Green Synthesis and Characterizations of Cobalt Oxide Nanoparticles and Their Coherent Photocatalytic and Antibacterial Investigations
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plant Extract Preparation
2.3. Biosynthesis of Co3O4 Nanoparticles
2.4. Characterization of Nanoparticles
2.5. Photocatalytic Activity
2.6. Antibacterial Activity
3. Result and Discussions
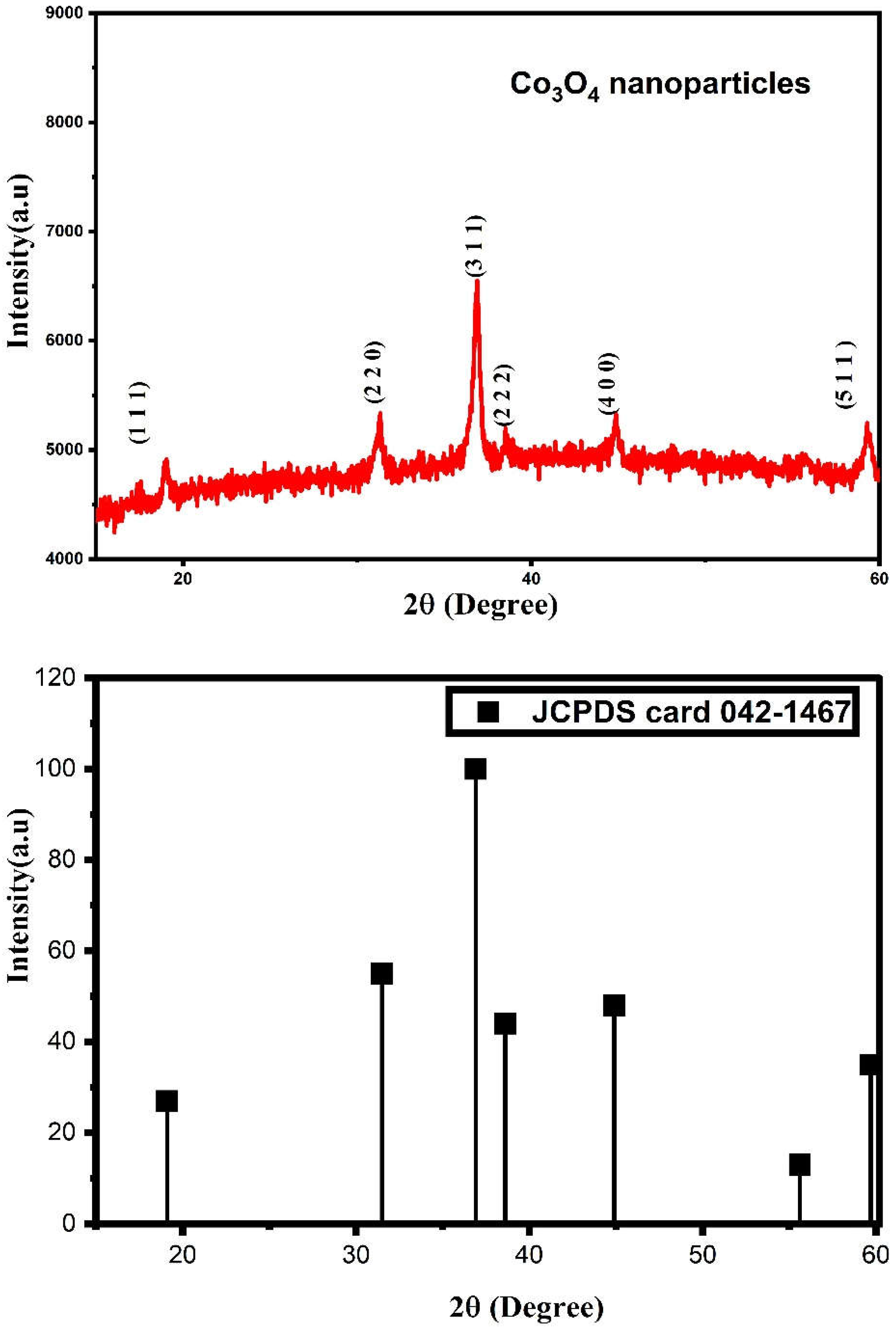
3.1. XRD Analysis
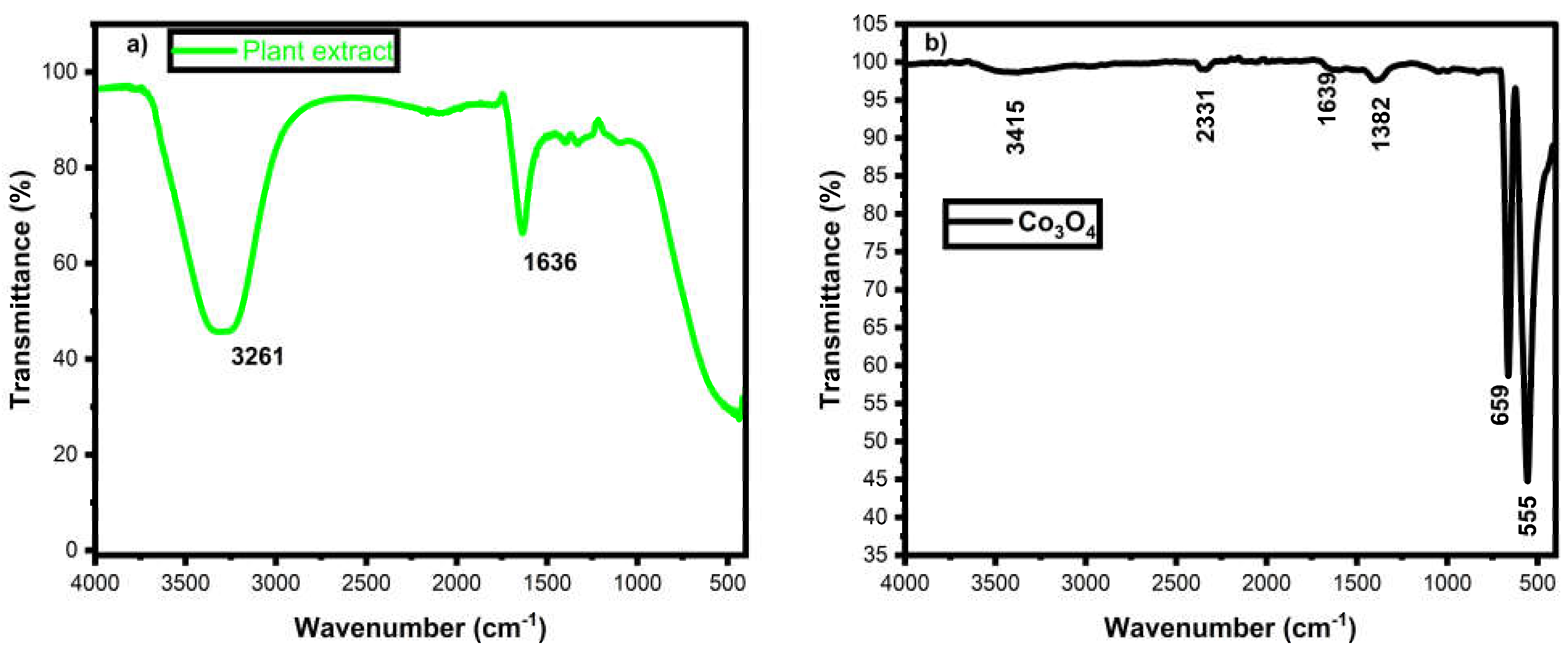
3.2. FTIR Analysis
3.3. UV-DRS Analysis
3.4. FESEM with EDX Analysis
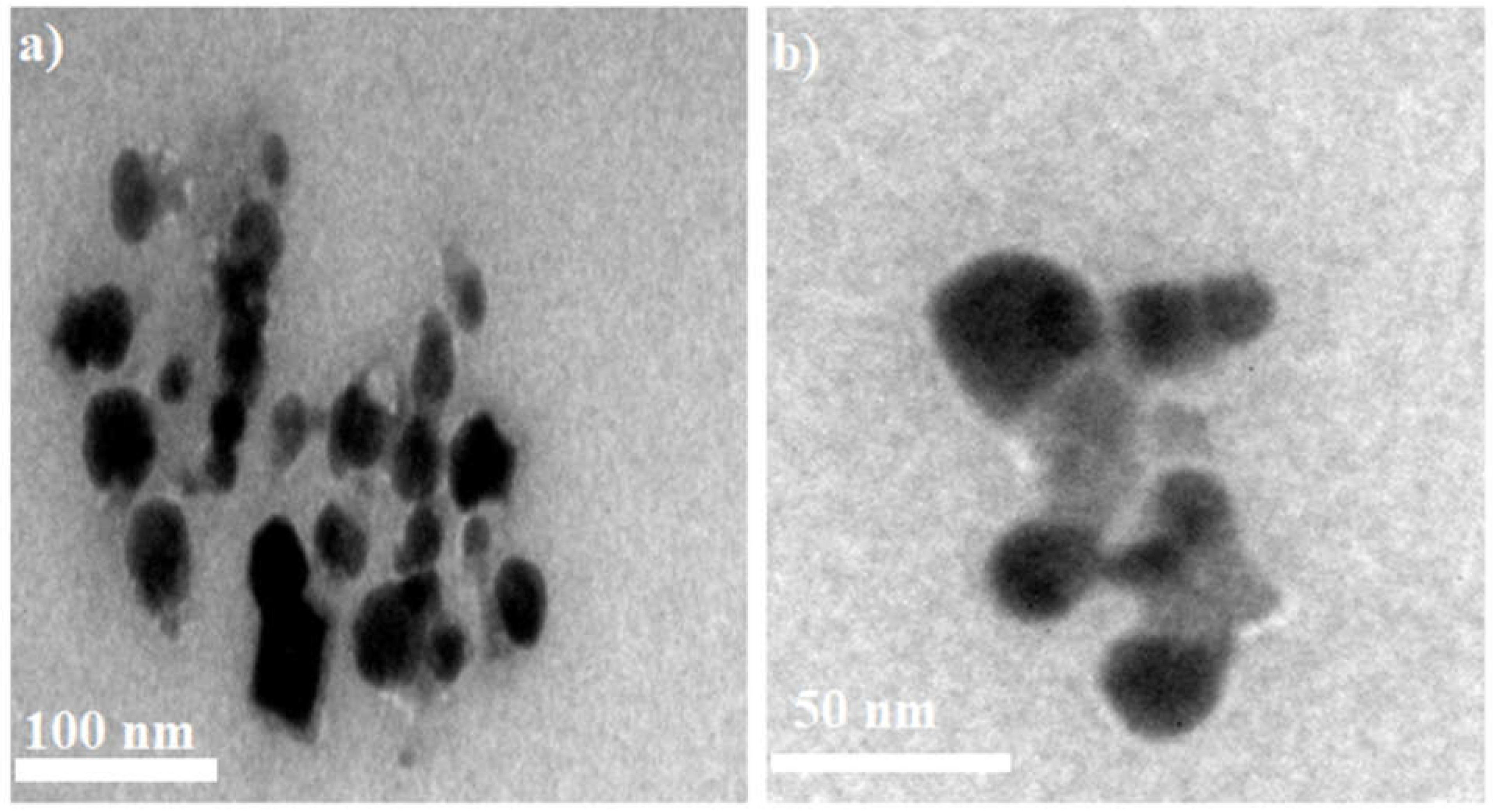
3.5. TEM Analysis
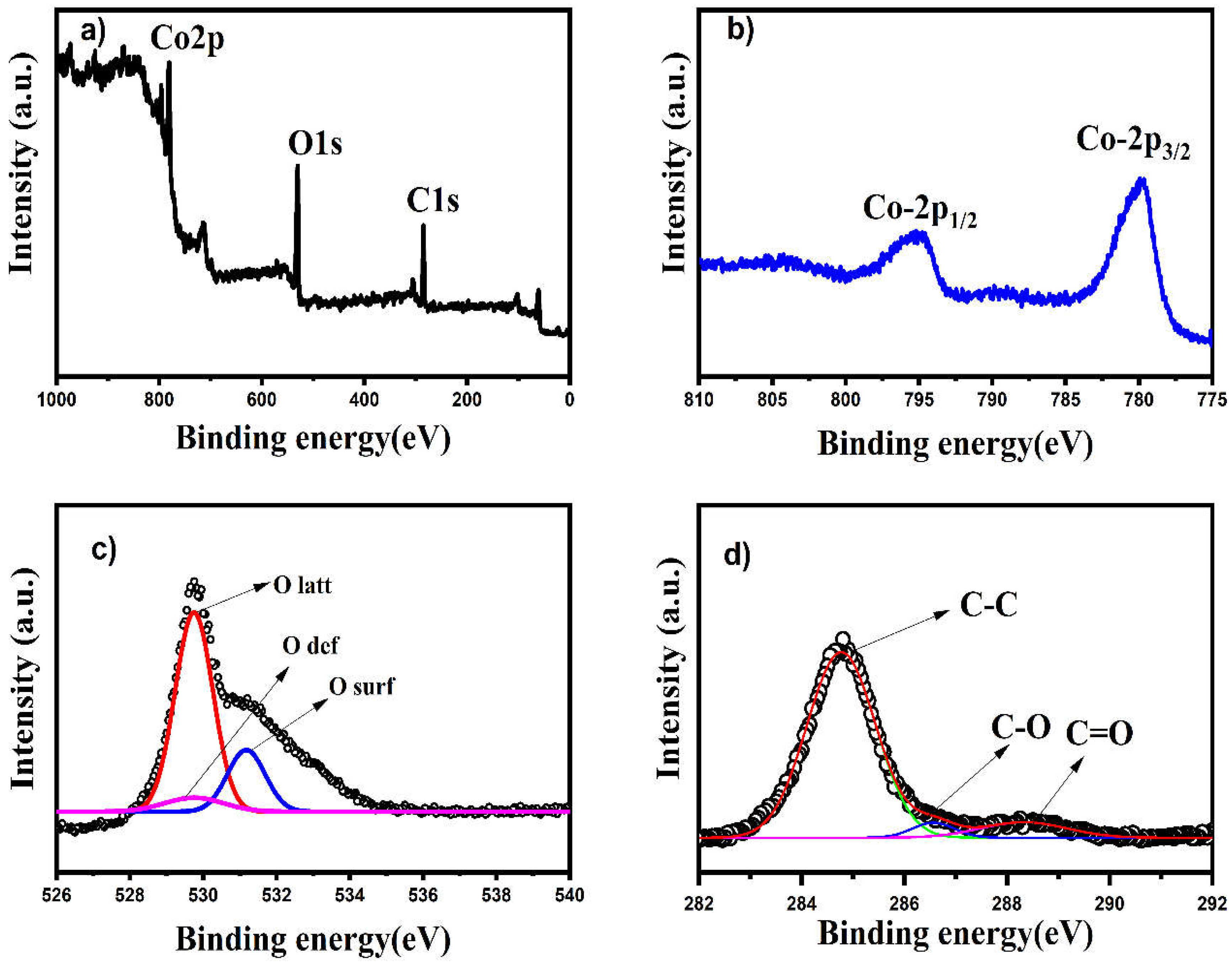
3.6. XPS Analysis
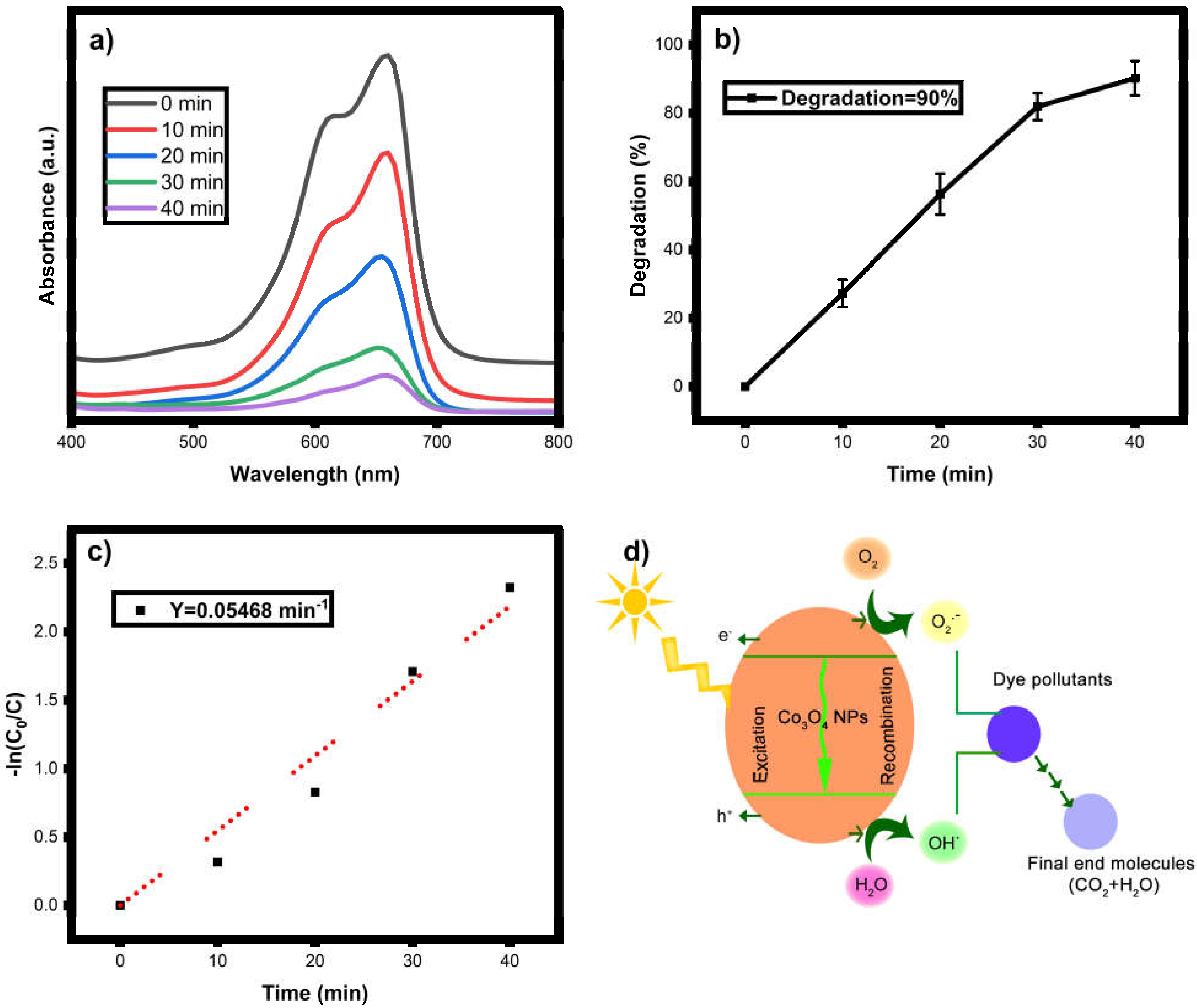
3.7. Photocatalytic Dye Degradation
3.8. Photodegradation Mechanism
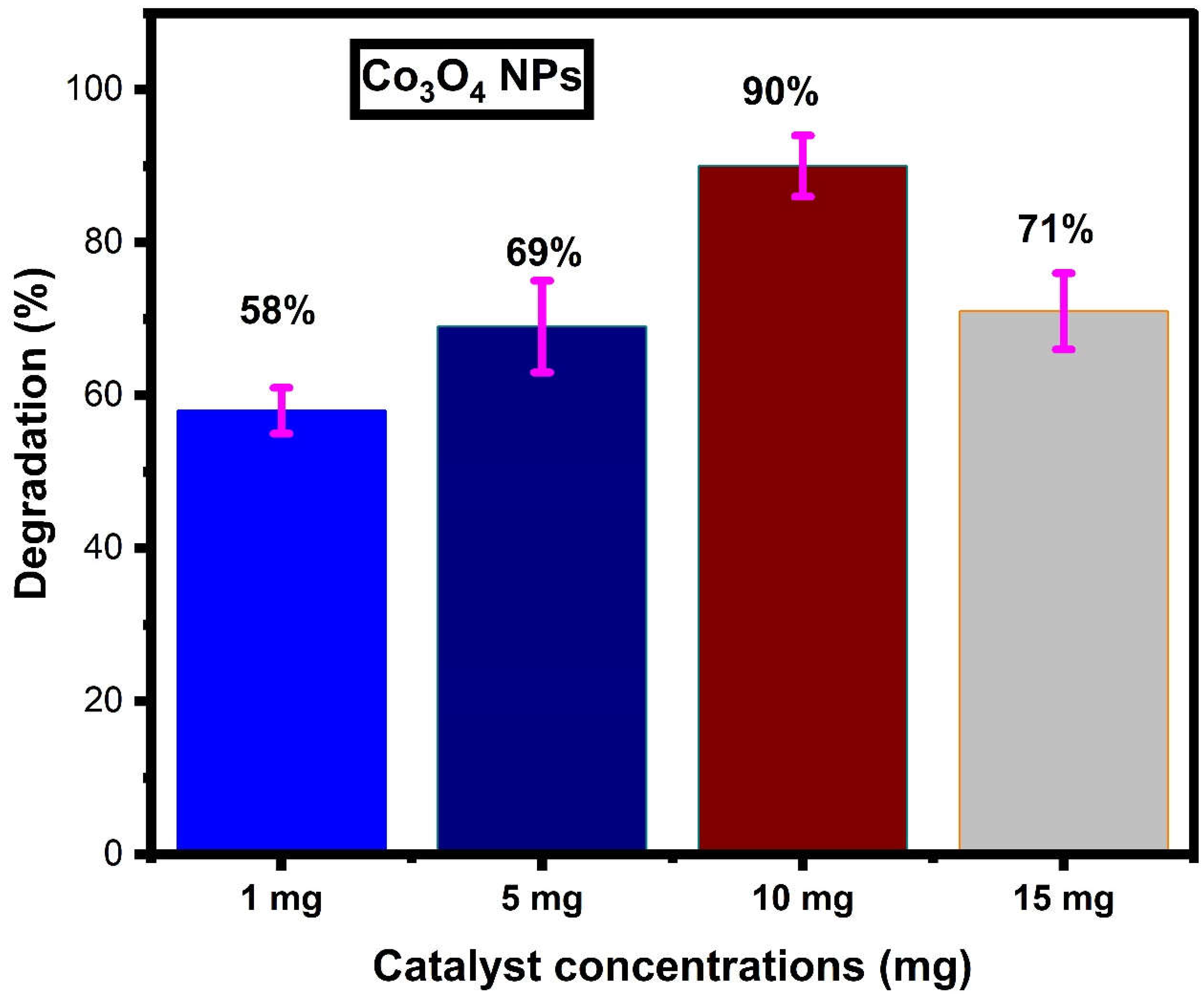
Effect of Catalyst Dosage
3.9. Antibacterial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ismail, M.; Akhtar, K.; Khan, M.I.; Kamal, T.; Khan, M.A.; Asiri, A.M.; Seo, J.; Khan, S.B. Pollution, Toxicity and Carcinogenicity of Organic Dyes and their Catalytic Bio-Remediation. Curr. Pharm. Des. 2019, 25, 3645–3663. [Google Scholar] [CrossRef] [PubMed]
- Burkinshaw, S.M. Application of dyes. In The Chemistry and Application of Dyes; Springer: Boston, MA, USA, 1990; pp. 237–379. [Google Scholar]
- Gregory, P. Classification of Dyes by Chemical Structure. In The Chemistry and Application of Dyes; Springer: Boston, MA, USA, 1990; pp. 17–47. [Google Scholar] [CrossRef]
- Mondal, P.; Baksi, S.; Bose, D. Study of environmental issues in textile industries and recent wastewater treatment technology. World Sci. News 2017, 61, 94–105. [Google Scholar]
- Moradi, O.; Pudineh, A.; Sedaghat, S. Synthesis and characterization Agar/GO/ZnO NPs nanocomposite for removal of methylene blue and methyl orange as azo dyes from food industrial effluents. Food Chem. Toxicol. 2022, 169, 113412. [Google Scholar] [CrossRef]
- Harvey, J.W.; Keitt, A.S. Studies of the efficacy and potential hazards of methylene blue therapy in aniline-induced methaemoglobinaemia. Br. J. Haematol. 1983, 54, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.; Shitu, A.; Ibrahim, A. Removal of methylene blue using low cost adsorbent: A review. Res. J. Chem. Sci. ISSN 2014, 2231, 606X. [Google Scholar]
- El-Sharkawy, E.; Soliman, A.Y.; Al-Amer, K.M. Comparative study for the removal of methylene blue via adsorption and photocatalytic degradation. J. Colloid Interface Sci. 2007, 310, 498–508. [Google Scholar] [CrossRef]
- Entezari, M.; Sharifalhoseini, Z. Sono-sorption as a new method for the removal of methylene blue from aqueous solution. Ultrason. Sonochem. 2007, 14, 599–604. [Google Scholar] [CrossRef]
- Sarioglu, M.; Atay, U.A. Removal of methylene blue by using biosolid. Glob. Nest J. 2006, 8, 113–120. [Google Scholar]
- Julkapli, N.M.; Bagheri, S.; Hamid, S.B.A. Recent Advances in Heterogeneous Photocatalytic Decolorization of Synthetic Dyes. Sci. World J. 2014, 2014, 692307. [Google Scholar] [CrossRef]
- Ahuja, P.; Ujjain, S.; Kanojia, R.; Attri, P. Transition Metal Oxides and Their Composites for Photocatalytic Dye Degradation. J. Compos. Sci. 2021, 5, 82. [Google Scholar] [CrossRef]
- Suresh, S.; Vennila, S.; Anita Lett, J.; Fatimah, I.; Mohammad, F.; Al-Lohedan, H.A.; Alshahateet, S.F.; Motalib Hossain, M.A.; Rafie Johan, M. Star fruit extract-mediated green synthesis of metal oxide nanoparticles. Inorg. Nano-Met. Chem. 2022, 52, 173–180. [Google Scholar] [CrossRef]
- Huang, H.; Wang, J.; Zhang, J.; Cai, J.; Pi, J.; Xu, J.-F. Inspirations of Cobalt Oxide Nanoparticle Based Anticancer Therapeutics. Pharmaceutics 2021, 13, 1599. [Google Scholar] [CrossRef] [PubMed]
- Parveen, K.; Banse, V.; Ledwani, L. Green synthesis of nanoparticles: Their advantages and disadvantages. In Proceedings of the AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2016; Volume 1724, p. 020048. [Google Scholar] [CrossRef]
- Thema, F.; Manikandan, E.; Dhlamini, M.; Maaza, M. Green synthesis of ZnO nanoparticles via Agathosma betulina natural extract. Mater. Lett. 2015, 161, 124–127. [Google Scholar] [CrossRef]
- Manikandan, V.; Jayanthi, P.; Priyadharsan, A.; Vijayaprathap, E.; Anbarasan, P.M.; Velmurugan, P. Green synthesis of pH-responsive Al2O3 nanoparticles: Application to rapid removal of nitrate ions with enhanced antibacterial activity. J. Photochem. Photobiol. A Chem. 2019, 371, 205–215. [Google Scholar] [CrossRef]
- Fadus, M.C.; Lau, C.; Bikhchandani, J.; Lynch, H.T. Curcumin: An age-old anti-inflammatory and anti-neoplastic agent. J. Tradit. Complement. Med. 2016, 7, 339–346. [Google Scholar] [CrossRef]
- Alsamydai, A.; Jaber, N. Pharmacological aspects of curcumin. Int. J. Pharm. 2018, 5, 313–326. [Google Scholar]
- Fouda, A.; Al-Otaibi, W.A.; Saber, T.; AlMotwaa, S.M.; Alshallash, K.S.; Elhady, M.; Badr, N.F.; Abdel-Rahman, M.A. Antimicrobial, antiviral, and in-vitro cytotoxicity and mosquitocidal activities of Portulaca oleracea-based green synthesis of selenium nanoparticles. J. Funct. Biomater. 2018, 13, 157. [Google Scholar] [CrossRef] [PubMed]
- Fouda, A.; Hassan SE, D.; Eid, A.M.; Awad, M.A.; Althumayri, K.; Badr, N.F.; Hamza, M.F. Endophytic bacterial strain, Brevibacillus brevis-mediated green synthesis of copper oxide nanoparticles, characterization, antifungal, in vitro cytotoxicity, and larvicidal activity. Green Process. Synth. 2022, 11, 931–950. [Google Scholar] [CrossRef]
- Fouda, A.; Eid, A.M.; Guibal, E.; Hamza, M.F.; Hassan SE, D.; Alkhalifah DH, M.; El-Hossary, D. Green Synthesis of Gold Nanoparticles by Aqueous Extract of Zingiber officinale: Characterization and Insight into Antimicrobial, Antioxidant, and In Vitro Cytotoxic Activities. Appl. Sci. 2022, 12, 12879. [Google Scholar] [CrossRef]
- Fouda, A.; Awad, M.A.; Eid, A.M.; Saied, E.; Barghoth, M.G.; Hamza, M.F.; Awad, M.F.; Abdelbary, S.; Hassan, S.E.D. An Eco-friendly approach to the control of pathogenic microbes and anopheles stephensi malarial vector using Magnesium Oxide Nanoparticles (Mg-NPs) fabricated by Penicillium chrysogenum. Int. J. Mol. Sci. 2021, 22, 5096. [Google Scholar] [CrossRef]
- Fouda, A.; Eid, A.M.; Abdel-Rahman, M.A.; El-Belely, E.F.; Awad, M.A.; Hassan, S.E.D.; Al-Faifi, Z.E.; Hamza, M.F. Enhanced Antimicrobial, Cytotoxicity, Larvicidal, and Repellence Activities of Brown Algae, Cystoseira crinita-Mediated Green Synthesis of Magnesium Oxide Nanoparticles. Front. Bioeng. Biotechnol. 2022, 10, 849921. [Google Scholar] [CrossRef]
- Parvathiraja, C.; Shailajha, S.; Shanavas, S.; Gurung, J. Biosynthesis of silver nanoparticles by Cyperus pangorei and its potential in structural, optical and catalytic dye degradation. Appl. Nanosci. 2020, 11, 477–491. [Google Scholar] [CrossRef]
- Parvathiraja, C.; Shailajha, S. Bioproduction of CuO and Ag/CuO heterogeneous photocatalysis-photocatalytic dye degradation and biological activities. Appl. Nanosci. 2021, 11, 1411–1425. [Google Scholar] [CrossRef]
- Fouda, A.; Eid, A.M.; Abdelkareem, A.; Said, H.A.; El-Belely, E.F.; Alkhalifah, D.H.M.; Alshallash, K.S.; Hassan, S.E.-D. Phyco-Synthesized Zinc Oxide Nanoparticles Using Marine Macroalgae, Ulva fasciata Delile, Characterization, Antibacterial Activity, Photocatalysis, and Tanning Wastewater Treatment. Catalysts 2022, 12, 756. [Google Scholar] [CrossRef]
- Pallavolu, M.R.; Krishna, K.G.; Nagaraju, G.; Babu, P.S.; Sambasivam, S.; Sreedhar, A. Rational design of Cu-doped Co3O4@carbon nanocomposite and agriculture crop-waste derived activated carbon for high-performance hybrid supercapacitors. J. Ind. Eng. Chem. 2022, 116, 428–437. [Google Scholar] [CrossRef]
- Kéranguéven, G.; Filimonenkov, I.S.; Savinova, E.R. Investigation of the stability of the boron-doped diamond support for Co3O4-based oxygen evolution reaction catalysts synthesized through in situ autocombustion method. J. Electroanal. Chem. 2022, 916, 116367. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, D.; Luo, Y.; Yang, W.; Zhan, X.; Yang, W.; Hou, H. Highly Efficient and Selective Visible-Light Driven Photoreduction of CO2 to CO by Metal–Organic Frameworks-Derived Ni—Co—O Porous Microrods. Small 2022, 18, 2202939. [Google Scholar] [CrossRef]
- Bahadoran, A.; Ramakrishna, S.; Oryani, B.; Al-Keridis, L.A.; Nodeh, H.R.; Rezania, S. Biodiesel production from waste cooking oil using heterogeneous nanocatalyst-based magnetic polyaniline decorated with cobalt oxide. Fuel 2022, 319, 123858. [Google Scholar] [CrossRef]
- Tang, C.-W.; Wang, C.-B.; Chien, S.-H. Characterization of cobalt oxides studied by FT-IR, Raman, TPR and TG-MS. Thermochim. Acta 2008, 473, 68–73. [Google Scholar] [CrossRef]
- Puspalak, A.; Chinnadurai, P.; Prathibha, R.; Kumar, M.P.; Manjushree, S.G.; UdayaKumar, V.; Adarakatti, P.S. Cobalt oxide nanoparticles based carbon electrode for the detection of residual nitrite in the soil of agricultural fields. Mater. Res. Innov. 2022, 27, 100–109. [Google Scholar] [CrossRef]
- Asha, G.; Rajeshwari, V.; Stephen, G.; Gurusamy, S.; Rachel, D.C.J. Eco-friendly synthesis and characterization of cobalt oxide nanoparticles by sativum species and its photo-catalytic activity. Mater. Today: Proc. 2021, 48, 486–493. [Google Scholar] [CrossRef]
- Reena, R.S.; Aslinjensipriya, A.; Infantiya, S.G.; Britto JD, J.; Jose, M.; Das, S.J. Visible-light active zinc doped cobalt oxide (Zn-Co3O4) nanoparticles for photocatalytic and photochemical activity. Mater. Today Proc. 2022, 68, 269–275. [Google Scholar] [CrossRef]
- Gopinath, S.; Mayakannan, M.; Vetrivel, S. Structural, optical, morphological properties of silver doped cobalt oxide nanoparticles by microwave irradiation method. Ceram. Int. 2021, 48, 6103–6115. [Google Scholar] [CrossRef]
- Maharani, N.Y. Structural, morphological, optical properties of Zr-doped Co3O4 nanoparticles. Part. Sci. Technol. 2022, 40, 662–674. [Google Scholar]
- Feng, Z.; Zhu, X.; Yang, J.; Zhong, K.; Jiang, Z.; Yu, Q.; Song, Y.; Hua, Y.; Li, H.; Xu, H. Inherent Facet-Dominant effect for cobalt oxide nanosheets to enhance photocatalytic CO2 reduction. Appl. Surf. Sci. 2022, 578, 151848. [Google Scholar] [CrossRef]
- Gouasmia, A.; Zouaoui, E.; Mekkaoui, A.A.; Haddad, A.; Bousba, D. Highly efficient photocatalytic degradation of malachite green dye over copper oxide and copper cobaltite photocatalysts under solar or microwave irradiation. Inorg. Chem. Commun. 2022, 145, 110066. [Google Scholar] [CrossRef]
- Gopinath, S.; Mayakannan, M.; Vetrivel, S. Analysis on the effect of doping in structural, morphological, and antibacterial properties of Yttrium doped cobalt oxide nanoparticles. Mater. Technol. 2022, 37, 2425–2435. [Google Scholar] [CrossRef]
- Samer, M.; Abdelsalam, E.M.; Mohamed, S.; Elsayed, H.; Attia, Y. Impact of photoactivated cobalt oxide nanoparticles addition on manure and whey for biogas production through dry anaerobic co-digestion. Environ. Dev. Sustain. 2021, 24, 7776–7793. [Google Scholar] [CrossRef]
- Haase, F.T.; Bergmann, A.; Jones, T.E.; Timoshenko, J.; Herzog, A.; Jeon, H.S.; Rettenmaier, C.; Cuenya, B.R. Size effects and active state formation of cobalt oxide nanoparticles during the oxygen evolution reaction. Nat. Energy 2022, 7, 765–773. [Google Scholar] [CrossRef]
- Natarajan, K. Photodegradation studies of pure and cobalt doped zinc oxide nanoparticles. Mater. Res. Innov. 2022, 27, 69–74. [Google Scholar] [CrossRef]
- Singh, A.K. A review on plant extract-based route for synthesis of cobalt nanoparticles: Photocatalytic, electrochemical sensing and antibacterial applications. Curr. Res. Green Sustain. Chem. 2022, 5, 100270. [Google Scholar] [CrossRef]
- Rathika, G.; Suba, V.; Lakshmi, D.S.; Rani, R. Exploring the Biosynthesized Metal Nanoparticles for their Catalytic Degradation of Toxic Water Wastes and Antimicrobial Potential. J. Inorg. Organomet. Polym. Mater. 2022, 32, 3153–3169. [Google Scholar] [CrossRef]
- Alagumalai, K.; Shanmugam, R.; Chen, T.W.; Chen, S.M.; Balamurugan, M.; Choi, S.S.; Ali, M.A.; Al-Mohaimeed, A.M.; Fan, C.H. The electrochemical evaluation of antipsychotic drug (promethazine) in biological and environmental samples through samarium cobalt oxide nanoparticles. Mater. Today Chem. 2022, 25, 100961. [Google Scholar] [CrossRef]
- Tien, T.M.; Chen, C.H.; Huang, C.T.; Chen, E.L. Photocatalytic Degradation of Methyl Orange Dyes Using Green Synthesized MoS2/Co3O4 Nanohybrids. Catalysts 2022, 12, 1474. [Google Scholar] [CrossRef]
- Vinayagam, R.; Hebbar, A.; Kumar, P.S.; Rangasamy, G.; Varadavenkatesan, T.; Murugesan, G.; Srivastava, S.; Goveas, L.C.; Kumar, N.M.; Selvaraj, R. Green synthesized cobalt oxide nanoparticles with photocatalytic activity towards dye removal from water environment. Environ. Res. 2022, 216, 114766. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, T.A.; Mengting, Z.; Fu, D.; Yeap, S.K.; Othman, M.H.D.; Avtar, R.; Ouyang, T. Functionalizing TiO2 with graphene oxide for enhancing photocatalytic degradation of methylene blue (MB) in contaminated wastewater. J. Environ. Manag. 2020, 270, 110871. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tian, B.; Wang, L.; Xing, M.; Lei, J. Photocatalysis; Springer: Singapore, 2018; pp. 1–15. [Google Scholar] [CrossRef]
- Tomar, R.; Abdala, A.A.; Chaudhary, R.G.; Singh, N. Photocatalytic degradation of dyes by nanomaterials. Mater. Today Proc. 2020, 29, 967–973. [Google Scholar] [CrossRef]
- Vennela, A.B.; Mangalaraj, D.; Muthukumarasamy, N.; Agilan, S.; Hemalatha, K.V. Structural and optical properties of Co3O4 nanoparticles prepared by sol-gel technique for photocatalytic application. Int. J. Electrochem. Sci. 2019, 14, 3535–3552. [Google Scholar] [CrossRef]
- Chowdhury, B.; Pradhan, S.S.; Das, H.S.; Biswas, B. Visible Light Induced Photocatalytic Dye Degradation by Cobalt Oxide Nanoparticles. Fine Chem. Eng. 2020, 1, 104–117. [Google Scholar] [CrossRef]
- Bibi, I.; Nazar, N.; Iqbal, M.; Kamal, S.; Nawaz, H.; Nouren, S.; Safa, Y.; Jilani, K.; Sultan, M.; Ata, S.; et al. Green and eco-friendly synthesis of cobalt-oxide nanoparticle: Characterization and photo-catalytic activity. Adv. Powder Technol. 2017, 28, 2035–2043. [Google Scholar] [CrossRef]
- Saravan, R.S.; Muthukumaran, M.; Mubashera, S.M.; Abinaya, M.; Prasath, P.V.; Parthiban, R.; Mohammad, F.; Oh, W.C.; Sagadevan, S. Evaluation of the photocatalytic efficiency of cobalt oxide nanoparticles towards the degradation of crystal violet and methylene violet dyes. Optik 2020, 207, 164428. [Google Scholar] [CrossRef]
- Verma, M.; Mitan, M.; Kim, H.; Vaya, D. Efficient photocatalytic degradation of Malachite green dye using facilely synthesized cobalt oxide nanomaterials using citric acid and oleic acid. J. Phys. Chem. Solids 2021, 155, 110125. [Google Scholar] [CrossRef]
- Yousefi, S.R.; Alshamsi, H.A.; Amiri, O.; Salavati-Niasari, M. Synthesis, characterization and application of Co/Co3O4 nanocomposites as an effective photocatalyst for discoloration of organic dye contaminants in wastewater and antibacterial properties. J. Mol. Liq. 2021, 337, 116405. [Google Scholar] [CrossRef]
- Adekunle, A.S.; Oyekunle, J.A.; Durosinmi, L.M.; Oluwafemi, O.S.; Olayanju, D.S.; Akinola, A.S.; Obisesan, O.R.; Akinyele, O.F.; Ajayeoba, T.A. Potential of cobalt and cobalt oxide nanoparticles as nanocatalyst towards dyes degradation in wastewater. Nano-Struct. Nano-Objects 2020, 21, 100405. [Google Scholar] [CrossRef]
- Magdalane, C.M.; Kaviyarasu, K.; Arularasu, M.; Kanimozhi, K.; Ramalingam, G. Structural and morphological properties of Co3O4 nanostructures: Investigation of low temperature oxidation for photocatalytic application for waste water treatment. Surf. Interfaces 2019, 17, 100369. [Google Scholar] [CrossRef]
- Dhiman, S.; Gupta, B. Co3O4 nanoparticles synthesized from waste Li-ion batteries as photocatalyst for degradation of methyl blue dye. Environ. Technol. Innov. 2021, 23, 101765. [Google Scholar] [CrossRef]
- Siddique, M.; Khan, N.M.; Saeed, M.; Ali, S.; Shah, Z. Green synthesis of cobalt oxide nanoparticles using Citrus medica leaves extract: Characterization and photo-catalytic activity. Z. Für Phys. Chem. 2020, 235, 663–681. [Google Scholar] [CrossRef]
- Fouda, A.; Hassan SE, D.; Eid, A.M.; Abdel-Rahman, M.A.; Hamza, M.F. Light enhanced the antimicrobial, anticancer, and catalytic activities of selenium nanoparticles fabricated by endophytic fungal strain, Penicillium crustosum EP-1. Sci. Rep. 2022, 12, 11834. [Google Scholar] [CrossRef]
- Fouda, A.; Awad, M.A.; Al-Faifi, Z.E.; Gad, M.E.; Al-Khalaf, A.A.; Yahya, R.; Hamza, M.F. Aspergillus flavus-Mediated Green Synthesis of Silver Nanoparticles and Evaluation of Their Antibacterial, Anti-Candida, Acaricides, and Photocatalytic Activities. Catalysts 2022, 12, 462. [Google Scholar] [CrossRef]
- Fouda, A.; Hassan SE, D.; Saied, E.; Hamza, M.F. Photocatalytic degradation of real textile and tannery effluent using biosynthesized magnesium oxide nanoparticles (MgO-NPs), heavy metal adsorption, phytotoxicity, and antimicrobial activity. J. Environ. Chem. Eng. 2021, 9, 105346. [Google Scholar] [CrossRef]
- Singh, J.; Singh, G.P.; Jain, R.K.; Singh, B.; Singh, K.J.; Singh, R.C. Effect of calcination temperature on structural, optical and antibacterial properties of ball mill synthesized Co3O4 nanomaterials. J. Mater. Sci. Mater. Electron. 2022, 33, 3250–3266. [Google Scholar] [CrossRef]
- Anuradha, C.; Raji, P. Facile-synthesis and characterization of cobalt oxide (Co3O4) nanoparticles by using Arishta leaves assisted biological molecules and its antibacterial and antifungal activities. J. Mol. Struct. 2022, 1262, 133065. [Google Scholar] [CrossRef]



| S.No | Sample | Dye | Dye volume | Dosage | Degradation (%) | References |
|---|---|---|---|---|---|---|
| 1. | Co3O4 | MB | 50 mL | 0.1 mg | 93.8 | [52] |
| 2. | Co3O4 | TY | 100 mL | 0.0020 g | 51.4 | [53] |
| 3. | Co3O4 | BO | 100 mL | 0.5 g | 78.45 | [54] |
| 4. | Co3O4 | MV, CV | 50 mL | 1.0 g | 92 and 64 | [55] |
| 5. | Co3O4 | MG | 100 mL | 50 mg | 91.2 | [56] |
| 6. | Co/Co3O4 | AR and AB | 50 mL | 0.04 g | 93 and 69.5 | [57] |
| 7. | Co and Co3O4 | murexide dye and EBT | 100 mL | 0.1 gL−1 | 43 and 39 | [58] |
| 8. | Co3O4 | CR | 100 mL | 0.50 g | 98 | [59] |
| 9. | Co3O4 | MB | 20 mL | 5 mg | 86 | [60] |
| 10. | Co3O4 | MO | 100 mL | 6 mg | 95 | [61] |
| 11. | Co3O4 | MB | 100 mL | 10 mg | 90 | Present work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chelliah, P.; Wabaidur, S.M.; Sharma, H.P.; Jweeg, M.J.; Majdi, H.S.; AL. Kubaisy, M.M.R.; Iqbal, A.; Lai, W.-C. Green Synthesis and Characterizations of Cobalt Oxide Nanoparticles and Their Coherent Photocatalytic and Antibacterial Investigations. Water 2023, 15, 910. https://doi.org/10.3390/w15050910
Chelliah P, Wabaidur SM, Sharma HP, Jweeg MJ, Majdi HS, AL. Kubaisy MMR, Iqbal A, Lai W-C. Green Synthesis and Characterizations of Cobalt Oxide Nanoparticles and Their Coherent Photocatalytic and Antibacterial Investigations. Water. 2023; 15(5):910. https://doi.org/10.3390/w15050910
Chicago/Turabian StyleChelliah, Parvathiraja, Saikh Mohammad Wabaidur, Hari Prapan Sharma, Muhsin J. Jweeg, Hasan Sh. Majdi, Munthir Mohammed Radhy AL. Kubaisy, Amjad Iqbal, and Wen-Cheng Lai. 2023. "Green Synthesis and Characterizations of Cobalt Oxide Nanoparticles and Their Coherent Photocatalytic and Antibacterial Investigations" Water 15, no. 5: 910. https://doi.org/10.3390/w15050910
APA StyleChelliah, P., Wabaidur, S. M., Sharma, H. P., Jweeg, M. J., Majdi, H. S., AL. Kubaisy, M. M. R., Iqbal, A., & Lai, W.-C. (2023). Green Synthesis and Characterizations of Cobalt Oxide Nanoparticles and Their Coherent Photocatalytic and Antibacterial Investigations. Water, 15(5), 910. https://doi.org/10.3390/w15050910








