Natural Bioactive Phytocompounds to Reduce Toxicity in Common Carp Cyprinus carpio: A Challenge to Environmental Risk Assessment of Nanomaterials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Procurement and Acclimatization of Fish
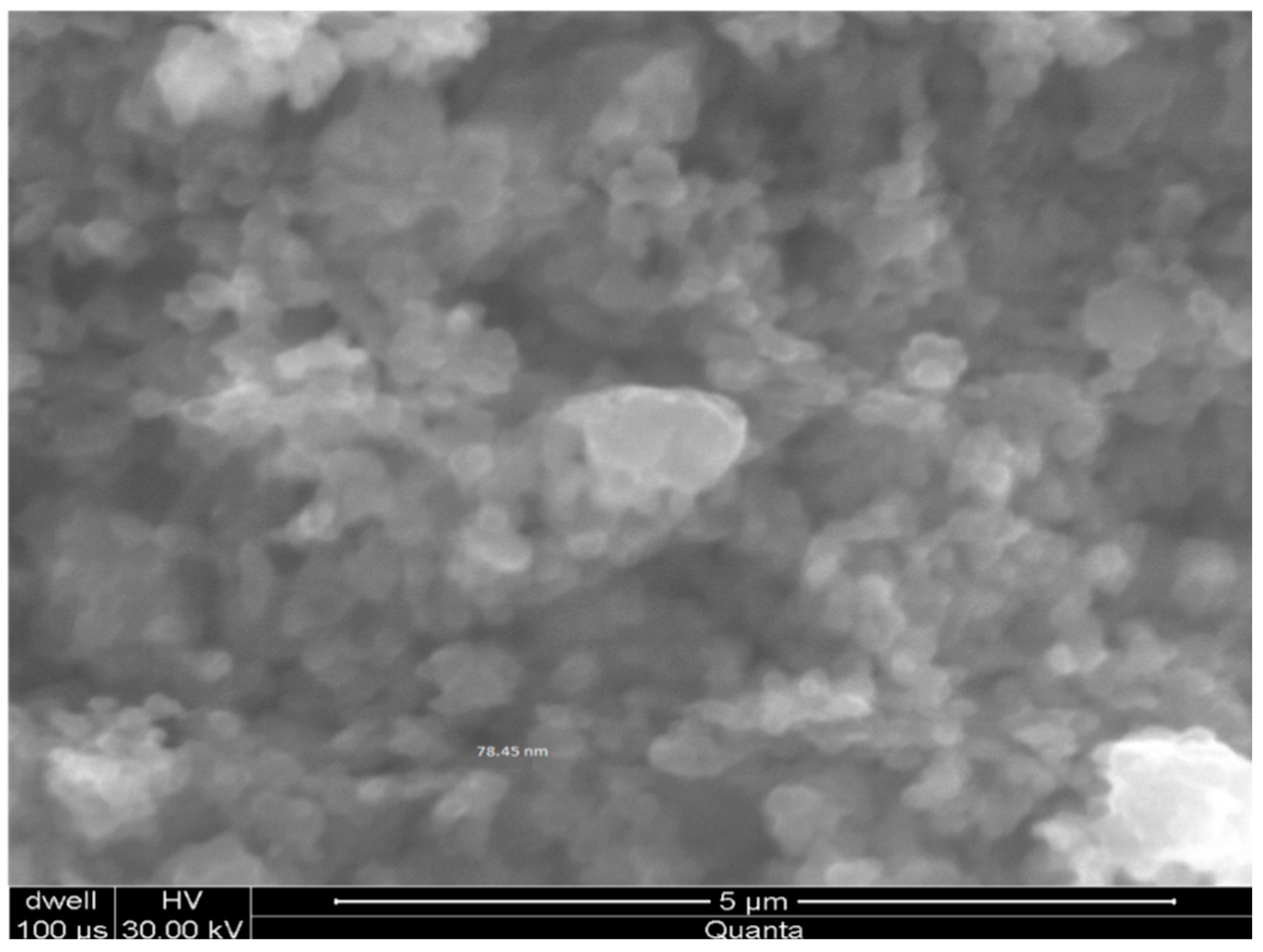
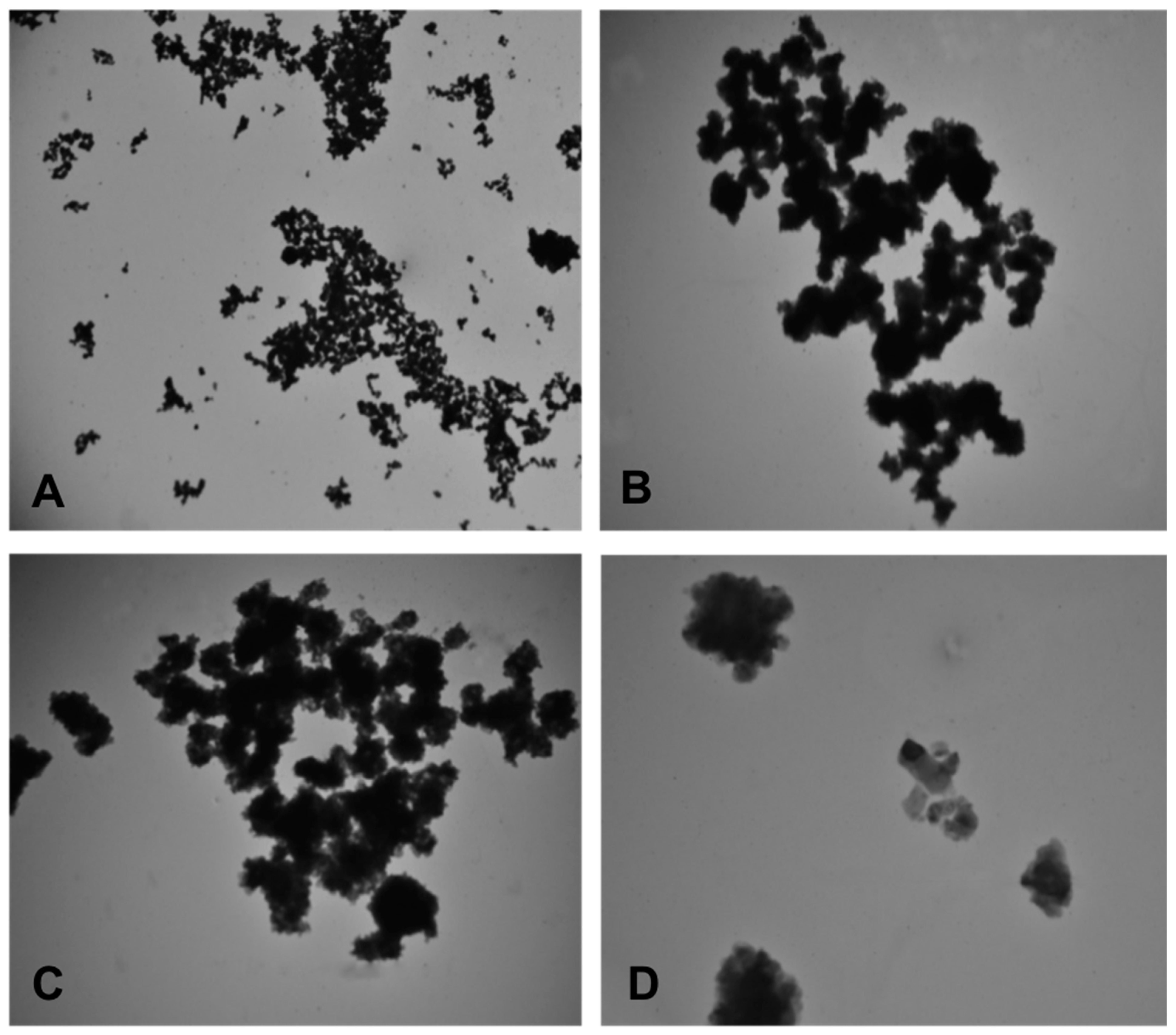
2.2. Characterization of CuO and Cu-NPs
- D = particle diameter (average crystallite size);
- Β = Full Width at Half Maximum (FWHM);
- θ = Bragg angle;
- λ = X-ray wavelength, Cu-Kα emission (λ = 1.54056 A°).
2.3. Preparation of the Nutmeg Extract
2.4. LC50 of MFSE for Common Carp
2.5. Experimental Design
2.6. Biochemical Analysis
2.6.1. Tissue Metal Analysis
2.6.2. Hematological Analysis
2.6.3. Histological Analysis
2.6.4. Analysis of Oxidative Stress Enzymes
2.7. Statistical Analysis
3. Results
3.1. LC50, Phytochemical and Proximate Composition of MFSE
3.2. Bioaccumulation of Cu in Fish Tissues
3.3. Hematological Assessment
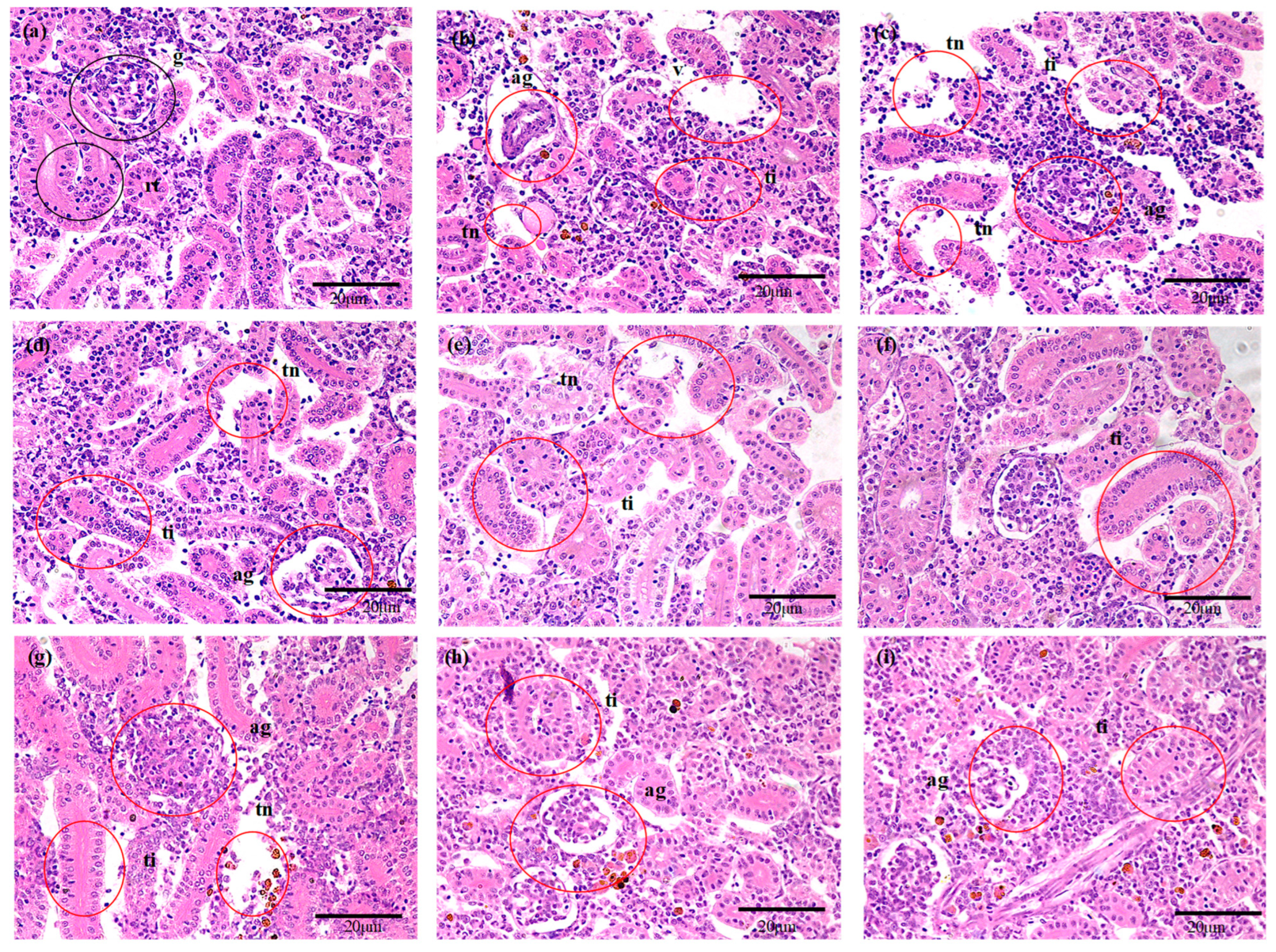
3.4. Histological Assessment
3.5. Oxidative Stress Enzymes (OSEs)
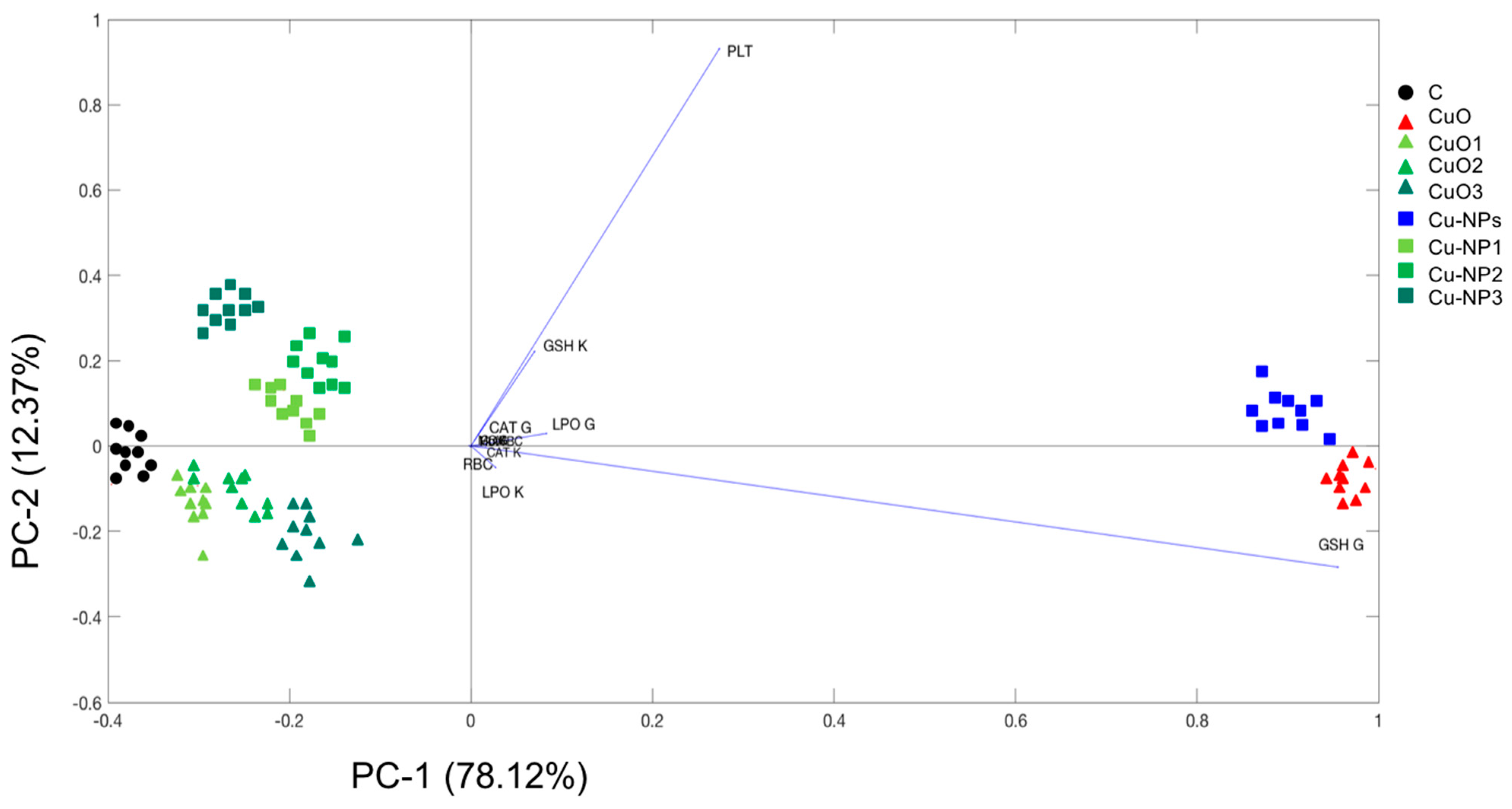
3.6. Principal Component Analysis (PCA)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iftikhar, M.; Noureen, A.; Uzair, M.; Jabeen, F.; Abdel Daim, M.; Cappello, T. Perspectives of nanoparticles in male infertility: Evidence for induced abnormalities in sperm production. Int. J. Environ. Res. Public Health 2021, 18, 1758. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Josic, U.; Delfi, M.; Pinelli, F.; Jahed, V.; Kaya, E.; Ashrafizadeh, M.; Zarepour, A.; Rossi, F.; Zarrabi, A.; et al. Drug delivery (nano) platforms for oral and dental applications: Tissue regeneration, infection control, and cancer management. Adv. Sci. 2021, 8, 2004014. [Google Scholar] [CrossRef] [PubMed]
- Sahu, T.; Ratre, Y.K.; Chauhan, S.; Bhaskar, L.V.K.S.; Nair, M.P.; Verma, H.K. Nanotechnology based drug delivery system: Current strategies and emerging therapeutic potential for medical science. J. Drug Delivery Sci. Technol. 2021, 63, 102487. [Google Scholar] [CrossRef]
- Hassanisaadi, M.; Barani, M.; Rahdar, A.; Heidary, M.; Thysiadou, A.; Kyzas, G.Z. Role of agrochemical-based nanomaterials in plants: Biotic and abiotic stress with germination improvement of seeds. Plant Growth Regul. 2022, 97, 375–418. [Google Scholar] [CrossRef]
- Mayaya, T. Nanotechnology applications in crop production and food systems. Int. J. Plant Breeding 2020, 7, 624–634. [Google Scholar]
- Chen, M.C.; Koh, P.W.; Ponnusamy, V.K.; Lee, S.L. Titanium dioxide and other nanomaterials based antimicrobial additives in functional paints and coatings. Progr. Organ. Coat. 2022, 163, 106660. [Google Scholar] [CrossRef]
- Zhu, Q.; Chua, M.H.; Ong, P.J.; Lee, J.J.C.; Chin, K.L.O.; Wang, S.; Kai, D.; Ji, R.; Kong, J.; Dong, Z.; et al. Recent advances in nanotechnology-based functional coatings for the built environment. Mater. Today Adv. 2022, 15, 100270. [Google Scholar] [CrossRef]
- Pandey, P.P. Role of nanotechnology in electronics: A review of recent developments and patents. Recent Patents Nanotechnol. 2022, 16, 45–66. [Google Scholar]
- Kumar, P.M.; Saminathan, R.; Sumayli, A. Experimental analysis of a heat sink for electronic chipset cooling using a nano improved PCM (NIPCM). Mater. Today Proceed. 2022, 56, 1527–1531. [Google Scholar] [CrossRef]
- Quezada, G.A.; Ingle, A.; Golińska, P.; Rai, M. Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks. Nanotechnol. Rev. 2022, 11, 2123–2140. [Google Scholar] [CrossRef]
- Joshi, H.; Somdutt; Choudhary, P.; Mundra, S.L. Future prospects of nanotechnology in agriculture. Int. J. Chem. Stud. 2019, 7, 957–963. [Google Scholar]
- Kah, M.; Kookana, R.S.; Gogos, A.; Bucheli, T.D. A critical evaluation of nanopesticides and nanofertilizers against their conventional analogues. Nature Nanotechnol. 2018, 13, 677–684. [Google Scholar] [CrossRef]
- Adil, M.; Bashir, S.; Bashir, S.; Aslam, Z.; Ahmad, N.; Younas, T.; Asghar, R.M.A.; Alkahtani, J.; Dwiningsih, Y.; Elshikh, M.S. Zinc oxide nanoparticles improved chlorophyll contents, physical parameters, and wheat yield under salt stress. Front. Plant Sci. 2022, 13, 932861. [Google Scholar] [CrossRef]
- Kapoor, P.; Dhaka, R.K.; Sihag, P.; Mehla, S.; Sagwal, V.; Singh, Y.; Langaya, S.; Balyan, P.; Singh, K.P.; Xing, B.; et al. Nanotechnology-enabled biofortification strategies for micronutrients enrichment of food crops: Current understanding and future scope. NanoImpact 2022, 26, 100407. [Google Scholar] [CrossRef]
- Kalwani, M.; Chakdar, H.; Srivastava, A.; Pabbi, S.; Shukla, P. Effects of nanofertilizers on soil and plant-associated microbial communities: Emerging trends and perspectives. Chemosphere 2022, 287, 132107. [Google Scholar] [CrossRef] [PubMed]
- Zahra, Z.; Habib, Z.; Hyun, H.; Shahzad, H.M.A. Overview on recent developments in the design, application, and impacts of nanofertilizers in agriculture. Sustainability 2022, 14, 9397. [Google Scholar] [CrossRef]
- Beig, B.; Niazi, M.B.K.; Sher, F.; Jahan, Z.; Malik, U.S.; Khan, M.D.; Américo-Pinheiro, J.H.P.; Vo, D.-V.N. Nanotechnology-based controlled release of sustainable fertilizers. A review. Environ. Chem. Lett. 2022, 20, 2709–2726. [Google Scholar] [CrossRef]
- Elsayed, A.A.; El-Gohary, A.; Taha, Z.K.; Farag, H.M.; Hussein, M.S.; AbouAitah, K. Hydroxyapatite nanoparticles as novel nano-fertilizer for production of rosemary plants. Sci. Hortic. 2022, 295, 110851. [Google Scholar] [CrossRef]
- Chen, H.; Yada, R. Nanotechnologies in agriculture: New tools for sustainable development. Trends Food Sci. Technol. 2011, 22, 585–594. [Google Scholar] [CrossRef]
- Nasr, M. Nanotechnology application in agricultural sector. In Nanobiotechnology in Bioformulations; Springer: Berlin/Heidelberg, Germany, 2019; pp. 317–329. [Google Scholar]
- Abbasifar, A.; Shahrabadi, F.; ValizadehKaji, B. Effects of green synthesized zinc and copper nano-fertilizers on the morphological and biochemical attributes of basil plant. J. Plant Nutr. 2020, 43, 1104–1118. [Google Scholar] [CrossRef]
- Cota-Ruiz, K.; Ye, Y.; Valdes, C.; Deng, C.; Wang, Y.; Hernández-Viezcas, J.A.; Duarte-Gardea, M.; Gardea-Torresdey, J.L. Copper nanowires as nanofertilizers for alfalfa plants: Understanding nano-bio systems interactions from microbial genomics, plant molecular responses and spectroscopic studies. Sci. Total Environ. 2020, 742, 140572. [Google Scholar] [CrossRef]
- De Marchi, L.; Rocha, R.J.M.; Rodrigues, A.C.M.; Soares, A.M.V.M.; Pretti, C.; Chiellini, F.; Freitas, R. Environmental fate of multistressors on carpet shell clam Ruditapes decussatus: Carbon nanoparticles and temperature variation. Sustainability 2020, 12, 4939. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y.; O’Brien, A.M.; Lins, T.F.; Rochman, C.M.; Sinton, D. Biological responses to climate change and nanoplastics are altered in concert: Full-factor screening reveals effects of multiple stressors on primary producers. Environ. Sci. Technol. 2020, 54, 2401–2410. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Kaur, I.; Sehgal, A.; Singh, S.; Sharma, N.; Bhatia, S.; Al-Harrasi, A.; Bungau, S. The dichotomy of nanotechnology as the cutting edge of agriculture: Nano-farming as an asset versus nanotoxicity. Chemosphere 2022, 288, 132533. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.K.; Suar, M.; Mishra, Y.K. Green perspective of nano-biotechnology: Nanotoxicity horizon to biomedical applications. Front. Bioeng. Biotechnol. 2022, 10, 919226. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, S.; Gu, Z.; Chen, C.; Zhao, Y. Toxicity of manufactured nanomaterials. Particuology 2022, 69, 31–48. [Google Scholar] [CrossRef]
- Guerriero, G.; Trocchia, S.; Abdel-Gawad, F.K.; Ciarcia, G. Roles of reactive oxygen species in the spermatogenesis regulation. Front. Endocrinol. 2014, 5, 56. [Google Scholar] [CrossRef] [Green Version]
- Cappello, T.; Vitale, V.; Oliva, S.; Villari, V.; Mauceri, A.; Fasulo, S.; Maisano, M. Alteration of neurotransmission and skeletogenesis in sea urchin Arbacia lixula embryos exposed to copper oxide nanoparticles. Comp. Biochem. Physiol. C 2017, 199, 20–27. [Google Scholar] [CrossRef]
- D’Agata, A.; Fasulo, S.; Dallas, L.J.; Fisher, A.S.; Maisano, M.; Readman, J.W.; Jha, A.N. Enhanced toxicity of ‘bulk’ titanium dioxide compared to ‘fresh’ and ‘aged’ nano-TiO2 in marine mussels (Mytilus galloprovincialis). Nanotoxicology 2014, 8, 549–558. [Google Scholar] [CrossRef]
- Vandhana, S.; Nithya, M.; Deepak, A. Review on nano toxic effects in living organisms (mice and zebrafish). Int. J. Innov. Res. Sci. Technol. 2015, 1, 134–137. [Google Scholar]
- Noureen, A.; Jabeen, F.; Tabish, T.A.; Yaqub, S.; Ali, M.; Chaudhry, A.S. Assessment of copper nanoparticles (Cu-NPs) and copper (II) oxide (CuO) induced hemato-and hepatotoxicity in Cyprinus carpio. Nanotechnology 2018, 29, 144003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pormohammad, A.; Hansen, D.; Turner, R.J. Antibacterial, antibiofilm, and antioxidant activity of 15 different plant-based natural compounds in comparison with ciprofloxacin and gentamicin. Antibiotics 2022, 11, 1099. [Google Scholar] [CrossRef] [PubMed]
- Belhadj, S.; Gargouri, M.; Guerriero, G.; Hentati, O. Polysaccharides from the green alga Ulva lactuca improve antioxidant balance and bone mineral density in diabetic rats. Biomed. Environ. Sci. 2021, 34, 637–640. [Google Scholar]
- Khalil, W.K.B.; Bassem, S.M.; Sabry, N.M.; Eltawab, M.I.A.; Enshasy, H.E.; Atemraz, T.; Guerriero, G.; Abdel-Gawad, F.K. The prevention impact of the green algal extract against genetic toxicity and antioxidant enzyme alteration in the Mozambique tilapia. Egypt. J. Aquat. Biol. Fish. 2022, 26, 1103–1118. [Google Scholar] [CrossRef]
- Budiman, A.; Aulifa, D.L.; Kusuma, A. Antibacterial and antioxidant activity of black mulberry (Morus nigra L.) extract for acne treatment. Pharm. J. 2017, 9, 611–614. [Google Scholar] [CrossRef] [Green Version]
- Tan, K.; Khoo, H.; Azrina, A. Comparison of antioxidant components and antioxidant capacity in different parts of nutmeg (Myristica fragrans). Int. Food Res. J. 2013, 20, 1049–1052. [Google Scholar]
- Ginting, B.; Nurdin, N. Evaluation of antioxidant and anticancer activity of Myristica fragrans Houtt. Bark. Pharmac. J. 2021, 13, 780–786. [Google Scholar] [CrossRef]
- Nikolic, V.; Nikolic, L.; Dinc, A.; Gajic, I.; Urosevic, M.; Stanojevic, L.; Stanojevic, J.; Danilovic, B. Chemical composition, antioxidant and antimicrobial activity of nutmeg (Myristica fragrans Houtt.) seed essential oil. J. Essent. Oil Bearing Plants 2021, 24, 218–227. [Google Scholar] [CrossRef]
- Din, M.U.; Ali, A.; Yasir, M.; Jilani, M.I.; Shoaib, S.; Latif, M.; Ahmad, A.; Naz, S.; Aslam, F.; Iqbal, M.; et al. Chemical composition and in vitro evaluation of cytotoxicity, antioxidant and antimicrobial activities of essential oil extracted from Myristica Fragrans Houtt. Pol. J. Environ. Stud. 2021, 30, 1585–1590. [Google Scholar] [CrossRef]
- Li, C.-W.; Chu, Y.-C.; Huang, C.-Y.; Fu, S.-L.; Chen, J.-J. Evaluation of antioxidant and anti-α-glucosidase activities of various solvent extracts and major bioactive components from the seeds of Myristica fragrans. Molecules 2020, 25, 5198. [Google Scholar] [CrossRef]
- Sulaiman, S.F.; Ooi, K.L. Antioxidant and anti food-borne bacterial activities of extracts from leaf and different fruit parts of Myristica fragrans Houtt. Food Control 2012, 25, 533–536. [Google Scholar] [CrossRef]
- Reddy, B.C.; Noor, A.; Sarada, N.C.; Vijayalakshmi, M.A. Antioxidant properties of Cordyline terminalis (L.) Kunth and Myristica fragrans Houtt. encapsulated separately into casein beads. Curr. Sci. 2011, 101, 416–420. [Google Scholar]
- Lee, J.Y.; Park, W. Anti-inflammatory effect of myristicin on RAW 264.7 macrophages stimulated with polyinosinic-polycytidylic acid. Molecules 2011, 16, 7132–7142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima, R.K.; Cardoso, M.d.G.; Andrade, M.A.; Guimarães, P.L.; Batista, L.R.; Nelson, D.L. Bactericidal and antioxidant activity of essential oils from Myristica fragrans Houtt and Salvia microphylla HBK. J. Am. Oil Chem. Soc. 2012, 89, 523–528. [Google Scholar] [CrossRef]
- Dang, Z.; Su, S.; Jin, G.; Nan, X.; Ma, L.; Li, Z.; Lu, D.; Ge, R. Tsantan Sumtang attenuated chronic hypoxia-induced right ventricular structure remodeling and fibrosis by equilibrating local ACE-AngII-AT1R/ACE2-Ang1-7-Mas axis in rat. J. Ethnopharmacol. 2020, 250, 112470. [Google Scholar] [CrossRef]
- Nurjanah, S.; Putri, I.L.; Sugiarti, D.P. Antibacterial activity of nutmeg oil. KnE Life Sci. 2017, 2, 563–569. [Google Scholar] [CrossRef] [Green Version]
- Sripanidkulchai, B.; Suttajit, M.; Ratanavalachai, T. Anti-aging strategies, plant bioactives, and drug development: Current insights. In Plant Bioactives as Natural Panacea Against Age-Induced Diseases; Pandey, K.B., Suttajit, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 23–48. [Google Scholar]
- Noureen, A.; Jabeen, F.; Tabish, T.A.; Zahoor, M.K.; Ali, M.; Iqbal, R.; Yaqub, S.; Chaudhry, A.S. Ameliorative effects of Moringa oleifera on copper nanoparticle induced toxicity in Cyprinus carpio assessed by histology and oxidative stress markers. Nanotechnology 2018, 29, 464003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morsy, N.F.S. A comparative study of nutmeg (Myristica fragrans Houtt.) oleoresins obtained by conventional and green extraction techniques. J. Food Sci. Technol. 2016, 53, 3770–3777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben-Gigirey, B.; Rodríguez-Velasco, M.L.; Gago-Martínez, A. Extension of the validation of AOAC official method SM 2005.06 for dc-GTX2, 3: Interlaboratory study. J. AOAC Int. 2012, 95, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Assa, J.R.; Kusnadi, J.; Berhimpon, S. Antioxidant potential of flesh, seed and mace of nutmeg (Myristica fragrans Houtt). Int. J. Chem. Tech. Res. 2014, 6, 2460–2468. [Google Scholar]
- Hayfaa, A.A.-S.; Sahar, A.M.A.-S.; Awatif, M.A.-S. Evaluation of analgesic activity and toxicity of alkaloids in Myristica fragrans seeds in mice. J. Pain Res. 2013, 6, 611–615. [Google Scholar] [CrossRef] [Green Version]
- Rancy, A.; Krishnakumari, S. Phytochemical profiling of Myristica fragrans seed extract with different organic solvents. Asian J. Pharma. Clin. Res. 2015, 8, 303–307. [Google Scholar]
- Uddin, A.B.M.H.; Khalid, R.S.; Alaama, M.; Abdualkader, A.M. Comparative study of three digestion methods for elemental analysis in traditional medicine products using atomic absorption spectrometry. J. Anal. Sci. Technol. 2016, 7, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Noureen, A.; De Marco, G.; Rehman, N.; Jabeen, F.; Cappello, T. Ameliorative hematological and histomorphological effects of dietary Trigonella foenum-graecum seeds in common carp (Cyprinus carpio) exposed to copper oxide nanoparticles. Int. J. Environ. Res. Public Health 2022, 19, 13462. [Google Scholar] [CrossRef]
- Niki, E. Biomarkers of lipid peroxidation in clinical material. Biochim. Biophys. Acta (BBA)-Gen. Subjects 2014, 1840, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Lakritz, J.; Plopper, C.G.; Buckpitt, A.R. Validated high-performance liquid chromatography-electrochemical method for determination of glutathione and glutathione disulfide in small tissue samples. Anal. Biochem. 1997, 247, 63–68. [Google Scholar] [CrossRef]
- Olaleye, M.; Akinmoladun, C.; Akindahunsi, A. Antioxidant properties of Myristica fragrans (Houtt) and its effect on selected organs of albino rats. Afr. J. Biotechnol. 2006, 5, 1274–1278. [Google Scholar]
- Bamidele, O.; Akinnuga, A.M.; Alagbonsi, I.A.; Ojo, O.A.; Olorunfemi, J.O.; Akuyoma, M.A. Effects of ethanolic extract of Myristica fragrans Houtt.(nutmeg) on some heamatological indices in albino rats. Int. J. Med. Med. Sci. 2011, 3, 215–218. [Google Scholar]
- Nasreen, W.; Sarker, S.; Sufian, M.A.; Opo, F.A.D.M.; Shahriar, M.; Akhter, R.; Halim, M.A. A possible alternative therapy for type 2 diabetes using Myristica fragrans Houtt in combination with glimepiride: In vivo evaluation and in silico support. Zeitschrift für Naturforschung C J. Biosci. 2020, 75, 103–112. [Google Scholar] [CrossRef]
- Anaduaka, E.G.; Uchendu, N.O.; Ezeanyika, L.U.S. Mineral, amino acid and fatty acid evaluations of Myristica fragrans seeds extracts. Scient. Afr. 2020, 10, e00567. [Google Scholar] [CrossRef]
- Bhavan, P.S.; Devi, N.N.; Muralisankar, T.; Manickam, N.; Radhakrishnan, S.; Srinivasan, V. Effects of Myristica fragrans, Glycyrrhiza glabra and Quercus infectoria on growth promotion in the prawn Macrobrachium rosenbergii. Int. J. Life Sci. Biotech. Pharm. Res. 2013, 2, 169–182. [Google Scholar]
- Willis, B.E.; Bishop, W.M. Understanding fate and effects of copper pesticides in aquatic systems. J. Geosci. Environ. Protect. 2016, 4, 37–42. [Google Scholar] [CrossRef] [Green Version]
- Lotz, A. Marine coatings: Making sense of US, state, and local mandates of copper-based antifouling regulations. JCT Coatingstech 2016, 13, 50–54. [Google Scholar]
- Zhang, Y.; Xie, P.; Guo, X.; Kang, W. Procoagulant substance and mechanism of Myristica fragrans. J. Med. Food 2016, 19, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Gawad, F.K.; Khalil, W.K.B.; Bassem, S.M.; Kumar, V.; Parisi, C.; Inglese, S.; Temraz, T.A.; Nassar, H.F.; Guerriero, G. The duckweed, Lemna minor modulates heavy metal-induced oxidative stress in the Nile tilapia Oreochromis niloticus. Water 2020, 12, 2983. [Google Scholar] [CrossRef]
- Balakrishnan, S.; Sivaji, S.; Kandasamy, S.; Duraisamy, S.; Kumar, N.S.; Gurusubramanian, G. Biosynthesis of silver nanoparticles using Myristica fragrans seed (nutmeg) extract and its antibacterial activity against multidrug-resistant (MDR) Salmonella enterica serovar Typhi isolates. Environ. Sci. Pollut. Res. 2017, 24, 14758–14769. [Google Scholar] [CrossRef]
- Lei, L.; Xue, Y.-B.; Liu, Z.; Peng, S.-S.; He, Y.; Zhang, Y.; Fang, R.; Wang, J.-P.; Luo, Z.-W.; Yao, G.-M.; et al. Coumarin derivatives from Ainsliaea fragrans and their anticoagulant activity. Sci. Rep. 2015, 5, 13544. [Google Scholar] [CrossRef] [Green Version]
- Abdel Salam, R.G.; Bassem, S.M.; Abdel-Reheim, E.S.; Mahmoud, A.-L.; Guerriero, G.; Abdel-Gawad, F.K. Role of effective microorganisms on hematological and biochemical indices of cultured Oreochromis niloticus exposed to lead, copper, and cadmium under temperature variations. J. Appl. Biol. Biotechnol. 2023, 11, 153–160. [Google Scholar]
- Parrino, V.; De Marco, G.; Minutoli, R.; Lo Paro, G.; Giannetto, A.; Cappello, T.; De Plano, L.M.; Cecchini, S.; Fazio, F. Effects of pesticides on Chelon labrosus (Risso, 1827) evaluated by enzymatic activities along the north eastern Sicilian coastlines (Italy). Eur. Zool. J. 2021, 88, 540–548. [Google Scholar]
- Rashidian, G.; Shahin, K.; Elshopakey, G.E.; Mahboub, H.H.; Fahim, A.; Elabd, H.; Prokić, M.D.; Faggio, C. The dietary effects of nutmeg (Myristica fragrans) extract on growth, hematological parameters, immunity, antioxidant status, and disease resistance of common carp (Cyprinus carpio) against Aeromonas hydrophila. J. Mar. Sci. Eng. 2022, 10, 325. [Google Scholar] [CrossRef]
- Bachri, M.; Yuliani, S.; Sari, A. Effect of subchronic administration of nutmeg (Myristica fragrans Houtt) ethanolic extract to hematological parameters in rat. IOP Conf. Ser. Mater. Sci. Eng. 2017, 259, 012009. [Google Scholar] [CrossRef] [Green Version]
- Kapoor, I.; Singh, B.; Singh, G.; de Heluani, C.S.; Catalan, C.A. Chemical composition and antioxidant activity of essential oil and oleoresins of nutmeg (Myristica fragrans Houtt.) fruits. Int. J. Food Propr. 2013, 16, 1059–1070. [Google Scholar] [CrossRef] [Green Version]
- Orhan, I.E.; Belhattab, R.; Deniz, F.S.S.; Gulpinar, A.R.; Coskun, S.H.; Kartal, M. Profiling of cholinesterase inhibitory and antioxidant activities of Artemisia absinthium, A. herba-alba, A. fragrans, Marrubium vulgare, M. astranicum, Origanum vulgare subsp. glandulossum and essential oil analysis of two Artemisia species. Ind. Crops Prod. 2010, 32, 566–571. [Google Scholar]
- Liu, W.; Huang, F.; Liao, Y.; Zhang, J.; Ren, G.; Zhuang, Z.; Zhen, J.; Lin, Z.; Wang, C. Treatment of CrVI-containing Mg(OH)2 nanowaste. Angewandte Int. Ed. Chemie 2008, 47, 5619–5622. [Google Scholar] [CrossRef]
- Liu, W.; Huang, F.; Wang, Y.J.; Zou, T.; Zheng, J.; Lin, Z. Recycling Mg(OH)2 nanoadsorbent during treating the low concentration of CrVI. Environ. Sci. Technol. 2011, 45, 1955–1961. [Google Scholar] [CrossRef]
- De Marco, G.; Oliveri Conti, G.; Giannetto, A.; Cappello, T.; Galati, M.; Iaria, C.; Pulvirenti, E.; Capparucci, F.; Mauceri, A.; Ferrante, M.; et al. Embryotoxicity of polystyrene microplastics in zebrafish Danio rerio. Environ. Res. 2022, 208, 112552. [Google Scholar] [CrossRef]
- Maisano, M.; Cappello, T.; Catanese, E.; Vitale, V.; Natalotto, A.; Giannetto, A.; Barreca, D.; Brunelli, E.; Mauceri, A.; Fasulo, S. Developmental abnormalities and neurotoxicological effects of CuO NPs on the black sea urchin Arbacia lixula by embryotoxicity assay. Mar. Environ. Res. 2015, 111, 121–127. [Google Scholar] [CrossRef]
- Giannetto, A.; Cappello, T.; Oliva, S.; Parrino, V.; De Marco, G.; Fasulo, S.; Mauceri, A.; Maisano, M. Copper oxide nanoparticles induce the transcriptional modulation of oxidative stress-related genes in Arbacia lixula embryos. Aquat. Toxicol. 2018, 201, 187–197. [Google Scholar] [CrossRef]


| Water Quality Parameters | |
|---|---|
| pH | 6.9–7.2 |
| Temperature (°C) | 25 |
| Dissolved oxygen (mg/L) | 6.8–7.4 |
| NH3 (ppm) | 0.4–0.6 |
| Total hardness (ppm) | 47–52 |
| Total dissolved solids (ppt) | 6.8–7.5 |
| D A° | 2 Θ Degree | FWHM Rad | K Constant | D nm |
|---|---|---|---|---|
| 2.08600 | 43.341 | 0.294 | 0.89 | 84 |
| 1.80650 | 50.479 | 0.356 | 0.89 | 72 |
| 1.27740 | 74.173 | 0.388 | 0.89 | 79 |
| Bioactive compounds | Total phenolics (mg/100 g) | 0.41 ± 0.01 |
| Tannins (mg/100 g) | 2.53 ± 0.02 | |
| Alkaloids (mg/100 g) | 365.6 ± 10.15 | |
| Saponins (%) | 0.03 ± 0.01 | |
| Flavonoids (mg/100 g) | 0.33 ± 0.02 | |
| Biochemistry | Moisture (%) | 10.12 ± 1.21 |
| Ash (%) | 3.17 ± 0.32 | |
| Oil contents (%) | 29.54 ± 2.07 | |
| Carbohydrates (%) | 59.07 ± 5.06 | |
| Proteins (%) | 8.23 ± 1.22 | |
| Fiber (%) | 10.87 ± 1.01 |
| Groups | CuO or Cu-NPs (mg/L) | MFSE (mg/L) | Gills (µg/Kg w.w.) | Kidney (µg/Kg w.w.) |
|---|---|---|---|---|
| C | 0 | 0.0 | 0.11 ± 0.004 d | 0.14 ± 0.008 d |
| CuO | 1.5 | 0.0 | 2.46 ± 0.006 a | 2.44 ± 0.006 a |
| CuO1 | 1.5 | 4.0 | 1.23 ± 0.010 b | 1.24 ± 0.010 b |
| CuO2 | 1.5 | 8.0 | 1.10 ± 0.010 c | 1.13 ± 0.015 b |
| CuO3 | 1.5 | 12.0 | 1.03 ± 0.010 c | 1.06 ± 0.010 c |
| Cu-NPs | 1.5 | 0.0 | 2.44 ± 0.006 a | 2.47 ± 0.006 a |
| Cu-NP1 | 1.5 | 4.0 | 1.22 ± 0.010 b | 1.27 ± 0.010 b |
| Cu-NP2 | 1.5 | 8.0 | 1.33 ± 0.010 c | 1.16 ± 0.010 c |
| Cu-NP3 | 1.5 | 12.0 | 1.23 ± 0.010 b | 1.09 ± 0.010 b |
| Groups | CuO or Cu-NPs (mg/L) | MFSE (mg/L) | Hb (g/dL) | Hct (%) | RBC (×106/M) | WBC (×103/µL) | MCV (fl) | PLT (×103/µL) |
|---|---|---|---|---|---|---|---|---|
| C | 0 | 0.0 | 9.21 | 24.62 | 2.82 | 3.34 | 139.41 | 181.22 |
| CuO | 1.5 | 0.0 | 5.81 | 19.73 | 1.18 | 45.85 | 130.42 | 1252.34 |
| CuO1 | 1.5 | 4.0 | 6.03 | 18.15 | 1.57 | 5.95 | 134.21 | 171.55 |
| CuO2 | 1.5 | 8.0 | 6.23 | 18.97 | 1.54 | 4.45 | 135.23 | 167.32 |
| CuO3 | 1.5 | 12.0 | 6.21 | 19.15 | 1.58 | 3.55 | 137.22 | 156.53 |
| Cu-NPs | 1.5 | 0.0 | 5.62 | 18.16 | 1.08 | 68.98 | 129.42 | 1272.33 |
| Cu-NP1 | 1.5 | 4.0 | 6.11 | 18.16 | 1.56 | 5.95 | 135.21 | 799.36 |
| Cu-NP2 | 1.5 | 8.0 | 6.12 | 19.26 | 1.58 | 5.85 | 136.22 | 792.31 |
| Cu-NP3 | 1.5 | 12.0 | 6.31 | 20.15 | 1.64 | 4.45 | 137.24 | 789.54 |
| Groups | CuO or Cu-NPs (mg/L) | LPO (nmol/mg of Protein) | GSH (µM/g) | CAT (mol/min/mg) | |||
|---|---|---|---|---|---|---|---|
| Kidney | Gills | Kidney | Gills | Kidney | Gills | ||
| C | 0 | 319.11 ± 18.08 d | 423 ± 19.02 d | 1235 ± 28.11 d | 2239 ± 41.08 d | 2.37 ± 0.22 a | 2.54 ± 11.02 a |
| CuO | 1.5 | 517.70 ± 19.05 a | 718.7 ± 21.09 a | 1455 ± 25.01 b | 5587 ± 45.08 a | 1.13 ± 0.41 d | 1.57 ± 0.24 d |
| CuO1 | 1.5 | 472.10 ± 17.06 c | 433.4 ± 18.08 d | 1564 ± 27.05 c | 2465 ± 41.08 b | 2.17 ± 0.22 b | 2.08 ± 0.13 b |
| CuO2 | 1.5 | 480.40 ± 16.04 c | 487.8 ± 19.02 c | 1591 ± 28.03 c | 2484 ± 40.09 b | 2.12 ± 0.19 b | 2.01 ± 11.04 c |
| CuO3 | 1.5 | 570.70 ± 19.07 b | 505.1 ± 18.01 b | 1598 ± 28.13 c | 2489 ± 41.81 b | 2.08 ± 0.17 c | 2.03 ± 0.13 c |
| Cu-NPs | 1.5 | 545.80 ± 20.03 a | 759.4 ± 22.05 a | 2040 ± 31.04 a | 5407 ± 56.11 a | 1.11 ± 0.21 d | 1.93 ± 0.05 d |
| Cu-NP1 | 1.5 | 397.70 ± 17.08 c | 472.1 ± 16.08 c | 1562 ± 27.09 c | 2455 ± 40.12 c | 2.17 ± 0.23 b | 2.08 ± 0.12 b |
| Cu-NP2 | 1.5 | 409.70 ± 18.03 c | 487.8 ± 15.09 c | 1601 ± 28.03 b | 2488 ± 40.23 b | 2.11 ± 0.22 b | 2.03 ± 0.11 c |
| Cu-NP3 | 1.5 | 503.40 ± 18.08 b | 530.6 ± 20.03 b | 1617 ± 28.11 b | 2527 ± 41.08 c | 2.06 ± 0.15 c | 2.03 ± 0.11 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noureen, A.; Jabeen, F.; Wajid, A.; Kazim, M.Z.; Safdar, N.; Cappello, T. Natural Bioactive Phytocompounds to Reduce Toxicity in Common Carp Cyprinus carpio: A Challenge to Environmental Risk Assessment of Nanomaterials. Water 2023, 15, 1152. https://doi.org/10.3390/w15061152
Noureen A, Jabeen F, Wajid A, Kazim MZ, Safdar N, Cappello T. Natural Bioactive Phytocompounds to Reduce Toxicity in Common Carp Cyprinus carpio: A Challenge to Environmental Risk Assessment of Nanomaterials. Water. 2023; 15(6):1152. https://doi.org/10.3390/w15061152
Chicago/Turabian StyleNoureen, Aasma, Farhat Jabeen, Abdul Wajid, Muhammad Zafarullah Kazim, Nafeesa Safdar, and Tiziana Cappello. 2023. "Natural Bioactive Phytocompounds to Reduce Toxicity in Common Carp Cyprinus carpio: A Challenge to Environmental Risk Assessment of Nanomaterials" Water 15, no. 6: 1152. https://doi.org/10.3390/w15061152
APA StyleNoureen, A., Jabeen, F., Wajid, A., Kazim, M. Z., Safdar, N., & Cappello, T. (2023). Natural Bioactive Phytocompounds to Reduce Toxicity in Common Carp Cyprinus carpio: A Challenge to Environmental Risk Assessment of Nanomaterials. Water, 15(6), 1152. https://doi.org/10.3390/w15061152







