Recent Advances on Chemically Functionalized Cellulose-Based Materials for Arsenic Removal in Wastewater: A Review
Abstract
:1. Introduction
2. Selective Studies on the Extraction of Cellulose from Various Sources
3. Brief History of Arsenic
4. Functionalised Cellulose for Removal of Arsenic
4.1. Preparation of Functionalised Cellulose
4.2. Adsorption Capacity of Functionalised Cellulose Materials
5. Conclusions and Future Recommendations
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ng, H.-M.; Sin, L.T.; Tee, T.-T.; Bee, S.-T.; Hui, D.; Low, C.-Y.; Rahmat, A. Extraction of cellulose nanocrystals from plant sources for application as reinforcing agent in polymers. Compos. Part B Eng. 2015, 75, 176–200. [Google Scholar] [CrossRef]
- Razali, N.A.M.; Sohaimi, R.M.; Othman, R.N.I.R.; Abdullah, N.; Demon, S.Z.N.; Jasmani, L.; Yunus, W.M.Z.W.; Ya’Acob, W.M.H.W.; Salleh, E.M.; Norizan, M.N.; et al. Comparative Study on Extraction of Cellulose Fiber from Rice Straw Waste from Chemo-Mechanical and Pulping Method. Polymers 2022, 14, 387. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.; Hamad, W.Y. Cellulose reinforced polymer composites and nanocomposites: A critical review. Cellulose 2013, 20, 2221–2262. [Google Scholar] [CrossRef]
- Karim, Z.; Hakalahti, M.; Tammelin, T.; Mathew, A.P. In situ TEMPO surface functionalization of nanocellulose membranes for enhanced adsorption of metal ions from aqueous medium. RSC Adv. 2017, 7, 5232–5241. [Google Scholar] [CrossRef] [Green Version]
- Pettignano, A.; Charlot, A.; Fleury, E. Carboxyl-functionalized derivatives of carboxymethyl cellulose: Towards advanced biomedical applications. Polym. Rev. 2019, 59, 510–560. [Google Scholar] [CrossRef]
- Kanikireddy, V.; Varaprasad, K.; Jayaramudu, T.; Karthikeyan, C.; Sadiku, R. Carboxymethyl cellulose-based materials for infection control and wound healing: A review. Int. J. Biol. Macromol. 2020, 164, 963–975. [Google Scholar] [CrossRef]
- Nicu, R.; Ciolacu, F.; Ciolacu, D.E. Advanced functional materials based on nanocellulose for pharmaceutical/medical applications. Pharmaceutics 2021, 13, 1125. [Google Scholar] [CrossRef]
- Long, W.; Ouyang, H.; Hu, X.; Liu, M.; Zhang, X.; Feng, Y.; Wei, Y. State-of-art review on preparation, surface functionalization and biomedical applications of cellulose nanocrystals-based materials. Int. J. Biol. Macromol. 2021, 186, 591–615. [Google Scholar] [CrossRef]
- Koshani, R.; Zhang, J.; van de Ven, T.G.M.; Lu, X.; Wang, Y. Modified Hairy Nanocrystalline Cellulose as Photobactericidal Nanofillers for Food Packaging Application. ACS Sustain. Chem. Eng. 2021, 9, 10513–10523. [Google Scholar] [CrossRef]
- Yu, H.; Yan, C.; Yao, J. Fully biodegradable food packaging materials based on functionalized cellulose nanocrystals/poly(3-hydroxybutyrate-co-3-hydroxyvalerate) nanocomposites. RSC Adv. 2014, 4, 59792–59802. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Barud, H.; Farinas, C.S.; Vasconcellos, V.M.; Claro, A.M. Bacterial Cellulose as a Raw Material for Food and Food Packaging Applications. Front. Sustain. Food Syst. 2019, 3, 7. [Google Scholar] [CrossRef] [Green Version]
- Azeredo, H.M.C.; Rosa, M.F.; Mattoso, L.H.C. Nanocellulose in bio-based food packaging applications. Ind. Crops Prod. 2017, 97, 664–671. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Ahmed, S.; Sameen, D.E.; Wang, Y.; Lu, R.; Dai, J.; Li, S.; Qin, W. A review of cellulose and its derivatives in biopol-ymer-based for food packaging application. Trends Food Sci. Technol. 2021, 112, 532–546. [Google Scholar] [CrossRef]
- Jiang, Z.; Ngai, T. Recent Advances in Chemically Modified Cellulose and Its Derivatives for Food Packaging Applications: A Review. Polymers 2022, 14, 1533. [Google Scholar] [CrossRef]
- Li, F.; Biagioni, P.; Bollani, M.; Maccagnan, A.; Piergiovanni, L. Multi-functional coating of cellulose nanocrystals for flexible packaging applications. Cellulose 2013, 20, 2491–2504. [Google Scholar] [CrossRef] [Green Version]
- Saedi, S.; Garcia, C.V.; Kim, J.T.; Shin, G.H. Physical and chemical modifications of cellulose fibers for food packaging ap-plications. Cellulose 2021, 28, 8877–8897. [Google Scholar] [CrossRef]
- Obey, G.; Adelaide, M.; Ramaraj, R. Biochar derived from non-customized matamba fruit shell as an adsorbent for wastewater treatment. J. Bioresour. Bioprod. 2022, 7, 109–115. [Google Scholar] [CrossRef]
- Bethke, K.; Palantöken, S.; Andrei, V.; Roß, M.; Raghuwanshi, V.S.; Kettemann, F.; Greis, K.; Ingber, T.T.K.; Stückrath, J.B.; Valiyaveettil, S.; et al. Functionalized Cellulose for Water Purification, Antimicrobial Applications, and Sensors. Adv. Funct. Mater. 2018, 28, 1800409. [Google Scholar] [CrossRef]
- Jjagwe, J.; Olupot, P.W.; Menya, E.; Kalibbala, H.M. Synthesis and Application of Granular Activated Carbon from Biomass Waste Materials for Water Treatment: A Review. J. Bioresour. Bioprod. 2021, 6, 292–322. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, C.; Chen, W.; Pastel, G.; Guo, X.; Liu, S.; Wang, Q.; Liu, Y.; Li, J.; Yu, H.; et al. Nanocellulose-enabled, all-nanofiber, high-performance supercapacitor. ACS Appl. Mater. Interfaces 2019, 11, 5919–5927. [Google Scholar]
- Jiang, Q.; Kacica, C.; Soundappan, T.; Liu, K.K.; Tadepalli, S.; Biswas, P.; Singamaneni, S. An in situ grown bacterial nano-cellulose/graphene oxide composite for flexible supercapacitors. J. Mater. Chem. 2017, 5, 13976–13982. [Google Scholar] [CrossRef]
- Li, Z.; Liu, J.; Jiang, K.; Thundat, T. Carbonized nanocellulose sustainably boosts the performance of activated carbon in ionic liquid supercapacitors. Nano Energy 2016, 25, 161–169. [Google Scholar] [CrossRef]
- Xiao, J.; Li, H.; Zhang, H.; He, S.; Zhang, Q.; Liu, K.; Jiang, S.; Duan, G.; Zhang, K. Nanocellulose and its derived composite electrodes toward supercapacitors: Fabrication, properties, and challenges. J. Bioresour. Bioprod. 2022, 7, 245–269. [Google Scholar] [CrossRef]
- Virtanen, J.; Pammo, A.; Keskinen, J.; Sarlin, E.; Tuukkanen, S. Pyrolysed cellulose nanofibrils and dandelion pappus in su-percapacitor application. Cellulose 2017, 24, 3387–3397. [Google Scholar] [CrossRef]
- Shankaran, D.R. Cellulose nanocrystals for health care applications. In Applications of Nanomaterials: Advances and Key Technologies; Woodhead Publishing: Sawston, UK, 2018; pp. 415–459. [Google Scholar]
- Heinze, T. Cellulose: Structure and properties. In Cellulose Chemistry and Properties: Fibers, Nanocelluloses and Advanced Materials; Springer: Berlin/Heidelberg, Germany, 2016; Volume 271, pp. 1–52. [Google Scholar]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef]
- Nishiyama, Y. Structure and properties of the cellulose microfibril. J. Wood Sci. 2009, 55, 241–249. [Google Scholar] [CrossRef]
- Naderi, A. Nanofibrillated cellulose: Properties reinvestigated. Cellulose 2017, 24, 1933–1945. [Google Scholar] [CrossRef]
- Peng, B.L.; Yao, Z.L.; Wang, X.C.; Crombeen, M.; Sweeney, D.G.; Tam, K.C. Cellulose-based materials in wastewater treatment of petroleum industry. Green Energy Environ. 2020, 5, 37–49. [Google Scholar] [CrossRef]
- Liimatainen, H.; Sirviö, J.; Sundman, O.; Visanko, M.; Hormi, O.; Niinimäki, J. Flocculation performance of a cationic bi-opolymer derived from a cellulosic source in mild aqueous solution. Bioresour. Technol. 2011, 102, 9626–9632. [Google Scholar] [CrossRef]
- Yan, M.; Li, S.; Zhang, M.; Li, C.; Dong, F.; Li, W. Characterization of Surface Acetylated Nanocrystalline Cellulose by Single-Step Method. Bioresources 2013, 8, 6330–6341. [Google Scholar] [CrossRef]
- Zhang, Z.; Sèbe, G.; Rentsch, D.; Zimmermann, T.; Tingaut, P. Ultralightweight and Flexible Silylated Nanocellulose Sponges for the Selective Removal of Oil from Water. Chem. Mater. 2014, 26, 2659–2668. [Google Scholar] [CrossRef]
- Abdel-Halim, E.S. Chemical modification of cellulose extracted from sugarcane bagasse: Preparation of hydroxyethyl cel-lulose. Arab. J. Chem. 2014, 7, 362–371. [Google Scholar] [CrossRef] [Green Version]
- Belgacem, M.N.; Salon-Brochier, M.C.; Krouit, M.; Bras, J. Recent advances in surface chemical modification of cellulose fibres. J. Adhes. Sci. Technol. 2011, 25, 661–684. [Google Scholar] [CrossRef]
- Yagyu, H.; Saito, T.; Isogai, A.; Koga, H.; Nogi, M. Chemical Modification of Cellulose Nanofibers for the Production of Highly Thermal Resistant and Optically Transparent Nanopaper for Paper Devices. ACS Appl. Mater. Interfaces 2015, 7, 22012–22017. [Google Scholar] [CrossRef] [PubMed]
- Heise, K.; Delepierre, G.; King, A.W.T.; Kostiainen, M.A.; Zoppe, J.; Weder, C.; Kontturi, E. Chemical Modification of Reducing End-Groups in Cellulose Nanocrystals. Angew. Chem. Int. Ed. 2020, 60, 66–87. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, H.; Li, X.; Gibril, M.E.; Yu, M. Chemical modification of cellulose by in situ reactive extrusion in ionic liq-uid. Carbohydr. Polym. 2014, 99, 126–131. [Google Scholar] [CrossRef]
- Durán, V.L.; Larsson, P.A.; Wågberg, L. Chemical modification of cellulose-rich fibres to clarify the influence of the chemical structure on the physical and mechanical properties of cellulose fibres and thereof made sheets. Carbohydr. Polym. 2018, 182, 1–7. [Google Scholar] [CrossRef]
- Zhou, L.; Ke, K.; Yang, M.B.; Yang, W. Recent progress on chemical modification of cellulose for high mechanical-performance Poly (lactic acid)/Cellulose composite: A review. Compos. Commun. 2021, 23, 100548. [Google Scholar] [CrossRef]
- Taczała, J.; Sawicki, J.; Pietrasik, J. Chemical Modification of Cellulose Microfibres to Reinforce Poly(methyl methacrylate) Used for Dental. Appl. Mater. 2020, 13, 3807. [Google Scholar] [CrossRef]
- Aguado, R.; Lourenço, A.F.; Ferreira, P.J.T.; Moral, A.; Tijero, A. The relevance of the pretreatment on the chemical modification of cellulosic fibers. Cellulose 2019, 26, 5925–5936. [Google Scholar] [CrossRef]
- Sirviö, J.A.; Heiskanen, J.P. Room-temperature dissolution and chemical modification of cellulose in aqueous tetrae-thylammonium hydroxide–carbamide solutions. Cellulose 2020, 27, 1933–1950. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Lu, M.; Li, Q.; Jiang, H.; Yin, S. Research progress of arsenic removal from wastewater. IOP Conf. Ser. Earth Environ. Sci. 2019, 218, 012142. [Google Scholar]
- Chen, H.; Sharma, S.K.; Sharma, P.R.; Yeh, H.; Johnson, K.; Hsiao, B.S. Arsenic(III) Removal by Nanostructured Dialdehyde Cellulose–Cysteine Microscale and Nanoscale Fibers. ACS Omega 2019, 4, 22008–22020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, Y.; Wu, M.; Lin, X.; Huang, P.; Huang, Y. Synthesis of magnetic wheat straw for arsenic adsorption. J. Hazard. Mater. 2011, 193, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw materi-al. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Aziz, T.; Farid, A.; Haq, F.; Kiran, M.; Ullah, A.; Zhang, K.; Li, C.; Ghazanfar, S.; Sun, H.; Ullah, R.; et al. A Review on the Modification of Cellulose and Its Applications. Polymers 2022, 14, 3206. [Google Scholar] [CrossRef] [PubMed]
- Hokkanen, S.; Repo, E.; Sillanpää, M. Removal of heavy metals from aqueous solutions by succinic anhydride modified mercerized nanocellulose. J. Chem. Eng. 2013, 223, 40–47. [Google Scholar] [CrossRef]
- Yang, R.; Aubrecht, K.B.; Ma, H.; Wang, R.; Grubbs, R.B.; Hsiao, B.S.; Chu, B. Thiol-modified cellulose nanofibrous composite membranes for chromium (VI) and lead (II) adsorption. Polymer 2014, 55, 1167–1176. [Google Scholar] [CrossRef]
- Alatalo, S.-M.; Pileidis, F.; Mäkilä, E.; Sevilla, M.; Repo, E.; Salonen, J.; Sillanpää, M.; Titirici, M.-M. Versatile Cellulose-Based Carbon Aerogel for the Removal of Both Cationic and Anionic Metal Contaminants from Water. ACS Appl. Mater. Interfaces 2015, 7, 25875–25883. [Google Scholar] [CrossRef]
- Ma, H.; Hsiao, B.S.; Chu, B. Ultrafine cellulose nanofibers as efficient adsorbents for removal of UO22+ in water. ACS Macro Lett. 2012, 1, 213–216. [Google Scholar] [CrossRef]
- Sharma, P.R.; Chattopadhyay, A.; Sharma, S.K.; Hsiao, B.S. Efficient Removal of UO22+ from Water Using Carboxycellulose Nanofibers Prepared by the Nitro-Oxidation Method. Ind. Eng. Chem. Res. 2017, 56, 13885–13893. [Google Scholar] [CrossRef]
- Sharma, P.R.; Chattopadhyay, A.; Zhan, C.; Sharma, S.K.; Geng, L.; Hsiao, B.S. Lead removal from water using carboxycel-lulose nanofibers prepared by nitro-oxidation method. Cellulose 2018, 25, 1961–1973. [Google Scholar] [CrossRef]
- Sharma, P.R.; Chattopadhyay, A.; Sharma, S.K.; Geng, L.; Amiralian, N.; Martin, D.; Hsiao, B.S. Nanocellulose from Spinifex as an Effective Adsorbent to Remove Cadmium(II) from Water. ACS Sustain. Chem. Eng. 2018, 6, 3279–3290. [Google Scholar] [CrossRef]
- Cui, G.; Liu, M.; Chen, Y.; Zhang, W.; Zhao, J. Synthesis of a ferric hydroxide-coated cellulose nanofiber hybrid for effective removal of phosphate from wastewater. Carbohydr. Polym. 2016, 154, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, C.; Zhao, L.; Meng, G.; Wu, J.; Liu, Z. Cellulose-based porous adsorbents with high capacity for methylene blue adsorption from aqueous solutions. Fibers Polym. 2017, 18, 891–899. [Google Scholar] [CrossRef]
- Sharma, P.R.; Sharma, S.K.; Antoine, R.; Hsiao, B.S. Efficient Removal of Arsenic Using Zinc Oxide Nanocrystal-Decorated Regenerated Microfibrillated Cellulose Scaffolds. ACS Sustain. Chem. Eng. 2019, 7, 6140–6151. [Google Scholar] [CrossRef]
- Cullen, L.E.; Macfarlane, C. Comparison of cellulose extraction methods for analysis of stable isotope ratios of carbon and oxygen in plant material. Tree Physiol. 2005, 25, 563–569. [Google Scholar] [CrossRef] [Green Version]
- Ohwoavworhua, F.; Adelakun, T.; Okhamafe, A. Processing pharmaceutical grade microcrystalline cellulose from groundnut husk: Extraction methods and characterization. Int. J. Green Pharm. 2009, 3, 97–104. [Google Scholar] [CrossRef]
- Vu, N.D.; Tran, H.T.; Bui, N.D.; Vu, C.D.; Nguyen, H.V. Lignin and Cellulose Extraction from Vietnam’s Rice Straw Using Ultrasound-Assisted Alkaline Treatment Method. Int. J. Polym. Sci. 2017, 2017, 1063695. [Google Scholar]
- Menon, M.P.; Selvakumar, R.; Kumar, P.S.; Ramakrishna, S. Extraction and modification of cellulose nanofibers derived from biomass for environmental application. RSC Adv. 2017, 7, 42750–42773. [Google Scholar] [CrossRef] [Green Version]
- Park, N.-M.; Choi, S.; Oh, J.E.; Hwang, D.Y. Facile extraction of cellulose nanocrystals. Carbohydr. Polym. 2019, 223, 115114. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Y.; Wang, S.; Ma, L.; Yu, Y.; Dai, H.; Zhang, Y. Extraction and comparison of cellulose nanocrystals from lemon (Citrus limon) seeds using sulfuric acid hydrolysis and oxidation methods. Carbohydr. Polym. 2020, 238, 116180. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Huang, Y.; Yu, W. Effects of extraction methods on morphology, structure and properties of bamboo cellulose. Ind. Crop. Prod. 2021, 169, 113640. [Google Scholar] [CrossRef]
- He, C.; Li, H.; Hong, J.; Xiong, H.; Ni, H.; Zheng, M. Characterization and Functionality of Cellulose from Pomelo Fruitlets by Different Extraction Methods. Polymers 2022, 14, 518. [Google Scholar] [CrossRef] [PubMed]
- Amin, K.N.M.; Hosseinmardi, A.; Martin, D.J.; Annamalai, P.K. A mixed acid methodology to produce thermally stable cellulose nanocrystal at high yield using phosphoric acid. J. Bioresour. Bioprod. 2022, 7, 99–108. [Google Scholar] [CrossRef]
- Teisala, H.; Tuominen, M.; Kuusipalo, J. Superhydrophobic coatings on cellulose-based materials: Fabrication, properties, and applications. Adv. Mater. Interfaces 2014, 1, 1300026. [Google Scholar] [CrossRef]
- Chopra, L. Manikanika Extraction of cellulosic fibers from the natural resources: A short review. Mater. Today Proc. 2021, 48, 1265–1270. [Google Scholar]
- de Oliveira, J.P.; Bruni, G.P.; Lima, K.O.; El Halal, S.L.M.; da Rosa, G.S.; Dias, A.R.G.; da Rosa Zavareze, E. Cellulose fibers extracted from rice and oat husks and their application in hydrogel. Food Chem. 2017, 221, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Reiniati, I.; Hrymak, A.N.; Margaritis, A. Recent developments in the production and applications of bacterial cellulose fibers and nanocrystals. Crit. Rev. Biotechnol. 2016, 37, 510–524. [Google Scholar] [CrossRef]
- Iglesias, M.C.; Gomez-Maldonado, D.; Via, B.K.; Jiang, Z.; Peresin, M.S. Pulping Processes and Their Effects on Cellulose Fibers and Nanofibrillated Cellulose Properties: A Review. For. Prod. J. 2020, 70, 10–21. [Google Scholar] [CrossRef]
- Harini, K.; Ramya, K.; Sukumar, M. Extraction of nano cellulose fibers from the banana peel and bract for production of acetyl and lauroyl cellulose. Carbohydr. Polym. 2018, 201, 329–339. [Google Scholar] [CrossRef]
- Ilangovan, M.; Guna, V.; Hu, C.; Nagananda, G.; Reddy, N. Curcuma longa L. plant residue as a source for natural cellulose fibers with antimicrobial activity. Ind. Crop. Prod. 2018, 112, 556–560. [Google Scholar] [CrossRef]
- Orelma, H.; Hokkanen, A.; Leppänen, I.; Kammiovirta, K.; Kapulainen, M.; Harlin, A. Optical cellulose fiber made from regenerated cellulose and cellulose acetate for water sensor applications. Cellulose 2019, 27, 1543–1553. [Google Scholar] [CrossRef] [Green Version]
- Lavanya, D.K.P.K.; Kulkarni, P.K.; Dixit, M.; Raavi, P.K.; Krishna, L.N.V. Sources of cellulose and their applications—A review. Int. J. Drug Dev. Res. 2011, 2, 19–38. [Google Scholar]
- Morán, J.I.; Álvarez, V.; Cyras, V.P.; Vázquez, A. Extraction of cellulose and preparation of nanocellulose from sisal fibers. Cellulose 2007, 15, 149–159. [Google Scholar] [CrossRef]
- Song, K.; Zhu, X.; Zhu, W.; Li, X. Preparation and characterization of cellulose nanocrystal extracted from Calotropis procera biomass. Bioresour. Bioprocess 2019, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Azum, N.; Jawaid, M.; Kian, L.K.; Khan, A.; Alotaibi, M.M. Extraction of Microcrystalline Cellulose from Washingtonia Fibre and Its Characterization. Polymers 2021, 13, 3030. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Jiang, W.; Zhang, Y.; Wang, H.; Zou, F.; Yu, K.; Han, G. A novel process of nanocellulose extraction from kenaf bast. Mater. Res. Express 2018, 5, 085032. [Google Scholar] [CrossRef]
- Lam, E.; Male, K.B.; Chong, J.H.; Leung, A.C.; Luong, J.H. Applications of functionalized and nanoparticle-modified nano-crystalline cellulose. Trends Biotechnol. 2012, 30, 283–290. [Google Scholar] [CrossRef]
- Luzi, F.; Fortunati, E.; Puglia, D.; Lavorgna, M.; Santulli, C.; Kenny, J.M.; Torre, L. Optimized extraction of cellulose nano-crystals from pristine and carded hemp fibres. Ind. Crops Prod. 2014, 56, 175–186. [Google Scholar] [CrossRef]
- Das, A.M.; Hazarika, M.P.; Goswani, M.; Yadav, A.; Khound, P. Extraction of cellulose from agricultural waste using mont-morillonite K-10/LiOH and its conversion to renewable energy: Biofuel by using myrothecium gramineum. Carbohydr. Polym. 2016, 141, 20–27. [Google Scholar] [CrossRef]
- Romruen, O.; Karbowiak, T.; Tongdeesoontorn, W.; Shiekh, K.A.; Rawdkuen, S. Extraction and Characterization of Cellulose from Agricultural By-Products of Chiang Rai Province, Thailand. Polymers 2022, 14, 1830. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Cai, Z.; Lin, F.; Tang, L.; Wang, S.; Huang, B. Extraction of cellulose nanocrystals with a high yield of 88% by simul-taneous mechanochemical activation and phosphotungstic acid hydrolysis. ACS Sustain. Chem. Eng. 2016, 4, 2165–2172. [Google Scholar] [CrossRef]
- Singh, A.; Ranawat, B.; Meena, R. Extraction and characterization of cellulose from halophytes: Next generation source of cellulose fibre. SN Appl. Sci. 2019, 1, 1311. [Google Scholar] [CrossRef] [Green Version]
- Wahib, S.A.; Da’Na, D.A.; Al-Ghouti, M.A. Insight into the extraction and characterization of cellulose nanocrystals from date pits. Arab. J. Chem. 2022, 15, 103650. [Google Scholar] [CrossRef]
- Al-Ghamdi, Y.O. Immobilization of cellulose extracted from Robinia Pseudoacacia seed fibers onto chitosan: Chemical characterization and study of methylene blue removal. Arab. J. Chem. 2022, 15, 104066. [Google Scholar] [CrossRef]
- Judith, R.B.D.; Pámanes-Carrasco, G.A.; Delgado, E.; Rodríguez-Rosales, M.D.J.; Medrano-Roldán, H.; Reyes-Jáquez, D. Ex-traction optimization and molecular dynamic simulation of cellulose nanocrystals obtained from bean forage. Biocatal. Agric. Biotechnol. 2022, 43, 102443. [Google Scholar] [CrossRef]
- Mohamad, N.A.N.; Jai, J. Response surface methodology for optimization of cellulose extraction from banana stem using NaOH-EDTA for pulp and papermaking. Heliyon 2022, 8, e09114. [Google Scholar] [CrossRef]
- Gao, A.; Chen, H.; Tang, J.; Xie, K.; Hou, A. Efficient extraction of cellulose nanocrystals from waste Calotropis gigantea fiber by SO42-/TiO2 nano-solid superacid catalyst combined with ball milling exfoliation. Ind. Crops Prod. 2020, 152, 112524. [Google Scholar] [CrossRef]
- Chin, K.-M.; Sam, S.T.; Ong, H.L.; Wong, Y.S.; Tan, W.K.; Vannaladsaysy, V. Bioinspired Crosslinked Nanocomposites of Polyvinyl Alcohol-Reinforced Cellulose Nanocrystals Extracted from Rice Straw with Ethanedioic Acid. J. Nanomater. 2022, 2022, 3225211. [Google Scholar] [CrossRef]
- Khan, A.; Raghunathan, V.; Singaravelu, D.L.; Sanjay, M.; Siengchin, S.; Jawaid, M.; Alamry, K.A.; Asiri, A.M. Extraction and Characterization of Cellulose Fibers from the Stem of Momordica Charantia. J. Nat. Fibers 2020, 19, 2232–2242. [Google Scholar] [CrossRef]
- Lemita, N.; Deghboudj, S.; Rokbi, M.; Rekbi, F.M.L.; Halimi, R. Characterization and analysis of novel natural cellulosic fiber extracted from Strelitzia reginae plant. J. Compos. Mater. 2021, 56, 99–114. [Google Scholar] [CrossRef]
- Pota, G.; Salerno, A.S.; Costantini, A.; Silvestri, B.; Passaro, J.; Califano, V. Co-immobilization of Cellulase and β-Glucosidase into Mesoporous Silica Nanoparticles for the Hydrolysis of Cellulose Extracted from Eriobotrya japonica Leaves. Langmuir 2022, 38, 5481–5493. [Google Scholar] [CrossRef] [PubMed]
- Lou, C.; Zhou, Y.; Yan, A.; Liu, Y. Extraction cellulose from corn-stalk taking advantage of pretreatment technology with immobilized enzyme. RSC Adv. 2021, 12, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Ma, T.; Hu, X.; Zhao, J.; Liao, X.; Song, Y.; Hu, X. Facile extraction and characterization of cellulose nanocrystals from agricultural waste sugarcane straw. J. Sci. Food Agric. 2021, 102, 312–321. [Google Scholar] [CrossRef]
- Almutairi, F.M.; El-Ghoul, Y.; Jabli, M. Extraction of Cellulose Polymeric Material from Populus tremula Fibers: Character-ization and Application to the Adsorption of Methylene Blue and Crystal Violet. Polymers 2021, 13, 3334. [Google Scholar] [CrossRef]
- Nasution, H.; Yahya, E.B.; Khalil, H.P.S.A.; Shaah, M.A.; Suriani, A.B.; Mohamed, A.; Alfatah, T.; Abdullah, C.K. Extraction and Isolation of Cellulose Nanofibers from Carpet Wastes Using Supercritical Carbon Dioxide Approach. Polymers 2022, 14, 326. [Google Scholar] [CrossRef]
- Mitbumrung, W.; Rungraung, N.; Muangpracha, N.; Akanitkul, P.; Winuprasith, T. Approaches for Extracting Nanofibrillated Cellulose from Oat Bran and Its Emulsion Capacity and Stability. Polymers 2022, 14, 327. [Google Scholar] [CrossRef]
- Perumal, A.B.; Nambiar, R.B.; Sellamuthu, P.S.; Sadiku, E.R.; Li, X.; He, Y. Extraction of cellulose nanocrystals from areca waste and its application in eco-friendly biocomposite film. Chemosphere 2021, 287, 132084. [Google Scholar] [CrossRef]
- Sambu, S.; Wilson, R. Arsenic in food and water–a brief history. Toxicol. Ind. Health 2008, 24, 217–226. [Google Scholar] [CrossRef]
- Hughes, M.F.; Beck, B.D.; Chen, Y.; Lewis, A.S.; Thomas, D.J. Arsenic Exposure and Toxicology: A Historical Perspective. Toxicol. Sci. 2011, 123, 305–332. [Google Scholar] [CrossRef] [Green Version]
- Vahidnia, A.; van der Voet, G.B.; de Wolff, F.A. Arsenic neurotoxicity—A review. Hum. Exp. Toxicol. 2007, 26, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Ye, Z.; Huang, L.; Zhang, C.; Guo, Y.; Zhang, W. Arsenic Occurrence and Cycling in the Aquatic Environment: A Comparison between Freshwater and Seawater. Water 2022, 15, 147. [Google Scholar] [CrossRef]
- Waxman, S.; Anderson, K.C. History of the Development of Arsenic Derivatives in Cancer Therapy. Oncologist 2001, 6, 3–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.S.; Wai, C.M. Arsenic in Drinking Water—A Global Environmental Problem. J. Chem. Educ. 2004, 81, 207. [Google Scholar] [CrossRef]
- Cullen, W.R. Is Arsenic an Aphrodisiac? The Sociochemistry of an Element; Royal Society of Chemistry: London, UK, 2008; p. 428. [Google Scholar]
- Arifullah Changsheng, H.; Akram, W.; Rashid, A.; Ullah, Z.; Shah, M.; Alrefaei, A.F.; Kamel, M.; Aleya, L.; Abdel-Daim, M.M. Quality Assessment of Groundwater Based on Geochemical Modelling and Water Quality Index (WQI). Water 2022, 14, 3888. [Google Scholar] [CrossRef]
- Kumar, A.; Joshi, H.; Kumar, A. Arsenate Removal from the Groundwater Employing Maghemite Nanoparticles. Water 2022, 14, 3617. [Google Scholar] [CrossRef]
- 40 CFR 141.11; Code of Federal Regulations. U.S. Government Publishing Office (GPO): Washington, DC, USA, 1992.
- De Klerk, R.J.; Feldmann, T.; Daenzer, R.; Demopoulos, G.P. Continuous circuit coprecipitation of arsenic (V) with ferric iron by lime neutralization: The effect of circuit staging, co-ions and equilibration pH on long-term arsenic reten-tion. Hydrometallurgy 2015, 151, 42–50. [Google Scholar] [CrossRef]
- Laatikainen, M.; Sillanpää, M.; Sainio, T. Comparison of ion exchange process configurations for arsenic removal from natural waters. Desalination Water Treat. 2016, 57, 13770–13781. [Google Scholar] [CrossRef]
- Bahmani, P.; Maleki, A.; Daraei, H.; Khamforoush, M.; Rezaee, R.; Gharibi, F.; Tkachev, A.G.; Burakov, A.E.; Agarwal, S.; Gupta, V.K. High-flux ultrafiltration membrane based on electrospun polyacrylonitrile nanofibrous scaffolds for arsenate removal from aqueous solutions. J. Colloid Interface Sci. 2017, 506, 564–571. [Google Scholar] [CrossRef]
- Hao, L.; Liu, M.; Wang, N.; Li, G. A critical review on arsenic removal from water using iron-based adsorbents. RSC Adv. 2018, 8, 39545–39560. [Google Scholar] [CrossRef]
- Hao, L.; Wang, N.; Wang, C.; Li, G. Arsenic removal from water and river water by the combined adsorption—UF membrane process. Chemosphere 2018, 202, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Yang, Z.; Zeng, G.; Yang, X.; Xu, H.; Wang, L.; Xu, R.; Xiong, W.; Ahmad, K. Electrocoagulation treatment of arsenic in wastewaters: A comprehensive review. Chem. Eng. J. 2017, 317, 707–725. [Google Scholar] [CrossRef]
- Chowdhury, R. Using adsorption and sulphide precipitation as the principal removal mechanisms of arsenic from a con-structed wetland–a critical review. Chem. Ecol. 2017, 33, 560–571. [Google Scholar] [CrossRef]
- Driehaus, W.; Dupont, F. Arsenic removal: Solutions for a world wide health problem using iron based adsorbents. Eur. J. Water Qual. 2005, 36, 119–132. [Google Scholar] [CrossRef]
- Pintor, A.M.; Vieira, B.R.; Santos, S.C.; Boaventura, R.A.; Botelho, C.M. Arsenate and arsenite adsorption onto iron-coated cork granulates. Sci. Total Environ. 2018, 642, 1075–1089. [Google Scholar] [CrossRef]
- Yang, G.; Liu, Y.; Song, S. Competitive adsorption of As(V) with co-existing ions on porous hematite in aqueous solutions. J. Environ. Chem. Eng. 2015, 3, 1497–1503. [Google Scholar] [CrossRef]
- Lalley, J.; Han, C.; Li, X.; Dionysiou, D.D.; Nadagouda, M.N. Phosphate adsorption using modified iron oxide-based sorbents in lake water: Kinetics, equilibrium, and column tests. Chem. Eng. J. 2016, 284, 1386–1396. [Google Scholar] [CrossRef]
- Fakour, H.; Pan, Y.-F.; Lin, T.-F. Effect of Humic Acid on Arsenic Adsorption and Pore Blockage on Iron-Based Adsorbent. Water Air Soil Pollut. 2015, 226, 14. [Google Scholar] [CrossRef]
- Feng, Z.; Ning, Y.; Yang, S.; Yu, J.; Ouyang, W.; Li, Y. A novel strategy for arsenic removal from acid wastewater via strong reduction processing. Environ. Sci. Pollut. Res. 2023, 30, 1–15. [Google Scholar] [CrossRef]
- Akin, I.; Arslan, G.; Tor, A.; Cengeloglu, Y.; Ersoz, M. Removal of arsenate [As(V)] and arsenite [As(III)] from water by SWHR and BW-30 reverse osmosis. Desalination 2011, 281, 88–92. [Google Scholar] [CrossRef]
- Gecol, H.; Ergican, E.; Fuchs, A. Molecular level separation of arsenic (V) from water using cationic surfactant micelles and ultrafiltration membrane. J. Membr. Sci. 2004, 241, 105–119. [Google Scholar] [CrossRef]
- Figoli, A.; Cassano, A.; Criscuoli, A.; Mozumder, M.S.I.; Uddin, M.T.; Islam, M.A.; Drioli, E. Influence of operating parameters on the arsenic removal by nanofiltration. Water Res. 2010, 44, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Hokkanen, S.; Repo, E.; Lou, S.; Sillanpää, M. Removal of arsenic (V) by magnetic nanoparticle activated microfibrillated cellulose. Chem. Eng. J. 2015, 260, 886–894. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, Y.; Liu, Z.; Huang, Q. Characteristics of equilibrium, kinetics studies for adsorption of Hg (II), Cu (II), and Ni (II) ions by thiourea-modified magnetic chitosan microspheres. J. Hazard. Mater. 2009, 161, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Hafez, I.; Tajvidi, M.; Amirbahman, A. Highly Efficient Iron Oxide Nanoparticles Immobilized on Cellulose Nanofibril Aerogels for Arsenic Removal from Water. Nanomaterials 2021, 11, 2818. [Google Scholar] [CrossRef]
- Chai, F.; Zhang, R.; Min, X.; Yang, Z.; Chai, L.; Zhao, F. Highly efficient removal of arsenic (III/V) from groundwater using nZVI functionalized cellulose nanocrystals fabricated via a bioinspired strategy. Sci. Total Environ. 2022, 842, 156937. [Google Scholar] [CrossRef]
- Yu, X.; Tong, S.; Ge, M.; Zuo, J.; Cao, C.; Song, W. One-step synthesis of magnetic composites of cellulose@ iron oxide nano-particles for arsenic removal. J. Mater. Chem. A 2013, 1, 959–965. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, M.; Wu, W.; Jin, W. Synthesis of the cotton cellulose based Fe(III)-loaded adsorbent for arsenic(V) removal from drinking water. Desalination 2009, 249, 1006–1011. [Google Scholar] [CrossRef]
- Taleb, K.; Markovski, J.; Veličković, Z.; Rusmirović, J.; Rančić, M.; Pavlović, V.; Marinković, A. Arsenic removal by magnet-ite-loaded amino modified nano/microcellulose adsorbents: Effect of functionalization and media size. Arab. J. Chem. 2019, 12, 4675–4693. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, S.; Kumar, A.A.; Sudhakar, C.; Kumar, R.; Ahuja, T.; Mondal, B.; Srikrishnarka, P.; Philip, L.; Pradeep, T. Sustainable and Affordable Composites Built Using Microstructures Performing Better than Nanostructures for Arsenic Removal. ACS Sustain. Chem. Eng. 2018, 7, 3222–3233. [Google Scholar] [CrossRef]
- Deng, S.; Chen, S.; Xue, Y.; Du, Z.; Wang, P. Rapid and effective preparation of a HPEI modified biosorbent based on cellulose fiber with a microwave irradiation method for enhanced arsenic removal in water. J. Mater. Chem. A 2016, 441, 15851–15860. [Google Scholar] [CrossRef]
- Chai, F.; Wang, R.; Yan, L.; Li, G.; Cai, Y.; Xi, C. Facile fabrication of pH-sensitive nanoparticles based on nanocellulose for fast and efficient As(V) removal. Carbohydr. Polym. 2020, 245, 116511. [Google Scholar] [CrossRef] [PubMed]
- Anirudhan, T.; Nima, J.; Sandeep, S.; Ratheesh, V.R.N. Development of an amino functionalized glycidylmethacry-late-grafted-titanium dioxide densified cellulose for the adsorptive removal of arsenic (V) from aqueous solutions. Chem. Eng. J. 2012, 209, 362–371. [Google Scholar] [CrossRef]
- Pramanik, K.; Sarkar, P.; Bhattacharyay, D. 3-Mercapto-propanoic acid modified cellulose filter paper for quick removal of arsenate from drinking water. Int. J. Biol. Macromol. 2018, 122, 185–194. [Google Scholar] [CrossRef]
- Nagarajan, D.; Venkatanarasimhan, S. Magnetite microparticles decorated cellulose sponge as an efficacious filter for im-proved arsenic (V) removal. J. Environ. Chem. Eng. 2019, 7, 103386. [Google Scholar] [CrossRef]
- Singh, K.; Sinha, T.; Srivastava, S. Functionalized nanocrystalline cellulose: Smart biosorbent for decontamination of arsenic. Int. J. Miner. Process. 2015, 139, 51–63. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Senan, P.; Suchithra, P.S. Evaluation of iron (III)-coordinated amino-functionalized poly (glycidyl methac-rylate)-grafted cellulose for arsenic (V) adsorption from aqueous solutions. Wat. Air Soil Pollut. 2011, 220, 101–116. [Google Scholar] [CrossRef]
- Nakakubo, K.; Endo, M.; Sakai, Y.; Biswas, F.B.; Wong, K.H.; Mashio, A.S.; Taniguchi, T.; Nishimura, T.; Maeda, K.; Hasegawa, H. Cross-linked dithiocarbamate-modified cellulose with enhanced thermal stability and dispersibility as a sorbent for ar-senite removal. Chemosphere 2022, 307, 135671. [Google Scholar] [CrossRef]
- Tewatia, P.; Kumar, V.; Samota, S.; Singhal, S.; Kaushik, A. Sensing and annihilation of ultra-trace level arsenic (III) using fluoranthene decorated fluorescent nanofibrous cellulose probe. J. Hazard. Mater. 2022, 424, 127722. [Google Scholar] [CrossRef]
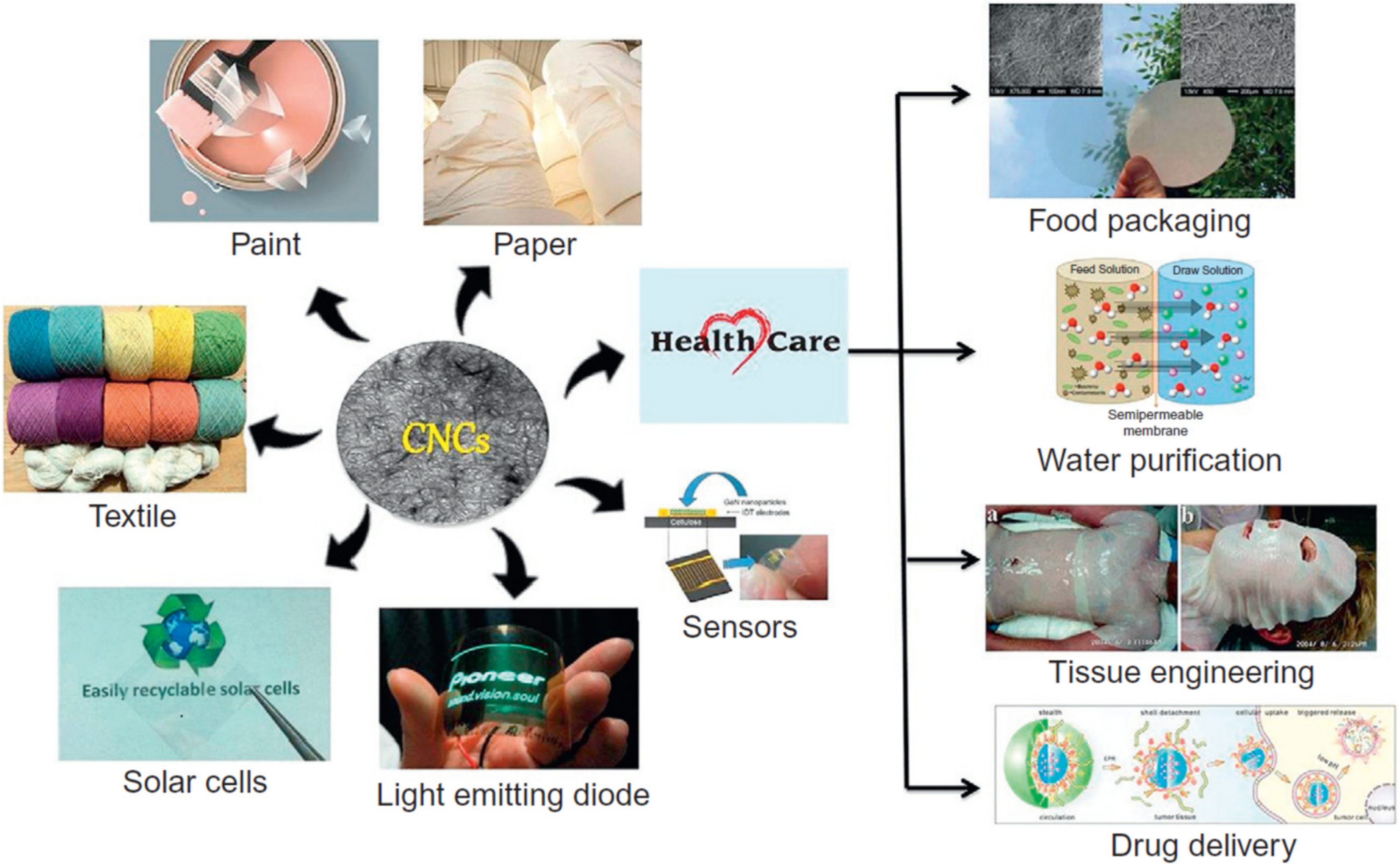

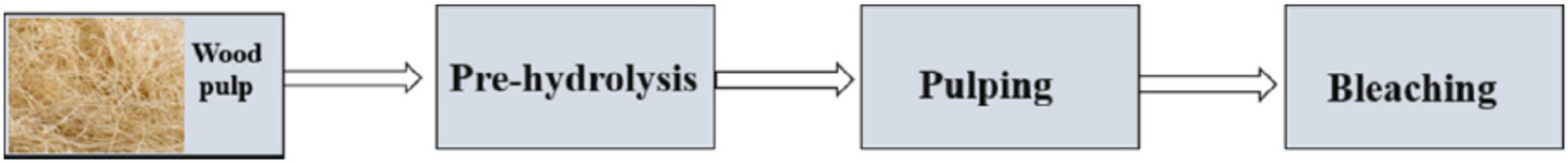
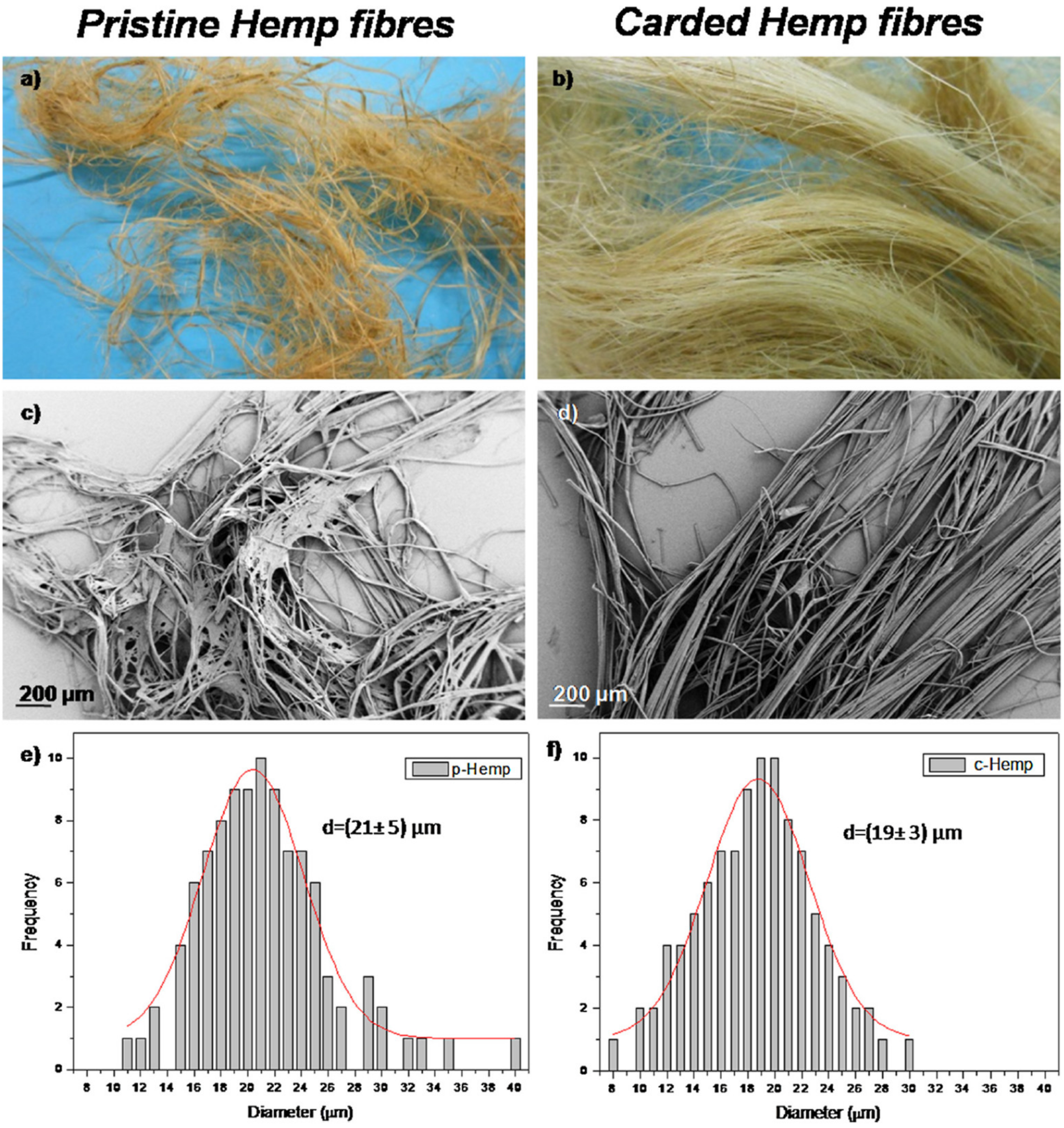




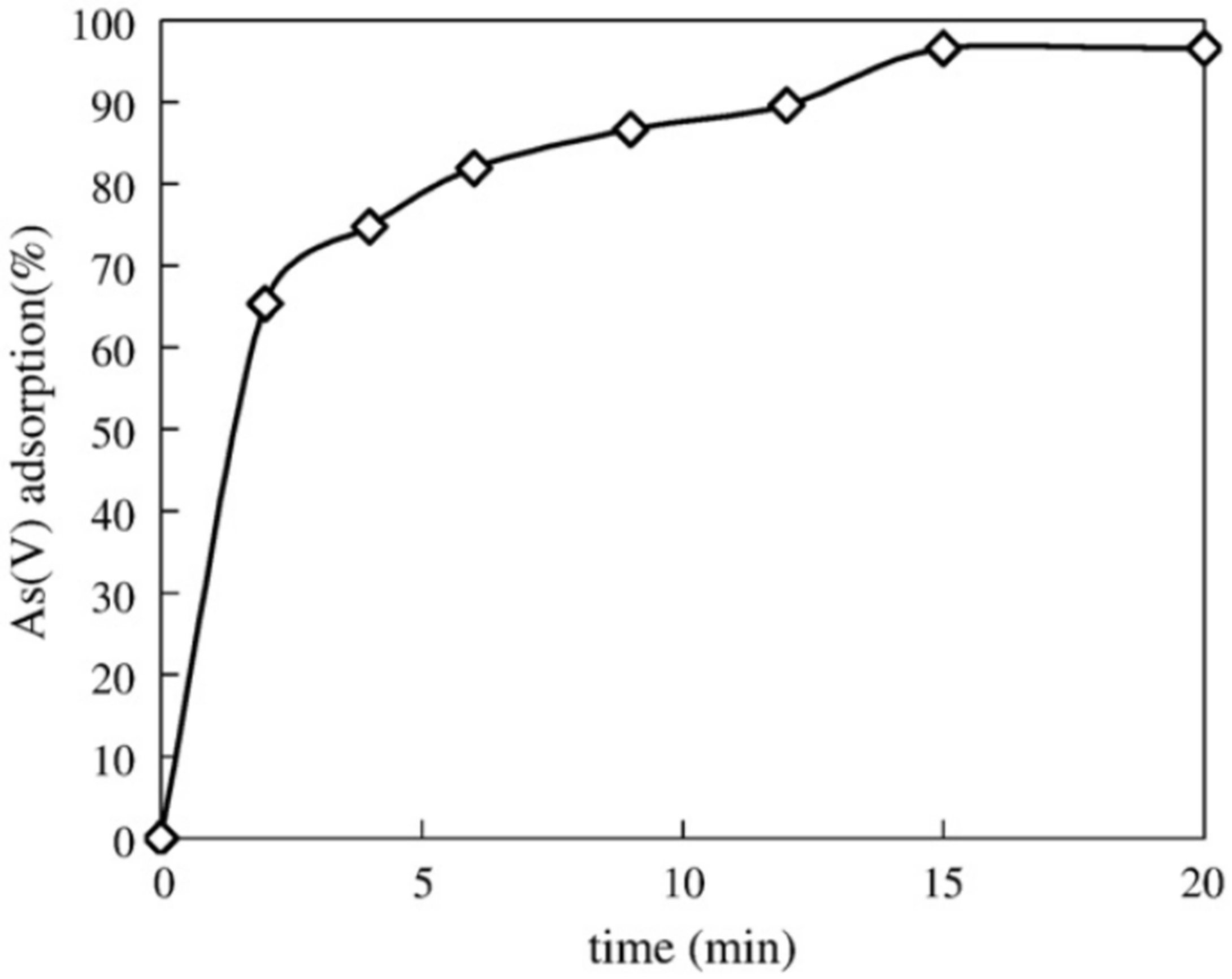
| Source of Cellulose | Type of Cellulose | Cellulose Extraction Method | Extraction Conditions | Results Obtained | Refs. |
|---|---|---|---|---|---|
| Date pits (DP) | Cellulose nanocrystals (CNCs) | Two methods were used: mechanical stirrer method (CNCs1) and soxhlet apparatus method (CNCs2) |
|
| [87] |
| Robinia pseudoacacia seed fibres | Cellulose fibres immobilised onto chitosan beads | Cellulose was extracted using methods by Gao et al. [25] and Almutairi et al. [32], with slight modifications; extracted cellulose was filtered and blended with chitosan at variable composition ratios |
|
| [88] |
| Bean forage | Cellulose nanocrystals | Two response surface superimposition methodologies were used with a rotatable composite central design (CCD) for both |
|
| [89] |
| Banana stem | Cellulose pulp | Alkaline pulping using sodium hydroxide (NaOH) | 17.7% NaOH and 10% EDTA, pulping of banana stem at 100 ± 5 °C for 30 min |
| [90] |
| Calotropis gigantea fibre (CGF) | Cellulose nanocrystals (CNCs) | Combined ball milling defibrillation and SO42−/TiO2 nanosolid superacid catalyst-assisted method | Bleached CGF was milled with superfine zirconium beads in the presence of diluted H2SO4 and the synthesised SO42−/TiO2 catalyst |
| [91] |
| Rice straw | Microcrystalline cellulose (MCC) and cellulose nanocrystals (CNCs) | Extracted through alkaline treatment, bleaching, and acid hydrolysis, with slight modification to the process described by Chin et al. [26] |
| Extracted CNC appear as long, well-defined rodlike crystals with a high aspect ratio (41). | [92] |
| Momordica Charantia plant stem | Cellulose fibres | Manual retting process | Fibres were soaked in water for 4 h, retted, washed with distilled water, sun-dried for 48 h, and oven-dried at 50 °C for 2 h |
| [93] |
| Strelitzia reginae (SR) plant stem | Cellulose fibres | Retting process |
|
| [94] |
| Eriobotrya japonica leaves | Cellulose | Chemical extraction |
| The final product of the extraction was a crispy and fragile white film. | [95] |
| Corn stalk | Corn stalk cellulose (CSC) | Pretreatment technology with immobilised enzyme |
| The cellulose content obtained by immobilised enzyme pretreatment was 96.72%. | [96] |
| Sugarcane straw | Cellulose nanocrystals | Purification processes, followed by subsequent preparation of CNCs via sulfuric acid hydrolysis |
|
| [97] |
| Populus tremula seed fibres | Cellulose polymeric material | Chemical extraction |
|
| [98] |
| Carpet wastes | Cellulose nanofibres (CNFs) | Supercritical carbon dioxide (Sc.CO2) treatment approach |
|
| [99] |
| Oat bran fibres | Nanofibrillated cellulose (NFC) | Chemical (i.e., acid, base, and bleach) or hydrothermal (i.e., microwave pretreatments and autoclave), followed by disintegration using high-pressure homogenisation from oat bran fibres |
|
| [100] |
| Areca nut husk fibres | Cellulose nanocrystals (CNCs) | Sulfuric acid hydrolysis |
|
| [101] |
| Cellulose Type(s) | Cellulose Functionalisation Procedure | Cellulose-Based Adsorbent | Arsenic Adsorption Capacity | Refs |
|---|---|---|---|---|
| Microfibrillated cellulose (MC), nanocellulose (NC) | Precipitation of magnetite (MG) on an amino terminal branched organic structure (L), either linked by maleic acid residue on NC surface (NC-MA/L) or linked by oxalyl bridge on MC surface (MC-O/L) to produce NC-MA/L-MG and MC-O/L-MG adsorbents, respectively | Magnetite-loaded amino modified nano/microcellulose adsorbents |
| [134] |
| Carboxymethyl cellulose (CMC), microcrystalline cellulose (MCC), hydroxyethyl cellulose (HEC), industrial grade cellulose powder (CP) | 2-line ferrihydrite (FH) nanoparticles were incorporated in the biopolymeric confinement of microcellulose to act as active sites for As(III) and As(V) adsorption | Functionalised microcellulose-reinforced 2-line ferrihydrite composites |
| [135] |
| Cellulose fibre | Microwave irradiation (MW) was used to facilitate the grafting of the hyperbranched polyethylenimine (HPEI) coating agent with a large molecular size | Hyperbranched polyethylenimine (HPEI)-modified cellulose fibre (CellMW-HPEI) adsorbent |
| [136] |
| Cellulose nanocrystals (CNCs) | By using the “bridge joint” effect of iron ions, the cellulose-nanocrystal-containing high-performance adsorbents were synthesised via the co-precipitation method, which enhanced the cross-linking action of cellulose nanocrystal and polyethyleneimine (PEI) | Three cellulose nanocrystal-containing adsorbents were prepared |
| |
| Nanocellulose | pH-sensitive nanoparticles were synthesized via a simple single-graft method, in which nanocellulose treated with 2,2,6,6-tetramethylpiperidine 1-oxyl (TEMPO-NC) was cross-linked with glutaraldehyde (GA) and polyethyleneimine (PEI) | pH-sensitive nanoparticle based on nanocellulose, involving cross-linking polyethyleneimine and glutaraldehyde | The As(V) adsorption capacity of the nanoparticles reached approximately 255.19 mg g−1 at pH 3, which was five times greater than that achieved with the As(V) solution at its initial pH (44.33 mg g−1). | [137] |
| Cellulose | Ethylation of glycidylmethacrylate grafted aminated titanium dioxide densified cellulose (Et-AMPGDC) | Amino-functionalised glycidylmethacrylate-grafted-titanium dioxide densified cellulose for the adsorptive removal of arsenic(V) from aqueous solutions | The maximum adsorption capacity was evaluated to be 108.70 mg/g. | [138] |
| Cellulose filter paper | 3-mercapto-propanoic acid (MPA) was covalently grafted to the cellulose filter paper (Cell) by esterification process through the formation of O-acylisourea intermediate | 3-mercapto-propanoic acid (MPA)-modified cellulose filter paper (MPA–Cell paper) for arsenate removal from drinking water | The modified cellulose filter paper performed well at nearly a neutral pH, for arsenate removal through adsorption, and demonstrated a significant arsenate uptake capacity of 92.59 mg/g. | [139] |
| Cellulose sponge | Cellulose sponge was modified by coating with magnetite microparticles and then used as an effective adsorbent for the elimination of As(V) from aqueous solution | Magnetite microparticles decorated cellulose sponge as an efficacious filter for improved arsenic(V) removal | The maximum adsorption capacity of the adsorbent was 349.9 mg/g, which is substantially higher than the results from previous reports. | [140] |
| Nanocrystalline cellulose (NCC) | Nanocrystalline cellulose (NCC) was selectively oxidised using sodium periodate followed by grafting of diethylene triamine (DETA) to obtain their amine derivatives in 80–85% yield | Diethylene triamine grafted dialdehyde nanocrystalline cellulose (DETA-g-DA-NCC) for As(III and V) removal from aqueous solution | The adsorption capacities were 10.56 and 12.06 mg/g for As(III) and As(V), respectively. | [141] |
| Cellulose | The Fe(III)-AM-PGMACell was prepared through graft copolymerisation of glycidyl methacrylate (GMA) onto cellulose (Cell) in the presence of N,N′-methylenebisacrylamide (MBA) as a cross linker using benzoyl peroxide initiator, followed by treatment with ethylenediamine and ferric chloride in the presence of HCl | Iron(III)-coordinated amino-functionalised poly(glycidyl methacrylate)-grafted cellulose (Fe(III)-AM-PGMACell) for the adsorption of arsenic(V) from aqueous solutions |
| [142] |
| Cellulose | Cross-linked dithiocarbamate-modified cellulose sorbents cross-linked with epoxy or complexed with iron, and the quantities of the modifiers were varied between 3.0 and 10 mol% | Dithiocarbamate (DTC) modified cellulose sorbents that can selectively arsenite AsIII ions separate from water | The maximum sorption capacity of the epoxy-cross-linked sorbent, calculated from the Langmuir isotherm equation, was 600 μmol g−1 (45 mg g−1) at 25 °C. | [143] |
| Wheat straw cellulose | Fluorophore, 3-bromofluoranthene was grafted on cellulose (BF@CFs) for removal of arsenite As(III) ions in water | Fluoranthene decorated fluorescent nanofibrous cellulose probe (BF@CFs) |
| [144] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motloung, M.T.; Magagula, S.I.; Kaleni, A.; Sikhosana, T.S.; Lebelo, K.; Mochane, M.J. Recent Advances on Chemically Functionalized Cellulose-Based Materials for Arsenic Removal in Wastewater: A Review. Water 2023, 15, 793. https://doi.org/10.3390/w15040793
Motloung MT, Magagula SI, Kaleni A, Sikhosana TS, Lebelo K, Mochane MJ. Recent Advances on Chemically Functionalized Cellulose-Based Materials for Arsenic Removal in Wastewater: A Review. Water. 2023; 15(4):793. https://doi.org/10.3390/w15040793
Chicago/Turabian StyleMotloung, Mary T., Sifiso I. Magagula, Andiswa Kaleni, Tlholohelo S. Sikhosana, Kgomotso Lebelo, and Mokgaotsa J. Mochane. 2023. "Recent Advances on Chemically Functionalized Cellulose-Based Materials for Arsenic Removal in Wastewater: A Review" Water 15, no. 4: 793. https://doi.org/10.3390/w15040793
APA StyleMotloung, M. T., Magagula, S. I., Kaleni, A., Sikhosana, T. S., Lebelo, K., & Mochane, M. J. (2023). Recent Advances on Chemically Functionalized Cellulose-Based Materials for Arsenic Removal in Wastewater: A Review. Water, 15(4), 793. https://doi.org/10.3390/w15040793







