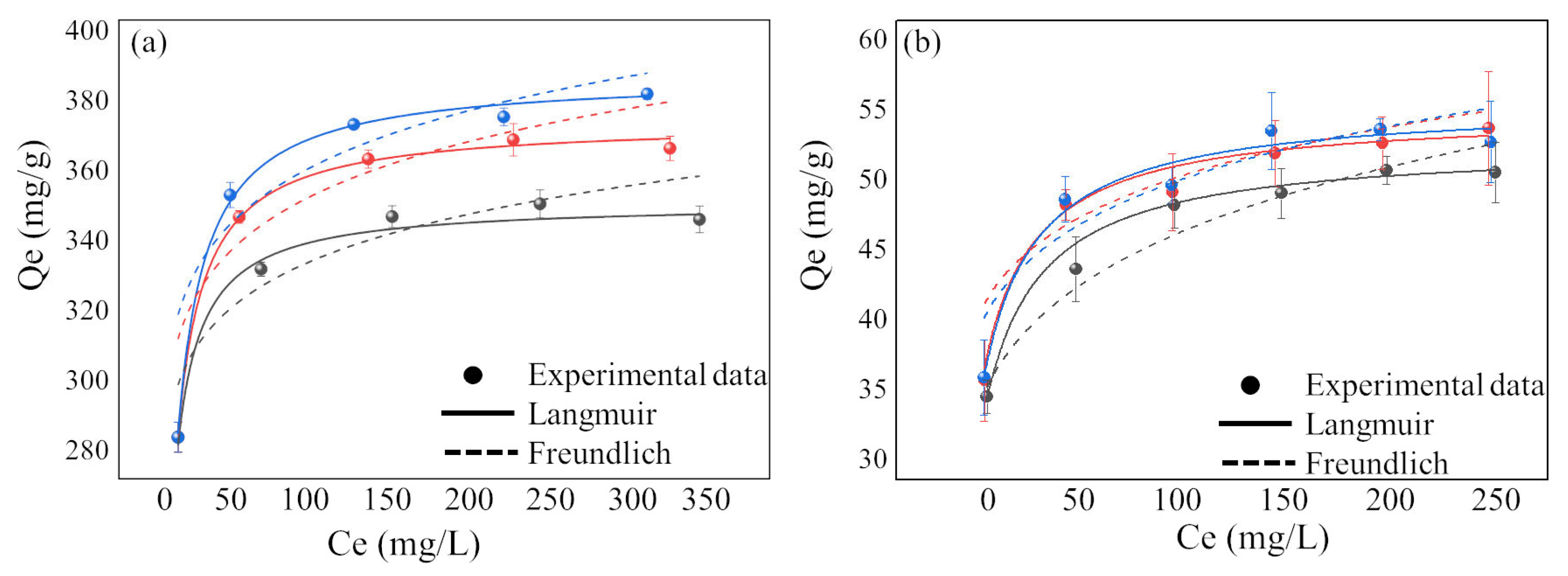
3.3. Adsorption Isotherm
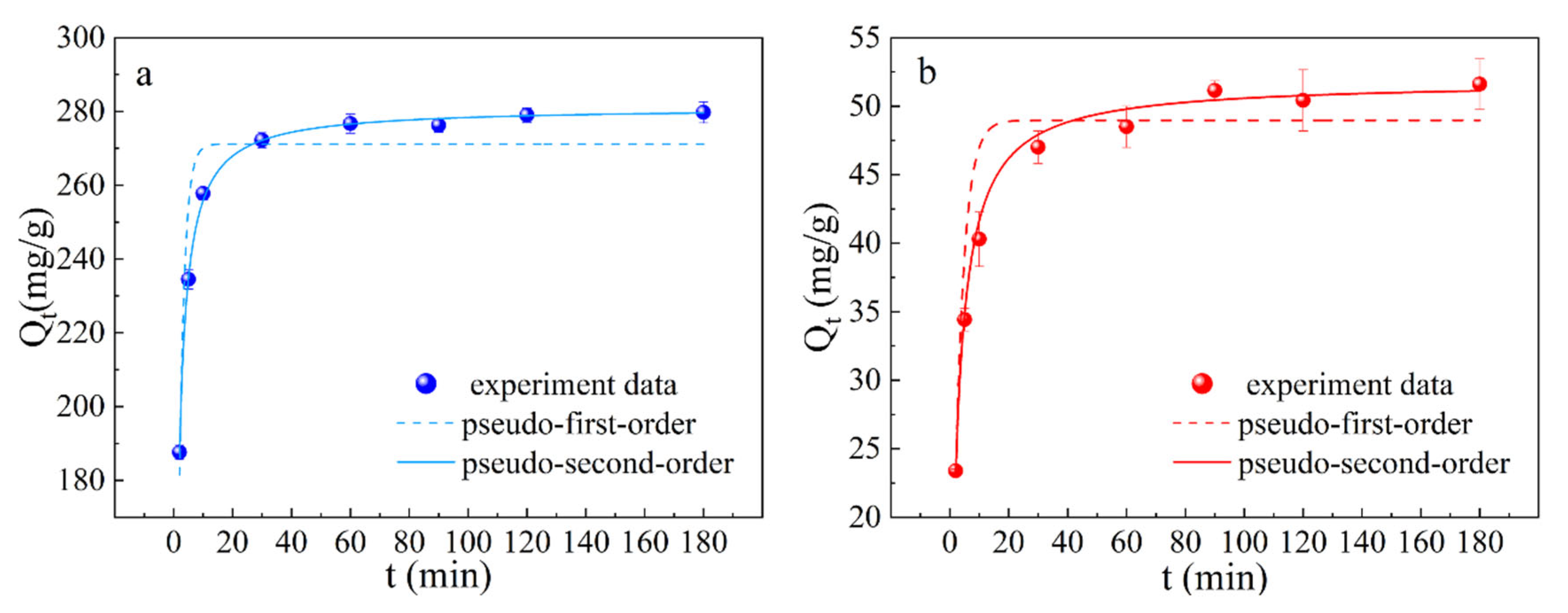
3.4. The Adsorption Kinetics
3.5. Desorption and Reusability of ZMA
3.6. Mechanism Analysis
3.7. Comparison of Synthesised ZMA with Other Adsorbents for PB(II) and Cu(II) Adsorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, G.; Shah, K.J.; Shi, L.; Chiang, P.-C.; You, Z. Red Soil Amelioration and Heavy Metal Immobilization by a Multi-Element Mineral Amendment: Performance and Mechanisms. Environ. Pollut. 2019, 254, 112964. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Shah, K.J.; Shi, L.; Chiang, P.-C. Removal of Cd (II) and Pb (II) Ions from Aqueous Solutions by Synthetic Mineral Adsorbent: Performance and Mechanisms. Appl. Surf. Sci. 2017, 409, 296–305. [Google Scholar] [CrossRef]
- Pawar, R.R.; Lalhmunsiama; Bajaj, H.C.; Lee, S.M. Activated Bentonite as a Low-Cost Adsorbent for the Removal of Cu(II) and Pb(II) from Aqueous Solutions: Batch and Column Studies. J. Ind. Eng. Chem. 2016, 34, 213–223. [Google Scholar] [CrossRef] [Green Version]
- Ates, N.; Uzal, N. Removal of Heavy Metals from Aluminum Anodic Oxidation Wastewaters by Membrane Filtration. Environ. Sci. Pollut. Res. 2018, 25, 22259–22272. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.T.; Khaleefa Ali, S.A. Removal of Heavy Metal by Ion Exchange Using Bentonite Clay. J. Ecol. Eng. 2020, 22, 104–111. [Google Scholar] [CrossRef]
- Wang, Z.; Tan, Z.; Li, H.; Yuan, S.; Zhang, Y.; Dong, Y. Direct Current Electrochemical Method for Removal and Recovery of Heavy Metals from Water Using Straw Biochar Electrode. J. Clean. Prod. 2022, 339, 130746. [Google Scholar] [CrossRef]
- Zhang, L.; Li, W.; Cao, H.; Hu, D.; Chen, X.; Guan, Y.; Tang, J.; Gao, H. Ultra-Efficient Sorption of Cu2+ and Pb2+ Ions by Light Biochar Derived from Medulla Tetrapanacis. Bioresour. Technol. 2019, 291, 121818. [Google Scholar] [CrossRef]
- Gholami, L.; Rahimi, G. The Efficiency of Potato Peel Biochar for the Adsorption and Immobilization of Heavy Metals in Contaminated Soil. Int. J. Phytoremed. 2023, 25, 263–273. [Google Scholar] [CrossRef]
- Xiong, W.; Tong, J.; Yang, Z.; Zeng, G.; Zhou, Y.; Wang, D.; Song, P.; Xu, R.; Zhang, C.; Cheng, M. Adsorption of Phosphate from Aqueous Solution Using Iron-Zirconium Modified Activated Carbon Nanofiber: Performance and Mechanism. J. Colloid Interface Sci. 2017, 493, 17–23. [Google Scholar] [CrossRef]
- Gul Zaman, H.; Baloo, L.; Kutty, S.R.; Aziz, K.; Altaf, M.; Ashraf, A.; Aziz, F. Insight into Microwave-Assisted Synthesis of the Chitosan-MOF Composite: Pb(II) Adsorption. Environ. Sci. Pollut. Res. 2023, 30, 6216–6233. [Google Scholar] [CrossRef]
- Boujelben, R.; Ellouze, M.; Aziz, F.; Ouazzani, N.; Sayadi, S. Box-Behnken Approach for Optimization of Cr(III) Removal from a Real Tanning Effluent Using Powdered Marble. Int. J. Environ. Sci. Technol. 2022, 19, 4305–4320. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, X.; Yan, Y.; Chen, D.; Huang, L.; Zhang, J.; Ke, Y.; Tan, S. The Utilization of a Three-Dimensional Reduced Graphene Oxide and Montmorillonite Composite Aerogel as a Multifunctional Agent for Wastewater Treatment. RSC Adv. 2018, 8, 4239–4248. [Google Scholar] [CrossRef] [Green Version]
- Lehman, S.E.; Larsen, S.C. Zeolite and Mesoporous Silica Nanomaterials: Greener Syntheses, Environmental Applications and Biological Toxicity. Environ. Sci. Nano 2014, 1, 200–213. [Google Scholar] [CrossRef]
- Rayalu, S.; Meshram, S.U.; Hasan, M.Z. Highly Crystalline Faujasitic Zeolites from Flyash. J. Hazard. Mater. 2000, 77, 123–131. [Google Scholar] [CrossRef]
- Keane, M.A. The Removal of Copper and Nickel from Aqueous Solution Using Y Zeolite Ion Exchangers. Colloids Surfaces A Physicochem. Eng. Asp. 1998, 138, 11–20. [Google Scholar] [CrossRef]
- Ahmed, S.; Chughtai, S.; Keane, M.A. The Removal of Cadmium and Lead from Aqueous Solution by Ion Exchange with Na-Y Zeolite. Sep. Purif. Technol. 1998, 13, 57–64. [Google Scholar] [CrossRef]
- Kim, J.S.; Keane, M.A. Ion Exchange of Divalent Cobalt and Iron with Na-Y Zeolite: Binary and Ternary Exchange Equilibria. J. Colloid Interface Sci. 2000, 232, 126–132. [Google Scholar] [CrossRef]
- Khalil, A.; Hashaikeh, R.; Hilal, N. 3D Printed Zeolite-Y for Removing Heavy Metals from Water. J. Water Process Eng. 2021, 42, 102187. [Google Scholar] [CrossRef]
- Yusof, A.M.; Malek, N.A.N.N. Removal of Cr(VI) and As(V) from Aqueous Solutions by HDTMA-Modified Zeolite Y. J. Hazard. Mater. 2009, 162, 1019–1024. [Google Scholar] [CrossRef]
- Pikus, S.; Zienkiewicz-Strzałka, M.; Skibińska, M. Synthesis of New Mesostructured Cellular Foams (MCFs) with NaY Zeolite and Their Application to Sorption of Thorium Ions. Mater. Sci. Pol. 2019, 37, 488–495. [Google Scholar] [CrossRef] [Green Version]
- Agbendeh, Z.M.; Gimba, C.E.; Ande, S.; Ekanem, S.F. Synthesis and Characterization of Zeolite Y From Kankara Clay Using Alkaline Fusion Method. J. Chem. Soc. Niger. 2021, 46, 1016–1031. [Google Scholar] [CrossRef]
- Li, X.Y.; Jiang, Y.; Liu, X.Q.; Shi, L.Y.; Zhang, D.Y.; Sun, L.B. Direct Synthesis of Zeolites from a Natural Clay, Attapulgite. ACS Sustain. Chem. Eng. 2017, 5, 6124–6130. [Google Scholar] [CrossRef]
- Li, X.; Han, S.; Guan, D.; Jiang, N.; Xu, J.; Park, S.E. Rapid Direct Synthesis of Nano-H-ZSM-5 from Leached Illite via Solid-like-State Conversion-Based Crystallization. Appl. Clay Sci. 2021, 203, 106028. [Google Scholar] [CrossRef]
- Yue, Y.; Kang, Y.; Bai, Y.; Gu, L.; Liu, H.; Bao, J.; Wang, T.; Yuan, P.; Zhu, H.; Bai, Z.; et al. Seed-Assisted, Template-Free Synthesis of ZSM-5 Zeolite from Natural Aluminosilicate Minerals. Appl. Clay Sci. 2018, 158, 177–185. [Google Scholar] [CrossRef]
- Sellaoui, L.; Hessou, E.P.; Badawi, M.; Netto, M.S.; Dotto, G.L.; Silva, L.F.O.; Tielens, F.; Ifthikar, J.; Bonilla-Petriciolet, A.; Chen, Z. Trapping of Ag+, Cu2+, and Co2+ by Faujasite Zeolite Y: New Interpretations of the Adsorption Mechanism via DFT and Statistical Modeling Investigation. Chem. Eng. J. 2021, 420, 127712. [Google Scholar] [CrossRef]
- Pinheiro, D.R.; Neves, R.d.F.; Paz, S.P.A. A Sequential Box-Behnken Design (BBD) and Response Surface Methodology (RSM) to Optimize SAPO-34 Synthesis from Kaolin Waste. Microporous Mesoporous Mater. 2021, 323, 111250. [Google Scholar] [CrossRef]
- Sivalingam, S.; Sen, S. Optimization of Synthesis Parameters and Characterization of Coal Fly Ash Derived Microporous Zeolite X. Appl. Surf. Sci. 2018, 455, 903–910. [Google Scholar] [CrossRef]
- Chang, Y.C.; Chen, D.H. Preparation and Adsorption Properties of Monodisperse Chitosan-Bound Fe3O4 Magnetic Nanoparticles for Removal of Cu(II) Ions. J. Colloid Interface Sci. 2005, 283, 446–451. [Google Scholar] [CrossRef]
- Sun, Z.; Song, H.; Xing, Y.; Li, J.; Lu, Y.; Wang, J. Simulation of rainwater infiltration and heavy metal pollutant migration in permeable brick pavement system in sponge City. Environ. Eng. 2020, 38, 46–52+100. [Google Scholar]
- Wang, H. Migration and Transformation of Dissolved Organic Matter and Heavy Metals in Runoff Rainwater in LID Facilities. Master’s Thesis, Beijing University of Architecture, Beijing, China, 2019; p. 78. [Google Scholar]
- Yang, K.; Li, Y.; Guo, H.; Liu, X.; Chen, X.; Cao, J. Rapid Synthesis of Zeolite P from Potassic Rocks by Gel-like-Solid Phase Method. Asia-Pacific J. Chem. Eng. 2021, 16, e2641. [Google Scholar] [CrossRef]
- Belviso, C.; Cavalcante, F.; Niceforo, G.; Lettino, A. Sodalite, Faujasite and A-Type Zeolite from 2:1dioctahedral and 2:1:1 Trioctahedral Clay Minerals. A Singular Review of Synthesis Methods through Laboratory Trials at a Low Incubation Temperature. Powder Technol. 2017, 320, 483–497. [Google Scholar] [CrossRef]
- Belviso, C.; Giannossa, L.C.; Huertas, F.J.; Lettino, A.; Mangone, A.; Fiore, S. Synthesis of Zeolites at Low Temperatures in Fly Ash-Kaolinite Mixtures. MicroporMesopor Mat. 2015, 212, 35–47. [Google Scholar] [CrossRef]
- Cai, X.; Yu, X.; Yu, X.; Wu, Z.; Li, S.; Yu, C. Synthesis of Illite/Iron Nanoparticles and Their Application as an Adsorbent of Lead Ions. Environ. Sci. Pollut. Res. 2019, 26, 29449–29459. [Google Scholar] [CrossRef] [PubMed]
- Nezamzadeh-Ejhieh, A.; Kabiri-Samani, M. Effective Removal of Ni(II) from Aqueous Solutions by Modification of Nano Particles of Clinoptilolite with Dimethylglyoxime. J. Hazard. Mater. 2013, 260, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Caballero, I.; Colina, F.G.; Costa, J. Synthesis of X-Type Zeolite from Dealuminated Kaolin by Reaction with Sulfuric Acid at High Temperature. Ind. Eng. Chem. Res. 2007, 46, 1029–1038. [Google Scholar] [CrossRef]
- Aly, H.M.; Moustafa, M.E.; Abdelrahman, E.A. Synthesis of Mordenite Zeolite in Absence of Organic Template. Adv. Powder Technol. 2012, 23, 757–760. [Google Scholar] [CrossRef]
- Abdelrahman, E.A.; Tolan, D.A.; Nassar, M.Y. A Tunable Template-Assisted Hydrothermal Synthesis of Hydroxysodalite Zeolite Nanoparticles Using Various Aliphatic Organic Acids for the Removal of Zinc(II) Ions from Aqueous Media. J. Inorg. Organomet. Polym. Mater. 2019, 29, 229–247. [Google Scholar] [CrossRef]
- Dos Santos, M.B.; Vianna, K.C.; Pastore, H.O.; Andrade, H.M.C.; Mascarenhas, A.J.S. Studies on the Synthesis of ZSM-5 by Interzeolite Transformation from Zeolite Y without Using Organic Structure Directing Agents. Microporous Mesoporous Mater. 2020, 306, 110413. [Google Scholar] [CrossRef]
- Shariatinia, Z.; Bagherpour, A. Synthesis of Zeolite NaY and Its Nanocomposites with Chitosan as Adsorbents for Lead(II) Removal from Aqueous Solution. Powder Technol. 2018, 338, 744–763. [Google Scholar] [CrossRef]
- Doan, T.; Nguyen, K.; Dam, P.; Vuong, T.H.; Le, M.T.; Thanh, H.P. Synthesis of SAPO-34 Using Different Combinations of Organic Structure-Directing Agents. J. Chem. 2019, 2019, 6197527. [Google Scholar] [CrossRef] [Green Version]
- Shah, K.J.; Mishra, M.K.; Shukla, A.D.; Imae, T.; Shah, D.O. Controlling Wettability and Hydrophobicity of Organoclays Modified with Quaternary Ammonium Surfactants. J. Colloid Interface Sci. 2013, 407, 493–499. [Google Scholar] [CrossRef]
- Mezni, M.; Hamzaoui, A.; Hamdi, N.; Srasra, E. Synthesis of Zeolites from the Low-Grade Tunisian Natural Illite by Two Different Methods. Appl. Clay Sci. 2011, 52, 209–218. [Google Scholar] [CrossRef]
- Khalifa, A.Z.; Pontikes, Y.; Elsen, J.; Cizer, Ö. Comparing the Reactivity of Different Natural Clays under Thermal and Alkali Activation. RILEM Tech. Lett. 2019, 4, 74–80. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Nong, S.; Zhao, Y.; Riaz, M.S.; Xiao, Y.; Molokeev, M.S.; Huang, F. Renewable P-Type Zeolite for Superior Absorption of Heavy Metals: Isotherms, Kinetics, and Mechanism. Sci. Total Environ. 2020, 726, 138535. [Google Scholar] [CrossRef]
- Gupta, V.K.; Fakhri, A.; Rashidi, S.; Ibrahim, A.A.; Asif, M.; Agarwal, S. Optimization of Toxic Biological Compound Adsorption from Aqueous Solution onto Silicon and Silicon Carbide Nanoparticles through Response Surface Methodology. Mater. Sci. Eng. C 2017, 77, 1128–1134. [Google Scholar] [CrossRef]
- Li, Y.; Bai, P.; Yan, Y.; Yan, W.; Shi, W.; Xu, R. Removal of Zn2+, Pb2+, Cd2+, and Cu2+ from Aqueous Solution by Synthetic Clinoptilolite. Microporous Mesoporous Mater. 2019, 273, 203–211. [Google Scholar] [CrossRef]
- El-Mekkawi, D.M.; Selim, M.M. Removal of Pb2+ from Water by Using Na-Y Zeolites Prepared from Egyptian Kaolins Collected from Different Sources. J. Environ. Chem. Eng. 2014, 2, 723–730. [Google Scholar] [CrossRef]
- Nibou, D.; Mekatel, H.; Amokrane, S.; Barkat, M.; Trari, M. Adsorption of Zn2+ Ions onto NaA and NaX Zeolites: Kinetic, Equilibrium and Thermodynamic Studies. J. Hazard. Mater. 2010, 173, 637–646. [Google Scholar] [CrossRef]
- Apiratikul, R.; Pavasant, P. Sorption of Cu2+, Cd2+, and Pb2+ Using Modified Zeolite from Coal Fly Ash. Chem. Eng. J. 2008, 144, 245–258. [Google Scholar] [CrossRef]
- Han, S.; Liu, Y.; Yin, C.; Jiang, N. Fast Synthesis of Submicron ZSM-5 Zeolite from Leached Illite Clay Using a Seed-Assisted Method. Microporous Mesoporous Mater. 2019, 275, 223–228. [Google Scholar] [CrossRef]
- Abdelrahman, E.A.; Alharbi, A.; Subaihi, A.; Hameed, A.M.; Almutairi, M.A.; Algethami, F.K.; Youssef, H.M. Facile Fabrication of Novel Analcime/Sodium Aluminum Silicate Hydrate and Zeolite Y/Faujasite Mesoporous Nanocomposites for Efficient Removal of Cu(II) and Pb(II) Ions from Aqueous Media. J. Mater. Res. Technol. 2020, 9, 7900–7914. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, G.; Luo, Q.; Li, X.; Wang, Z. The Thermodynamics and Kinetics for the Removal of Copper and Nickel Ions by the Zeolite y Synthesized from Fly Ash. Mater. Res. Express 2019, 6, 025001. [Google Scholar] [CrossRef]
- Ge, Q.; Tian, Q.; Hou, R.; Wang, S. Combing Phosphorus-Modified Hydrochar and Zeolite Prepared from Coal Gangue for Highly Effective Immobilization of Heavy Metals in Coal-Mining Contaminated Soil. Chemosphere 2022, 291, 132835. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-P.; Kim, G.-C.; Go, M.-S. A Study on the Removal of Heavy Metal with Mg-Modified Zeolite. J. Korean Powder Metall. Inst. 2020, 27, 287–292. [Google Scholar] [CrossRef]
- Chen, X.; Tao, J.; Sun, P.; Yu, F.; Li, B.; Dun, L. Effect of Calcination on the Adsorption of Chifeng Zeolite on Pb2+and Cu2+. Int. J. Low-Carbon Technol. 2022, 17, 462–468. [Google Scholar] [CrossRef]
- Elboughdiri, N. The Use of Natural Zeolite to Remove Heavy Metals Cu (II), Pb (II) and Cd (II), from Industrial Wastewater. Cogent Eng. 2020, 7, 1782623. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Ogata, F.; Nakamura, T.; Kawasaki, N. Synthesis of Novel Zeolites Produced from Fly Ash by Hydrothermal Treatment in Alkaline Solution and Its Evaluation as an Adsorbent for Heavy Metal Removal. J. Environ. Chem. Eng. 2020, 8, 103687. [Google Scholar] [CrossRef]
- Yulianti, E.; Abdullah; Yusniyyah, S.I.; Aini, N.; Khalifah, S.N.; Istighfarini, V.N. Removal of Cu and Pb from Wastewater Using Modified Natural Zeolite. Proc. Int. Conf. Eng. Technol. Soc. Sci. 2021, 529, 363–369. [Google Scholar] [CrossRef]
- Shirendev, N.; Bat-Amgalan, M.; Kano, N.; Kim, H.J.; Gunchin, B.; Ganbat, B.; Yunden, G. A Natural Zeolite Developed with 3-Aminopropyltriethoxysilane and Adsorption of Cu(II) from Aqueous Media. Appl. Sci. 2022, 12, 11344. [Google Scholar] [CrossRef]
- Joseph, I.V.; Tosheva, L.; Miller, G.; Doyle, A.M. Fau—Type Zeolite Synthesis from Clays and Its Use for the Simultaneous Adsorption of Five Divalent Metals from Aqueous Solutions. Materials 2021, 14, 3738. [Google Scholar] [CrossRef]
- Chen, Y.; Armutlulu, A.; Sun, W.; Jiang, W.; Jiang, X.; Lai, B.; Xie, R. Ultrafast Removal of Cu(II) by a Novel Hierarchically Structured Faujasite-Type Zeolite Fabricated from Lithium Silica Fume. Sci. Total Environ. 2020, 714, 136724. [Google Scholar] [CrossRef]




| Original Variable | Encoding Variable | Coded Level of Variables | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| NaOH/illite | A | 0.8 | 1.0 | 1.2 |
| Temperature (°C) | B | 1.0 | 2.0 | 3.0 |
| Time (h) | C | 1.2 | 1.4 | 1.6 |
| Standard | Coding Variables | Original Variables | ||||
|---|---|---|---|---|---|---|
| x1 | x2 | x3 | NaOH/Illite | Temperature (°C) | Time (h) | |
| 1 | −1 | −1 | 0 | 0.8 | 100 | 2 |
| 2 | 1 | −1 | 0 | 1.2 | 100 | 2 |
| 3 | −1 | 1 | 0 | 0.8 | 200 | 2 |
| 4 | 1 | 1 | 0 | 1.2 | 200 | 2 |
| 5 | −1 | 0 | −1 | 0.8 | 150 | 1 |
| 6 | 1 | 0 | −1 | 1.2 | 150 | 1 |
| 7 | −1 | 0 | 1 | 0.8 | 150 | 3 |
| 8 | 1 | 0 | 1 | 1.2 | 150 | 3 |
| 9 | 0 | −1 | −1 | 1 | 100 | 1 |
| 10 | 0 | 1 | −1 | 1 | 200 | 1 |
| 11 | 0 | −1 | 1 | 1 | 100 | 3 |
| 12 | 0 | 0 | 1 | 1 | 200 | 3 |
| 13 | 0 | 0 | 0 | 1 | 150 | 2 |
| 14 | 0 | 0 | 0 | 1 | 150 | 2 |
| 15 | 0 | 0 | 0 | 1 | 150 | 2 |
| 16 | 0 | 0 | 0 | 1 | 150 | 2 |
| 17 | 0 | 0 | 0 | 1 | 150 | 2 |
| Standard | A | B | C | %R | Standard | A | B | C | %R |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.8 | 100 | 2 | 61 | 10 | 1 | 100 | 1 | 82 |
| 2 | 1.2 | 100 | 2 | 66 | 11 | 1 | 200 | 3 | 70 |
| 3 | 0.8 | 200 | 2 | 83 | 12 | 1 | 150 | 3 | 92 |
| 4 | 1.2 | 200 | 2 | 93 | 13 | 1 | 150 | 2 | 85 |
| 5 | 0.8 | 150 | 1 | 67 | 14 | 1 | 150 | 2 | 85 |
| 6 | 1.2 | 150 | 1 | 78 | 15 | 1 | 150 | 2 | 85 |
| 7 | 0.8 | 150 | 3 | 79 | 16 | 1 | 150 | 2 | 85 |
| 8 | 1.2 | 150 | 3 | 91 | 17 | 1 | 150 | 2 | 85 |
| 9 | 1 | 100 | 1 | 62 |
| Source | DF | SS | MS | F-Value | p-Value | Remark |
|---|---|---|---|---|---|---|
| Model | 8 | 1699.38 | 212.42 | 82.90 | <0.0001 | Significant |
| A-NaOH/illite ratio | 1 | 180.50 | 180.50 | 70.44 | <0.0001 | Significant |
| B-Activation temperature (°C) | 1 | 1035.13 | 1035.13 | 403.95 | <0.0001 | Significant |
| C-Activation time (h) | 1 | 231.13 | 231.13 | 90.20 | <0.0001 | Significant |
| AB | 1 | 6.25 | 6.25 | 2.44 | 0.1570 | |
| BC | 1 | 1.00 | 1.00 | 0.39 | 0.5496 | |
| A2 | 1 | 51.58 | 51.58 | 20.13 | 0.0020 | Significant |
| B2 | 1 | 139.21 | 139.21 | 54.33 | <0.0001 | Significant |
| C2 | 1 | 31.84 | 31.84 | 12.43 | 0.0078 | Significant |
| Residual | 8 | 20.50 | 2.56 | |||
| Lack of Fit | 4 | 20.50 | 5.12 | |||
| Pure Error | 4 | 0.000 | 0.000 | |||
| Cor Total | 16 | 1719.88 |
| T | qe.exp(mg/g) | Langmuir | Freundlich | ||||||
|---|---|---|---|---|---|---|---|---|---|
| qe,cal(mg/g) | KL | R2 | χ2 | KF | n | R2 | χ2 | ||
| Pb(II) | |||||||||
| 15 °C | 345.69 | 350.15 | 0.32 | 0.9672 | 0.2388 | 261.59 | 18.79 | 0.7724 | 1.1985 |
| 25 °C | 334.95 | 372.16 | 0.25 | 0.9959 | 0.0299 | 268.66 | 17.06 | 0.6666 | 2.9341 |
| 35 °C | 379.83 | 384.75 | 0.21 | 0.9930 | 0.0446 | 273.73 | 16.84 | 0.6694 | 3.9399 |
| Cu(II) | |||||||||
| 15 °C | 49.25 | 51.17 | 0.11 | 0.9907 | 0.0868 | 22.27 | 6.62 | 0.9674 | 0.2361 |
| 25 °C | 52.37 | 53.47 | 0.12 | 0.9698 | 0.0449 | 29.90 | 9.45 | 0.7947 | 0.8478 |
| 35 °C | 52.27 | 54.14 | 0.12 | 0.9387 | 0.1049 | 28.40 | 8.63 | 0.8438 | 0.7354 |
| Zeolite Type | Modifier | Synthetic Method | Type and Capacity of Heavy Metal Adsorption | Reference |
|---|---|---|---|---|
| Y-type zeolite | glutamic acid or L-arginine | Hydrothermal method | Cu 105.82 mg/g Pb 83.26 mg/g | [52] |
| Y-type zeolite | prepared seed gel of sodium aluminate and sodium silicate | Hydrothermal synthesis | Ag 320.91 mg/g Cu 86.32 mg/g Co 124.82 mg/g | [25] |
| Y-type zeolite | Class C fly ash | Fusion-hydrothermal method | Cu 235 mg/g Ni 170 mg/g | [53] |
| P-type zeolite | Aluminum nitrate nonahydrate and Sodium metasilicate nonahydrate | Facial hydrothermal method | Pb 649 mg/g Cd 210 mg/g Cu 90 mg/g Zn 88 mg/g | [45] |
| Na-X zeolite | H3PO4 modified hydrochar | Hydrothermal carbonization method | Cd 67.01% Cu 57.01% Pb 78.72% | [54] |
| Mg-Zeolite | Magnesium chloride | Ion-exchange | Pb 99.03 mg/L 99.86% removal | [55] |
| Chifeng zeolite (Natural-China) | - | Calcination | Pb 75 mg/L (75%) Cu 40 mg/L (40%) | [56] |
| Natural zeolite | - | - | Pb 6.5 mg/g Cu 2.2 mg/g Cd 1.4 mg/g | [57] |
| Zeolite | JIS Type-II fly ash | Alkaly hydrothermal treatment | Pb 18.1 mg/g Hg 5.6 mg/g | [58] |
| Natural zeolite (Indonetian) | HCl acid activation | Pb 71 mg/g Cu 61.56 mg/g | [59] | |
| Natural zeolite | 3-Aminopropyltriethoxysilane | Cu 20.66 mg/g | [60] | |
| FAU-type zeolite | Vermiculite-kaolinite clay | Hydrothermal crystalization | Pb 100 mg/g Cu 46.7 mg/g Cd 41.9 mg/g | [61] |
| FAU-type zeolite | Lithium silica fumes | Hydrothermal treatment | Cu 94.46 mg/g | [62] |
| Y-type zeolite (present study) | Illite clay | Hydrothermal treatment | Pb 372.1 mg/g Cu 53.46 mg/g | Present study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, K.J.; Yu, J.; Zhang, T.; You, Z. Y-Type Zeolite Synthesized from an Illite Applied for Removal of Pb(II) and Cu(II) Ions from Aqueous Solution: Box-Behnken Design and Kinetics. Water 2023, 15, 1171. https://doi.org/10.3390/w15061171
Shah KJ, Yu J, Zhang T, You Z. Y-Type Zeolite Synthesized from an Illite Applied for Removal of Pb(II) and Cu(II) Ions from Aqueous Solution: Box-Behnken Design and Kinetics. Water. 2023; 15(6):1171. https://doi.org/10.3390/w15061171
Chicago/Turabian StyleShah, Kinjal J., Jiacheng Yu, Ting Zhang, and Zhaoyang You. 2023. "Y-Type Zeolite Synthesized from an Illite Applied for Removal of Pb(II) and Cu(II) Ions from Aqueous Solution: Box-Behnken Design and Kinetics" Water 15, no. 6: 1171. https://doi.org/10.3390/w15061171
APA StyleShah, K. J., Yu, J., Zhang, T., & You, Z. (2023). Y-Type Zeolite Synthesized from an Illite Applied for Removal of Pb(II) and Cu(II) Ions from Aqueous Solution: Box-Behnken Design and Kinetics. Water, 15(6), 1171. https://doi.org/10.3390/w15061171