Competitive Adsorption of Quaternary Metal Ions, Ni2+, Mn2+, Cr6+, and Cd2+, on Acid-Treated Activated Carbon
Abstract
1. Introduction
2. Materials and Methods
2.1. Adsorbents
2.2. Characterization of Adsorbents
2.3. Adsorption Isotherms
3. Results and Discussion
3.1. Properties of the Adsorbents
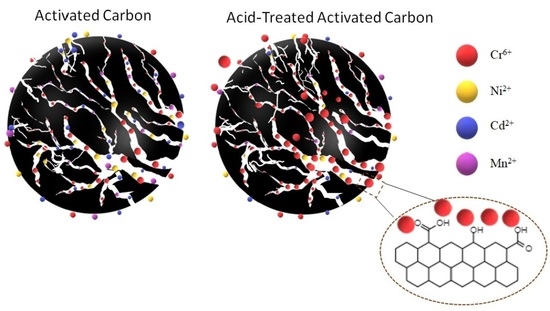
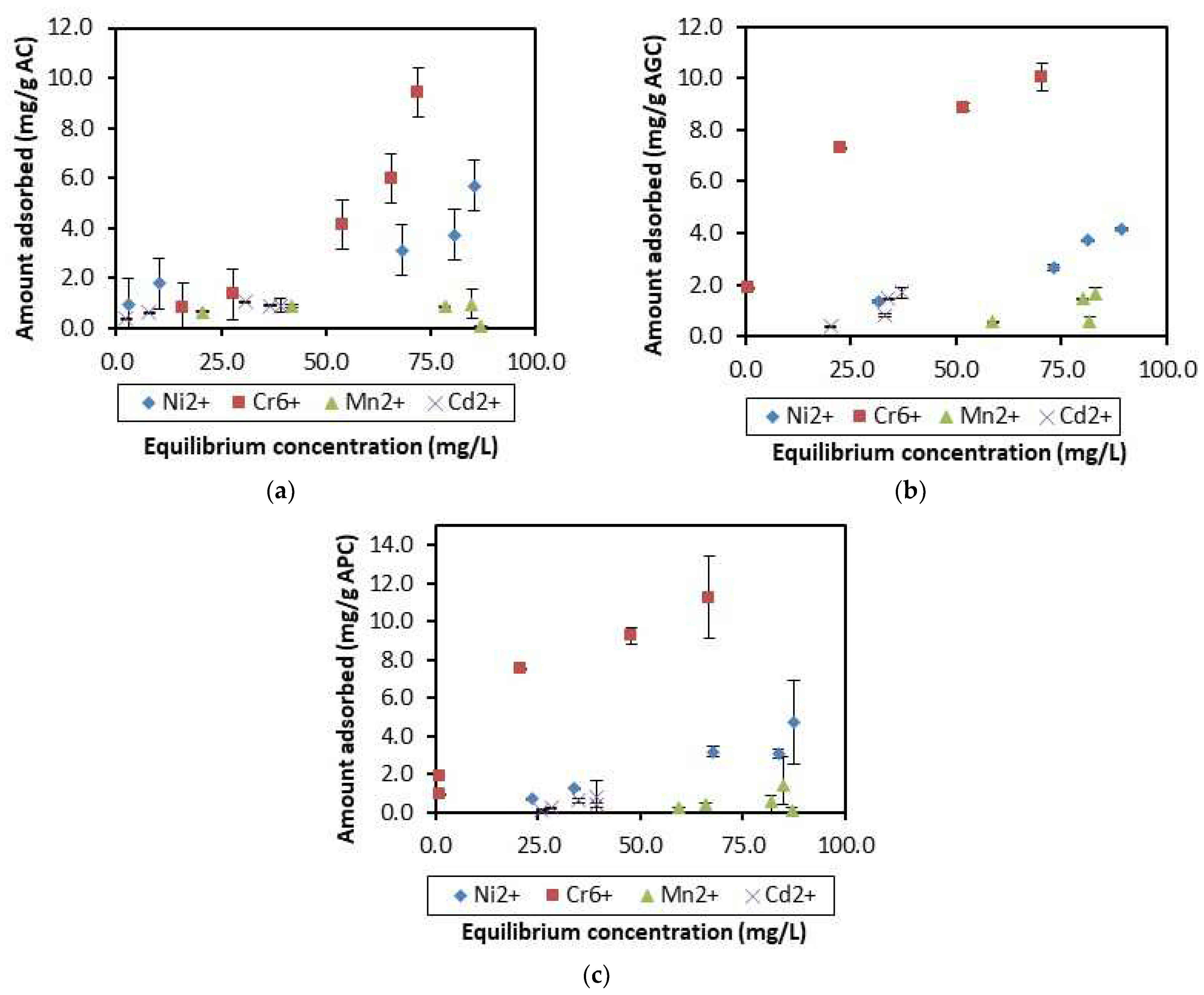
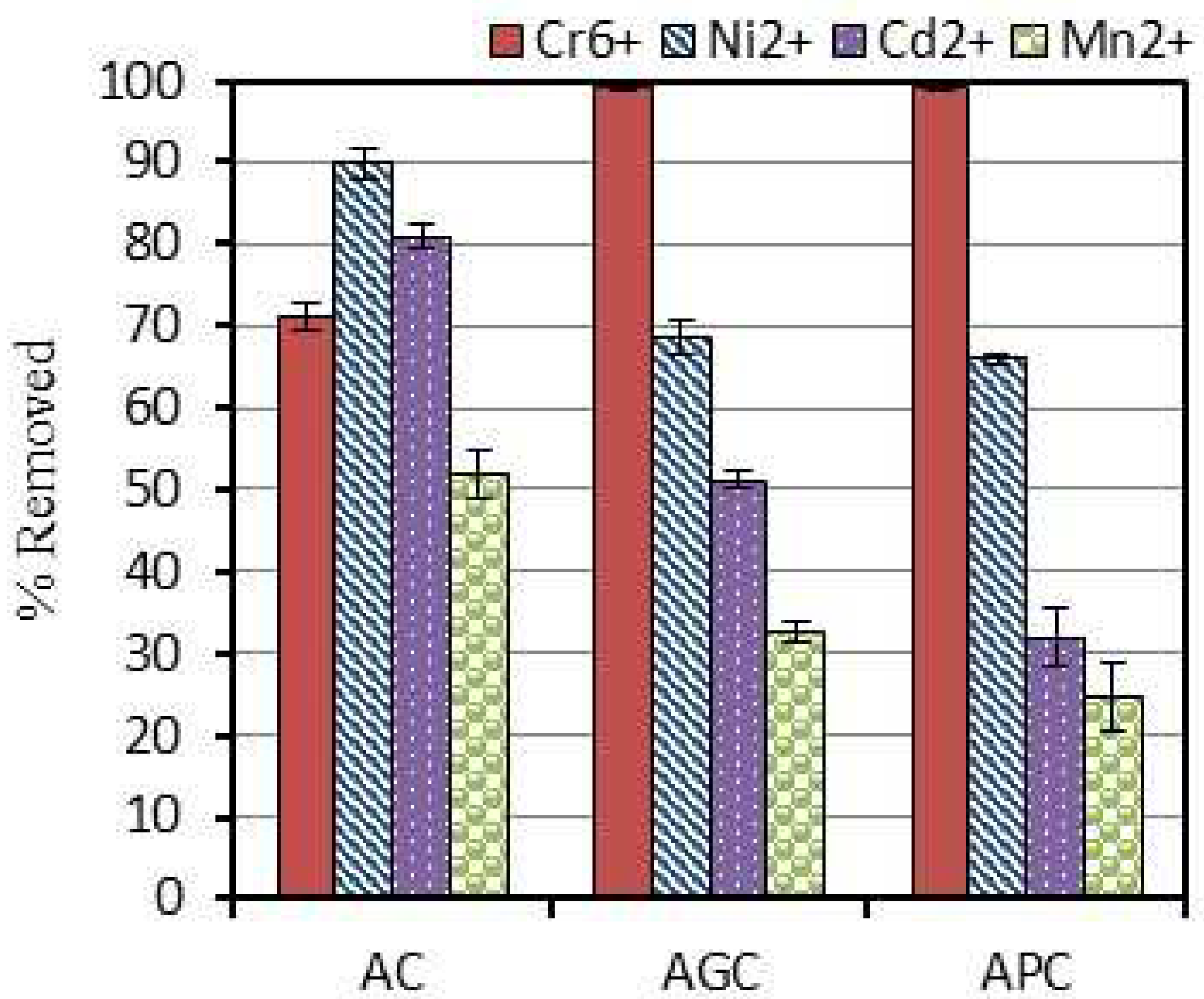
3.2. Competitive Adsorption of Heavy Metals
| Element | Ionic Radius (Å) [52] | Hydrated Ionic Radius (Å) [53] | Ionic Potential (z/r) (A−1) | Pauling Electronegativity Values [54] |
|---|---|---|---|---|
| Cr6+ | 0.44 | 4.61 * for Cr3+ | 13.64 (calculated) | 1.66 |
| Ni2+ | 0.69 | 4.04 | 2.90 (calculated) | 1.91 |
| Mn2+ | 0.83 | 4.38 | 2.41 (calculated) | 1.55 |
| Cd2+ | 0.95 | 4.26 | 2.11 [49] | 1.69 |
3.3. Modeling Competitive Metal Ions Adsorption Isotherms
3.3.1. Langmuir Model
3.3.2. Freundlich Model
3.3.3. The Dubinin–Kaganer–Radushkevich (DKR) Model
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Patel, N.; Chauhan, D.; Shahane, S.; Rai, D.; Ali Khan, M.Z.; Mishra, U.; Chaudhary, V.K. Contamination and Health Impact of Heavy Metals. In Water Pollution and Remediation: Heavy Metals; Inamuddin, A.M.I., Lichtfouse, E., Eds.; Environmental Chemistry for a Sustainable World; Springer International Publishing: Cham, Switzerland, 2021; pp. 259–280. ISBN 978-3-030-52421-0. [Google Scholar]
- Jaramillo, J.; Gómez-Serrano, V.; Álvarez, P.M. Enhanced Adsorption of Metal Ions onto Functionalized Granular Activated Carbons Prepared from Cherry Stones. J. Hazard. Mater. 2009, 161, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Obasi, P.N.; Akudinobi, B.B. Potential Health Risk and Levels of Heavy Metals in Water Resources of Lead–Zinc Mining Communities of Abakaliki, Southeast Nigeria. Appl. Water Sci. 2020, 10, 184. [Google Scholar] [CrossRef]
- Fallahzadeh, R.A.; Ghaneian, M.T.; Miri, M.; Dashti, M.M. Spatial Analysis and Health Risk Assessment of Heavy Metals Concentration in Drinking Water Resources. Environ. Sci. Pollut. Res. 2017, 24, 24790–24802. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K. Heavy Metals in Water: Presence, Removal and Safety; Royal Society of Chemistry: London, UK, 2014; ISBN 978-1-78262-017-4. [Google Scholar]
- Alfvén, T.; Elinder, C.-G.; Hellström, L.; Lagarde, F.; Järup, L. Cadmium Exposure and Distal Forearm Fractures. J. Bone Miner. Res. 2004, 19, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Järup, L.; Åkesson, A. Current Status of Cadmium as an Environmental Health Problem. Toxicol. Appl. Pharmacol. 2009, 238, 201–208. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.; Shi, W.; Zhang, D.; Zhu, T.; Li, X. Establishing a Human Health Risk Assessment Methodology for Metal Species and Its Application of Cr6+ in Groundwater Environments. Chemosphere 2017, 189, 525–537. [Google Scholar] [CrossRef]
- Das, N.; Mathew, L. Chromium Pollution and Bioremediation: An Overview. In Biomanagement of Metal-Contaminated Soils; Springer: Berlin/Heidelberg, Germany, 2011; pp. 297–321. [Google Scholar]
- National Toxicology Program. Report on Carcinogens; U.S. Department of Health and Human Services, Public Health Service: Research Triangle Park, NC, USA, 2016.
- Das, K.K.; Reddy, R.C.; Bagoji, I.B.; Das, S.; Bagali, S.; Mullur, L.; Khodnapur, J.P.; Biradar, M.S. Primary Concept of Nickel Toxicity—An Overview. J. Basic Clin. Physiol. Pharmacol. 2018, 30, 141–152. [Google Scholar] [CrossRef]
- O’Neal, S.L.; Zheng, W. Manganese Toxicity Upon Overexposure: A Decade in Review. Curr. Environ. Health Rep. 2015, 2, 315–328. [Google Scholar] [CrossRef]
- Michalke, B.; Halbach, S.; Nischwitz, V. Speciation and Toxicological Relevance of Manganese in Humans. J. Environ. Monit. 2007, 9, 650–656. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, N.; Li, Y.; Ren, B.; Ding, X.; Bian, H.; Yao, X. Total Concentrations and Sources of Heavy Metal Pollution in Global River and Lake Water Bodies from 1972 to 2017. Glob. Ecol. Conserv. 2020, 22, e00925. [Google Scholar] [CrossRef]
- Xará, S.; Delgado, J.; Almeida, M.F.; Costa, C. Laboratory Study on the Leaching Potential of Spent Alkaline Batteries Using a MSW Landfill Leachate. J. Mater. Cycles Waste Manag. 2013, 15, 61–72. [Google Scholar] [CrossRef]
- Karnchanawong, S.; Limpiteeprakan, P. Evaluation of Heavy Metal Leaching from Spent Household Batteries Disposed in Municipal Solid Waste. Waste Manag. 2009, 29, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Cai, X.; Xia, Z.; Jin, X.; Wu, H. Contamination Characteristics of Heavy Metals in a Small-Scale Tanning Area of Southern China and Their Source Analysis. Environ. Geochem. Health 2020. [Google Scholar] [CrossRef] [PubMed]
- Soliman, N.K.; Moustafa, A.F. Industrial Solid Waste for Heavy Metals Adsorption Features and Challenges; a Review. J. Mater. Res. Technol. 2020, 9, 10235–10253. [Google Scholar] [CrossRef]
- Oruko, R.O.; Selvarajan, R.; Ogola, H.J.O.; Edokpayi, J.N.; Odiyo, J.O. Contemporary and Future Direction of Chromium Tanning and Management in Sub Saharan Africa Tanneries. Process Saf. Environ. Prot. 2020, 133, 369–386. [Google Scholar] [CrossRef]
- Gotvajn, A.Ž.; Tišler, T.; Zagorc-Končan, J. Comparison of Different Treatment Strategies for Industrial Landfill Leachate. J. Hazard. Mater. 2009, 162, 1446–1456. [Google Scholar] [CrossRef]
- Wang, S.; Kalkhajeh, Y.K.; Qin, Z.; Jiao, W. Spatial Distribution and Assessment of the Human Health Risks of Heavy Metals in a Retired Petrochemical Industrial Area, South China. Environ. Res. 2020, 188, 109661. [Google Scholar] [CrossRef]
- Kadhum, S.A. A Preliminary Study of Heavy Metals Pollution in the Sandy Dust Storms and Its Human Risk Assessment from Middle and South of Iraq. Environ. Sci. Pollut. Res. 2020, 27, 8570–8579. [Google Scholar] [CrossRef]
- Aghel, B.; Mohadesi, M.; Gouran, A.; Razmegir, M.H. Use of Modified Iranian Clinoptilolite Zeolite for Cadmium and Lead Removal from Oil Refinery Wastewater. Int. J. Environ. Sci. Technol. 2020, 17, 1239–1250. [Google Scholar] [CrossRef]
- Salem, M.A.; Bedade, D.K.; Al-Ethawi, L.; Al-waleed, S.M. Assessment of Physiochemical Properties and Concentration of Heavy Metals in Agricultural Soils Fertilized with Chemical Fertilizers. Heliyon 2020, 6, e05224. [Google Scholar] [CrossRef]
- Song, Y.; Li, H.; Li, J.; Mao, C.; Ji, J.; Yuan, X.; Li, T.; Ayoko, G.A.; Frost, R.L.; Feng, Y. Multivariate Linear Regression Model for Source Apportionment and Health Risk Assessment of Heavy Metals from Different Environmental Media. Ecotoxicol. Environ. Saf. 2018, 165, 555–563. [Google Scholar] [CrossRef]
- Alvarez-Bastida, C.; Martínez-Miranda, V.; Solache-Ríos, M.; Linares-Hernández, I.; Teutli-Sequeira, A.; Vázquez-Mejía, G. Drinking Water Characterization and Removal of Manganese. Removal of Manganese from Water. J. Environ. Chem. Eng. 2018, 6, 2119–2125. [Google Scholar] [CrossRef]
- Cerrato, J.M.; Reyes, L.P.; Alvarado, C.N.; Dietrich, A.M. Effect of PVC and Iron Materials on Mn(II) Deposition in Drinking Water Distribution Systems. Water Res. 2006, 40, 2720–2726. [Google Scholar] [CrossRef]
- Li, W.; Gu, K.; Yu, Q.; Sun, Y.; Wang, Y.; Xin, M.; Bian, R.; Wang, H.; Wang, Y.; Zhang, D. Leaching Behavior and Environmental Risk Assessment of Toxic Metals in Municipal Solid Waste Incineration Fly Ash Exposed to Mature Landfill Leachate Environment. Waste Manag. 2021, 120, 68–75. [Google Scholar] [CrossRef]
- Vongdala, N.; Tran, H.-D.; Xuan, T.D.; Teschke, R.; Khanh, T.D. Heavy Metal Accumulation in Water, Soil, and Plants of Municipal Solid Waste Landfill in Vientiane, Laos. Int. J. Environ. Res. Public Health 2019, 16, 22. [Google Scholar] [CrossRef]
- Abu-Daabes, M.; Qdais, H.A.; Alsyouri, H. Assessment of Heavy Metals and Organics in Municipal Solid Waste Leachates from Landfills with Different Ages in Jordan. J. Environ. Prot. 2013, 2013, 344–352. [Google Scholar] [CrossRef]
- Bolisetty, S.; Peydayesh, M.; Mezzenga, R. Sustainable Technologies for Water Purification from Heavy Metals: Review and Analysis. Chem. Soc. Rev. 2019, 48, 463–487. [Google Scholar] [CrossRef]
- Lin, S.-H.; Juang, R.-S. Heavy Metal Removal from Water by Sorption Using Surfactant-Modified Montmorillonite. J. Hazard. Mater. 2002, 92, 315–326. [Google Scholar] [CrossRef]
- Erdem, B.; Özcan, A.; Gök, Ö.; Özcan, A.S. Immobilization of 2,2′-Dipyridyl onto Bentonite and Its Adsorption Behavior of Copper(II) Ions. J. Hazard. Mater. 2009, 163, 418–426. [Google Scholar] [CrossRef]
- Akar, S.T.; Sayin, F.; Yilmazer, D.; Akar, T. Removal of Cadmium and Manganese by an Ecofriendly Biomass. CLEAN—Soil Air Water 2016, 44, 202–210. [Google Scholar] [CrossRef]
- Cao, F.; Lian, C.; Yu, J.; Yang, H.; Lin, S. Study on the Adsorption Performance and Competitive Mechanism for Heavy Metal Contaminants Removal Using Novel Multi-Pore Activated Carbons Derived from Recyclable Long-Root Eichhornia Crassipes. Bioresour. Technol. 2019, 276, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.N.; Kim, D.-G.; Ko, S.-O. Adsorption Mechanisms of Manganese (II) Ions onto Acid-Treated Activated Carbon. KSCE J. Civ. Eng. 2018, 22, 3772–3782. [Google Scholar] [CrossRef]
- Mohan, D.; Chander, S. Single Component and Multi-Component Adsorption of Metal Ions by Activated Carbons. Colloids Surf. A Physicochem. Eng. Asp. 2001, 177, 183–196. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Gómez-Serrano, V.; Álvarez, P.M.; Alvim-Ferraz, M.C.M.; Dias, J.M. Activated Carbon Modifications to Enhance Its Water Treatment Applications. An Overview. J. Hazard. Mater. 2011, 187, 1–23. [Google Scholar] [CrossRef]
- Liu, X.; Xu, X.; Dong, X.; Park, J. Competitive Adsorption of Heavy Metal Ions from Aqueous Solutions onto Activated Carbon and Agricultural Waste Materials. Pol. J. Environ. Stud. 2019, 29, 749–761. [Google Scholar] [CrossRef]
- Berber-Mendoza, M.S.; Martínez-Costa, J.I.; Leyva-Ramos, R.; Amezquita Garcia, H.J.; Medellín Castillo, N.A. Competitive Adsorption of Heavy Metals from Aqueous Solution onto Oxidized Activated Carbon Fiber. Water Air Soil Pollut. 2018, 229, 257. [Google Scholar] [CrossRef]
- Kavand, M.; Kaghazchi, T.; Soleimani, M. Optimization of Parameters for Competitive Adsorption of Heavy Metal Ions (Pb+2, Ni+2, Cd+2) onto Activated Carbon. Korean J. Chem. Eng. 2014, 31, 692–700. [Google Scholar] [CrossRef]
- Coughlin, R.W.; Ezra, F.S. Role of Surface Acidity in the Adsorption of Organic Pollutants on the Surface of Carbon. Environ. Sci. Technol. 1968, 2, 291–297. [Google Scholar] [CrossRef]
- Franz, M.; Arafat, H.A.; Pinto, N.G. Effect of Chemical Surface Heterogeneity on the Adsorption Mechanism of Dissolved Aromatics on Activated Carbon. Carbon 2000, 38, 1807–1819. [Google Scholar] [CrossRef]
- Abu-Daabes, M.A.; Pinto, N.G. Effect of Surface Oxygen Complexes of Activated Carbon on Phenol Adsorption from Single and Mixed Non-Aqueous Solvents. Sep. Sci. Technol. 2004, 39, 2997–3009. [Google Scholar] [CrossRef]
- Radovic, L.R.; Moreno-Castilla, C.; Rivera-Utrilla, J. Carbon Materials as Adsorbents in Aqueous Solutions. Chem. Phys. Carbon 2000, 27, 227–405. [Google Scholar]
- Rivera-Utrilla, J.; Sánchez-Polo, M. Adsorption of Cr(III) on Ozonised Activated Carbon. Importance of Cπ—Cation Interactions. Water Res. 2003, 37, 3335–3340. [Google Scholar] [CrossRef]
- Covelo, E.F.; Couce, M.L.A.; Vega, F.A. Competitive Adsorption and Desorption of Cadmium, Chromium, Copper, Nickel, Lead, and Zinc by Humic Umbrisols. Commun. Soil Sci. Plant Anal. 2004, 35, 2709–2729. [Google Scholar] [CrossRef]
- Sposito, G. The Chemistry of Soils; Oxford University Press: Cambridge, MA, USA, 2008; ISBN 978-0-19-531369-7. [Google Scholar]
- McBride, M.B. Environmental Chemistry of Soils; Oxford University Press: New York, NY, USA, 1994; ISBN 978-0-19-507011-8. [Google Scholar]
- Iwanaga, M.; Yoshida, H.; Amano, Y.; Machida, M. Reduction of Cr(VI) Varying with the Surface Properties of Activated Carbon. J. Environ. Chem. 2013, 23, 19–23. [Google Scholar] [CrossRef]
- Módenes, A.N.; Espinoza-Quiñones, F.R.; Palácio, S.M.; Kroumov, A.D.; Stutz, G.; Tirao, G.; Camera, A.S. Cr(VI) Reduction by Activated Carbon and Non-Living Macrophytes Roots as Assessed by Kβ Spectroscopy. Chem. Eng. J. 2010, 162, 266–272. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Nightingale, E.R. Phenomenological Theory of Ion Solvation. Effective Radii of Hydrated Ions. J. Phys. Chem. 1959, 63, 1381–1387. [Google Scholar] [CrossRef]
- Pauling, L. The Nature of the Chemical Bond…; Cornell University Press: Ithaca, NY, USA, 1960; Volume 260. [Google Scholar]
- El-Khaiary, M.I. Least-Squares Regression of Adsorption Equilibrium Data: Comparing the Options. J. Hazard. Mater. 2008, 158, 73–87. [Google Scholar] [CrossRef]
- Dhaouadi, F.; Sellaoui, L.; Reynel-Ávila, H.E.; Landín-Sandoval, V.; Mendoza-Castillo, D.I.; Jaime-Leal, J.E.; Lima, E.C.; Bonilla-Petriciolet, A.; Lamine, A.B. Adsorption Mechanism of Zn2+, Ni2+, Cd2+, and Cu2+ Ions by Carbon-Based Adsorbents: Interpretation of the Adsorption Isotherms via Physical Modelling. Environ. Sci. Pollut. Res. 2021, 28, 30943–30954. [Google Scholar] [CrossRef]
- Al-Anber, M.A. Thermodynamics Approach in the Adsorption of Heavy Metals. In Thermodynamics—Interaction Studies—Solids, Liquids and Gases; Piraján, J.C.M., Ed.; IntechOpen Limited: London, UK, 2011; pp. 737–764. ISBN 978-953-307-563-1. [Google Scholar]
- Dubinin, M.M.; Stoeckli, H.F. Homogeneous and Heterogeneous Micropore Structures in Carbonaceous Adsorbents. J. Colloid Interface Sci. 1980, 75, 34–42. [Google Scholar] [CrossRef]
- Hutson, N.D.; Yang, R.T. Theoretical Basis for the Dubinin-Radushkevitch (D-R) Adsorption Isotherm Equation. Adsorption 1997, 3, 189–195. [Google Scholar] [CrossRef]
- Igberase, E.; Osifo, P.; Ofomaja, A. The Adsorption of Pb, Zn, Cu, Ni, and Cd by Modified Ligand in a Single Component Aqueous Solution: Equilibrium, Kinetic, Thermodynamic, and Desorption Studies. Int. J. Anal. Chem. 2017, 2017, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Krishna, B.S.; Murty, D.S.R.; Jai Prakash, B.S. Thermodynamics of Chromium(VI) Anionic Species Sorption onto Surfactant-Modified Montmorillonite Clay. J. Colloid Interface Sci. 2000, 229, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Mobasherpour, I.; Salahi, E.; Pazouki, M. Comparative of the Removal of Pb2+, Cd2+ and Ni2+ by Nano Crystallite Hydroxyapatite from Aqueous Solutions: Adsorption Isotherm Study. Arab. J. Chem. 2012, 5, 439–446. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Padmesh, T.; Palanivelu, K.; Velan, M. Biosorption of Nickel(II) Ions onto Sargassum Wightii: Application of Two-Parameter and Three-Parameter Isotherm Models. J. Hazard. Mater. 2006, 133, 304–308. [Google Scholar] [CrossRef]
- Wang, C.-C.; Juang, L.-C.; Lee, C.-K.; Hsu, T.-C.; Lee, J.-F.; Chao, H.-P. Effects of Exchanged Surfactant Cations on the Pore Structure and Adsorption Characteristics of Montmorillonite. J. Colloid Interface Sci. 2004, 280, 27–35. [Google Scholar] [CrossRef]

| Adsorbent | BET Surface Area | HK Cumulative Pore Volume | HK Pore Width |
|---|---|---|---|
| (m2/g) | (cm3/g) | (nm) | |
| AC | 635 | 2.99 × 10−1 | 1.398 |
| AGC | 591 | 2.79 × 10−1 | 1.388 |
| APC | 892 | 4.22 × 10−1 | 1.388 |
| Ions | Langmuir | Freundlich | DKR | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (mg/g) | b (L/mg) | R2 | n | R2 | Xm (mg/g) | β (mol/kJ)2 | E (kJ/mol) | R2 | |||
| AC | |||||||||||
| Cr6+ | Negative slope | - | - | 0.9331 | 0.01 | 0.63 | 0.9675 | 759.77 | 0.0175 | 5.35 | 0.959 |
| Ni2+ | 3.98 | 0.088 | 0.102 | 0.9793 | 0.68 | 2.63 | 0.9846 | 13.22 | 0.0043 | 10.78 | 0.9213 |
| Cd2+ | 1.04 | 0.270 | 0.082 | 0.9804 | 0.32 | 3.19 | 0.9296 | 2.90 | 0.0028 | 13.36 | 0.9422 |
| Mn2+ | 1.05 | 0.099 | 0.104 | 0.9805 | 0.35 | 4.55 | 0.7929 | 1.85 | 0.0026 | 13.87 | 0.81 |
| AGC | |||||||||||
| Cr6+ | 10.47 | 0.160 | 0.061 | 0.9849 | 2.24 | 2.79 | 0.9963 | 23.52 | 0.0032 | 12.50 | 0.9992 |
| Ni2+ | Negative slope | - | - | 0.019 | 0.04 | 0.98 | 0.9495 | 80.85 | 0.0118 | 6.51 | 0.9452 |
| Cd2+ | Negative slope | - | - | 0.7352 | 0.00 | 0.47 | 0.8671 | 5723.39 | 0.0209 | 4.89 | 0.8647 |
| Mn2+ | Negative slope | - | - | 0.0473 | 0.00 | 0.49 | 0.3216 | 7.30 × 102 | 0.0249 | 4.48 | 0.3216 |
| APC | |||||||||||
| Cr6+ | 12.092 | 0.105 | 0.0904 | 0.9769 | 2.02 | 2.45 | 0.995 | 30.01 | 0.0037 | 11.62 | 0.9974 |
| Ni2+ | Negative slope | - | - | 0.4969 | 0.02 | 0.80 | 0.9652 | 166.56 | 0.0143 | 5.91 | 0.9666 |
| Cd2+ | Negative slope | - | - | 0.6087 | 0.00 | 0.32 | 0.7558 | 1.20 × 105 | 0.0311 | 4.01 | 0.7587 |
| Mn2+ | Negative slope | - | - | 0.712 | 0.00 | 0.28 | 0.7539 | 14.1 | 0.013 | 6.20 | 0.0465 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu-Daabes, M.A.; Abu Zeitoun, E.; Mazi, W. Competitive Adsorption of Quaternary Metal Ions, Ni2+, Mn2+, Cr6+, and Cd2+, on Acid-Treated Activated Carbon. Water 2023, 15, 1070. https://doi.org/10.3390/w15061070
Abu-Daabes MA, Abu Zeitoun E, Mazi W. Competitive Adsorption of Quaternary Metal Ions, Ni2+, Mn2+, Cr6+, and Cd2+, on Acid-Treated Activated Carbon. Water. 2023; 15(6):1070. https://doi.org/10.3390/w15061070
Chicago/Turabian StyleAbu-Daabes, Malyuba A., Edrees Abu Zeitoun, and Wafa Mazi. 2023. "Competitive Adsorption of Quaternary Metal Ions, Ni2+, Mn2+, Cr6+, and Cd2+, on Acid-Treated Activated Carbon" Water 15, no. 6: 1070. https://doi.org/10.3390/w15061070
APA StyleAbu-Daabes, M. A., Abu Zeitoun, E., & Mazi, W. (2023). Competitive Adsorption of Quaternary Metal Ions, Ni2+, Mn2+, Cr6+, and Cd2+, on Acid-Treated Activated Carbon. Water, 15(6), 1070. https://doi.org/10.3390/w15061070