Competitive Adsorption of Quaternary Metal Ions, Ni2+, Mn2+, Cr6+, and Cd2+, on Acid-Treated Activated Carbon
Abstract
:1. Introduction
2. Materials and Methods
2.1. Adsorbents
2.2. Characterization of Adsorbents
2.3. Adsorption Isotherms
3. Results and Discussion
3.1. Properties of the Adsorbents
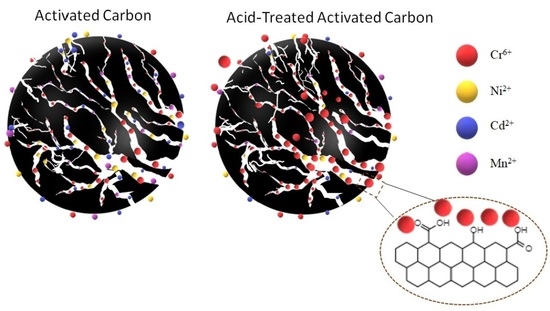
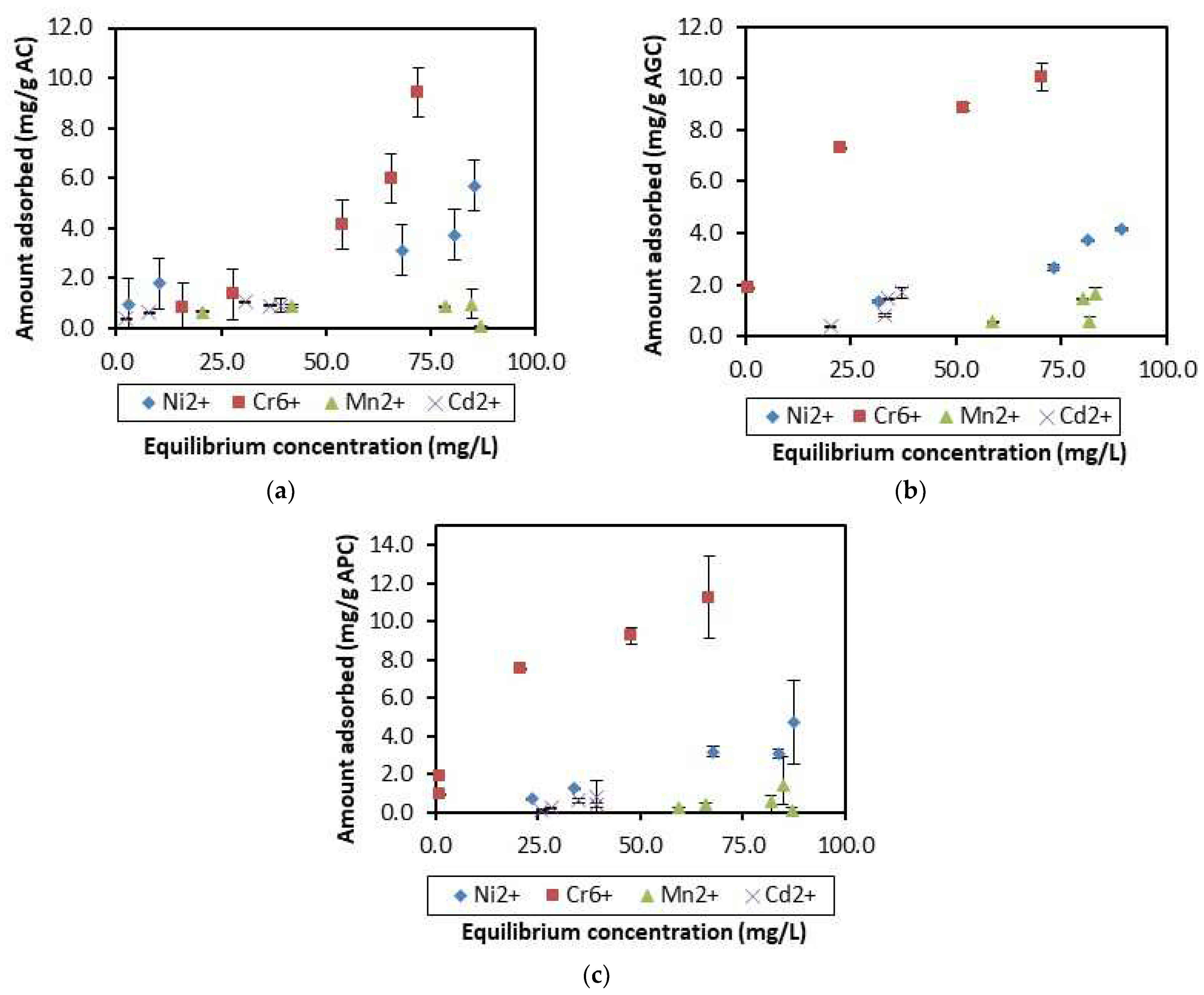
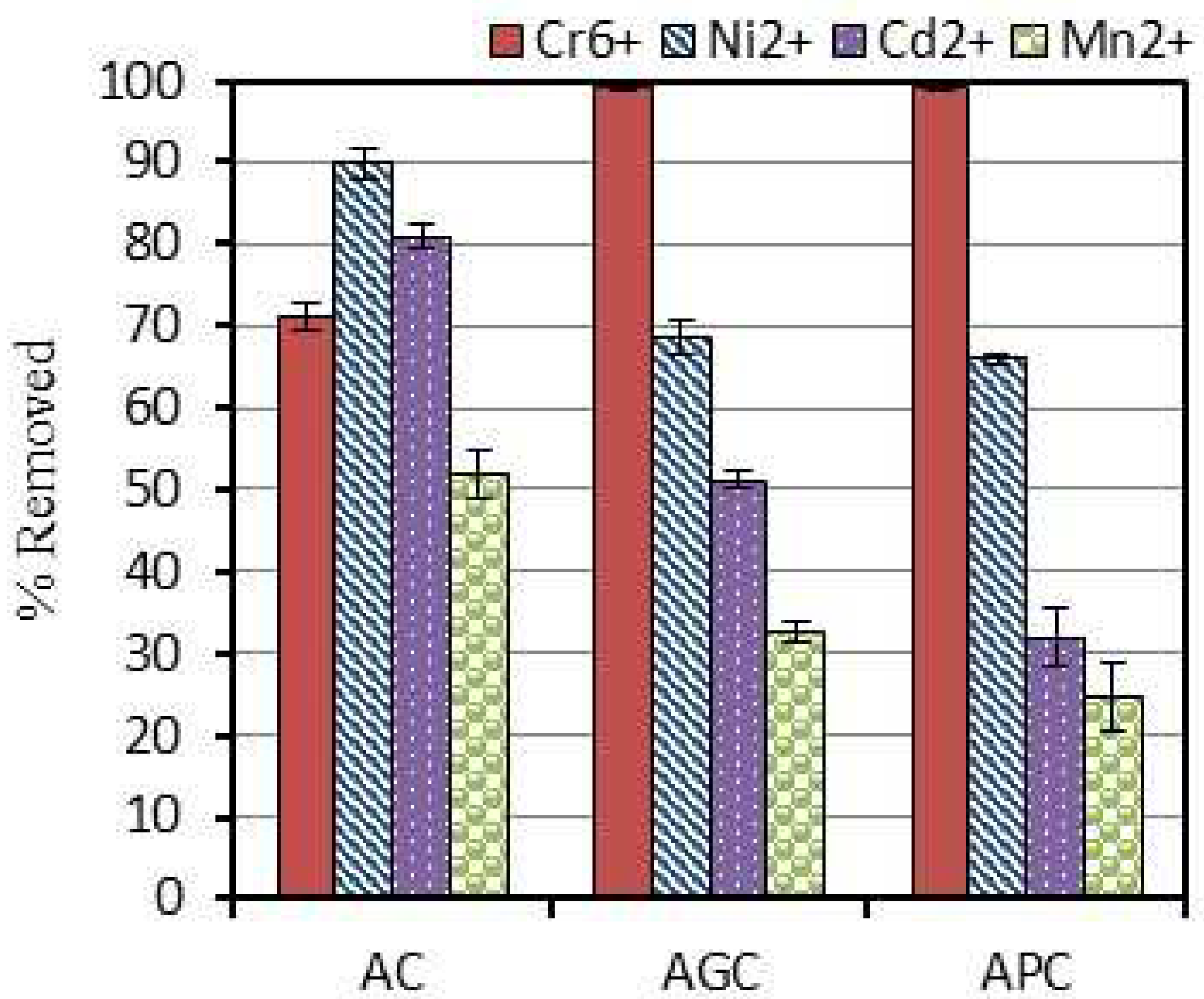
3.2. Competitive Adsorption of Heavy Metals
| Element | Ionic Radius (Å) [52] | Hydrated Ionic Radius (Å) [53] | Ionic Potential (z/r) (A−1) | Pauling Electronegativity Values [54] |
|---|---|---|---|---|
| Cr6+ | 0.44 | 4.61 * for Cr3+ | 13.64 (calculated) | 1.66 |
| Ni2+ | 0.69 | 4.04 | 2.90 (calculated) | 1.91 |
| Mn2+ | 0.83 | 4.38 | 2.41 (calculated) | 1.55 |
| Cd2+ | 0.95 | 4.26 | 2.11 [49] | 1.69 |
3.3. Modeling Competitive Metal Ions Adsorption Isotherms
3.3.1. Langmuir Model
3.3.2. Freundlich Model
3.3.3. The Dubinin–Kaganer–Radushkevich (DKR) Model
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Patel, N.; Chauhan, D.; Shahane, S.; Rai, D.; Ali Khan, M.Z.; Mishra, U.; Chaudhary, V.K. Contamination and Health Impact of Heavy Metals. In Water Pollution and Remediation: Heavy Metals; Inamuddin, A.M.I., Lichtfouse, E., Eds.; Environmental Chemistry for a Sustainable World; Springer International Publishing: Cham, Switzerland, 2021; pp. 259–280. ISBN 978-3-030-52421-0. [Google Scholar]
- Jaramillo, J.; Gómez-Serrano, V.; Álvarez, P.M. Enhanced Adsorption of Metal Ions onto Functionalized Granular Activated Carbons Prepared from Cherry Stones. J. Hazard. Mater. 2009, 161, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Obasi, P.N.; Akudinobi, B.B. Potential Health Risk and Levels of Heavy Metals in Water Resources of Lead–Zinc Mining Communities of Abakaliki, Southeast Nigeria. Appl. Water Sci. 2020, 10, 184. [Google Scholar] [CrossRef]
- Fallahzadeh, R.A.; Ghaneian, M.T.; Miri, M.; Dashti, M.M. Spatial Analysis and Health Risk Assessment of Heavy Metals Concentration in Drinking Water Resources. Environ. Sci. Pollut. Res. 2017, 24, 24790–24802. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K. Heavy Metals in Water: Presence, Removal and Safety; Royal Society of Chemistry: London, UK, 2014; ISBN 978-1-78262-017-4. [Google Scholar]
- Alfvén, T.; Elinder, C.-G.; Hellström, L.; Lagarde, F.; Järup, L. Cadmium Exposure and Distal Forearm Fractures. J. Bone Miner. Res. 2004, 19, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Järup, L.; Åkesson, A. Current Status of Cadmium as an Environmental Health Problem. Toxicol. Appl. Pharmacol. 2009, 238, 201–208. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.; Shi, W.; Zhang, D.; Zhu, T.; Li, X. Establishing a Human Health Risk Assessment Methodology for Metal Species and Its Application of Cr6+ in Groundwater Environments. Chemosphere 2017, 189, 525–537. [Google Scholar] [CrossRef]
- Das, N.; Mathew, L. Chromium Pollution and Bioremediation: An Overview. In Biomanagement of Metal-Contaminated Soils; Springer: Berlin/Heidelberg, Germany, 2011; pp. 297–321. [Google Scholar]
- National Toxicology Program. Report on Carcinogens; U.S. Department of Health and Human Services, Public Health Service: Research Triangle Park, NC, USA, 2016.
- Das, K.K.; Reddy, R.C.; Bagoji, I.B.; Das, S.; Bagali, S.; Mullur, L.; Khodnapur, J.P.; Biradar, M.S. Primary Concept of Nickel Toxicity—An Overview. J. Basic Clin. Physiol. Pharmacol. 2018, 30, 141–152. [Google Scholar] [CrossRef] [Green Version]
- O’Neal, S.L.; Zheng, W. Manganese Toxicity Upon Overexposure: A Decade in Review. Curr. Environ. Health Rep. 2015, 2, 315–328. [Google Scholar] [CrossRef] [Green Version]
- Michalke, B.; Halbach, S.; Nischwitz, V. Speciation and Toxicological Relevance of Manganese in Humans. J. Environ. Monit. 2007, 9, 650–656. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, N.; Li, Y.; Ren, B.; Ding, X.; Bian, H.; Yao, X. Total Concentrations and Sources of Heavy Metal Pollution in Global River and Lake Water Bodies from 1972 to 2017. Glob. Ecol. Conserv. 2020, 22, e00925. [Google Scholar] [CrossRef]
- Xará, S.; Delgado, J.; Almeida, M.F.; Costa, C. Laboratory Study on the Leaching Potential of Spent Alkaline Batteries Using a MSW Landfill Leachate. J. Mater. Cycles Waste Manag. 2013, 15, 61–72. [Google Scholar] [CrossRef]
- Karnchanawong, S.; Limpiteeprakan, P. Evaluation of Heavy Metal Leaching from Spent Household Batteries Disposed in Municipal Solid Waste. Waste Manag. 2009, 29, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Cai, X.; Xia, Z.; Jin, X.; Wu, H. Contamination Characteristics of Heavy Metals in a Small-Scale Tanning Area of Southern China and Their Source Analysis. Environ. Geochem. Health 2020. [Google Scholar] [CrossRef] [PubMed]
- Soliman, N.K.; Moustafa, A.F. Industrial Solid Waste for Heavy Metals Adsorption Features and Challenges; a Review. J. Mater. Res. Technol. 2020, 9, 10235–10253. [Google Scholar] [CrossRef]
- Oruko, R.O.; Selvarajan, R.; Ogola, H.J.O.; Edokpayi, J.N.; Odiyo, J.O. Contemporary and Future Direction of Chromium Tanning and Management in Sub Saharan Africa Tanneries. Process Saf. Environ. Prot. 2020, 133, 369–386. [Google Scholar] [CrossRef]
- Gotvajn, A.Ž.; Tišler, T.; Zagorc-Končan, J. Comparison of Different Treatment Strategies for Industrial Landfill Leachate. J. Hazard. Mater. 2009, 162, 1446–1456. [Google Scholar] [CrossRef]
- Wang, S.; Kalkhajeh, Y.K.; Qin, Z.; Jiao, W. Spatial Distribution and Assessment of the Human Health Risks of Heavy Metals in a Retired Petrochemical Industrial Area, South China. Environ. Res. 2020, 188, 109661. [Google Scholar] [CrossRef]
- Kadhum, S.A. A Preliminary Study of Heavy Metals Pollution in the Sandy Dust Storms and Its Human Risk Assessment from Middle and South of Iraq. Environ. Sci. Pollut. Res. 2020, 27, 8570–8579. [Google Scholar] [CrossRef]
- Aghel, B.; Mohadesi, M.; Gouran, A.; Razmegir, M.H. Use of Modified Iranian Clinoptilolite Zeolite for Cadmium and Lead Removal from Oil Refinery Wastewater. Int. J. Environ. Sci. Technol. 2020, 17, 1239–1250. [Google Scholar] [CrossRef]
- Salem, M.A.; Bedade, D.K.; Al-Ethawi, L.; Al-waleed, S.M. Assessment of Physiochemical Properties and Concentration of Heavy Metals in Agricultural Soils Fertilized with Chemical Fertilizers. Heliyon 2020, 6, e05224. [Google Scholar] [CrossRef]
- Song, Y.; Li, H.; Li, J.; Mao, C.; Ji, J.; Yuan, X.; Li, T.; Ayoko, G.A.; Frost, R.L.; Feng, Y. Multivariate Linear Regression Model for Source Apportionment and Health Risk Assessment of Heavy Metals from Different Environmental Media. Ecotoxicol. Environ. Saf. 2018, 165, 555–563. [Google Scholar] [CrossRef]
- Alvarez-Bastida, C.; Martínez-Miranda, V.; Solache-Ríos, M.; Linares-Hernández, I.; Teutli-Sequeira, A.; Vázquez-Mejía, G. Drinking Water Characterization and Removal of Manganese. Removal of Manganese from Water. J. Environ. Chem. Eng. 2018, 6, 2119–2125. [Google Scholar] [CrossRef]
- Cerrato, J.M.; Reyes, L.P.; Alvarado, C.N.; Dietrich, A.M. Effect of PVC and Iron Materials on Mn(II) Deposition in Drinking Water Distribution Systems. Water Res. 2006, 40, 2720–2726. [Google Scholar] [CrossRef]
- Li, W.; Gu, K.; Yu, Q.; Sun, Y.; Wang, Y.; Xin, M.; Bian, R.; Wang, H.; Wang, Y.; Zhang, D. Leaching Behavior and Environmental Risk Assessment of Toxic Metals in Municipal Solid Waste Incineration Fly Ash Exposed to Mature Landfill Leachate Environment. Waste Manag. 2021, 120, 68–75. [Google Scholar] [CrossRef]
- Vongdala, N.; Tran, H.-D.; Xuan, T.D.; Teschke, R.; Khanh, T.D. Heavy Metal Accumulation in Water, Soil, and Plants of Municipal Solid Waste Landfill in Vientiane, Laos. Int. J. Environ. Res. Public Health 2019, 16, 22. [Google Scholar] [CrossRef] [Green Version]
- Abu-Daabes, M.; Qdais, H.A.; Alsyouri, H. Assessment of Heavy Metals and Organics in Municipal Solid Waste Leachates from Landfills with Different Ages in Jordan. J. Environ. Prot. 2013, 2013, 344–352. [Google Scholar] [CrossRef] [Green Version]
- Bolisetty, S.; Peydayesh, M.; Mezzenga, R. Sustainable Technologies for Water Purification from Heavy Metals: Review and Analysis. Chem. Soc. Rev. 2019, 48, 463–487. [Google Scholar] [CrossRef]
- Lin, S.-H.; Juang, R.-S. Heavy Metal Removal from Water by Sorption Using Surfactant-Modified Montmorillonite. J. Hazard. Mater. 2002, 92, 315–326. [Google Scholar] [CrossRef]
- Erdem, B.; Özcan, A.; Gök, Ö.; Özcan, A.S. Immobilization of 2,2′-Dipyridyl onto Bentonite and Its Adsorption Behavior of Copper(II) Ions. J. Hazard. Mater. 2009, 163, 418–426. [Google Scholar] [CrossRef]
- Akar, S.T.; Sayin, F.; Yilmazer, D.; Akar, T. Removal of Cadmium and Manganese by an Ecofriendly Biomass. CLEAN—Soil Air Water 2016, 44, 202–210. [Google Scholar] [CrossRef]
- Cao, F.; Lian, C.; Yu, J.; Yang, H.; Lin, S. Study on the Adsorption Performance and Competitive Mechanism for Heavy Metal Contaminants Removal Using Novel Multi-Pore Activated Carbons Derived from Recyclable Long-Root Eichhornia Crassipes. Bioresour. Technol. 2019, 276, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.N.; Kim, D.-G.; Ko, S.-O. Adsorption Mechanisms of Manganese (II) Ions onto Acid-Treated Activated Carbon. KSCE J. Civ. Eng. 2018, 22, 3772–3782. [Google Scholar] [CrossRef]
- Mohan, D.; Chander, S. Single Component and Multi-Component Adsorption of Metal Ions by Activated Carbons. Colloids Surf. A Physicochem. Eng. Asp. 2001, 177, 183–196. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Gómez-Serrano, V.; Álvarez, P.M.; Alvim-Ferraz, M.C.M.; Dias, J.M. Activated Carbon Modifications to Enhance Its Water Treatment Applications. An Overview. J. Hazard. Mater. 2011, 187, 1–23. [Google Scholar] [CrossRef]
- Liu, X.; Xu, X.; Dong, X.; Park, J. Competitive Adsorption of Heavy Metal Ions from Aqueous Solutions onto Activated Carbon and Agricultural Waste Materials. Pol. J. Environ. Stud. 2019, 29, 749–761. [Google Scholar] [CrossRef]
- Berber-Mendoza, M.S.; Martínez-Costa, J.I.; Leyva-Ramos, R.; Amezquita Garcia, H.J.; Medellín Castillo, N.A. Competitive Adsorption of Heavy Metals from Aqueous Solution onto Oxidized Activated Carbon Fiber. Water Air Soil Pollut. 2018, 229, 257. [Google Scholar] [CrossRef]
- Kavand, M.; Kaghazchi, T.; Soleimani, M. Optimization of Parameters for Competitive Adsorption of Heavy Metal Ions (Pb+2, Ni+2, Cd+2) onto Activated Carbon. Korean J. Chem. Eng. 2014, 31, 692–700. [Google Scholar] [CrossRef]
- Coughlin, R.W.; Ezra, F.S. Role of Surface Acidity in the Adsorption of Organic Pollutants on the Surface of Carbon. Environ. Sci. Technol. 1968, 2, 291–297. [Google Scholar] [CrossRef]
- Franz, M.; Arafat, H.A.; Pinto, N.G. Effect of Chemical Surface Heterogeneity on the Adsorption Mechanism of Dissolved Aromatics on Activated Carbon. Carbon 2000, 38, 1807–1819. [Google Scholar] [CrossRef]
- Abu-Daabes, M.A.; Pinto, N.G. Effect of Surface Oxygen Complexes of Activated Carbon on Phenol Adsorption from Single and Mixed Non-Aqueous Solvents. Sep. Sci. Technol. 2004, 39, 2997–3009. [Google Scholar] [CrossRef]
- Radovic, L.R.; Moreno-Castilla, C.; Rivera-Utrilla, J. Carbon Materials as Adsorbents in Aqueous Solutions. Chem. Phys. Carbon 2000, 27, 227–405. [Google Scholar]
- Rivera-Utrilla, J.; Sánchez-Polo, M. Adsorption of Cr(III) on Ozonised Activated Carbon. Importance of Cπ—Cation Interactions. Water Res. 2003, 37, 3335–3340. [Google Scholar] [CrossRef]
- Covelo, E.F.; Couce, M.L.A.; Vega, F.A. Competitive Adsorption and Desorption of Cadmium, Chromium, Copper, Nickel, Lead, and Zinc by Humic Umbrisols. Commun. Soil Sci. Plant Anal. 2004, 35, 2709–2729. [Google Scholar] [CrossRef]
- Sposito, G. The Chemistry of Soils; Oxford University Press: Cambridge, MA, USA, 2008; ISBN 978-0-19-531369-7. [Google Scholar]
- McBride, M.B. Environmental Chemistry of Soils; Oxford University Press: New York, NY, USA, 1994; ISBN 978-0-19-507011-8. [Google Scholar]
- Iwanaga, M.; Yoshida, H.; Amano, Y.; Machida, M. Reduction of Cr(VI) Varying with the Surface Properties of Activated Carbon. J. Environ. Chem. 2013, 23, 19–23. [Google Scholar] [CrossRef] [Green Version]
- Módenes, A.N.; Espinoza-Quiñones, F.R.; Palácio, S.M.; Kroumov, A.D.; Stutz, G.; Tirao, G.; Camera, A.S. Cr(VI) Reduction by Activated Carbon and Non-Living Macrophytes Roots as Assessed by Kβ Spectroscopy. Chem. Eng. J. 2010, 162, 266–272. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Nightingale, E.R. Phenomenological Theory of Ion Solvation. Effective Radii of Hydrated Ions. J. Phys. Chem. 1959, 63, 1381–1387. [Google Scholar] [CrossRef]
- Pauling, L. The Nature of the Chemical Bond…; Cornell University Press: Ithaca, NY, USA, 1960; Volume 260. [Google Scholar]
- El-Khaiary, M.I. Least-Squares Regression of Adsorption Equilibrium Data: Comparing the Options. J. Hazard. Mater. 2008, 158, 73–87. [Google Scholar] [CrossRef]
- Dhaouadi, F.; Sellaoui, L.; Reynel-Ávila, H.E.; Landín-Sandoval, V.; Mendoza-Castillo, D.I.; Jaime-Leal, J.E.; Lima, E.C.; Bonilla-Petriciolet, A.; Lamine, A.B. Adsorption Mechanism of Zn2+, Ni2+, Cd2+, and Cu2+ Ions by Carbon-Based Adsorbents: Interpretation of the Adsorption Isotherms via Physical Modelling. Environ. Sci. Pollut. Res. 2021, 28, 30943–30954. [Google Scholar] [CrossRef]
- Al-Anber, M.A. Thermodynamics Approach in the Adsorption of Heavy Metals. In Thermodynamics—Interaction Studies—Solids, Liquids and Gases; Piraján, J.C.M., Ed.; IntechOpen Limited: London, UK, 2011; pp. 737–764. ISBN 978-953-307-563-1. [Google Scholar]
- Dubinin, M.M.; Stoeckli, H.F. Homogeneous and Heterogeneous Micropore Structures in Carbonaceous Adsorbents. J. Colloid Interface Sci. 1980, 75, 34–42. [Google Scholar] [CrossRef] [Green Version]
- Hutson, N.D.; Yang, R.T. Theoretical Basis for the Dubinin-Radushkevitch (D-R) Adsorption Isotherm Equation. Adsorption 1997, 3, 189–195. [Google Scholar] [CrossRef]
- Igberase, E.; Osifo, P.; Ofomaja, A. The Adsorption of Pb, Zn, Cu, Ni, and Cd by Modified Ligand in a Single Component Aqueous Solution: Equilibrium, Kinetic, Thermodynamic, and Desorption Studies. Int. J. Anal. Chem. 2017, 2017, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Krishna, B.S.; Murty, D.S.R.; Jai Prakash, B.S. Thermodynamics of Chromium(VI) Anionic Species Sorption onto Surfactant-Modified Montmorillonite Clay. J. Colloid Interface Sci. 2000, 229, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Mobasherpour, I.; Salahi, E.; Pazouki, M. Comparative of the Removal of Pb2+, Cd2+ and Ni2+ by Nano Crystallite Hydroxyapatite from Aqueous Solutions: Adsorption Isotherm Study. Arab. J. Chem. 2012, 5, 439–446. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Padmesh, T.; Palanivelu, K.; Velan, M. Biosorption of Nickel(II) Ions onto Sargassum Wightii: Application of Two-Parameter and Three-Parameter Isotherm Models. J. Hazard. Mater. 2006, 133, 304–308. [Google Scholar] [CrossRef]
- Wang, C.-C.; Juang, L.-C.; Lee, C.-K.; Hsu, T.-C.; Lee, J.-F.; Chao, H.-P. Effects of Exchanged Surfactant Cations on the Pore Structure and Adsorption Characteristics of Montmorillonite. J. Colloid Interface Sci. 2004, 280, 27–35. [Google Scholar] [CrossRef]

| Adsorbent | BET Surface Area | HK Cumulative Pore Volume | HK Pore Width |
|---|---|---|---|
| (m2/g) | (cm3/g) | (nm) | |
| AC | 635 | 2.99 × 10−1 | 1.398 |
| AGC | 591 | 2.79 × 10−1 | 1.388 |
| APC | 892 | 4.22 × 10−1 | 1.388 |
| Ions | Langmuir | Freundlich | DKR | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (mg/g) | b (L/mg) | R2 | n | R2 | Xm (mg/g) | β (mol/kJ)2 | E (kJ/mol) | R2 | |||
| AC | |||||||||||
| Cr6+ | Negative slope | - | - | 0.9331 | 0.01 | 0.63 | 0.9675 | 759.77 | 0.0175 | 5.35 | 0.959 |
| Ni2+ | 3.98 | 0.088 | 0.102 | 0.9793 | 0.68 | 2.63 | 0.9846 | 13.22 | 0.0043 | 10.78 | 0.9213 |
| Cd2+ | 1.04 | 0.270 | 0.082 | 0.9804 | 0.32 | 3.19 | 0.9296 | 2.90 | 0.0028 | 13.36 | 0.9422 |
| Mn2+ | 1.05 | 0.099 | 0.104 | 0.9805 | 0.35 | 4.55 | 0.7929 | 1.85 | 0.0026 | 13.87 | 0.81 |
| AGC | |||||||||||
| Cr6+ | 10.47 | 0.160 | 0.061 | 0.9849 | 2.24 | 2.79 | 0.9963 | 23.52 | 0.0032 | 12.50 | 0.9992 |
| Ni2+ | Negative slope | - | - | 0.019 | 0.04 | 0.98 | 0.9495 | 80.85 | 0.0118 | 6.51 | 0.9452 |
| Cd2+ | Negative slope | - | - | 0.7352 | 0.00 | 0.47 | 0.8671 | 5723.39 | 0.0209 | 4.89 | 0.8647 |
| Mn2+ | Negative slope | - | - | 0.0473 | 0.00 | 0.49 | 0.3216 | 7.30 × 102 | 0.0249 | 4.48 | 0.3216 |
| APC | |||||||||||
| Cr6+ | 12.092 | 0.105 | 0.0904 | 0.9769 | 2.02 | 2.45 | 0.995 | 30.01 | 0.0037 | 11.62 | 0.9974 |
| Ni2+ | Negative slope | - | - | 0.4969 | 0.02 | 0.80 | 0.9652 | 166.56 | 0.0143 | 5.91 | 0.9666 |
| Cd2+ | Negative slope | - | - | 0.6087 | 0.00 | 0.32 | 0.7558 | 1.20 × 105 | 0.0311 | 4.01 | 0.7587 |
| Mn2+ | Negative slope | - | - | 0.712 | 0.00 | 0.28 | 0.7539 | 14.1 | 0.013 | 6.20 | 0.0465 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu-Daabes, M.A.; Abu Zeitoun, E.; Mazi, W. Competitive Adsorption of Quaternary Metal Ions, Ni2+, Mn2+, Cr6+, and Cd2+, on Acid-Treated Activated Carbon. Water 2023, 15, 1070. https://doi.org/10.3390/w15061070
Abu-Daabes MA, Abu Zeitoun E, Mazi W. Competitive Adsorption of Quaternary Metal Ions, Ni2+, Mn2+, Cr6+, and Cd2+, on Acid-Treated Activated Carbon. Water. 2023; 15(6):1070. https://doi.org/10.3390/w15061070
Chicago/Turabian StyleAbu-Daabes, Malyuba A., Edrees Abu Zeitoun, and Wafa Mazi. 2023. "Competitive Adsorption of Quaternary Metal Ions, Ni2+, Mn2+, Cr6+, and Cd2+, on Acid-Treated Activated Carbon" Water 15, no. 6: 1070. https://doi.org/10.3390/w15061070
APA StyleAbu-Daabes, M. A., Abu Zeitoun, E., & Mazi, W. (2023). Competitive Adsorption of Quaternary Metal Ions, Ni2+, Mn2+, Cr6+, and Cd2+, on Acid-Treated Activated Carbon. Water, 15(6), 1070. https://doi.org/10.3390/w15061070