Geochemical Features of River Runoff and Their Effect on the State of the Aquatic Environment of Lake Onego
Abstract
:1. Introduction
2. Material and Methods
3. Results and Discussion
3.1. Chemical Composition of River Water
3.2. Suspended Matter in River Waters
3.3. Content of Metals in Suspended Matter from the Water of the Tributaries of Lake Onego
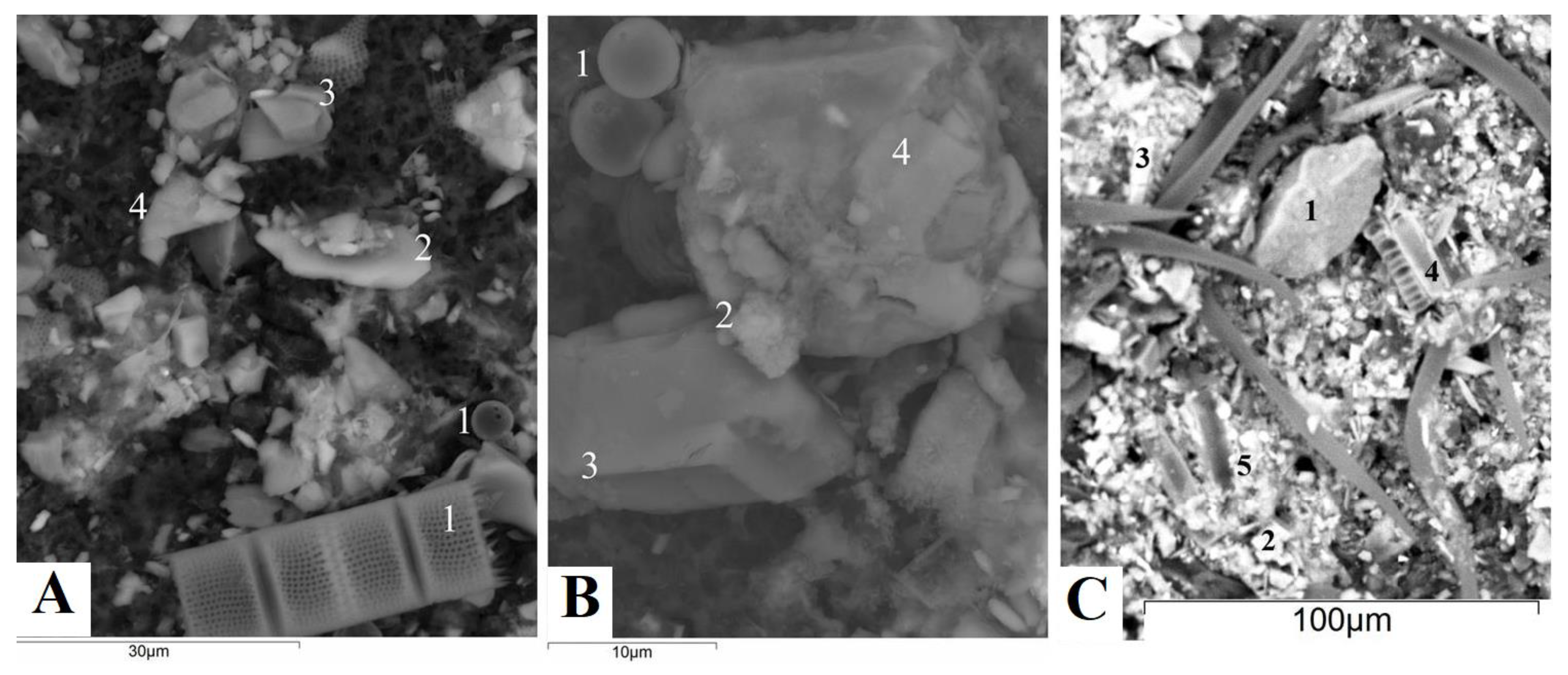
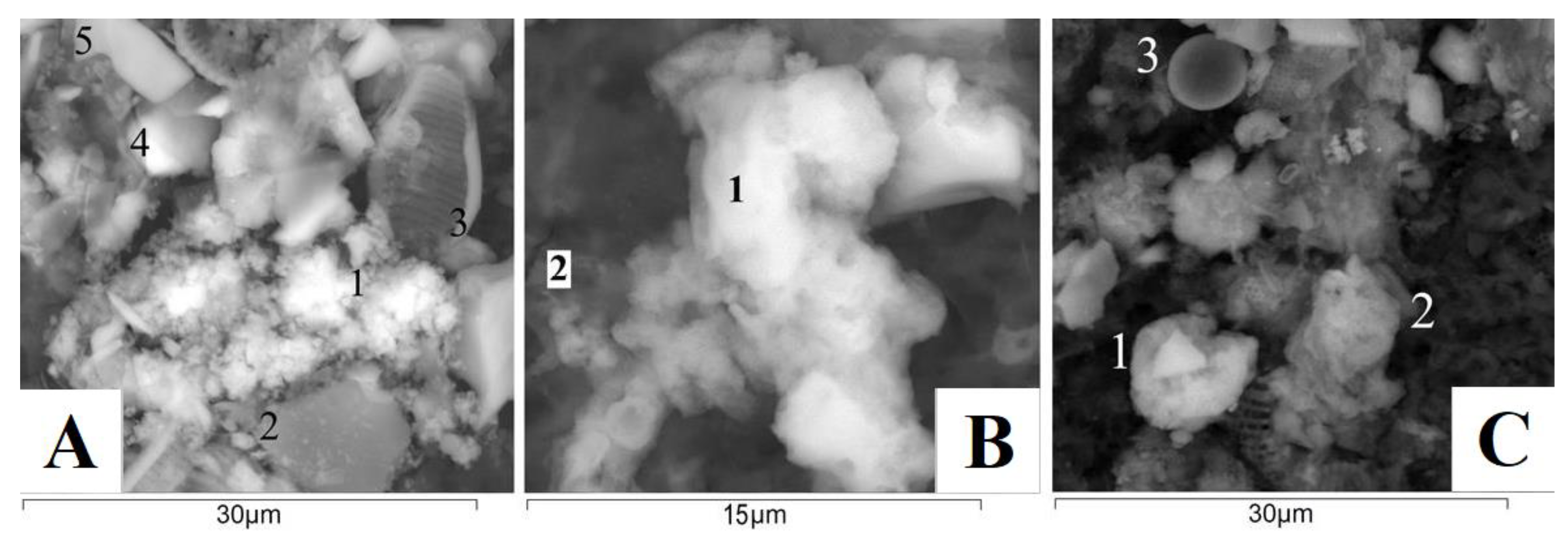
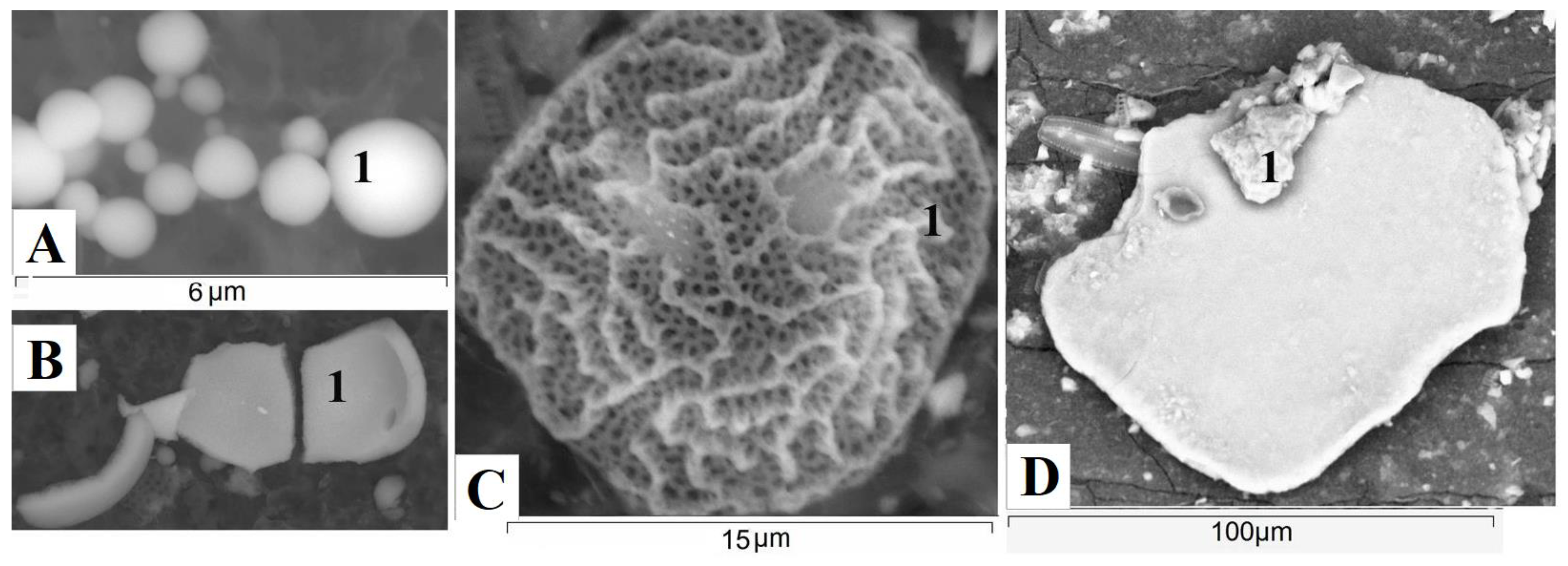
3.4. Mineral Composition of Suspended Matter of Rivers
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Moore, J.W.; Ramamoorthy, S. Heavy Metals in Natural Waters. In Applied Monitoring and Impact Assessment; Springer: New York, NY, USA, 1984; 268p, ISBN 978-1-4612-5210-8. [Google Scholar]
- Reimann, C.; Ayras, M.; Chekushin, V.; Bogatyrev, I.; Boyd, R.; de Caritat, P.; Dutter, R.; Finne, T.E.; Halleraker, J.H.; Jaeger, O.; et al. A Geochemical Atlas of the Central Parts of the Barents Region; NorgesGeologiskeUndersokelse (NGU) Geological Survey of Norway: Trondheim, Norway, 1998; 745p, ISBN 82-7385-176-1. [Google Scholar]
- Marx, S.K.; Rashid, S.; Stromsoe, N. Global-scale patterns in anthropogenic Pb contamination reconstructed from natural archives. Environ. Pollut. 2016, 213, 283–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pratte, S.; Bao, K.; Shen, J.; Mackenzie, L.; Klamt, A.M.; Wang, G.; Xing, W. Recent atmospheric metal deposition in peatlands of Northeast China: A review. Sci. Total Environ. 2018, 626, 1284–1294. [Google Scholar] [CrossRef]
- Zubova, E.M.; Kashulin, N.A.; Dauvalter, V.A.; Denisov, D.B.; Valkova, S.A.; Vandysh, O.I.; Slukovskii, Z.I.; Terentyev, P.M.; Cherepanov, A.A. Long-Term Environmental Monitoring in an Arctic Lake Polluted by Metals under Climate Change. Environments 2020, 7, 34. [Google Scholar] [CrossRef]
- Denisov, D.; Terentjev, P.; Valkova, S.; Kudryavtzeva, L. Small Lakes Ecosystems under the Impact of Non-Ferrous Metallurgy (Russia, Murmansk Region). Environments 2020, 7, 29. [Google Scholar] [CrossRef] [Green Version]
- Malakhov, S.G.; Makhon’ko, E.P. Emission of toxic metals into the atmosphere and their accumulation in the surface layer of the Earth. Russ. Chem. Rev. 1990, 59, 1777–1798. [Google Scholar] [CrossRef]
- Fedorets, N.G. Geochemical features of sandy illuvial-humus-ferruginous podzols on binomial deposits. In Ecological-Geochemical and Biological Patterns in Soil Formation in Taiga Forest Ecosystems; Fedorets, N.G., Bakhmet, O.N., Eds.; KarRC RAS Publishing House: Petrozavodsk, Russia, 2009; pp. 18–30. ISBN 978-5-9274-0379-0. [Google Scholar]
- Ryanzhin, S.V.; Subetto, D.A.; Klochkov, N.V.; Akhmetova, N.S.; Weinmeister, N.A. Polar lakes of the world: Current data and state of research. Water Resour. 2010, 37, 387–397. [Google Scholar] [CrossRef]
- Maslov, A.V.; Ronkin, Y.L.; Lepikhina, O.P.; Shevchenko, V.P.; Novigatsky, A.N.; Filippov, A.S.; Shevchenko, N.V. Peculiarities of the rare-earth element distribution in the modern bottom sediments of the White Sea and the lower reaches of the Severnaya Dvina river. Oceanology 2013, 53, 702–714. [Google Scholar] [CrossRef]
- Filatov, N.N.; Kulikova, T.P.; Lozovik, P.A. (Eds.) The current state of the water bodies of the Republic of Karelia. In Based on the Results of Monitoring 1992–1997; KarRC RAS Publishing House: Petrozavodsk, Russia, 1998; 30p, ISBN 5-201-07969-5. [Google Scholar]
- Onega Lake. Ecological Problem; Filatov, N.N., Ed.; KarRC RAS Publishing House: Petrozavodsk, Russia, 1999; 292p, ISBN 5-88741-016-7. [Google Scholar]
- Lozovik, P.A.; Kulikova, T.P.; Martynova, N.N. (Eds.) Status of Water Objects in Republic of Karelia. In Based on the Results of Monitoring in 1998–2006; KarRC RAS Publishing House: Petrozavodsk, Russia, 2007; 210p, ISBN 978-5-9274-0299-1. [Google Scholar]
- Filatov, N.N.; Kalinkina, N.M.; Kulikova, T.P.; Litvinenko, A.V.; Lozovik, P.A. (Eds.) The Largest Lakes-Reservoirs of the North-West European Part of Russia: Current State and Changes of Ecosystems under Climate Variability and Antropogenic Impact; KarRC RAS Publishing House: Petrozavodsk, Russia, 2015; 375p, ISBN 978-5-9274-666-1. [Google Scholar]
- Lozovik, P.A.; Kulik, N.V.; Efremenko, N.A. Lithophile elements and heavy metals in lake Onego: Sources, concentrations and transformation. Trans. Karelian Res. Cent. Russ. Acad. Sci. 2020, 4, 62–74. [Google Scholar] [CrossRef]
- Kulik, N.V.; Belkina, N.A.; Efremenko, N.A. Introduction, transformation and distribution of Manganese in lake Onego. Mosc. Econ. J. 2020, 1, 13. [Google Scholar] [CrossRef]
- Andronikov, A.V.; Novak, M.; Kram, P.; Sebek, O.; Andronikova, I.E.; Efremenko, N.A.; Borodulina, G.S.; Subetto, D.A.; Stepanova, M.; Antalova, E.; et al. Behaviour of Cr in runoff from two catchments underlain by felsic bedrock. Hydrol. Sci. J. 2020, 65, 2765–2782. [Google Scholar] [CrossRef]
- Shvets, P.D. Water balance of Lake Onego. In Studies of the Regime and Calculations of the Water Balance of Lakes-Reservoirs of Karelia; Smirnova, A.V., Shvets, P.D., Karaeva, Y.K., Eds.; Hydrometeoizdat: Leningrad, Russia, 1977; Volume 11, pp. 25–53. [Google Scholar]
- Arestova, N.A.; Chekulaev, V.P.; Lobach-Zhuchenko, S.B.; Kucherovskii, G.A. Formation of the Archean crust of the ancient Vodlozero domain (Baltic shield). Stratigr. Geol. Correl. 2015, 23, 119–130. [Google Scholar] [CrossRef]
- Glushanin, L.V.; Sharov, N.V.; Shchiptsov, V.V. (Eds.) Palaeoproterozoic Onega Structure (Geology, Tectonics, Deep Structure and Mineralogeny); KarRC RAS Publishing House: Petrozavodsk, Russia, 2011; 430p, ISBN 978-5-9274-0456-8. [Google Scholar]
- Saarnisto, M.; Saarinen, T. Deglaciation chronology of the Scandinavian Ice Sheet from the lake Onega basin to the Salpausselkya End Moraine. Glob. Planet. Chang. 2001, 31, 387–405. [Google Scholar] [CrossRef]
- Filatov, N.N.; Bakhmet, O.N. (Eds.) Atlas of the Republic of Karelia; Verso Publishing House: Petrozavodsk, Russia, 2021; pp. 12–13. ISBN 978-5-91997-395-9. [Google Scholar]
- Onego Lake. Atlas; Filatov, N.N., Ed.; KarRC RAS Publishing House: Petrozavodsk, Russia, 2010; 151p, ISBN 978-5-9274-0432-2. [Google Scholar]
- Boeva, L.V. (Ed.) Guidelines for the Chemical Analysis of Land Surface Waters; NOC Publishing House: Rostov-on-Don, Russia; NOC Publishing House: Novocherkassk, Russia, 2009; Volume 1, 21p, ISBN 978-5-8431-01428. [Google Scholar]
- Lozovik, P.A.; Efremenko, N.A. (Eds.) Analytical, Kinetic and Computational Methods in Hydrochemical Practice; Nestor-History: St. Petersburg, Russia, 2017; p. 272. ISBN 978-5-4469-1207-0. [Google Scholar]
- Zobkov, M.; Zobkova, M.; Galakhina, N.; Efremova, T.; Efremenko, N.; Kulik, N. Data on the Chemical Composition of Lake Onego Water in 2019–2021. Data Brief 2022, 42, 108079. [Google Scholar] [CrossRef] [PubMed]
- Alekin, O.A. Fundamentals of Hydrochemistry; Bruevich, S.V., Ed.; Hydrometeorological Publishing House: Leningrad, Russia, 1970; pp. 120–121. [Google Scholar]
- Kulik, N.V.; Efremenko, N.A.; Strakhovenko, V.D.; Belkina, N.A. Features of migration of Fe, Mn, Al, Cu and Zn in the Onego Lake. Limnol. Freshw. Biol. 2020, 4, 505–506. [Google Scholar] [CrossRef]
- Lozovik, P.A. Hydrogeochemical Criteria of the State of Surface Waters of the Humid Zone and Their Resistance to Anthropogenic Impact. Ph.D. Thesis, Vernadsky Institute, Moscow, Russia, 2006; 56p. [Google Scholar]
- Ryzhakov, A.V.; Vapirov, V.V.; Stepanova, I.A. Silicon in surface waters of the humid zone (the case of Karelian waterbodies). Trans. Karelian Res. Cent. Russ. Acad. Sci. 2019, 3, 52–60. [Google Scholar] [CrossRef] [Green Version]
- Lozovik, P.A.; Zobkov, M.B.; Borodulina, G.S.; Tokarev, I.V. Assessing external water exchange of lake bays by water chemistry characteristics. Water Resour. 2019, 46, 94–102. [Google Scholar] [CrossRef]
- Borodulina, G.; Tokarev, I.; Avramenko, I. Investigation of small river watershed hydrology in Karelia (north-west Russia) by high-resolution record of δ2H and δ18O in precipitation and river discharge, including experimental estimates of evaporation. In Book of Extended Synopses, Proceedings of the International Symposium on Isotope Hydrology: Revisiting Foundations and Exploring Frontiers, Vienna, Austria, 11–15 May 2015; IAEA: Vienna, Austria, 2015; pp. 174–176. [Google Scholar]
- Ferronsky, V.I.; Dubinchuk, V.T.; Polyakov, V.A.; Seletsky, Y.B.; Kuptsov, V.M.; Yakubovsky, A.V. Natural Isotopes of the Hydrosphere; Nedra: Moscow, Russia, 1975; 280p. [Google Scholar]
- Gordeev, V.V. Geochemistry of the River—Sea System; Publishing house Matushkina, I.I.: Moscow, Russia, 2012; 452p, ISBN 978-5-94101-261-9. [Google Scholar]
- Gaillardet, J.; Viers, J.; Dupré, B. Trace Elements in River Waters. In Treatise on Geochemistry. Surface and Ground Water, Weathering, and Soils; Drever, J.I., Holland, H.D., Turekian, K.K., Eds.; Elsevier Science: Oxford, UK, 2005; Volume 5, pp. 225–272. ISBN 0-08-044719-8. [Google Scholar]
- Savenko, A.V.; Savenko, V.S.; Pokrovsky, O.S. New data on the concentrations of dissolved trace elements in waters of Russian Arctic rivers. Dokl. Earth Sci. 2020, 491, 257–263. [Google Scholar] [CrossRef]
- Strakhovenko, V.D.; Belkina, N.A.; Efremenko, N.A.; Potakhin, M.S.; Subetto, D.A.; Frolova, L.A.; Nigamatzyanova, G.R.; Ludikova, A.V.; Ovdina, E.A. The First Data on the Mineralogy and Geochemistry of the Suspension of Lake Onego. Russ. Geol. Geophys. 2022, 63, 55–71. [Google Scholar] [CrossRef]
- Chudaeva, V.A.; Shesterkin, V.P.; Chudaev, O.V. Trace elements in surface water in Amur river basin. Water Resour. 2011, 38, 650–661. [Google Scholar] [CrossRef]
- Gordeev, V.V.; Lisitsyn, A.P. Average chemical composition of suspensions of the rivers of the world and the supply of oceans with river sedimentary material. Dokl. Akad. Nauk SSSR 1978, 238, 255–258. [Google Scholar]
- Savenko, V.S. Chemical Composition of Suspended Sediments of the Rivers of the World; GEOS: Moscow, Russia, 2006; 174p, ISBN 5-89118-345-5. [Google Scholar]
- Viers, J.; Dupré, B.; Gaillardet, J. Chemical composition of suspended sediments in World Rivers: New insights from a new database. Sci. Total Environ. 2009, 407, 853–868. [Google Scholar] [CrossRef]
- Chudaeva, V.A.; Chudaev, O.V. Specific features of chemical composition of the water and suspended matter of Primorye rivers (Far East Russia). Russ. J. Pac. Geol. 2011, 30, 102–119. [Google Scholar]
- Shevchenko, V.P.; Filippov, A.S.; Lisitsyn, A.P.; Zolotykh, E.O.; Isaeva, A.B.; Kravchishina, M.D.; Novigatsky, A.N.; Politova, N.V.; Pokrovsky, O.S.; Bobrov, V.A.; et al. On the elemental composition of suspended matter of the Severnaya Dvina river (White Sea region). Dokl. Earth Sci. 2010, 430, 228–234. [Google Scholar] [CrossRef]
- Puzanov, A.V.; Baboshkina, S.V. Microelements in surface waters of Altai. Izv. Samara Sci. Cent. Russ. Acad. Sci. 2009, 11, 344–346. [Google Scholar]
- Taylor, S.R.; McLennan, S.M. The Continental Crust: Its Composition and Evolution; Blackwell Scientific: Oxford, UK; Boston, MA, USA, 1985; 312p, ISBN 0-632-01148-3. [Google Scholar]
- Fedorets, N.G.; Bakhmet, O.N.; Medvedeva, M.V.; Akhmetova, G.V.; Novikov, S.G.; Tkachenko, Y.N.; Solodovnikov, A.N. Heavy Metals in Soils of Karelia; Akhmetova, G.V., Ed.; KarRC RAS Publishing House, Petrozavodsk, Russia, 2015; 220p, ISBN 978-5-9274-0674-6. [Google Scholar]
- Belkina, N.A.; Efremenko, N.A.; Kulik, N.V. Specifics of iron migration, transformation, and accumulation in the Vygozero reservoir. Water Resour. 2018, 45, 738–745. [Google Scholar] [CrossRef]
- Strakhov, N.M. Fundamentals of the Theory of Lithogenesis, Publishing House of the USSR Academy of Sciences: Moscow, Russia, 1960–1962; Volume 1; Volume 2; Volume 3, 212p; 574p; 550p.
- Sawhney, B.L. Selective Sorption and Fixation of Cations by Clay Minerals: A Review. Clays Clay Miner 1972, 20, 93–100. [Google Scholar] [CrossRef]
- Elbaz-Poulichet, F.; Seyler, P.; Maurice-Bourgoin, L.; Guyot, J.-L.; Dupuy, C. Trace element geochemistry in the upper Amazon drainage basin (Bolivia). Chem. Geol. 1999, 157, 319–334. [Google Scholar] [CrossRef]
- Belkina, N.A.; Vapirov, V.V.; Efremenko, N.A.; Romanova, T.N. On the question of how the natural migration of copper in lake Onega. Princ. Ecol. 2012, 1, 25–28. [Google Scholar] [CrossRef] [Green Version]
- Schafer, J.; Blanc, G. Relationship between ore deposits in river catchments and geochemistry of suspended matter from six rivers in southwest France. Sci. Total Environ. 2002, 298, 103–118. [Google Scholar] [CrossRef]
- Dauvalter, V.A. Biogeochemical Features of the Distribution of Chalcophilic Elements (Hg, Cd, Pb, As) in the Reservoirs of the North of the European Part of Russia; Bauman Press: Murmansk, Russia, 2015; 136p, ISBN 978-5-86185-836-6. [Google Scholar]
- Reeder, S.M.; Hitchon, B.; Levinson, A.A. Hydrogeochemistry of the surface waters of the Mackenzie River drainage basin, Canada-1. Factors controlling inorganic composition. Geochim. Et Cosmochim. Acta 1972, 36, 825–865. [Google Scholar] [CrossRef]
- Savenko, V.S. Chemical composition of sediment load carried by rivers. Geochem. Int. 2007, 45, 816–824. [Google Scholar] [CrossRef]
- Maslov, A.V.; Shevchenko, V.P. Ree–Th systematics of the suspended particulate matter and bottom sediments from the mouth zones of the world rivers of different categories/classes and some large Russian Arctic rivers. Geochem. Int. 2019, 57, 56–73. [Google Scholar] [CrossRef]
- Lisitzin, A.P. Arid sedimentation in the oceans and atmospheric particulate matter. Russ. Geol. Geophys. 2011, 52, 1100–1133. [Google Scholar] [CrossRef]
- Strakhovenko, V.; Belkina, N.; Subetto, D.; Rybalko, A.; Efremenko, N.; Kulik, N.; Potakhin, M.; Zobkov, M.; Ovdina, E.; Ludikova, A. Distribution of rare earth elements and yttrium in water, suspended matter and bottom sediments in Lake Onego: Evidence of the watershed transformation in the Late Pleistocene. Quat. Int. 2023, 644–645, 120–133. [Google Scholar] [CrossRef]
- Kulik, N.; Efremenko, N.; Belkina, N.; Strakhovenko, V.; Gatalskaya, E.; Orlov, A. Fe, Mn, Al, Cu, Zn, and Cr in the sedimentary matter of Lake Onego. Quat. Int. 2023, 644–645, 134–144. [Google Scholar] [CrossRef]
- Kulik, N.; Efremenko, N.; Strakhovenko, V.; Belkina, N.; Borodulina, G.; Gatalskaya, E.; Malov, V.; Tokarev, I. Geochemical features of river runoff and their effect on the state of the aquatic environment of Lake Onego. Mendeley Data 2023, V2. [Google Scholar] [CrossRef]
- Kulik, N.; Efremenko, N.; Belkina, N.; Strakhovenko, V.; Gatalskaya, E. Sedimentogenesis of Fe, Mn, Al, Cu, Zn and Cr in the lake Onego. Mendeley Data 2021, v1. [Google Scholar] [CrossRef]
- Strakhovenko, V.; Belkina, N.; Subetto, D.; Rybalko, A.; Efremenko, N.; Kulik, N.; Potakhin, M.; Zobkov, M.; Ovdina, E.; Ludicova, A. Distribution of Rare Earth Elements and Yttrium in Water, Suspended Matter and Bottom Sediments in Lake Onego: Evidence of the Watershed Transformation in the Late Pleistocene. Mendeley Data 2021, 1. [Google Scholar] [CrossRef]







| Geological Structure | Coast | River | Flow Volume, km3/Year | Length, km | Watershed | ||
|---|---|---|---|---|---|---|---|
| Area, Thousand km2 | Lacustrine Nature of the Territory, % | Swampiness of the Territory, % | |||||
| Fennoscandian Crystalline Shield (FCS) (I) | Northwest | Lososinka | 0.12 | 25 | 0.3 | 5.7 | 10 |
| Shuya | 3.09 | 279 | 10.3 | 10.4 | ~20 | ||
| Suna | 2.34 | 282 | 7.67 | 12.5 | 19 | ||
| North | Kumsa | 0.23 | 67 | 0.74 | 8.5 | 7 | |
| Eastern | Vodla | 4.63 | 406 | 13.7 | 5.6 | 24 | |
| Southeastern | Andoma | 1.03 | 142 | 2.57 | 1.3 | 12 | |
| Southwest | Derevyanka | 0.03 | 20 | 0.093 | 0.4 | – | |
| Sheltozerka | 0.03 | 11 | 0.069 | 3.9 | – | ||
| The central part of the East European Platform (II) | Southern | Vytegra | 0.52 | 40 | 1.67 | <1 | 12 |
| Svir’ | 24.9 | 224 | 84.4 | – | – | ||
| Geological Structure | Coast | River | Season | pH | Ntot | Norg | Pmin | Ptot | BOD | PI, mgO/L | COD, mgO/L | Si (Filtered) | Color | Σions | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mgN/L | mgN/L | mgN/L | mgN/L | μg/L | μg/L | mgO/L | Not Filtered | Filtered | Not Filtered | mg/L | °Pt | mg/L | |||||
| The Fennoscandian Crystalline Shield (FCS) (I) | Northwest | Lososinka (before the city) | Spring | 7.01 | 0.013 | <0.01 | 0.40 | 0.39 | 17 | 46 | 1.91 | 18.8 | 27.9 | 29.4 | 2.67 | 136 | 26 |
| Summer | 7.37 | 0.051 | 0.009 | 0.60 | 0.54 | 48 | 91 | 1.74 | 19.9 | 43.4 | 45.4 | 2.68 | 154 | 39 | |||
| Autumn | 7.01 | 0.046 | 0.042 | 0.54 | 0.45 | 26 | 50 | 1.57 | 23.5 | 44.8 | 45.6 | 2.74 | 169 | 30 | |||
| Winter | 6.59 | 0.055 | 0.104 | 0.67 | 0.51 | 15 | 155 | 2.67 | 26.3 | 53.9 | 62.5 | 3.46 | 170 | 22 | |||
| Lososinka (the mouth of the river) | Spring | 7.14 | 0.024 | 0.017 | 0.43 | 0.39 | 22 | 53 | 2.01 | 18.8 | 30.8 | 33.5 | 2.7 | 135 | 32 | ||
| Summer | 7.29 | 0.108 | 0.093 | 0.66 | 0.46 | 61 | 103 | 2.04 | 18.3 | 41.9 | 46.2 | 2.64 | 137 | 51 | |||
| Autumn | 7.26 | 0.076 | 0.072 | 0.68 | 0.53 | 38 | 69 | 2.12 | 22.7 | 45.2 | 48.8 | 2.52 | 169 | 38 | |||
| Winter | 7.28 | 0.070 | 0.136 | 0.46 | 0.25 | 18 | 62 | 1.98 | 14.6 | 35.3 | 36.5 | 3.05 | 117 | 60 | |||
| Shuya | Spring | 6.39 | 0.013 | 0.029 | 0.38 | 0.34 | 4 | 31 | 2.31 | 19.6 | 36.8 | 38.7 | 2.70 | 152 | 15 | ||
| Summer | 7.01 | 0.032 | 0.009 | 0.43 | 0.39 | 9.8 | 45 | 1.36 | 12.6 | 27.5 | 31.0 | 1.22 | 78 | 23 | |||
| Autumn | 6.58 | 0.041 | 0.038 | 0.54 | 0.46 | 14 | 36 | 1.63 | 22.7 | 42.5 | 48.8 | 2.2 | 153 | 18 | |||
| Winter | 6.06 | 0.038 | 0.182 | 0.82 | 0.60 | 8 | 53 | 1.82 | 20.2 | 45.7 | 47.7 | 3.39 | 27 | ||||
| Suna | Spring | 7.13 | 0.009 | 0.055 | 0.35 | 0.29 | 1 | 11 | 1.49 | 10.0 | 21.6 | 24.6 | 1.94 | 60 | 17 | ||
| Summer | 6.70 | 0.024 | 0.041 | 0.35 | 0.29 | 1 | 12 | 0.74 | 10.9 | 19.9 | 20.7 | 2.02 | 62 | 16 | |||
| Autumn | 6.91 | 0.024 | 0.082 | 0.30 | 0.19 | 1 | 1 | 1.68 | 10.1 | 19.1 | 21.9 | 2.09 | 61 | 11 | |||
| Winter | 6.75 | 0.045 | 0.089 | 0.33 | 0.20 | 0 | 1 | 0.55 | 11 | 27.7 | 25.9 | 2.12 | 77 | 14 | |||
| North | Kumsa | Spring | 7.19 | 0.010 | 0.010 | 0.34 | 0.32 | 1 | 9 | 2.22 | 12.6 | 25.5 | 25.5 | 2.10 | 68 | 30 | |
| Summer | 7.30 | 0.019 | 0.021 | 0.38 | 0.34 | 0.3 | 12 | 1.34 | 10.7 | 25.1 | 30.2 | 2.00 | 59 | 40 | |||
| Autumn | 7.09 | 0.046 | 0.038 | 0.41 | 0.33 | 1 | 10 | 1.07 | 11.8 | 29.1 | 31.2 | 2.45 | 75 | 37 | |||
| Winter | 7.22 | 0.047 | 0.065 | 0.46 | 0.35 | 0 | 24 | 1.81 | 12.2 | 28.2 | 30.9 | 2.7 | 65 | 42 | |||
| Eastern | Vodla | Spring | 6.83 | 0.010 | <0.01 | 0.37 | 0.36 | 9 | 45 | 2.48 | 18.8 | 36.8 | 38.9 | 2.15 | 120 | 21 | |
| Summer | 6.33 | 0.030 | 0.006 | 0.47 | 0.43 | 2.3 | 43 | 2.86 | 12.6 | 23.3 | 29.5 | 0.84 | 71 | 30 | |||
| Autumn | 6.67 | 0.066 | 0.026 | 0.68 | 0.59 | 8 | 34 | 3.65 | 24.7 | 48.6 | 52 | 2.08 | 149 | 27 | |||
| Winter | 6.83 | 0.090 | 0.132 | 0.78 | 0.56 | 10 | 44 | 1.61 | 16.9 | 3.85 | 40.2 | 2.43 | 92 | 39 | |||
| Southeastern | Andoma | Spring | 6.89 | 0.016 | 0.041 | 0.51 | 0.45 | 11 | 33 | 1.65 | 18.3 | 29.0 | 37.9 | 1.62 | 140 | 42 | |
| Summer | 6.99 | 0.025 | 0.026 | 0.85 | 0.80 | 11 | 62 | 2.98 | 26.4 | 46.0 | 54.5 | 2.06 | 196 | 57 | |||
| Autumn | 6.16 | 0.050 | 0.019 | 0.97 | 0.90 | 14 | 44 | 2.84 | 34.3 | 67.4 | 70.6 | 2.91 | 215 | 19 | |||
| Winter | 6.91 | 0.097 | 0.163 | 0.84 | 0.58 | 8 | 61 | 3.45 | 17.7 | 39.5 | 40.9 | 2.97 | 127 | 33 | |||
| Southwest | Derevyanka | Spring | 7.20 | 0.010 | 0.043 | 0.55 | 0.50 | 25 | 67 | 3.10 | 29.3 | 45.7 | 48.9 | 3.09 | 208 | 44 | |
| Summer | 7.21 | 0.021 | 0.076 | 1.24 | 1.14 | 58 | 127 | 2.84 | 54.2 | 78.0 | 94.7 | 4.73 | 358 | 53 | |||
| Autumn | 7.21 | 0.043 | 0.104 | 1.13 | 0.98 | 37 | 78 | 2.36 | 45 | 72.6 | 751 | 2.09 | 271 | 53 | |||
| Winter | 7.11 | 0.218 | 0.667 | 1.55 | 0.66 | 36 | 168 | 2.84 | 14.1 | 37.2 | 423 | 3.56 | 107 | 92 | |||
| Sheltozerka | Spring | 6.89 | 0.010 | <0.01 | 0.58 | 0.57 | 9 | 39 | 1.96 | 22.0 | 42.8 | 46.3 | 2.30 | 160 | 23 | ||
| Summer | 7.21 | 0.027 | 0.088 | 1.11 | 1.00 | 47 | 89 | – | 26.4 | 50.1 | 51.7 | 3.73 | 211 | 75 | |||
| Autumn | 6.65 | 0.025 | 0.028 | 0.93 | 0.88 | 11 | 36 | 7.3 | 33.8 | 65.7 | 70.4 | 3.1 | 213 | 26 | |||
| Winter | 6.82 | 0.103 | 0.508 | 1.33 | 0.74 | 26 | 76 | 2.21 | 14.5 | 46.3 | 50.2 | 4.05 | 151 | 48 | |||
| The central part of theEast European Platform (II) | Southern | Vytegra | Spring | 7.71 | 0.025 | 0.076 | 0.35 | 0.25 | 6 | 25 | 1.43 | 13.4 | 25.3 | 30.1 | 1.71 | 89 | 110 |
| Summer | 8.09 | 0.029 | 0.013 | 0.54 | 0.50 | 16 | 59 | 1.63 | 6.9 | 15.5 | 17.9 | 1.78 | 38 | 185 | |||
| Autumn | 7.87 | 0.109 | 0.061 | 0.76 | 0.59 | 14 | 45 | 2.91 | 16.4 | 32.6 | 34.7 | 2.31 | 102 | 156 | |||
| Winter | 7.54 | 0.079 | 0.215 | 0.80 | 0.50 | 15 | 61 | – | 17.7 | 24.8 | 26.1 | 3.38 | 59 | 193 | |||
| Svir’ (the source of the lake) | Spring | 7.40 | 0.009 | 0.121 | 0.28 | 0.15 | 11.3 | 14 | 0.94 | 6.4 | 19.3 | 20.5 | 0.21 | 28 | 40 | ||
| Summer | 7.27 | 0.017 | 0.119 | 0.41 | 0.27 | 0.7 | 10 | 0.44 | 7.8 | 19.1 | 19.6 | 0.14 | 28 | 38 | |||
| Autumn | 7.47 | 0.032 | 0.128 | 0.45 | 0.29 | 0.7 | 9 | 0.45 | 8.2 | 18.5 | 19.7 | 0.28 | 33 | 37 | |||
| Winter | 7.37 | 0.035 | 0.164 | 0.39 | 0.19 | 0.7 | 18 | 0.62 | 6.8 | 18.4 | 20.0 | 0.34 | 34 | 38 | |||
| Geological Structure | Coast | River | Spring | Summer | Autumn | Winter |
|---|---|---|---|---|---|---|
| I | Northwest | Lososinka (before the city) | ||||
| Lososinka (the mouth of the river) | ||||||
| Shuya | ||||||
| Suna | ||||||
| North | Kumsa | |||||
| Eastern | Vodla | |||||
| Southeastern | Andoma | – | ||||
| Southwest | Derevyanka | |||||
| Sheltozerka | ||||||
| II | Southern | Vytegra | – | |||
| Svir’ |
| Geological Structure | Coast | River | n | Range | Winter–Spring | Summer–Autumn | ||||
|---|---|---|---|---|---|---|---|---|---|---|
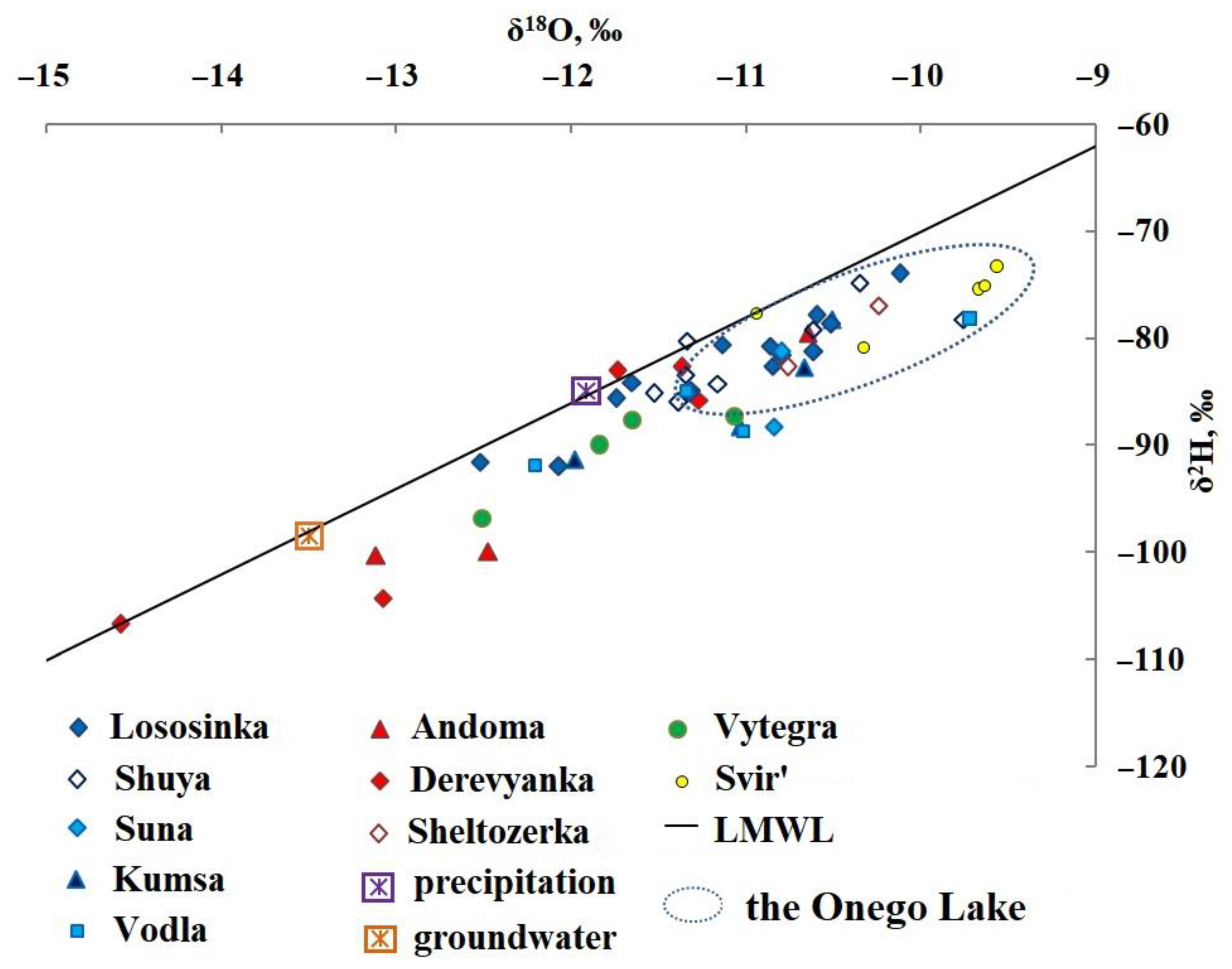
| δ18O, ‰ | δ2H, ‰ | d (2HExcess), ‰ | δ18O, ‰ | δ2H, ‰ | d (2HExcess), ‰ | |||||
| Fennoscandian Crystalline Shield (FCS)(I) | Northwest | Lososinka | 15 | Min. | −12.5 | −92 | 8.0 | −11.3 | −85 | 5.4 |
| Max. | −10.9 | −93 | −5.8 | −10.1 | −74 | 6.8 | ||||
| Shuya | 12 | Min. | −11.5 | −85 | 7.0 | −9.8 | −78 | 0.4 | ||
| Max. | −10.4 | −75 | 8.2 | |||||||
| Suna | 2 | Min. | – | – | – | −10.8 | −88 | −1.6 | ||
| Max. | – | – | – | −10.8 | −81 | 5.4 | ||||
| North | Kumsa | 4 | Min. | −12.0 | −91 | 5.0 | −10.7 | −83 | 2.6 | |
| Max. | −11.0 | −88 | 0.0 | −10.5 | −78 | 6.0 | ||||
| Eastern | Vodla | 4 | Min. | −12.2 | −92 | 5.6 | −11.3 | −85 | 5.4 | |
| Max. | −11.0 | −89 | −1.0 | −9.7 | −78 | −0.4 | ||||
| Southeastern | Andoma | 3 | Min. | −13.1 | −100 | 4.8 | −10.6 | −80 | 4.8 | |
| Max. | −12.5 | −100 | 0.0 | |||||||
| Southwest | Derevyanka | 4 | Min. | −14.6 | −107 | 9.8 | −11.7 | −83 | 10.6 | |
| Max. | −13.1 | −104 | 0.8 | −11.3 | −86 | 4.4 | ||||
| Sheltozerka | 2 | Min. | – | – | −10.8 | −83 | 3,4 | |||
| Max. | – | –- | −10.2 | −77 | 4,6 | |||||
| The central part of the East European Platform (EEP) (II) | Southern | Vytegra | 4 | Min. | −12.5 | −97 | 3.0 | −11.7 | −88 | 5.6 |
| Max. | −11.8 | −90 | 4.4 | −11.1 | −87 | 1.8 | ||||
| Svir’ (outflow from lake) | 5 | Min. | −10.9 | −78 | 9.2 | −9.7 | −75 | 2.6 | ||
| Max. | −10.3 | −81 | 1.4 | −9.6 | −73 | 3.8 | ||||
| Geological Structure | Coast | River | Season | Suspended Matter, mg/L | ||
|---|---|---|---|---|---|---|
| > 0.8 μm | 0.45 μm < Ø < 0.8 μm | > 0.45 μm | ||||
| The Fennoscandian Crystalline Shield (FCS) (I) | Northwest | Lososinka (before the city) | Spring | 2.96 | 1.80 | 12.26 |
| Summer | 6.25 | 0.48 | 11.67 | |||
| Autumn | 5.22 | 0.13 | – | |||
| Winter | 29.59 | 1.06 | 32.80 | |||
| Lososinka (the mouth of the river) | Spring | 3.49 | 2.50 | 5.37 | ||
| Summer | 7.60 | 0.89 | 1.88 | |||
| Autumn | 8.12 | 0.04 | – | |||
| Winter | 8.65 | 0.29 | 9.82 | |||
| Shuya | Spring | 7.36 | 0.75 | 7.12 | ||
| Summer | 9.82 | 0.59 | 4.47 | |||
| Autumn | 4.19 | 0.13 | 5.56 | |||
| Winter | 7.88 | 1.07 | 9.81 | |||
| Suna | Spring | 1.20 | 0.51 | 1.87 | ||
| Summer | 1.99 | 0.22 | 2.22 | |||
| Autumn | 0.87 | 0.03 | – | |||
| Winter | 0.72 | 0.23 | 0.88 | |||
| North | Kumsa | Spring | 1.50 | 0.37 | – | |
| Summer | 2.35 | 0.15 | 1.96 | |||
| Autumn | 0.93 | 0.12 | 1.18 | |||
| Winter | 2.11 | 0.29 | 2.60 | |||
| Eastern | Vodla | Spring | 7.76 | 1.35 | 3.72 | |
| Summer | 3.24 | 0.22 | 2.20 | |||
| Autumn | 3.93 | 0.24 | 2.29 | |||
| Winter | 4.22 | 5.94 | 0.98 | |||
| Southeastern | Andoma | Spring | 5.51 | 1.54 | 9.62 | |
| Summer | 9.16 | 0.60 | 8.10 | |||
| Autumn | 3.53 | 0.63 | 4.40 | |||
| Winter | 9.19 | 1.90 | 12.92 | |||
| Southwest | Derevyanka | Spring | 4.69 | 1.79 | 9.13 | |
| Summer | 4.83 | 1.10 | 14.17 | |||
| Autumn | 4.17 | 9.40 | – | |||
| Winter | 13.00 | 1.12 | 25.47 | |||
| Sheltozerka | Spring | 7.88 | 1.91 | 21.11 | ||
| Summer | 1.79 | 1.12 | 14.68 | |||
| Autumn | 2.08 | 0.23 | 5.60 | |||
| Winter | 22.77 | 1.40 | 16.59 | |||
| The central part of theEast European Platform (II) | Southern | Vytegra | Spring | 8.29 | 0.90 | – |
| Summer | 21.46 | 0.33 | – | |||
| Autumn | 5.80 | 0.30 | 4.53 | |||
| Winter | 6.15 | 0.70 | 6.75 | |||
| Svir’ (the source of the lake) | Spring | 2.15 | 0.28 | – | ||
| Summer | 0.80 | 0.08 | – | |||
| Autumn | 3.39 | 0.05 | – | |||
| Winter | 1.07 | 0.11 | 0.96 | |||
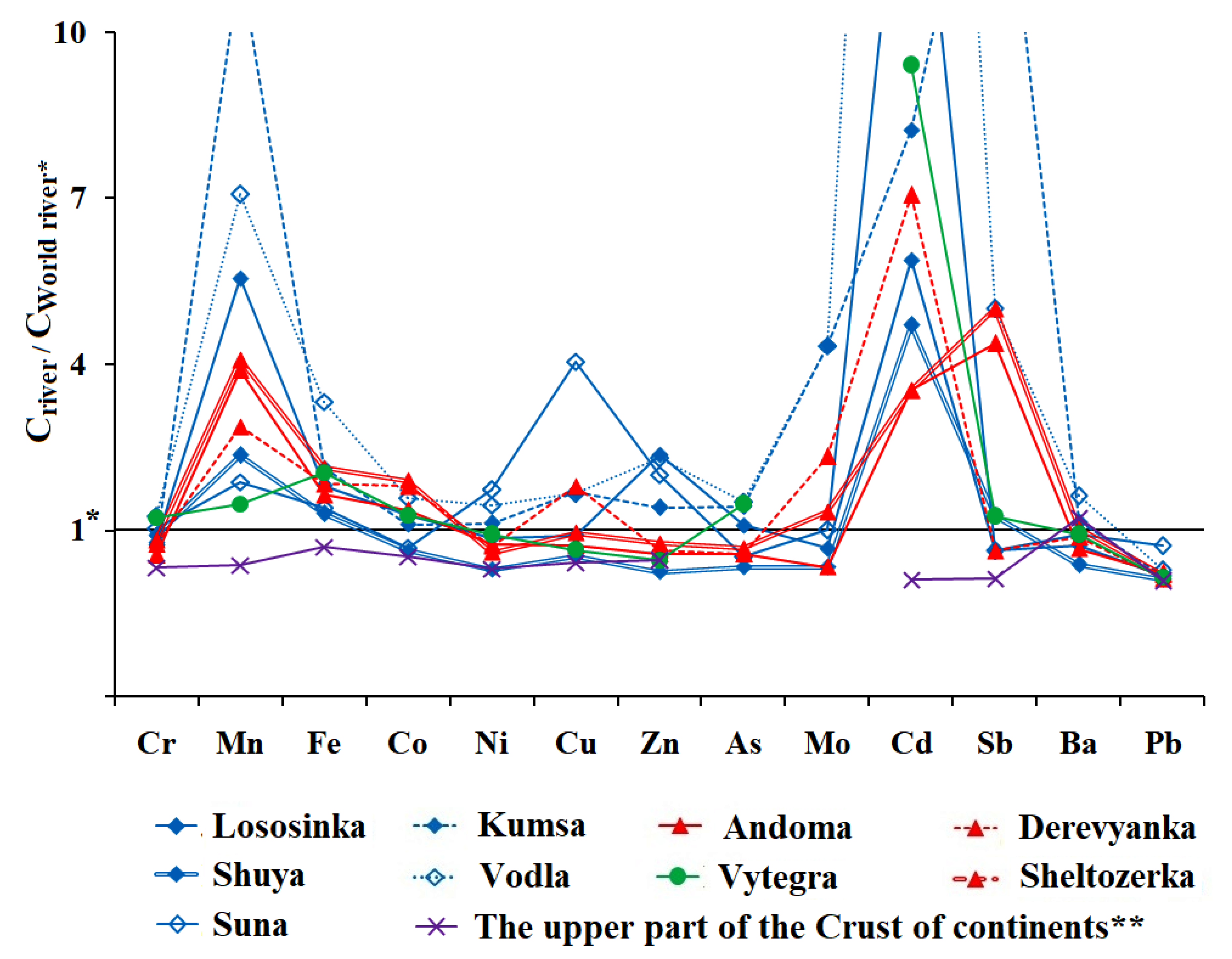
| Geological Structure | Coast | River | Series of Elements |
|---|---|---|---|
| I | Northwest | Lososinka | Fe > Mn > Zn > Ba > Cr > Ni > Cu > Pb > Co > As > Cd > Mo > Sb |
| Shuya | Fe > Mn > Ba > Cr > Zn > Cu = Pb > Ni > Co > As > Cd > Sb > Mo | ||
| Suna | Fe > Mn > Ba > Zn > Cu > Pb > Ni > Cr > Co = Cd > As > Mo > Sb | ||
| North | Kumsa | Fe > Mn > Ba > Zn > Cu > Cr > Ni > Pb > As > Sb > Co > Mo > Cd | |
| Eastern | Vodla | Fe > Mn > Ba > Zn > Cr > Cu > Ni > Pb > As > Co > Cd > Mo > Sb | |
| Southeastern | Andoma | Fe > Mn > Ba > Zn > Cr = Pb > Ni > Cu > Co > As > Sb > Cd > Mo | |
| Southwest | Derevyanka | Fe > Mn > Ba > Cu > Zn > Cr > Ni > Co = Pb > As > Mo > Cd > Sb | |
| Sheltozerka | Fe > Mn > Ba > Zn > Cr > Cu > Pb > Ni > Co > As > Sb > Mo > Cd | ||
| II | Southern | Vytegra | Fe > Mn > Ba > Cr > Zn > Ni > Cu > Pb > As > Co > Cd > Sb > Mo |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulik, N.; Efremenko, N.; Strakhovenko, V.; Belkina, N.; Borodulina, G.; Gatalskaya, E.; Malov, V.; Tokarev, I. Geochemical Features of River Runoff and Their Effect on the State of the Aquatic Environment of Lake Onego. Water 2023, 15, 964. https://doi.org/10.3390/w15050964
Kulik N, Efremenko N, Strakhovenko V, Belkina N, Borodulina G, Gatalskaya E, Malov V, Tokarev I. Geochemical Features of River Runoff and Their Effect on the State of the Aquatic Environment of Lake Onego. Water. 2023; 15(5):964. https://doi.org/10.3390/w15050964
Chicago/Turabian StyleKulik, Natalia, Natalia Efremenko, Vera Strakhovenko, Natalia Belkina, Galina Borodulina, Ekaterina Gatalskaya, Viktor Malov, and Igor Tokarev. 2023. "Geochemical Features of River Runoff and Their Effect on the State of the Aquatic Environment of Lake Onego" Water 15, no. 5: 964. https://doi.org/10.3390/w15050964






