Recent Advances and Challenges in the Inverse Identification of Thermal Diffusivity of Natural Ice in China
Abstract
:1. Introduction
2. Fundamentals of Physics and Mathematics
2.1. Physical Background of the Thermal Diffusivity of Ice
2.2. Non-Linearly Distributed Parameter System
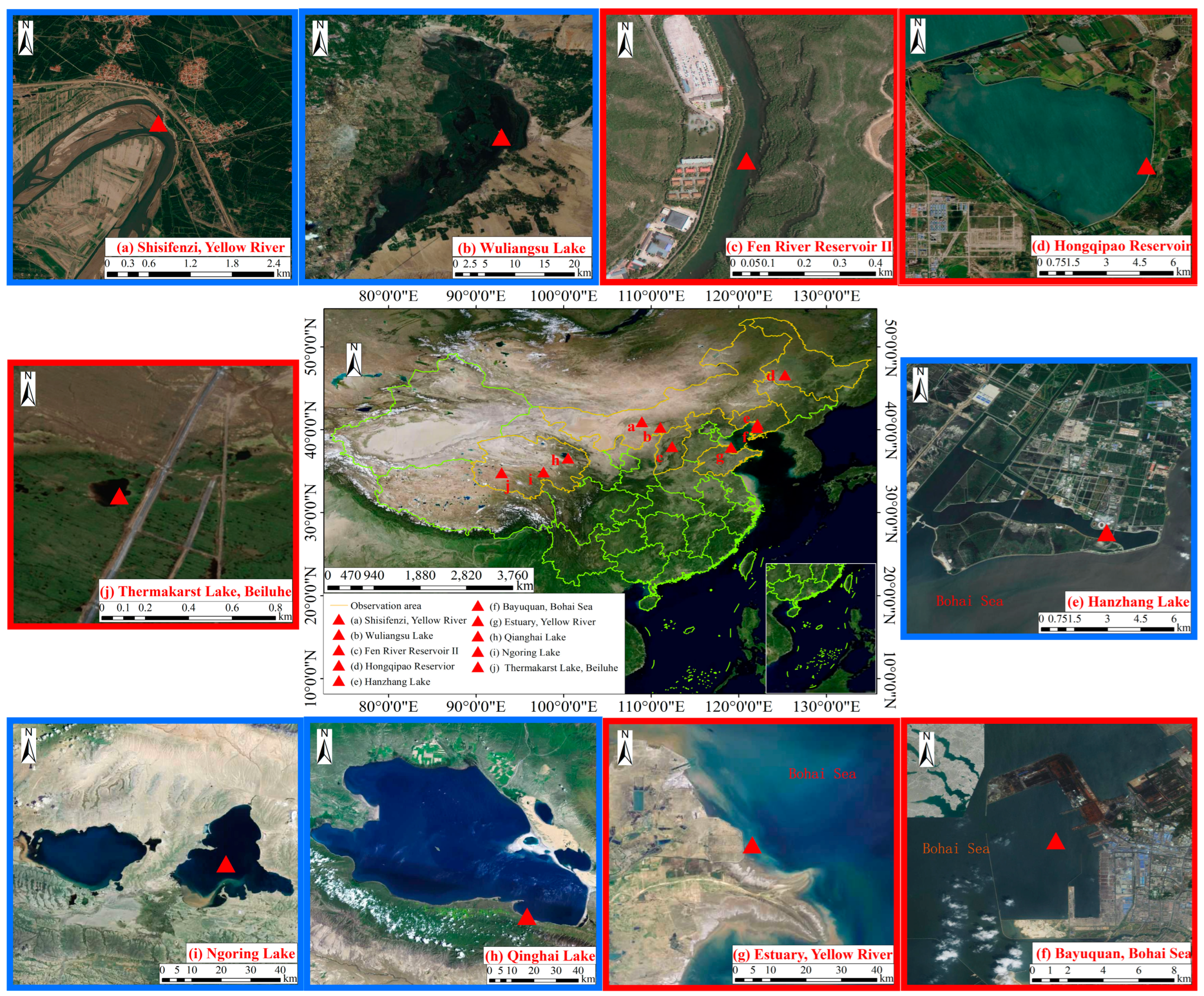
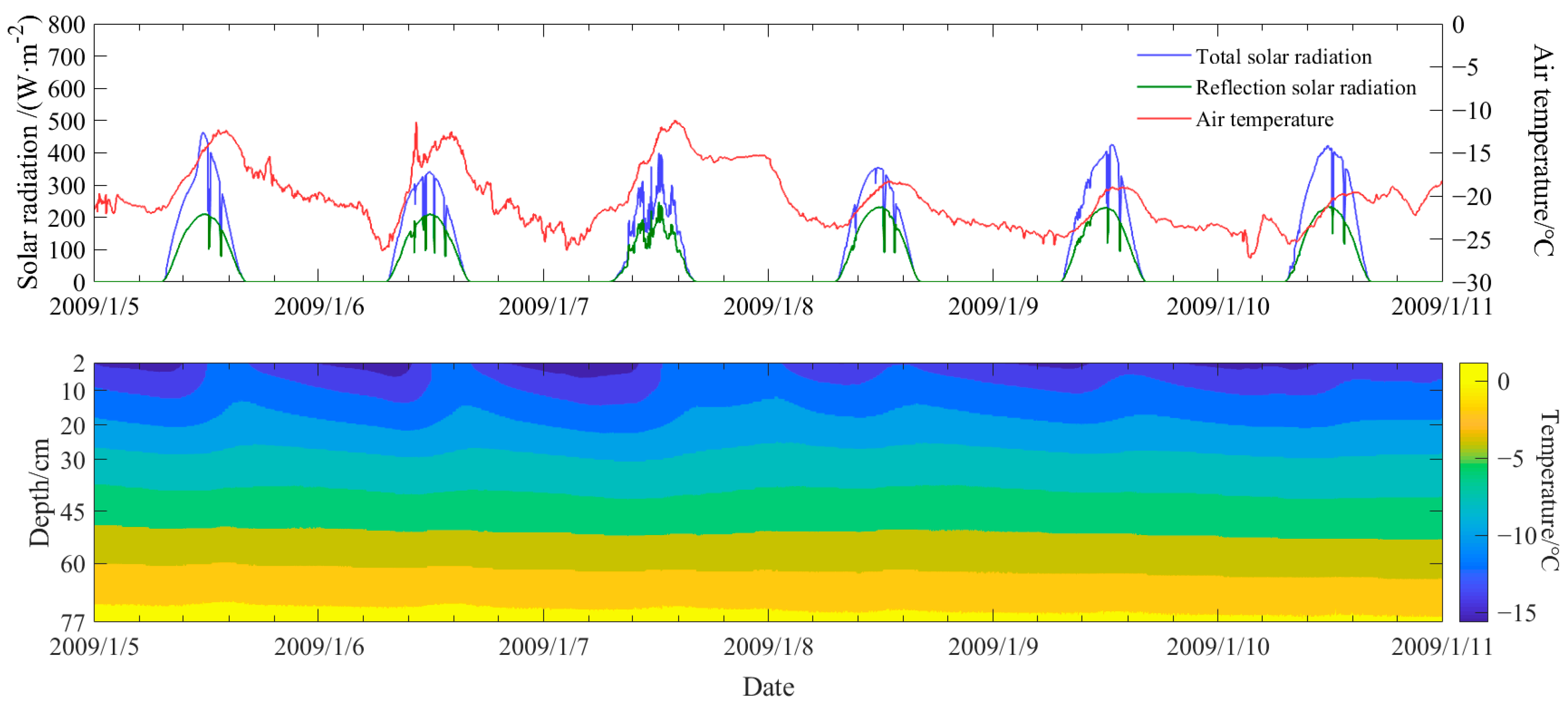
3. Field Survey of the Vertical Temperature Profile of Natural Ice
| Information | Hongqipao Reservoir | Thermakarst Lake, Beiluhe | Estuary, Yellow River | Fen River Reservoir II | Bayuquan, Bohai Sea | Zhongshan Station, Antarctica |
|---|---|---|---|---|---|---|
| Latitude and longitude/° | 112.27° E, 37.60° N | 92.92° E, 34.83° N | 119.12° E, 37.88° N | 112.38° E, 37.98° N | 122.07° E, 40.28° N | 76.37° E, 69.37° S |
| Elevation/m | 140 | 4640 | 2 | 800–1400 | 2 | 11 |
| Duration of freezing period/d | 150–180 | 150–210 | 80–100 | 80–100 | 110–120 | 300 |
| Ice thickness at severe ice period/m | 1.0–1.2 | 0.7–1.0 | 0.1–0.2 | 0.4–0.5 | 0.3–0.4 | 1.6–1.8 |
| Mode of Ice Formation | Thermodynamics | Thermodynamics | Thermodynamics | Thermodynamics | Thermodynamics | Thermodynamics |
| Ice classification | Lake Ice | Lake Ice | Saltwater ice | River ice | Sea ice | Sea ice |
| Ice crystals | Granular/columnar | Columnar | Columnar | Granular/columnar | Granular/columnar mixture | |
| Ice physical indicators | Density | Density, bubbles | Salinity, density | Salinity, Density | Salinity, density | |
| Ice thermal indicators | Laboratory thermal conductivity, identified thermal diffusivity | Laboratory thermal conductivity, identified thermal diffusivity | Laboratory thermal conductivity | Identified thermal diffusivity | Thermal conductivity in situ | Identified thermal diffusivity |
| Literatures | [40,41] | [16] | [44] | [38,39] | [44] | [43] |
4. Thermal Diffusivity Characteristics of Natural Freshwater Ice and Sea Ice
4.1. Thermal Diffusivity Characteristics of Freshwater Ice
4.2. Thermal Diffusivity Characteristics of Sea Ice
5. Future Working Directions and Considerations on the Relation between Thermal Conductivity and Ice Physical Parameters
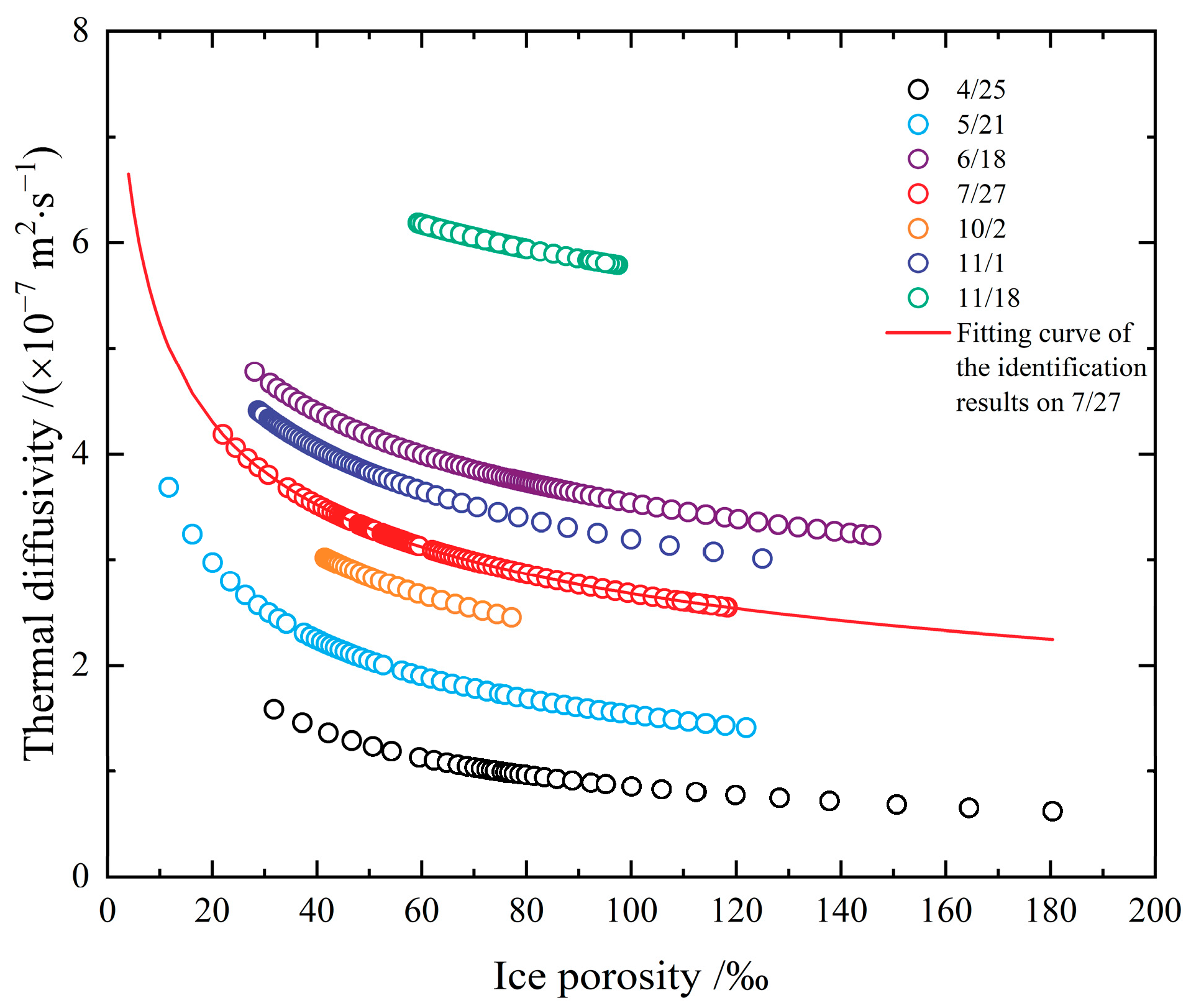
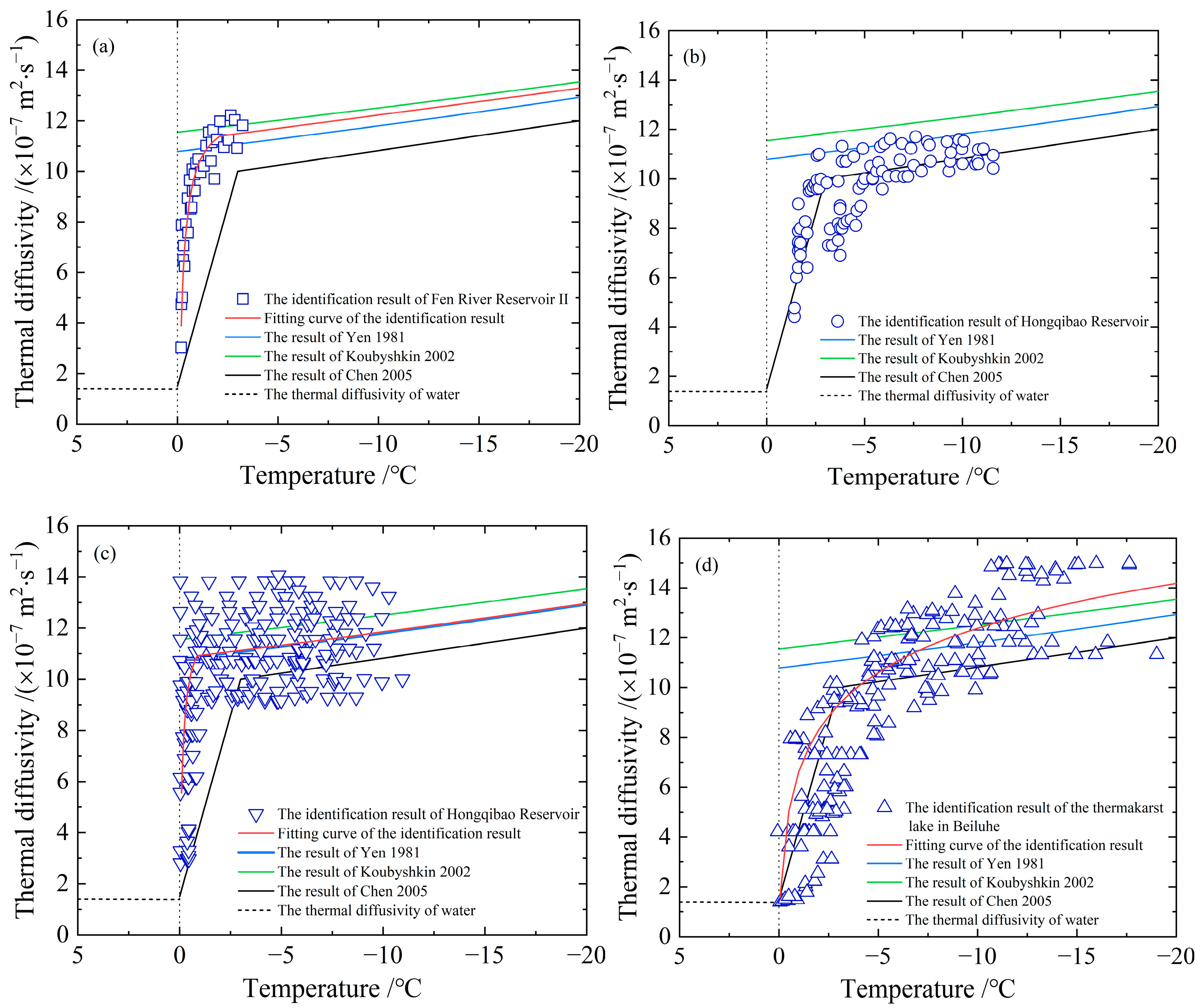
- The thermal diffusivities in Figure 5 are obtained based on measured data of ice temperature from different fields. The heat conduction equation was solved for numerical solutions during the inversion identification process, which relies on the initial boundary value conditions. Hence, different schemes and the initial conditions will generate various results of thermal diffusivity. The optimal parameters of the inversion identification model of the non-linearly distributed parameter system are not absolutely the best solution. However, comprehending from the measured data’s precision, on which the study is based, it will not make a difference in the identified thermal diffusivity. Figure 5a shows the results of a segmented discontinuous approach, which is to divide the temperature into multiple small ranges according to the measured ice temperature. It deems that the thermal diffusivity of ice varies as a linear variation or a power function variation with temperature within small temperature ranges, and the thermal diffusivity was recalculated again in the next temperature range. Although the results of these two calculations have the same function, their thermal diffusivities are different. Compared with other approaches, this method requires a lot of computation, but the resulting thermal diffusivities perform well in the sensitive high-temperature zone of phase transition, and even the smaller the temperature ranges, the better the results. Consequently, the points in the resulting scatter plot in Figure 5a are rather more concentrated, while the results in Figure 5b–d are relatively dispersed for the expansion of the selected temperature range in identification. Moreover, factors such as the time and space step length, interpolation method, and programming algorithm adopted in the identification calculation process can also influence the identification results in varying degrees. Figure 5b,c show the results from different scholars on account of the measurements taken at the same test site in different years, especially the data in Figure 5c covering the measured temperature data applied in Figure 5b. Nevertheless, the dispersion of the two identification results is apparently different because of the different methods. The steps, including how to unify the step length, interpolation, algorithm, etc., also need to be explored to acquire optimal results;
- As the global warming develops, lake ice, river ice, and sea ice are all reducing. In the Arctic area, except for the shortening of the freezing period on a macro level, thinner ice thickness, and a decline in the proportion of multi-year ice, there are also phenomena including an increase in ice temperature, a decrease in ice salinity, a reduction in ice density, and a widening of the varying range of ice density [15]. Likewise, the ice conditions in the Bohai Sea and inland China are also decreasing [54,55]. As the spatial and temporal proportions of comparatively “high-temperature” ice are growing worldwide, the simulation effects will be reduced in reality if the previous data on the relation between the thermal diffusivity of ice and temperature with no regard to the phase transition or constants are adopted for numerical simulations. If the thermal diffusivity of ice reduces, the heat storage capacity of the ice body will be strengthened, and it will cause an increase in entropy in the phase transition process from ice to water or from water to ice, which can moderate the melting or freezing rate of ice. In other words, despite the fact that the thermal conductivity of ice in the phase transition zone of the sea ice in the Bohai Sea was also relatively small in the 1980s [44], the ice in the Bohai Sea covers a comparatively small percentage of the global situation of sea ice, so that it is covered by a large amount of other low-temperature ice. However, the spatial and temporal proportions of ice within the phase transition zone have increased. In this case, previously adopted methods may still be fairly feasible on large-scale issues, but they may no longer be proper to describe the thermodynamic behavior of ice on a finite microscale;
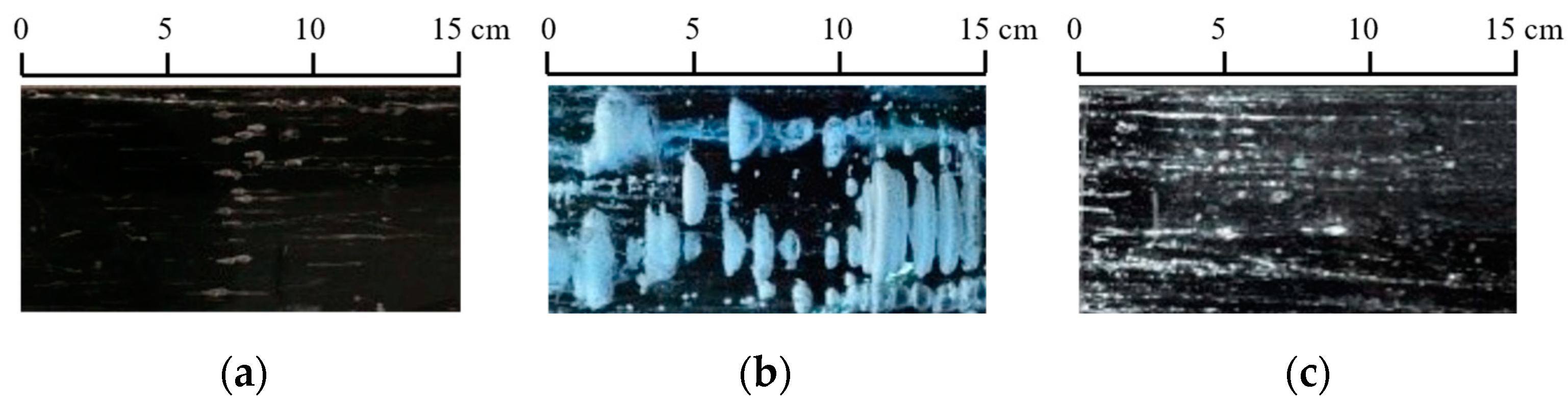
- In the inversion identification of thermal diffusivity, only the time-series data of ice temperature vertical profiles are used, without counting the types of ice crystals at the temperature measurement positions since these types cannot be expressed by numerical values directly. The crystals of ice frozen in calm waters (e.g., reservoir and lake ice) have a pattern of granular ice on the surface and then columnar ice [42]. While this is more complicated in the crystals of ice frozen from rivers and oceans. Granular ice’s properties are basically isotropic, while columnar ice exhibits anisotropy. This can result in a difference between the mathematical models and the calculated results, such as the spread velocity of radar waves in the ice as determined by permittivity [7,56]. Research shows that the thermal conductivities of natural columnar lake ice range from 1.60 W·m−1·°C−1 to 2.20 W·m−1·°C−1 in both the vertical and parallel long axes and are slightly higher (about 5%) in the vertical long axis, showing that the thermal conductivity of ice crystals only has weak anisotropy [16]. From the perspective of the dispersion of inversion-identified ice thermal diffusivities, the differences among the fitted curves and the data points are over 5%. The uncertainty in inversion-identified is larger than the difference in the anisotropy of ice crystals. If 5–10% of the error caused by thermal diffusivity can be accepted on the large scale simulated, the influence of ice crystals can be ignored. In the cases of transformation of ice crystals and overlapping or mixing of granular ice and columnar ice due to dynamics and thermodynamics, the differences of ice crystals can also be neglected. Otherwise, thermal diffusivity models of ice corresponding to various crystal structures should be selected. The ice crystal in the inversion identification of the thermal diffusivity is basically the columnar ice in China;
- If unfrozen water among ice crystals undergoes a phase transition, its mass will remain the same, but its volume ratio will be different, which is the same as the study of frozen soil [53]. If unfrozen water discharges under gravity, it is likely that the partial space originally occupied by unfrozen water will be replaced by gas. In general, the higher the content of bubbles, the lower the ice density. Therefore, ice density can reveal the content of bubbles [56,57] and is an ideal indicator reflecting the effect of bubbles on the thermal diffusivity of ice. If the content of bubbles is less than 3%, the laboratory-tested thermal conductivity of freshwater ice is close to the value [16] calculated by Hamilton and Crosser’s (1962) model [58]. When the content of bubbles is over 16%, any model of the porous medium’s thermal conductivity cannot accurately compute the thermal conductivity [16]. A joint computing model of the thermal diffusivity of lake ice must be built by introducing a shape factor that includes the content and shape of bubbles in the ice. In future ice investigations, promoting the ice density test is indispensable for all models. Meanwhile, focusing on ice density can also reflect two potential scientific issues: First, the ice temperature in the phase transition zone is relatively high, and the bubble content is high because of the discharge of unfrozen water. Secondly, as global warming develops, the plants under shallow lakes in mid-latitudes have higher activity, releasing gases under the ice in winter, and greenhouse gases contained in lake bottoms at high latitudes or high altitudes may be released, such as in the thermokarst lake ice of the Qinghai-Tibet Plateau. Since the thermal diffusivity of bubbles is much higher than that of pure ice, the thermal diffusivity of lake ice with bubbles is larger than the theoretical thermal diffusivity of pure ice. Particularly, the thermal diffusivity of bubble-containing lake ice with a relatively low temperature is more obviously higher than the value of pure ice because the content of unfrozen water reduces;
- Natural freshwater ice contains impurities, and the freezing temperature of unfrozen water is dynamic [31]. Meanwhile, the freezing and melting temperatures of ice with saline water. This shows an irreversible phenomenon in thermodynamics [31,59]. The salinity of sea ice is much higher than that of freshwater ice, and its influence is unmissable. When it comes to freshwater ice, the thermal diffusivity of ice can also be described as the relation between temperature and density if the influence of salinity is ignored, while this is impossible for sea ice because it might need to be an expression of the volume ratio of brine (temperature, salinity, density) and the volume ratio of bubbles (temperature, density). However, the inversion identification result for the thermal diffusivity of Antarctic sea ice (Figure 6) indicates that it is not that simple. It suggests that the refinement of the parameterization for the thermal diffusivity of sea ice is relatively difficult if the thermodynamic irreversible phenomenon is neglected, especially for sea ice in the melting period;
- The previous results of experimental [31] and inversion identification [48] are expressed as segmented functions instead of continuous functions for ice temperature. Since the thermal diffusivity of natural ice is mainly controlled by the thermal diffusivity of pure ice, bubbles, and saline or pure unfrozen water, with the thermal diffusivity of unfrozen water as the lower limit and the thermal diffusivity of bubbles as the upper limit. It is suggested that future development should be based on the logistic functional form, and the suggested Equation (15) form is as follows:where E is the lower limit of Equation (15), and it is close to the thermal diffusivity of unfrozen water (λw), which is 1.38 × 10−7 m2·s−1 at 0 °C. C + E is the upper limit of Equation (15), and it is close to the thermal diffusivity of bubbles (λa). Since the bubble volume ratio is related to the ice density, its expression is Equation (16) [56,57].where ρpi is the density of pure ice, 916.8 kg·m−3, and ρ is the density of natural ice. C is a nonlinear relation because the shape factor should be introduced when the bubble in ice is high in content and large in size. According to the model reported in the literature [17], the form of the power function (17) is suggested as follows.In (15) to (17), B is the maximum growth rate of the thermal diffusivity of natural ice at a certain temperature, A is also related to salinity, D/B is the ice-water phase transition temperature, which is also relatively complicated for unfrozen saline water not closed in the freezing and melting process, α, β, γ are fitting coefficients.
- This consideration is not proved by examples yet because of the sparse density data obtained from natural ice in the field. However, there were measured thermal conductivities at different temperatures (−5, −10, −15, −20, and −25 °C) and different densities (300, 350, 400, and 450 kg.m−3) of snow samples in the laboratory. Hence, this consideration was utilized to make a fitting (R2 = 0.906) analysis of 152 groups of data for the thermal conductivity of snow, indirectly proving the feasibility of this consideration. We look forward to continuing to accumulate field density test data on freshwater ice to confirm the validity of this research orientation;
- The expression of the relation between the thermal diffusivity and porosity of sea ice in Figure 6 is simple, but it exhibits great differences among ice cores. It is hard to explain the physical origin of these differences, either from the aspect of ice ages or bubble volume ratio. Maybe it is incorrect to use temperature and porosity to evaluate the thermal diffusivity of sea ice. The thermal diffusivity probably needs to be expressed as a multi-relation of ice temperature and the volume ratios of brine and bubbles. If this orientation is correct, it will be necessary to collect data on both salinity and density of sea ice. Here, the salinity is computed with electrical conductivity rather than being decided by a chemical analysis of specific substance composition. The laboratory measurements have found that the substance composition also influences thermal diffusivity [31]. The density of sea ice has been an indispensable factor in contemporary physical investigations of sea ice. In the future, we will be developing online measurement technologies for ice salinity and density and discovering refined expressions of the relation between the thermal diffusivity and physical indicators (e.g., temperature, salinity, and density of sea ice).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perovich, D.K.; Elder, B.C.; Richter-Menge, J.A. Observations of the annual cycle of sea ice temperature and mass balance. Geophys. Res. Lett. 1997, 24, 555–558. [Google Scholar] [CrossRef]
- Lu, P.; Cheng, B.; Leppäranta, M.; Li, Z. Modelling on seasonal lake ice evolution in central Asian arid climate zone: A case study. Adv. Polar Sci. 2021, 32, 356–363. [Google Scholar] [CrossRef]
- Magee, M.R.; Wu, C.H. Effects of changing climate on ice cover in three morphometrically different lakes. Hydrol. Process. 2017, 31, 308–323. [Google Scholar] [CrossRef]
- Semtner, A.J. A model for the thermodynamic growth of sea ice in numerical investigations of climate. J. Phys. Oceanogr. 1976, 6, 379–389. [Google Scholar] [CrossRef]
- Pringle, D.J.; Eicken, H.; Trodahl, H.J.; Backstrom, L. Thermal conductivity of landfast Antarctic and Arctic sea ice. J. Geophys. Res. Oceans 2007, 112, C04017. [Google Scholar] [CrossRef] [Green Version]
- Cheng, B.; Vihma, T.; Palo, T.; Nicolaus, M.; Gerland, S.; Rontu, L.; Haapala, J.; Perovich, D. Observation and modelling of snow and sea ice mass balance and its sensitivity to atmospheric forcing during spring and summer 2007 in the Central Arctic. Adv. Polar Sci. 2021, 32, 312–326. [Google Scholar] [CrossRef]
- Li, Z.; Li, C.; Yang, Y.; Zhang, B.; Deng, Y.; Wang, G. Physical scheme and parametrization by using air temperature and ice thickness to improve accuracy of GPR propagation velocity in Yellow River ice. J. Hydrau. Eng. 2022, 53, 902–913. (In Chinese) [Google Scholar] [CrossRef]
- Frankenstein, G.; Garner, R. Equations for determining the brine volume of sea ice from −0.5 °C to −22.9 °C. J. Glaciol. 1967, 6, 943–944. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Wu, Z. On the application of ice porosity in the analysis of ice compressive strength. In Proceedings of the 13th International Symposium on Ice, Beijing, China, 27–30 August 1996. [Google Scholar]
- Li, Z.; Zhang, L.; Lu, P.; Leppäranta, M.; Li, G. Experimental study on the effect of porosity on the uniaxial compressive strength of sea ice in Bohai Sea. Sci. China Technol. Sci. 2011, 54, 2429–2436. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Z.; Lu, P.; Xu, Y.; Li, Z. Flexural and compressive strength of the landfast sea ice in the Prydz Bay, East Antarctic. Cryosphere 2022, 16, 1941–1961. [Google Scholar] [CrossRef]
- Tarovik, O.; Yakimov, V.; Dobrodeev, A.; Li, F. Influence of seasonal and regional variation of ice properties on ship performance in the Arctic. Ocean Eng. 2022, 257, 111563. [Google Scholar] [CrossRef]
- Zhaka, V.; Bridges, R.; Riska, K.; Cwirzen, A. A review of level ice and brash ice growth models. J. Glaciol. 2022, 68, 685–704. [Google Scholar] [CrossRef]
- Karulina, M.; Marchenko, A.; Karulin, E.; Sodhi, D.S.; Sakharov, A.; Chistyakov, P. Full-scale flexural strength of sea ice and freshwater ice in Spitsbergen Fjords and North-West Barents Sea. Appl. Ocean Res. 2019, 90, 101853. [Google Scholar] [CrossRef]
- Wang, Q.; Lu, P.; Leppäranta, M.; Cheng, B.; Zhang, G.; Li, Z. Physical Properties of Summer Sea Ice in the Pacific Sector of the Arctic during 2008–2018. J. Geophys. Res. Oceans 2020, 125, e2020JC016371. [Google Scholar] [CrossRef]
- Huang, W.; Han, H.; Shi, L.; Niu, F.; Deng, Y.; Li, Z. Effective thermal conductivity of thermokarst lake ice in Beiluhe Basin, Qinghai-Tibet Plateau. Cold Reg. Sci. Technol. 2013, 85, 34–41. [Google Scholar] [CrossRef]
- Saito, T.; Hamamoto, S.; Mon, E.E.; Takemura, T.; Saito, H.; Komatsu, T.; Moldrup, P. Thermal properties of boring core samples from the Kanto area, Japan: Development of predictive models for thermal conductivity and diffusivity. Soils Found. 2014, 54, 116–125. [Google Scholar] [CrossRef] [Green Version]
- Dong, S.; Gao, X.; Ma, Z.; Wang, X.; Gao, C. Ice-templated porous silicate cement with hierarchical porosity. Mater. Lett. 2018, 217, 292–295. [Google Scholar] [CrossRef]
- Arkhangelskaya, T.; Lukyashchenko, K. Estimating soil thermal diffusivity at different water contents from easily available data on soil texture, bulk density, and organic carbon content. Biosyst. Eng. 2018, 168, 83–95. [Google Scholar] [CrossRef]
- Zhao, J.; Sun, S.; Liu, C.; Meng, Q. Thermal conductivity and thermal diffusivity of methane hydrate formed from compacted granular ice. Heat Mass Transf. 2018, 54, 3287–3295. [Google Scholar] [CrossRef]
- Hammerschmidt, U. Thermal transport properties of water and ice from one single experiment. Int. J. Thermophys. 2002, 23, 975–996. [Google Scholar] [CrossRef]
- Calonne, N.; Milliancourt, L.; Burr, A.; Philip, A.; Martin, C.; Flin, F.; Geindreau, C. Thermal conductivity of snow, firn, and porous ice from 3-D image-based computations. Geophys. Res. Lett. 2019, 46, 13079–13089. [Google Scholar] [CrossRef]
- Ling, F.; Zhang, T. Simulating heat source effect of a thermokarst lake in the first 540 years on the Alaskan Arctic using a simple lake expanding model. Cold Reg. Sci. Technol. 2019, 160, 176–183. [Google Scholar] [CrossRef]
- Zhang, T.; Osterkamp, T.E. Considerations in determining thermal diffusivity from temperature time series using finite difference methods. Cold Reg. Sci. Technol. 1995, 23, 333–341. [Google Scholar] [CrossRef]
- Tong, B.; Xu, H.; Horton, R.; Bian, L.; Guo, J. Determination of long-term soil apparent thermal diffusivity using near-surface soil temperature on the Tibetan Plateau. Remote Sens. 2022, 14, 4238. [Google Scholar] [CrossRef]
- Adams, W.M.; Watts, G.; Mason, G. Estimation of thermal diffusivity from field observations of temperature as a function of time and depth. Am. Mineral. 1976, 61, 560–568. [Google Scholar]
- Shi, L.; Li, Z.; Niu, F.; Hang, W.; Lu, P.; Feng, E.; Hang, H. Thermal diffusivity of thermokarst lake ice in the Beiluhe basin of the Qinghai-Tibetan Plateau. Ann. Glaciol. 2014, 55, 153–158. [Google Scholar] [CrossRef] [Green Version]
- Schwerdtfecer, P. The thermal properties of sea ice. J. Glaciol. 1963, 4, 789–807. [Google Scholar] [CrossRef] [Green Version]
- Yen, Y.C. Review of Thermal Properties of Snow, Ice and Sea Ice; CRREL Report 81-10; Cold Regions Research and Engineering Laboratory: Hanover, NH, USA, 1981. [Google Scholar]
- Koubyshkin, N.V.; Sazonov, K.E. Evaluation of loads due to partial freezing of seawater trapped in enclosed cavities. In Proceedings of the17th International Symposium on Ice, Saint Petersburg, Russia, 21–25 June 2002. [Google Scholar]
- Chen, N.J.; Morikawa, J.; Kishi, A.; Hashimoto, T. Thermal diffusivity of eutectic of alkali chloride and ice in the freezing-thawing process by temperature wave analysis. Thermochim. Acta 2005, 429, 73–79. [Google Scholar] [CrossRef]
- James, D.W. The thermal diffusivity of ice and water between −40 and +60 °C. J. Mater. Sci. 1968, 3, 540–543. [Google Scholar] [CrossRef]
- Cox, G.F.N.; Weeks, W.F. Equations for determining the gas and brine volumes in sea-ice samples. J. Glaciol. 1983, 29, 306–316. [Google Scholar] [CrossRef] [Green Version]
- Leppäranta, M.; Manninen, T. The Brine and Gas Content of Sea Ice with Attention to Low Salinities and High Temperatures; Finnish Institute of Marine Research: Helsinki, Finland, 1988. [Google Scholar]
- Reid, T.; Crout, N. A thermodynamic model of freshwater Antarctic lake ice. Ecol. Model. 2008, 210, 231–241. [Google Scholar] [CrossRef]
- Shi, L.; Li, Z.; Feng, E.; Bai, Y.; Yang, Y. Thermal diffusivity identification of distributed parameter systems to sea ice. J. Appl. Math. 2013, 2013, 760378. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.; Bai, Y.; Li, Z.; Cheng, B.; Leppäranta, M. Preliminary results on relationship between thermal diffusivity and porosity of sea ice in the Antarctic. Chin. J. Polar Sci. 2009, 20, 72–80. [Google Scholar] [CrossRef]
- Bai, Y. Parameter Identification of Non-Smooth Parameter Systems and Its Application; Dalian University of Technology: Dalian, China, 2006. (In Chinese) [Google Scholar]
- Bai, Y.; Li, Z.; Han, M.; Lu, P. Approaches to revise ice thermal diffusivity from measured time series of temperature in a river. In Proceedings of the 6th International Symposium on Test and Measurement, Dalian, China, 1–4 June 2005. [Google Scholar]
- Huang, W.; Li, Z.; Han, H.; Jia, Q. Limit resistive forces from ice frozen to concrete-revetment interface of an inclined dam wall. Cold Reg. Sci. Technol. 2017, 141, 181–187. [Google Scholar] [CrossRef]
- Huang, W.; Li, Z.; Liu, X.; Zhao, H.; Guo, S.; Jia, Q. Effective thermal conductivity of reservoir freshwater ice with attention to high temperature. Ann. Glaciol. 2013, 54, 189–195. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Huang, W.; Jia, Q.; Leppäranta, M. Distributions of crystals and gas bubbles in reservoir ice during growth period. Water Sci. Eng. 2011, 4, 204–211. [Google Scholar] [CrossRef]
- Lei, R.; Li, Z.; Zhang, Z.; Chen, Y. Thermodynamic processes of lake ice and landfast ice around Zhongshan Station, east Antarctica. Adv. Polar Sci. 2011, 22, 143–152. [Google Scholar] [CrossRef]
- LI, Z.; Yan, D.; Meng, G.; Zhang, M. Relation of thermal conductivity coefficient with temperature of sea ice in Bohai Sea. In Proceedings of the International Offshore Mechanics and Arctic Engineering, Montreal, QC, Canada, 7–12 June 1992. [Google Scholar]
- Li, Z.; Sui, J.; Yan, D.; Meng, G. Temperature, salinity, and density profiles in a fast ice sheet in Liao Dong Bay. In Proceedings of the 10th International Conference on Port an d Ocean Engineering under Artic Conditions, Luleå, Sweden, 12–16 June 1989. [Google Scholar]
- Shi, L.; Li, Z.; Lu, P.; Feng, E. Influence of ice temperature on thermal diffusivity of natural freshwater ice. J. Hydraul. Eng. 2013, 44, 1112–1117. (In Chinese) [Google Scholar] [CrossRef]
- Huang, W.; Li, Z.; Han, H.; Niu, F.; Lin, Z.; Leppäranta, M. Structural analysis of thermokarst lake ice in Beiluhe Basin, Qinghai-Tibet Plateau. Cold Reg. Sci. Technol. 2012, 72, 33–42. [Google Scholar] [CrossRef]
- Bai, Y.; Xu, H.; Shi, L. Research on the optimal identification of thermal diffusivity of fresh ice in reservoirs of cold regions. In Proceedings of the 21st IAHR International Symposium on Ice, Ice Research for a Sustainable Environment, Dalian, China, 11–15 June 2012. [Google Scholar]
- Nicolaus, M.; Wang, C.; Gerland, S.; Li, N.; Li, Z.; Cheng, B.; Perovich, D.; Granskog, M.; Shi, L.; Lei, R.; et al. Advancing the understanding of variations of Arctic sea ice optical and thermal behaviors through an international research and mobility project. Adv. Polar Sci. 2015, 26, 179–187. [Google Scholar] [CrossRef]
- Cheng, P.; Li, J.; Yu, D.; Hao, Z. Study of the ice cover temperature variation of the Kanas Lake. J. Glaciol. Geocryol. 2016, 38, 692–698. (In Chinese) [Google Scholar] [CrossRef]
- Congé, C.; Andrieu, J.; Laurent, P.; Ferrand, J. Experimental data and modelling of thermal properties of ice creams. J. Food Eng. 2003, 58, 331–341. [Google Scholar] [CrossRef]
- Shum, E.; Papangelakis, V. Water recovery from inorganic solutions via natural freezing and melting. J. Water Process Eng. 2019, 31, 100787. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Lai, Y.; Yu, F.; Li, F.; Yang, S. Estimating the freezing-thawing hysteresis of chloride saline soils based on the phase transition theory. Appl. Therm. Eng. 2018, 135, 22–33. [Google Scholar] [CrossRef]
- Ma, Y.; Cheng, B.; Xu, N.; Yuan, S.; Shi, H.; Shi, W. Long-term ice conditions in Yingkou, a coastal region northeast of the Bohai Sea, between 1951/1952 and 2017/2018: Modeling and observations. Remote Sens. 2022, 14, 182. [Google Scholar] [CrossRef]
- Yan, Y.; Uotila, P.; Huang, K.; Gu, W. Variability of sea ice area in the Bohai Sea from 1958 to 2015. Sci. Total Environ. 2020, 709, 136164. [Google Scholar] [CrossRef]
- Li, Z.; Jia, Q.; Zhang, B.; Matti, L.; Lu, P.; Lu, W. Influences of gas bubble and ice density on ice thickness measurement by GPR. Appl. Geophys. 2010, 7, 105–113. [Google Scholar] [CrossRef]
- Gherboudj, I.; Bernier, M.; Hicks, F.; Leconte, R. Physical characterization of air inclusions in river ice. Cold Reg. Sci. Technol. 2007, 49, 179–194. [Google Scholar] [CrossRef]
- Hamilton, R.L.; Crosser, O.K. Thermal conductivity of heterogeneous two-component systems. Ind. Eng. Chem. Fundam. 1962, 1, 187–191. [Google Scholar] [CrossRef]
- Overduin, P.P.; Kane, D.L.; van Loon, W.K.P. Measuring thermal conductivity in freezing and thawing soil using the soil temperature response to heating. Cold Reg. Sci. Technol. 2006, 45, 8–22. [Google Scholar] [CrossRef] [Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Fu, X.; Shi, L.; Huang, W.; Li, C. Recent Advances and Challenges in the Inverse Identification of Thermal Diffusivity of Natural Ice in China. Water 2023, 15, 1041. https://doi.org/10.3390/w15061041
Li Z, Fu X, Shi L, Huang W, Li C. Recent Advances and Challenges in the Inverse Identification of Thermal Diffusivity of Natural Ice in China. Water. 2023; 15(6):1041. https://doi.org/10.3390/w15061041
Chicago/Turabian StyleLi, Zhijun, Xiang Fu, Liqiong Shi, Wenfeng Huang, and Chunjiang Li. 2023. "Recent Advances and Challenges in the Inverse Identification of Thermal Diffusivity of Natural Ice in China" Water 15, no. 6: 1041. https://doi.org/10.3390/w15061041
APA StyleLi, Z., Fu, X., Shi, L., Huang, W., & Li, C. (2023). Recent Advances and Challenges in the Inverse Identification of Thermal Diffusivity of Natural Ice in China. Water, 15(6), 1041. https://doi.org/10.3390/w15061041