Research Progress of High-Salinity Wastewater Treatment Technology
Abstract
:1. Introduction
2. Water Quality Characteristics of High-Salinity Wastewater
| Types of Wastewater | Salt Content (mg/L) | Characteristics of Wastewater Quality | Chinese Discharge Standard of Water Pollutants | References |
|---|---|---|---|---|
| Tannery wastewater | 37,813 ± 32,041 | Chromium, salt, organic nitrogen, sulfide, phosphorus, and ammonium | GB 30486-2013 COD ≤ 100 mg/L NH4-N ≤ 25 mg/L | [7,8,9] |
| Printing and dyeing wastewater | 7256 ± 4489 | Chromium, SS, chlorides, nitrogen, heavy metals, sulfates, and organic pollutants | GB 4287-2012 COD ≤ 80 mg/L NH4-N ≤ 10 mg/L | [7,10,11] |
| Petrochemical wastewater | 35,026 ± 28,397 | Halogenated hydrocarbons, polycyclic aromatic hydrocarbons, aromatic amines, mercury, and lead | GB 31571-2015 COD ≤ 60 mg/L NH4-N ≤ 8 mg/L | [7,9,12] |
| Pharmaceutical wastewater | 35,499 ± 16,478 | Ammonia nitrogen, suspended solids, drug residues, drug intermediates, and waste solvents | GB 21904-2008 COD ≤ 120 mg/L NH4-N ≤ 25 mg/L | [7,13,14] |
3. Desalination Technology for High-Salinity Wastewater
3.1. Membrane Technology
| Types of Wastewater | Wastewater Quality | Membrane | Operating Conditions | Removal Efficiency | References |
|---|---|---|---|---|---|
| Printing and dyeing wastewater | NaCl: 1000–16,000 mg/L Dye: 100–6000 mg/L | NF | Transmembrane Pressure (TMP) = 0.4 MPa T = 25 ℃ | Dye: 91.4% NaCl: 95.3% | [17] |
| Textile wastewater | Electrical Conductivity (EC): 3275 μS/cm COD: 1771 mg/L NH3-N: 10.16 mg/L | NF | TMP = 12 bar pH = 8 | EC: 30.90 μS/cm COD: 34.35 mg/L NH3-N: 0 | [18] |
| Coal chemical wastewater | EC: 50,000–65,000 μS/cm Cl−: 12,000mg/L | Two-stage RO | TMP = 6.8–7.2 MPa T = 30 ℃ pH = 9–10 | EC: 200 μS/cm | [20] |
| Oily wastewater | NaCl = 2000 ppm Drop size: 300 nm | UF+RO | TMP = 1.6 MPa T = 24 h | Oil retention rate: 98% NaCl: 89.3% | [21] |
| Printing and dyeing wastewater | EC: 6500–7500 μS/cm TDS: 3500–4500 mg/L Cl-: 1600–1750 mg/L COD: 130–160 mg/L | ED | U = 80 V Q = 5.2 L/h I = 25 A | TDS: 78.07% Cl-: 88.50% | [25] |
| Glyphosate wastewater | EC: 23 mS/cm COD: 2800 mg/L Hardness: 570 mg/L | BMED | U = 24 V; V = 2.5 L S = 45 cm2 | EC: 1.5 mS/cm COD:364 mg/L Hardness: 0 | [26] |
| Petrochemical wastewater | EC: 82 mS/cm TDS: 84,000 mg/L Total Organic Carbon (TOC): 41 mg/L | Direct contact membrane distillation (DCMD) | Timport = 70 ℃; TInfiltration = 40 ℃ | TDS > 99.5% EC: 195 μS/cm TOC: 1.6 mg/L | [28] |
| Petrochemical wastewater | TOC: 127 ± 6 mg/L EC: 2400 μS/cm | DCMD | Timport = 60 ℃; TInfiltration = 20 ℃ | EC: 10 μS/cm; TOC: 8 mg/L | [29] |
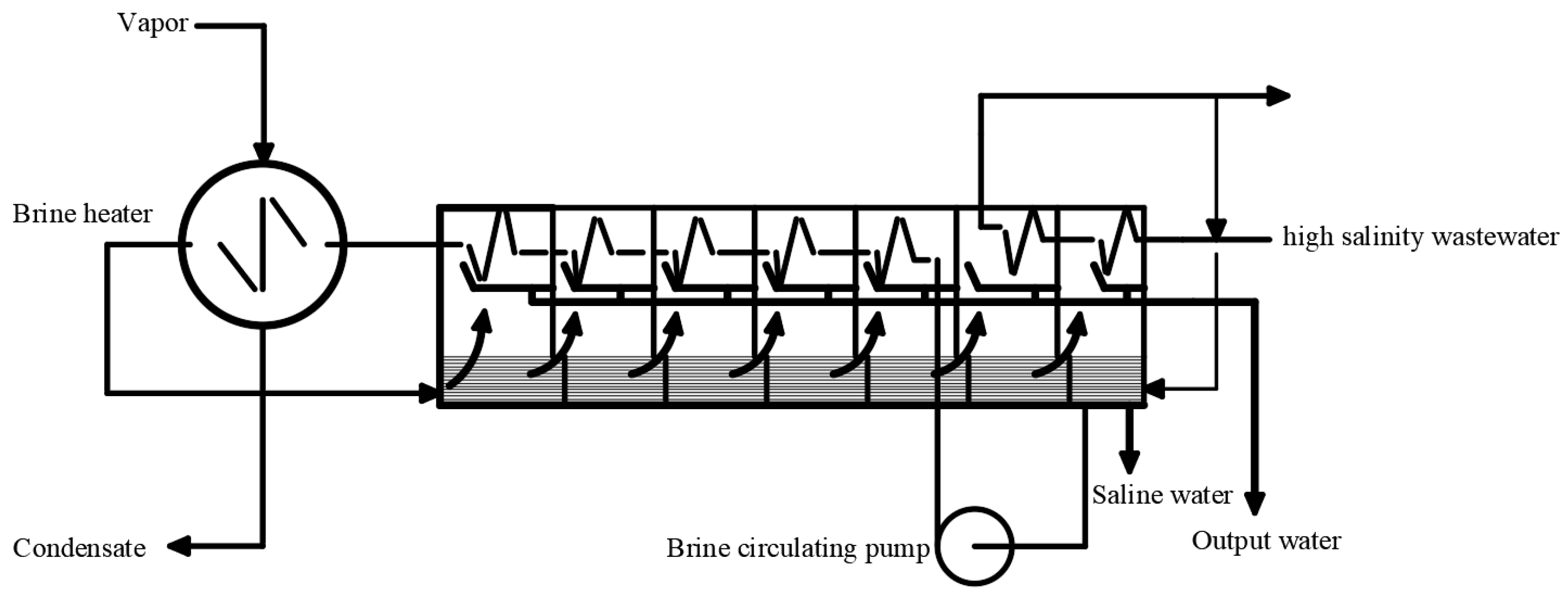
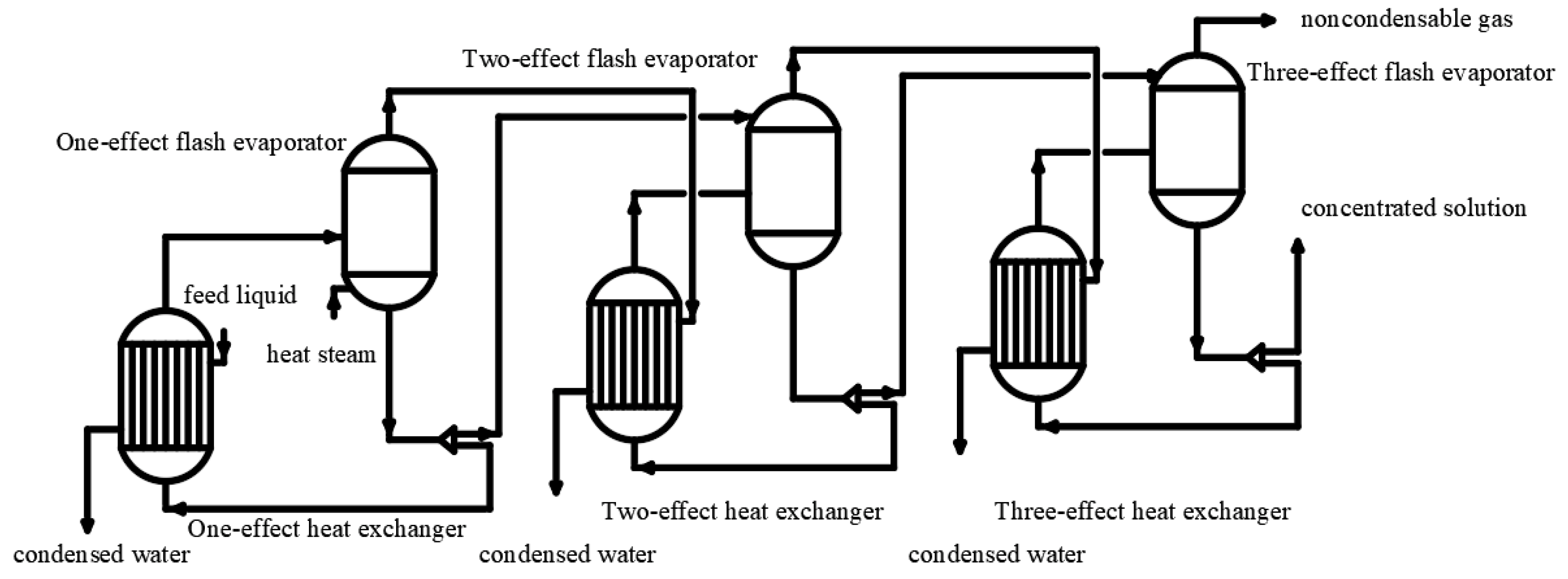
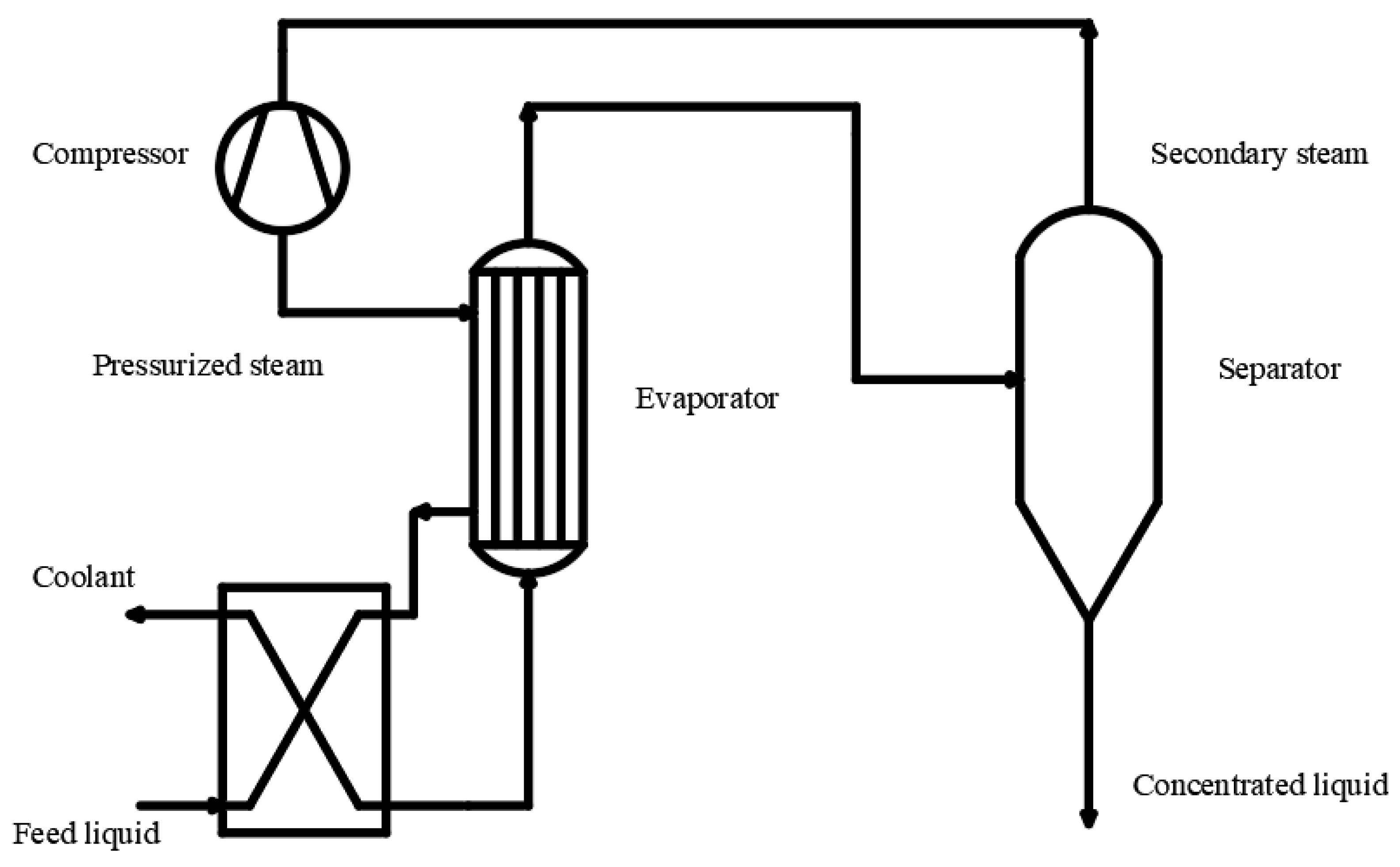
3.2. Thermal Concentration Method
3.3. Treatment of High-Salinity Concentrates
4. Treatment Technology of High-Salinity Wastewater
4.1. Advanced Oxidation Processes
| Types of Wastewater | Technology | Wastewater Quality | Operating Conditions | Removal Efficiency | References |
|---|---|---|---|---|---|
| Chemical wastewater | Activated carbon adsorption—Fenton | TDS: 20% COD: 13,650 mg/L | pH = 6 FeSO4 = 3.0 g/L H2O2 = 20 mL/L T: 30 min | COD: 84.4% | [41] |
| Synthetic wastewater | Electrochemical oxidation | TOC: 2000 mg/L COD: 3500 mg/L TDS: 30.94 g/L | pH = 7.69 T = 30 min U = 7.41 V | COD: 91.78% TOC: 68.49% | [43] |
| Petrochemical wastewater | Catalytic ozonation | COD = 362 ± 36 mg/L TOC: 42 mg/L EC: 59.9 ms/cm | Catalyst dosing: 0.45g/L pH = 7.2 Ozone dosing: 0.3 g/h | COD: 75.3% TOC: 50.3% | [45] |
| Pesticide wastewater | AC catalytic—photolysis of ozone | COD:15,000 mg/L Cl−: 15,000 mg/L NH3-N: 40–60 mg/L | AC dosing:40 g/L P: 14w Aeration volume: 800 L/h | COD: 70.9% NH3-N: 87.7% | [46] |
4.2. Membrane Bio-Reactor
| Types of Wastewater | Technology | Wastewater Quality | Operating Conditions | Removal Efficiency | References |
|---|---|---|---|---|---|
| Petrochemical wastewater | MSBR | TDS: 35,000 mg/L COD: 2250 mg/L | HRT = 48 h T = 30 °C Organic load = 1.124 kgCOD/(m3d) | COD: 97.5% TOC: 97.2% | [49] |
| Synthetic wastewater | two-phase MBR | NaCl: 100 g/L phenol: 2000 mg/L | T = 30 °C pH = 3 module area = 0.19 m2 | phenol: 95% | [50] |
| Synthetic wastewater | AnMBR | COD: 20,000 ± 410 mg/L EC: 1100 ± 100 mS/cm | MLVSS/MLSS = 0.78 T = 35 ± 1 °C HRT = 5 d SRT = 226 d | COD: 89.9–95.5% | [52] |
| Mustard production wastewater | AnMBR | COD: 7500 mg/L EC: 54 mS/cm | Organic load: Phase 1: 0.5–1.0 kgCOD/(m3·d) Phase 2: 7.6 kgCOD/(m3·d) | COD: 80% | [51] |
4.3. Sequencing Batch Reactor
4.4. Biological Contact Oxidation Process
4.5. Biological Aerated Filter
4.6. Up-flow Anaerobic Sludge Bed/Blanket
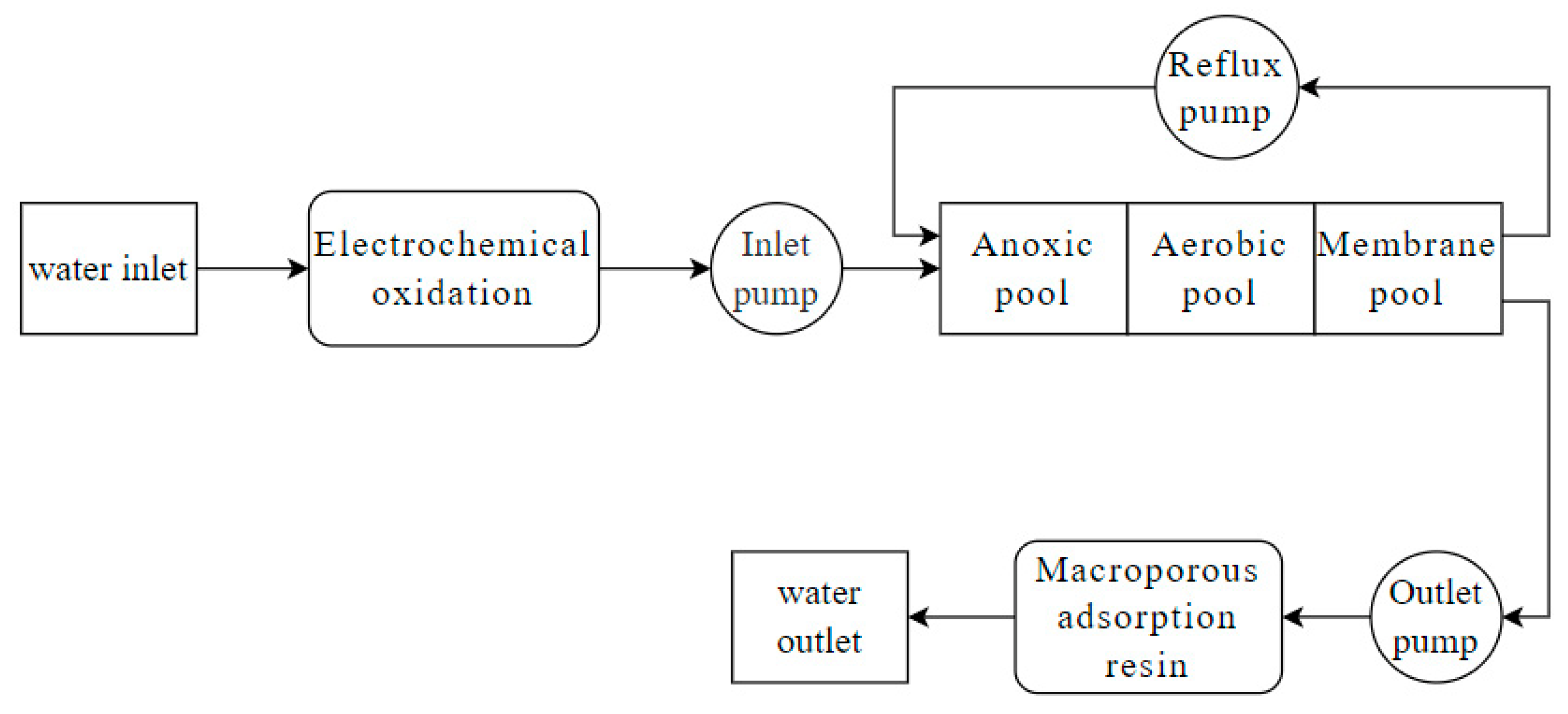
5. Hybrid Treatment Processes
6. Scaling and Corrosion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, S.; Liu, H.; Wang, M.; Xie, Y.; Ding, J.; Wang, Y. Research progress in new treatment technology for high-salinity wastewater. Mod. Chem. Ind. 2022, 42, 68–71. [Google Scholar]
- Singh, A. Soil salinization and waterlogging: A threat to environment and agricultural sustainability. Ecol. Indic. 2015, 57, 128–130. [Google Scholar] [CrossRef]
- Maharaja, P.; Boopathy, R.; Anushree, V.V.; Mahesh, M.; Swarnalatha, S.; Ravindran, B.; Chang, S.W.; Sekaran, G. Bio removal of proteins, lipids and mucopolysaccharides in tannery hyper saline wastewater using halophilic bacteria. J. Water Process Eng. 2020, 38, 101674. [Google Scholar] [CrossRef]
- Sforza, E.; Kumkum, P.; Barbera, E.; Kumar, S. Bioremediation of industrial effluents: How a biochar pretreatment may increase the microalgal growth in tannery wastewater. J. Water Process Eng. 2020, 37, 101431. [Google Scholar] [CrossRef]
- Liu, J.; Huang, Q.; Li, J.; Wu, T.; Zeng, G.; Yang, C. Research progress on the treatment technologies of industrial printing and dyeingwastewater. Technol. Water Treat. 2021, 47, 1–6. [Google Scholar]
- Jorfi, S.; Pourfadakari, S.; Ahmadi, M. Electrokinetic treatment of high saline petrochemical wastewater: Evaluation and scale-up. J. Environ. Manag. 2017, 204, 221–229. [Google Scholar] [CrossRef]
- Srivastava, A.; Parida, V.K.; Majumder, A.; Gupta, B.; Gupta, A.K. Treatment of saline wastewater using physicochemical, biological, and hybrid processes: Insights into inhibition mechanisms, treatment efficiencies and performance enhancement. J. Environ. Chem. Eng. 2021, 9, 105775. [Google Scholar] [CrossRef]
- Boopathy, R.; Karthikeyan, S.; Mandal, A.B.; Sekaran, G. Characterisation and recovery of sodium chloride from salt-laden solid waste generated from leather industry. Clean Technol. Environ. Policy 2013, 15, 117–124. [Google Scholar] [CrossRef]
- Xiao, Y.; Roberts, D.J. A review of anaerobic treatment of saline wastewater. Environ. Technol. 2010, 31, 1025–1043. [Google Scholar] [CrossRef]
- Yaseen, D.A.; Scholz, M. Textile dye wastewater characteristics and constituents of synthetic effluents: A critical review. Int. J. Environ. Sci. Technol. 2019, 16, 1193–1226. [Google Scholar] [CrossRef]
- Xu, H.; Yang, B.; Liu, Y.; Li, F.; Shen, C.; Ma, C.; Tian, Q.; Song, X.; Sand, W. Recent advances in anaerobic biological processes for textile printing and dyeing wastewater treatment: A mini-review. World J. Microbiol. Biotechnol. 2018, 34, 165. [Google Scholar] [CrossRef]
- Jia, X.; Jin, D.; Li, C.; Lu, W. Characterization and analysis of petrochemical wastewater through particle size distribution, biodegradability, and chemical composition. Chin. J. Chem. Eng. 2019, 27, 444–451. [Google Scholar] [CrossRef]
- Lefebvre, O.; Moletta, R. Treatment of organic pollution in industrial saline wastewater: A literature review. Water Res. 2006, 40, 3671–3682. [Google Scholar] [CrossRef]
- Ng, K.K.; Shi, X.; Kai, M. A novel application of anaerobic bio-entrapped membrane reactor for the treatment of chemical synthesis-based pharmaceutical wastewater. Sep. Purif. Technol. 2014, 132, 634–643. [Google Scholar] [CrossRef]
- Wang, B.; Shi, B.; Lai, J.; Xiong, M.; Wang, J. Research status and application of high-salt organic wastewater treatment. Technol. Water Treat. 2020, 46, 5–10. [Google Scholar]
- Wang, H.; Liu, Y.; Peng, D.; Wang, F.; Lu, M. The development of membrane separation technology and its application prospect. Appl. Chem. Ind. 2013, 42, 532–534. [Google Scholar]
- Wei, X.; Kong, X.; Sun, C.; Chen, J. Characterization and application of a thin-film composite nanofiltration hollow fiber membrane for dye desalination and concentration. Chem. Eng. J. 2013, 223, 172–182. [Google Scholar] [CrossRef]
- Couto, C.F.; Marques, L.S.; Amaral, M.C.S.; Moravia, W.G. Coupling of nanofiltration with microfiltration and membrane bioreactor for textile effluent reclamation. Sep. Sci. Technol. 2017, 52, 2150–2160. [Google Scholar] [CrossRef]
- Malaeb, L.; Ayoub, G.M. Reverse osmosis technology for water treatment: State of the art review. Desalination 2011, 267, 1–8. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, X.; Zhang, T. Application of the two-stage reverse osmosis system in the high-salt wastewater treatment of the coal chemical industry. Ind. Water Treat. 2020, 40, 115–117. [Google Scholar]
- Pei, B.; Chen, J.; Liu, P.; He, T.; Li, X.; Zhang, L. Hyperbranched poly (amidoamine)/tmc reverse osmosis membrane for oily saline water treatment. Environ. Technol. 2019, 40, 2779–2788. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Zhou, J.; He, Y.; Xu, Y.; Zhang, W. Fabrication of polyamide composite forward osmosis membranes and study on their desalination performance. Appl. Chem. Ind. 2018, 47, 1912–1916, 1921. [Google Scholar]
- Zhao, P.; Gao, B.; Yue, Q.; Liu, S.; Shon, H.K. The performance of forward osmosis in treating high-salinity wastewater containing heavy metal Ni2+. Chem. Eng. J. 2016, 288, 569–576. [Google Scholar] [CrossRef]
- Pan, H.; Chen, G.; Gao, Y.; Li, S. Progress of electrodialysis technology in high salinity wastewater treatment. Appl. Chem. Ind. 2021, 50, 2886–2891. [Google Scholar]
- Xu, Y.; Liu, Y.; Wang, N. Research progress on high salt treatment technology and dyeing wastewater. Appl. Chem. Ind. 2020, 49, 2859–2863. [Google Scholar]
- Shen, J.; Huang, J.; Liu, L.; Ye, W.; Lin, J.; Van der Bruggen, B. The use of bmed for glyphosate recovery from glyphosate neutralization liquor in view of zero discharge. J. Hazard. Mater. 2013, 260, 660–667. [Google Scholar] [CrossRef]
- Li, H.; Liu, H.; Shi, W.; Zhang, H. Recent research progress on the application of membrane distillation technology. New Chem. Mater. 2022, 50, 270–273, 277. [Google Scholar]
- Xu, J.; Srivatsa Bettahalli, N.M.; Chisca, S.; Khalid, M.K.; Ghaffour, N.; Vilagines, R.; Nunes, S.P. Polyoxadiazole hollow fibers for produced water treatment by direct contact membrane distillation. Desalination 2018, 432, 32–39. [Google Scholar] [CrossRef]
- Li, J.; Guo, S.; Xu, Z.; Li, J.; Pan, Z.; Du, Z.; Cheng, F. Preparation of omniphobic pvdf membranes with silica nanoparticles for treating coking wastewater using direct contact membrane distillation: Electrostatic adsorption vs. Chemical bonding. J. Membr. Sci. 2019, 574, 349–357. [Google Scholar] [CrossRef]
- Ma, X.; Liu, N. Study on zero discharge technologies of industrial wastewater in coal-fired power plants. Mod. Chem. Ind. 2020, 40, 45–49. [Google Scholar]
- Yuan, H.; Jin, C.; Fu, S. Research progress on evaporation techniques in the treatment of high salinity wastewater. Mod. Chem. Ind. 2017, 37, 50–54. [Google Scholar]
- Liu, T.; Zhang, H.; Zhao, D.; Xue, J.; Li, S.; Liu, W. Optimization and analysis on operation scheme of med concentrating saline wastewater of petrochemical enterprises. Mod. Chem. Ind. 2014, 34, 140–143, 145. [Google Scholar]
- Wei, F.; Jia, M.; Wang, X.; Men, J.; Du, Z.; Liang, C. Research progress in concentration treatment technologies for high salinity wastewater. Mod. Chem. Ind. 2019, 39, 21–25. [Google Scholar]
- Rui, Q.; Zhang, Z.; Ting, F. Pilot study on mechanical vapor recompression technology for treatment of saline wastewater. Chin. J. Environ. Eng. 2016, 10, 3671–3676. [Google Scholar]
- Liu, D.; Liu, Q.; Zhou, B.; Li, H.; Zhang, Y. Research progress on zero discharge and utilization of high salinity industrial wastewater. Mod. Chem. Ind. 2021, 41, 19–22. [Google Scholar]
- Chang, L.; Wei, J. Acclimation of salt-tolerant sludge for the biochemical treatment of salt-containing wastewater. Ind. Water Treat. 2009, 29, 34–37. [Google Scholar]
- Zhang, B.; Li, H.; Guo, D.; Liu, X.; Li, J. Pilot-scale research on enhanced treatment of high-salinity organic wastewater by complex halophilic bacteria. China Water Wastewater 2008, 24, 16–19. [Google Scholar]
- Zhao, Y.; Zhuang, X.; Ahmad, S.; Sung, S.; Ni, S. Biotreatment of high-salinity wastewater: Current methods and future directions. World J. Microbiol. Biotechnol. 2020, 36, 37. [Google Scholar] [CrossRef]
- Yang, H.; Zheng, X. Application and research progress of advanced oxidation process for degradation of organic pollutants. Technol. Water Treat. 2021, 47, 13–18. [Google Scholar]
- Wang, R.; Zeng, D.; Yang, Y.; Qin, R.; Ke, P.; Wang, G. Research progress of fenton reagent catalytic degradation of organic wastewater. Water Wastewater Eng. 2021, 47, 64–71. [Google Scholar]
- Yi, B.; Yang, C.; Guo, J.; Yang, Y.; Chen, X.; Li, X. Treatment of organic wastewater with high concentration of salts using coupling process of activated carbon adsorption and fenton oxidation. Chin. J. Environ. Eng. 2013, 7, 903–907. [Google Scholar]
- Zhou, Y.; Ji, Q.; Hu, C.; Qu, J. Recent advances in electro-oxidation technology for water treatment. J. Civ. Environ. Eng. 2022, 44, 104–118. [Google Scholar]
- Darvishmotevalli, M.; Zarei, A.; Moradnia, M.; Noorisepehr, M.; Mohammadi, H. Optimization of saline wastewater treatment using electrochemical oxidation process: Prediction by rsm method. Methodsx 2019, 6, 1101–1113. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, J.; Pan, L.; Cui, F.; Zhang, B.; Yang, L. Review on research of catalytic ozonation industrial wastewater. Appl. Chem. Ind. 2019, 48, 1914–1919. [Google Scholar]
- Ahmadi, M.; Kakavandi, B.; Jaafarzadeh, N.; Akbar Babaei, A. Catalytic ozonation of high saline petrochemical wastewater using PAC@ FeIIFe2IIIO4: Optimization, mechanisms and biodegradability studies. Sep. Purif. Technol. 2017, 177, 293–303. [Google Scholar] [CrossRef]
- Wen, D.; Chen, B.; Zheng, J.; Zeng, G. Atrazine manufacturing wastewater treatment by photocatalytic ozonization with activated carbon supported ferric iron. Chin. J. Environ. Eng. 2020, 14, 349–358. [Google Scholar]
- Li, J.; Guo, Y.; Wang, X.; Liang, S.; Zhang, Y. The research progress of activated carbon improving the performanceof membrane bioreactor. Technol. Water Treat. 2021, 47, 7–11, 26. [Google Scholar]
- Wang, Z.; Dai, R.; Zhang, X.; Wen, Y.; Chen, M.; Li, J. Recent advances and overview on sustainable development of membrane-based wastewater treatment technology. J. Civ. Environ. Eng. 2022, 44, 86–103. [Google Scholar]
- Pendashteh, A.R.; Abdullah, L.C.; Fakhru L-Razi, A.; Madaeni, S.S.; Zainal Abidin, Z.; Awang Biak, D.R. Evaluation of membrane bioreactor for hypersaline oily wastewater treatment. Process Saf. Environ. Prot. 2012, 90, 45–55. [Google Scholar] [CrossRef]
- Juang, R.; Huang, W.; Hsu, Y. Treatment of phenol in synthetic saline wastewater by solvent extraction and two-phase membrane biodegradation. J. Hazard. Mater. 2009, 164, 46–52. [Google Scholar] [CrossRef]
- Qi, J.; Xiao, X.; Zhang, R.; Ouyang, C.; Yan, X.; Ruan, W. Operation efficiency and membrane fouling characteristics of an anaerobic membrane reactor treating high-salt mustard tuber wastewater. Chin. J. Environ. Eng. 2021, 15, 553–562. [Google Scholar]
- Li, J.; Jiang, C.; Shi, W.; Song, F.; He, D.; Miao, H.; Wang, T.; Deng, J.; Ruan, W. Polytetrafluoroethylene (ptfe) hollow fiber anmbr performance in the treatment of organic wastewater with varying salinity and membrane cleaning behavior. Bioresour. Technol. 2018, 267, 363–370. [Google Scholar] [CrossRef]
- Li, B.; Wang, Z.; An, Y.; Wu, Z. Membrane surface fouling properties in mbrs for high-salinity wastewater treatment. Environ. Sci. 2014, 35, 643–650. [Google Scholar]
- Li, L.; Zhou, L.; Peng, Y.; Yang, Q. Research and application of sbr to refractory wastewater treatment. Ind. Water Treat. 2007, 27, 1–5. [Google Scholar]
- Mirbolooki, H.; Amirnezhad, R.; Pendashteh, A.R. Treatment of high saline textile wastewater by activated sludge microorganisms. J. Appl. Res. Technol. 2017, 15, 167–172. [Google Scholar] [CrossRef]
- Yang, H.; Chen, J.; Zhang, J. Effects of K+, Ca2+, Mg2+ on high salt heparin wastewater treatment. Chin. J. Environ. Eng. 2014, 8, 4267–4272. [Google Scholar]
- Jiang, C.; Sui, Q.; Chen, M.; Chai, Y.; Zhang, L.; Liu, M.; Zhang, Z.; Yang, J.; Wei, Y. Quick start of high saline wastewater biological treatment technology strengthened by composite salt-tolerant microbe. Chin. J. Environ. Eng. 2017, 11, 3929–3935. [Google Scholar]
- Ferrer-Polonio, E.; Carbonell-Alcaina, C.; Mendoza-Roca, J.A.; Iborra-Clar, A.; Álvarez-Blanco, S.; Bes-Piá, A.; Pastor-Alcañiz, L. Brine recovery from hypersaline wastewaters from table olive processing by combination of biological treatment and membrane technologies. J. Clean. Prod. 2017, 142, 1377–1386. [Google Scholar] [CrossRef]
- Wei, C.; Ru, X.; Yang, X.; Feng, C.; Wei, Y.; Li, F. Energy saving strategy based on oxygen control in wastewater bio-treatment. Chem. Ind. Eng. Prog. 2018, 37, 4121–4134. [Google Scholar]
- Lin, H.; Zhang, G.; Chen, Y.; Dong, Y.; Li, X.; Ji, Z. Start-up of a/o process in treating mixed chemical wastewater of high salinity. Environ. Sci. Technol. 2015, 38, 179–185. [Google Scholar]
- Yu, P.; Zhou, M.; Ji, X.; Sun, M.; Fu, J.; Ren, P. Low temperature starting and influence analysis of a/o biochemical filter for the treatment of high-salinity wastewater. Ind. Water Treat. 2016, 36, 80–84. [Google Scholar]
- Lv, B.; Xie, B.; Shao, C.; Huang, C. Treatment of saline organic wastewater by two-stage a/o biological contact oxidation process. China Water Wastewater 2011, 27, 102–105, 108. [Google Scholar]
- Yan, Z.; Ni, F.; Zhou, H.; Liu, P.; Ma, C. Application of biological treatment technology for the outflow of produced water from heavy mineralized heavy oil. Chem. Eng. Oil Gas 2020, 49, 124–130. [Google Scholar]
- Tang, S.; Zhou, R.; Zhong, H.; Qiu, S. Research progress and prospect of biological aerated filter. Mod. Chem. Ind. 2013, 33, 24–27. [Google Scholar]
- Wang, K.; Meng, R.; Cui, K. Study on advanced treatment of carboxymethyl cellulose wastewater by biological aerated filter. Water Wastewater Eng. 2016, 42, 56–59. [Google Scholar]
- Duan, J.; Jiang, X.; Chen, H.; Fan, S. Removal of ammonia nitrogen from mariculture wastewater by bioaugmented biofilter. Environ. Sci. Technol. 2019, 42, 37–42. [Google Scholar]
- Wang, J.; Zhang, X.; Wang, J.; Pan, X. Treatment of petrochemical high-salinity wastewater by sludge acclimation and bio-filter. Acta Pet. Sinica. Pet. Process. Sect. 2011, 27, 977–983. [Google Scholar]
- He, J.; Wei, J.; Zhang, J.; Liu, X.; Song, Y.; Yang, D.; Wang, J. Advanced treatment of antibiotic pharmaceutical wastewater by catalytic ozonation combined with baf process. Chin. J. Environ. Eng. 2019, 13, 2385–2392. [Google Scholar]
- Zhang, X.; Li, Q.; Wang, J.; Wang, X.; Lin, Y. Research progress process improvement of biological aerated filter: A review. Chem. Ind. Eng. Prog. 2015, 34, 2023–2030. [Google Scholar]
- Zou, X.; Yu, J.; Zhang, B. Research progress in treatment of hypersaline wastewater by anaerobic biological method. Mod. Chem. Ind. 2020, 40, 44–47. [Google Scholar]
- Liu, C.; Zhao, D.; Guo, Y.; Zhao, C. Performance and modeling of an up-flow anaerobic sludge blanket (uasb) reactor for treating high salinity wastewater from heavy oil production. China Pet. Process. Petrochem. Technol. 2012, 3, 90–95. [Google Scholar]
- Aslan, S.; Şekerdağ, N. Salt inhibition on anaerobic treatment of high salinity wastewater by upflow anaerobic sludge blanket (uasb) reactor. Desalination Water Treat. 2015, 57, 12998–13004. [Google Scholar] [CrossRef]
- Masoomeh, S.; Ali, A.M.; Sedigheh, A. Evaluation of biodecolorization of the textile azo dye by halophilic archaea. Biol. J. Microorg. 2017, 6, 1–17. [Google Scholar]
- Shi, X.; Lefebvre, O.; Ng, K.K.; Ng, H.Y. Sequential anaerobic–aerobic treatment of pharmaceutical wastewater with high salinity. Bioresour. Technol. 2014, 153, 79–86. [Google Scholar] [CrossRef]
- Zhan, Y.; Wei, R.; Zhou, H. Improvement on the treatment of thick oil sewage by using integrated biochemical treatment technology. Int. J. Environ. Sci. Technol. 2018, 15, 81–92. [Google Scholar] [CrossRef]
- Song, W.; Li, Z.; Ding, Y.; Liu, F.; You, H.; Qi, P.; Wang, F.; Li, Y.; Jin, C. Performance of a novel hybrid membrane bioreactor for treating saline wastewater from mariculture: Assessment of pollutants removal and membrane filtration performance. Chem. Eng. J. 2018, 331, 695–703. [Google Scholar] [CrossRef]
- Xu, F.; Ouyang, D.; Rene, E.R.; Ng, H.Y.; Guo, L.; Zhu, Y.; Zhou, L.; Yuan, Q.; Miao, M.; Wang, Q.; et al. Electricity production enhancement in a constructed wetland-microbial fuel cell system for treating saline wastewater. Bioresour. Technol. 2019, 288, 121462. [Google Scholar] [CrossRef]
- Liu, J.; Shi, S.; Ji, X.; Jiang, B.; Xue, L.; Li, M.; Tan, L. Performance and microbial community dynamics of electricity-assisted sequencing batch reactor (sbr) for treatment of saline petrochemical wastewater. Environ. Sci. Pollut. Res. Int. 2017, 24, 17556–17565. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, C.; Yuan, J.; Si, L.; Li, Y. Discussion on the reinjection disposal of tight gas produced water. Appl. Chem. Ind. 2022, 51, 1413–1417. [Google Scholar]
- Wang, S.; Lin, B.; Wu, T.; Wei, W.; Liu, X.; Chen, J.; Teng, H. Study on the characters of high-salinity wastewater from oil production. Ind. Water Treat. 2011, 31, 45–47. [Google Scholar]
- Zhang, J.; Qiao, W.; Pei, H.; Wang, X. Study on corrosion factor of wastewater re-injection well in shengli oilfield. Appl. Chem. Ind. 2021, 50, 1195–1198. [Google Scholar]
- Bu, K.; Yan, H.; Cheng, P.; Zhang, Y.; Liu, L.; Lin, H. Study on oilfield producedwater re-injection treatment process. Technol. Water Treat. 2022, 48, 104–107. [Google Scholar]
- Yang, D.; Shi, X.; Xu, Y.; Zhuo, M.; Cui, G.; Guo, J. Synthesis of corrosion inhibitor sdh-1 for high salinity wastewater gathering and transportation system in tahe oilfield. Fine Chem. 2018, 35, 326–332. [Google Scholar]
| Types of Wastewater | Technology | Wastewater Quality | Operating Conditions | Removal Efficiency | References |
|---|---|---|---|---|---|
| Textile wastewater | SBR | TDS: 1470 mg/L COD: 140.8 mg/L ABS: 1.387 | T = 27 ± 2 °C HRT = 24 h | Effluent COD = 50 mg/L ABS = 0.5 | [55] |
| Heparin sodium production wastewater | SBR | NaCl: 25,000–35,000 mg/L COD: 15,800–25,500 mg/L NH3-N: 1320–2650 mg/L | MLSS = 9000 mg/L DO = 3 mg/L T = 25 °C HRT = 12 h | COD = 80–85% NH3-N = 30–50% | [56] |
| domestic sewage | SBR | TOC: 133. 7 mg/L TDS: 471. 2 mg/L | HRT = 12 h T = 25–28 °C DO = 6–8 mg/L | TOC = 85% | [57] |
| Edible olive processing wastewater | SBR- membrane treatment | EC: 78.3 Ms/cm COD: 14.16 mg/L Phenolic compounds: 700–1500 mg TY/L | TMP = 15 bar HRT = 16.7 hPH = 8.2 T = 21.9 °C | COD: 80% Phenolic compounds:71% | [58] |
| Types of Wastewater | Technology | Wastewater Quality | Operating Conditions | Removal Efficiency | References |
|---|---|---|---|---|---|
| Synthetic wastewater | Biological contact oxidation | COD: 1500 mg/L NaCl: 14,000 mg/L | DO = 2–4 mg/L HRTA = 12 h HRTO = 24 h | COD: 83% | [60] |
| Ballast water | A/O biological contact oxidation | COD: 500 mg/L TN: 50 mg/L TDS: 3.5% | reflux ratio: 3:1 Filtration speed: 1.5 m/h | COD: 30.95 mg/L TN: 10.39 mg/L | [61] |
| Food manufacturing wastewater | Two-stage A/O biological contact oxidation | COD: 800–1500 mg/L NH3-N: 5–30 mg/L TDS: 2.8~4.7% | Vanaerobic:Vaerobic = 1:5 | COD: 96% NH3-N: 87.5% | [62] |
| Oil field wastewater | Preprocessing + A/O biological contact oxidation | COD: 476–682 mg/L Cl −: 11,042–14,725 mg/L Volatile phenol: 0.98–1.37 mg/L Petroleum: 1.33–11.65 mg/L | hydrolytic acidification: HRT = 6–8 h contact oxidation: HRT = 12 h | Petroleum: 81.82% Volatile phenol: 95.01% COD: 85.19% | [63] |
| Types of Wastewater | Technology | Wastewater Quality | Operating Conditions | Removal Efficiency | References |
|---|---|---|---|---|---|
| Carboxymethyl cellulose wastewater | Up-flow BAF | COD: 200 mg/L Cl-: 9850 mg/L TDS: 1.8 g/L | HRT = 10 h Gas-water ratio: 4:1 | COD: 60% | [65] |
| Aquaculture wastewater | BAF | TDS: 3–5% NH4+-N: 10 mg/L Permanganate index: 14 | DO = 4–5 mg/L T = 26 ± 2 °C HRT = 4 h | NH4+–N: 95% Permanganate index: 80% | [66] |
| Petrochemical wastewater | BAF | COD: 58.1–114.1 mg/L NH3-N:1.2–19.0 mg/L TDS: 18,000–35,000 mg/L | q0 = 1.1 m3/(m2·d) DO = 2.0 mg/L HRT = 2.7 h | COD: 43.7% NH3-N:74.2% | [67] |
| Pharmaceutical wastewater | Catalytic ozonation -BAF | COD: 203–262 mg/L TOC: 79–101 mg/L NH4+-N: 14 mg/L | HRT = 4 h Gas-water ratio: 4/1 | COD = 46 mg/L NH4+-N = 4.1 mg/L | [68] |
| Types of Wastewater | Technology | Wastewater Quality | Operating Conditions | Removal Efficiency | References |
|---|---|---|---|---|---|
| Heavy oil production wastewater | UASB | COD: 350–640 mg/L Oil content: 112.5–205.4 mg/L TDS: 11.5–14.6 g/L | T = 30.1 °C HRT ≥ 24 h | COD: 65.08% Oil content: 74.33% | [71] |
| Synthetic wastewater | UASB | COD: 2000 mg/L NaCl: 0, 10, 25, 50 g/L | T = 37 °C HRT = 1 d | COD: 72–92% | [72] |
| Dye wastewater | UASB | Dye concentration: 1000 mg/L TDS: 15–17. 5% | T = 30–50 °C pH = 7 | Rapid decolorization | [73] |
| Pharmaceutical wastewater | UASB + SBR/MBR | TDS: 4.96–24.90 g/L COD: 16,547 ± 1827 mg/L | OLR = 8.11 ± 0.31 g COD/L/d HRT = 48 h | COD: UASB + MBR: 94.7% UASB + SBR: 91.8% | [74] |
| Types of Wastewater | Technology | Wastewater Quality | Operating Conditions | Removal Efficiency | References |
|---|---|---|---|---|---|
| Oil field wastewater | Electrochemistry + Coagulation + MBBR + MBR + macroporous adsorption resin | EC: 4.5 mS/cm COD: 212 mg/L | PFS: 500 mg/L HRT = 12 h Adsorption resin: SD300 | COD: 20 mg/L | [75] |
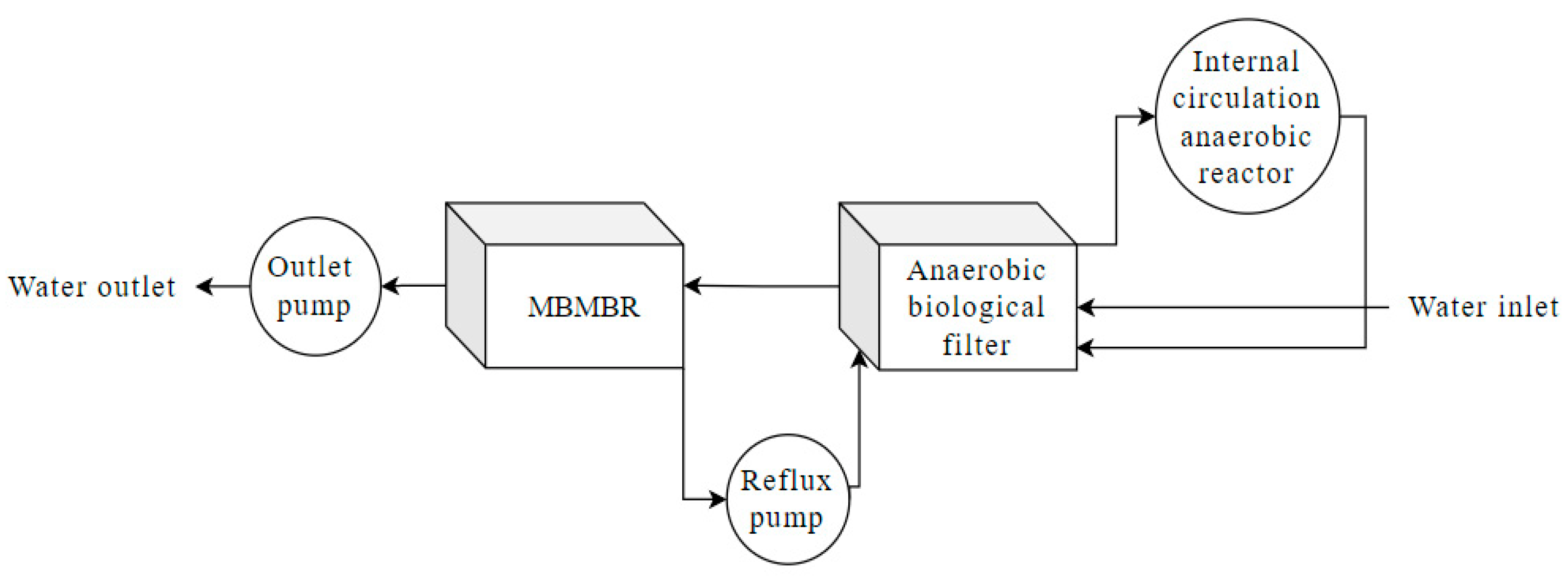
| Aquaculture wastewater | AF + MBMBR | TOC: 100–125 mg/L TN: 40–50 mg/L TDS: 34.5‰ | HRT: 134d Vaerobic:Vanaerobic = 10/4 Q = 10 (L/m2h) T = 25 °C | TOC: 92.8–96.2% TN: 93.2% | [76] |
| Synthetic wastewater | CW-MFC | NaCl: 5 g/L | T = 30±3 °C HRT = 3 d | TP: 86.64 ± 0.29% COD: 68.20 ± 1.15% | [77] |
| Petrochemical wastewater | Bioelectrochemical systems | COD: 1130 mg/L Phenol: 200 mg/L TDS: 12,000 mg/L | E = 0.1 V/cm T = 25 ± 2 °C, DO = 3.0 ± 0.5 mg/L HRT = 24 h | COD: 90.3% Phenol: 89.1% | [78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, L.; Xie, Y.; Sun, W.; Xu, Y.; Sun, Y. Research Progress of High-Salinity Wastewater Treatment Technology. Water 2023, 15, 684. https://doi.org/10.3390/w15040684
Guo L, Xie Y, Sun W, Xu Y, Sun Y. Research Progress of High-Salinity Wastewater Treatment Technology. Water. 2023; 15(4):684. https://doi.org/10.3390/w15040684
Chicago/Turabian StyleGuo, Lei, Yiming Xie, Wenquan Sun, Yanhua Xu, and Yongjun Sun. 2023. "Research Progress of High-Salinity Wastewater Treatment Technology" Water 15, no. 4: 684. https://doi.org/10.3390/w15040684
APA StyleGuo, L., Xie, Y., Sun, W., Xu, Y., & Sun, Y. (2023). Research Progress of High-Salinity Wastewater Treatment Technology. Water, 15(4), 684. https://doi.org/10.3390/w15040684







