Efficient Removal of Rhodamine B in Wastewater via Activation of Persulfate by MnO2 with Different Morphologies
Abstract
:1. Introduction
were carried out for three times to minimize the errors
2. Materials and Methods
2.1. Chemicals
2.2. Fabrication of MnO2 with Different Morphologies
2.2.1. Fabrication of Rod-Shaped MnO2
2.2.2. Fabrication of Acicular MnO2
2.2.3. Fabrication of Mixed MnO2
2.3. Characterizations
2.4. Catalytic Activity Tests
3. Results and Discussion
3.1. XRD and SEM Analysis
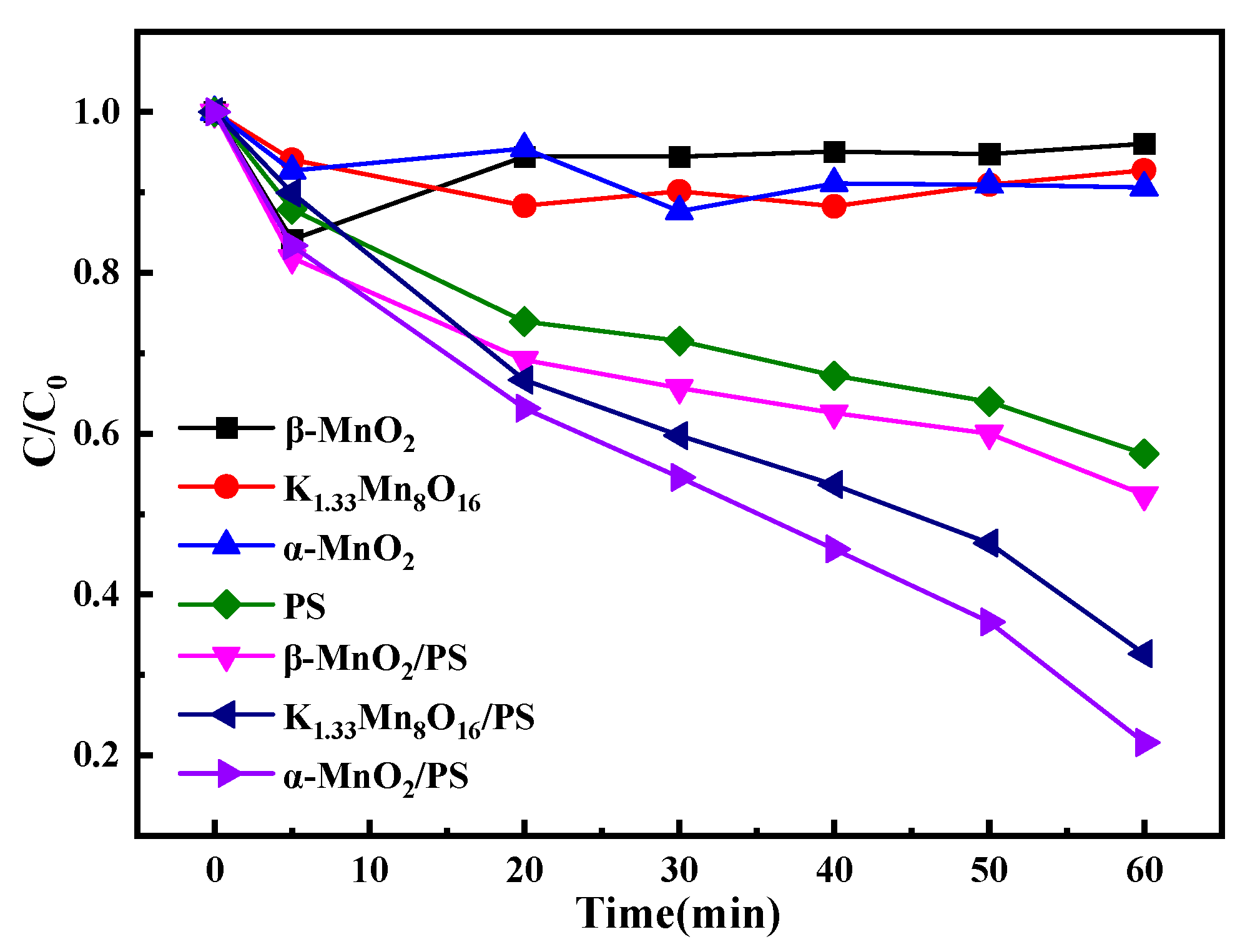
3.2. Organic Pollutants Degradation Performance
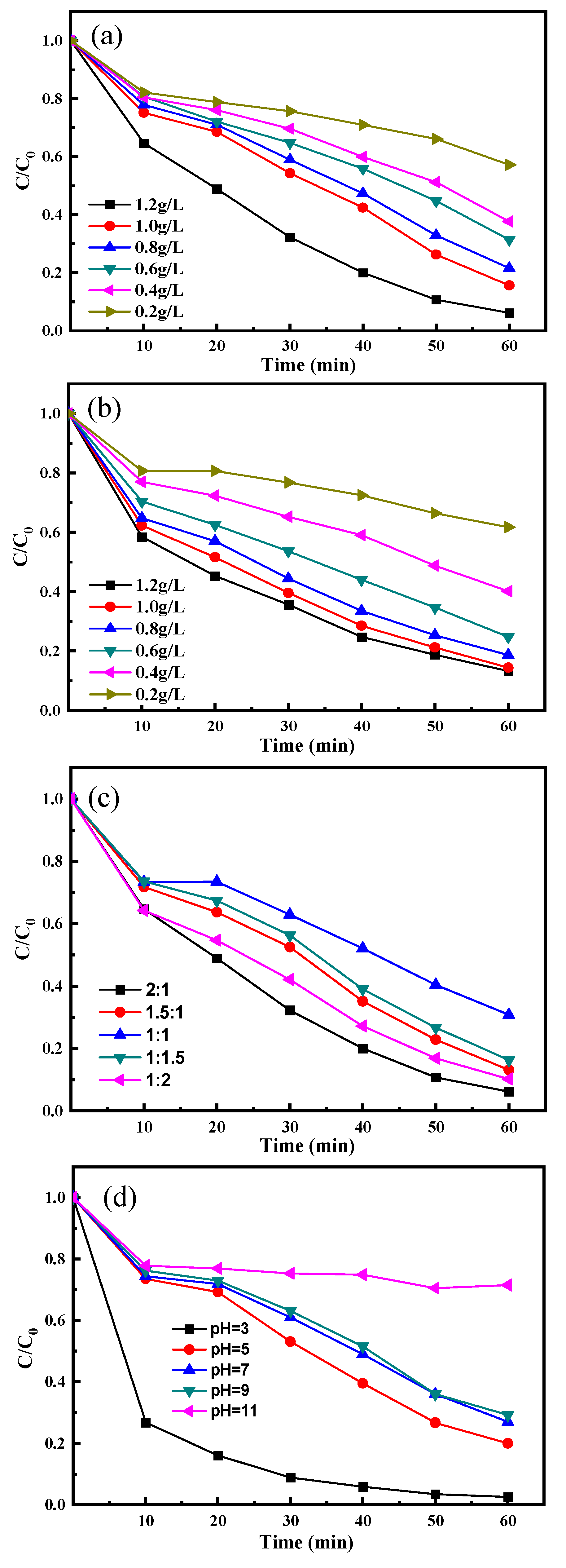
3.3. Optimization of Reaction Parameters
3.4. Mechanism Analysis
3.5. Reusability of α-MnO2
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, X.; Wang, J.; Dong, X.-X.; Lv, Y.-K. Functionalized metal-organic frameworks for photocatalytic degradation of organic pollutants in environment. Chemosphere 2020, 242, 125144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Tay, H.L.; Lim, S.S.; Wang, Y.; Zhong, Z.; Xu, R. Supported cobalt oxide on MgO: Highly efficient catalysts for degradation of organic dyes in dilute solutions. Appl. Catal. B Environ. 2010, 95, 93–99. [Google Scholar] [CrossRef]
- Guo, N.; Liu, H.; Fu, Y.; Hu, J. Preparation of Fe2O3 nanoparticles doped with In2O3 and photocatalytic degradation property for rhodamine B. Optik 2020, 201, 163537. [Google Scholar] [CrossRef]
- Ji, R.; Zhao, Z.; Yu, X.; Chen, M. Determination of rhodamine B in capsicol using the first derivative absorption spectrum. Optik 2019, 181, 796–801. [Google Scholar] [CrossRef]
- Pearce, C., Jr.; Lloyd, J.; Guthrie, J. The removal of colour from textile wastewater using whole bacterial cells: A review. Dye. Pigment. 2003, 58, 179–196. [Google Scholar] [CrossRef]
- Banat, I.M.; Nigam, P.; Singh, D.; Marchant, R. Microbial decolorization of textile-dyecontaining effluents: A review. Bioresour. Technol. 1996, 58, 217–227. [Google Scholar] [CrossRef]
- Foti, L.; Coviello, D.; Zuorro, A.; Lelario, F.; Bufo, S.A.; Scrano, L.; Sauvetre, A.; Chiron, S.; Brienza, M. Comparison of Sunlight-Aops for Levofloxacin Removal: Kinetics, Transformation Products, and Toxicity Assay on Escherichia Coli and Micrococcus Flavus. Environ. Sci. Pollut. Res. 2022, 29, 58201–58211. [Google Scholar] [CrossRef]
- Ennouri, R.; Lavecchia, R.; Zuorro, A.; Elaoud, S.C.; Petrucci, E. Degradation of chloramphenicol in water by oxidation on a boron-doped diamond electrode under UV irradiation. J. Water Process. Eng. 2021, 41, 101995. [Google Scholar] [CrossRef]
- Huixuan, Z.; Nengzi, L.-C.; Wang, Z.; Zhang, X.; Li, B.; Cheng, X. Construction of Bi2O3/Cunife Ldhs Composite and Its Enhanced Photocatalytic Degradation of Lomefloxacin with Persulfate under Simulated Sunlight. J. Hazard. Mater. 2020, 383, 121236. [Google Scholar]
- Pirsaheb, M.; Hossaini, H.; Janjani, H. Reclamation of hospital secondary treatment effluent by sulfate radicals based–advanced oxidation processes (SR-AOPs) for removal of antibiotics. Microchem. J. 2019, 153, 104430. [Google Scholar] [CrossRef]
- Lei, Z.; Yang, X.; Ji, Y.; Wei, J. Sulfate Radical-Based Oxidation of the Antibiotics Sulfamethoxazole, Sulfisoxazole, Sulfathiazole, and Sulfamethizole: The Role of Five-Membered Heterocyclic Rings. Sci. Total Environ. 2019, 692, 201–208. [Google Scholar]
- Chen, C.; Feng, H.; Deng, Y. Re-evaluation of sulfate radical based–advanced oxidation processes (SR-AOPs) for treatment of raw municipal landfill leachate. Water Res. 2019, 153, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Yang, L.; He, L.; Xue, J.; Ma, Y.; Xie, Z.; Wu, L.; Huang, M.; Zhang, Z. Persulfate-based degradation of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) in aqueous solution: Review on influences, mechanisms and prospective. J. Hazard. Mater. 2020, 393, 122405. [Google Scholar] [CrossRef]
- Matzek, L.W.; Carter, K.E. Activated persulfate for organic chemical degradation: A review. Chemosphere 2016, 151, 178–188. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, X.; Zhou, H.; Murugananthan, M.; Zhang, Y. Degradation of p-nitrophenol by heat and metal ions co-activated persulfate. Chem. Eng. J. 2015, 264, 39–47. [Google Scholar] [CrossRef]
- Ruonan, G.; Meng, Q.; Zhang, H.; Zhang, X.; Li, B.; Cheng, Q.; Cheng, X. Construction of Fe2O3/Co3O4/Exfoliated Graphite Composite and Its High Efficient Treatment of Landfill Leachate by Activation of Potassium Persulfate. Chem. Eng. J. 2019, 355, 952–962. [Google Scholar]
- Xing, S.; Li, W.; Liu, B.; Wu, Y.; Gao, Y. Removal of Ciprofloxacin by Persulfate Activation with Cuo: A Ph-Dependent Mechanism. Chem. Eng. J. 2020, 382, 122837. [Google Scholar] [CrossRef]
- Junjing, L.; Guo, R.; Ma, Q.; Nengzi, L.-C.; Cheng, X. Efficient Removal of Organic Contaminant via Activation of Potassium Persulfate by Γ-Fe2O3/A-MnO2 Nanocomposite. Sep. Purif. Technol. 2019, 227, 115669. [Google Scholar]
- Miao, L.; Wang, J.; Zhang, P. Review on manganese dioxide for catalytic oxidation of airborne formaldehyde. Appl. Surf. Sci. 2019, 466, 441–453. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, S. Droplets impact on textured surfaces: Mesoscopic simulation of spreading dynamics. Appl. Surf. Sci. 2015, 327, 159–167. [Google Scholar] [CrossRef]
- Ning, S.; Duan, Y.; Jiao, X.; Chen, D. Large-Scale Preparation and Catalytic Properties of One-Dimensional A/Β-MnO2 Nanostructures. J. Phys. Chem. C 2009, 113, 8560–8565. [Google Scholar]
- Xu, Y.; Lin, H.; Li, Y.; Zhang, H. The mechanism and efficiency of MnO2 activated persulfate process coupled with electrolysis. Sci. Total. Environ. 2017, 609, 644–654. [Google Scholar] [CrossRef]
- Zhengyu, D.; Zhang, Q.; Chen, B.-Y.; Hong, J. Oxidation of Bisphenol a by Persulfate via Fe3O4-A-MnO2 Nanoflower-Like Catalyst: Mechanism and Efficiency. Chem. Eng. J. 2019, 357, 337–347. [Google Scholar]
- Deshan, Z.; Sun, S.; Fan, W.; Yu, H.; Fan, C.; Cao, G.; Yin, Z.; Song, X. One-Step Preparation of Single-Crystalline Β-MnO2 Nanotubes. J. Phys. Chem. B 2005, 109, 16439–16443. [Google Scholar]
- Subramanian, V.; Zhu, H.; Vajtai, R.; Ajayan, P.M.; Wei, B. Hydrothermal Synthesis and Pseudocapacitance Properties of MnO2 Nanostructures. J. Phys. Chem. B 2005, 109, 20207–20214. [Google Scholar] [CrossRef] [PubMed]
- Jiechao, G.; Zhuo, L.; Yang, F.; Tang, B.; Wu, L.; Tung, C. One-Dimensional Hierarchical Layered KXMnO2 (X < 0.3) Nanoarchitectures: Synthesis, Characterization, and Their Magnetic Properties. J. Phys. Chem. B 2006, 110, 17854–17859. [Google Scholar]
- Gong, C.; Zhang, X.; Gao, Y.; Zhu, G.; Cheng, Q.; Cheng, X. Novel Magnetic MnO2 /MnFe2O4 Nanocomposite as a Heterogeneous Catalyst for Activation of Peroxymonosulfate (Pms) toward Oxidation of Organic Pollutants. Sep. Purif. Technol. 2019, 213, 456–464. [Google Scholar]
- Lavecchia, R.; Pugliese, A.; Zuorro, A. Removal of lead from aqueous solutions by spent tea leaves. Chem. Eng. Trans. 2010, 19, 73–78. [Google Scholar]
- Jain, N.; Roy, A. Phase & morphology engineered surface reducibility of MnO2 nano-heterostructures: Implications on catalytic activity towards CO oxidation. Mater. Res. Bull. 2019, 121, 110615. [Google Scholar]
- Rao, T.P.; Kumar, A.; Naik, V.M.; Naik, R. Effect of Carbon Nanofibers on Electrode Performance of Symmetric Supercapcitors with Composite A-MnO2 Nanorods. J. Alloys Compd. 2019, 789, 518–527. [Google Scholar] [CrossRef]
- Yang, W.; Su, Z.; Xu, Z.; Yang, W.; Peng, Y.; Li, J. Comparative Study of A-, Β-, Γ- and Δ-MnO2 on Toluene Oxidation: Oxygen Vacancies and Reaction Intermediates. Appl. Catal. B Environ. 2020, 260, 118150. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, H.; Zhang, X.; Li, B.; Guo, R.; Cheng, Q.; Cheng, X. Synthesis of magnetic CuO/MnFe2O4 nanocompisite and its high activity for degradation of levofloxacin by activation of persulfate. Chem. Eng. J. 2018, 360, 848–860. [Google Scholar] [CrossRef]
- Huixuan, Z.; Song, Y.; Nengzi, L.-c.; Gou, J.; Li, B.; Cheng, X. Activation of Persulfate by a Novel Magnetic CuFe2O4/Bi2O3 Composite for Lomefloxacin Degradation. Chem. Eng. J. 2020, 379, 122362. [Google Scholar]
- Zeming, W.; Wang, Z.; Li, W.; Lan, Y.; Chen, C. Performance Comparison and Mechanism Investigation of Co3O4-Modified Different Crystallographic MnO2 (A, Β, Γ, and Δ) as an Activator of Peroxymonosulfate (Pms) for Sulfisoxazole Degradation. Chem. Eng. J. 2022, 427, 130888. [Google Scholar]
- Gong, C.; Nengzi, L.-C.; Gao, Y.; Zhu, G.; Gou, J.; Cheng, X. Degradation of Tartrazine by Peroxymonosulfate through Magnetic Fe2O3/Mn2O3 Composites Activation. Chin. Chem. Lett. 2020, 31, 2730–2736. [Google Scholar]
- Hirakendu, B.; Singh, S.; Venkatesh, M.; Pimple, M.V.; Singhal, R.K. Graphene Oxide-MnO2-Goethite Microsphere Impregnated Alginate: A Novel Hybrid Nanosorbent for as (Iii) and as (V) Removal from Groundwater. J. Water Process Eng. 2021, 42, 102129. [Google Scholar]
- Shijun, Z.; Li, H.; Wang, L.; Cai, Z.; Wang, Q.; Shen, S.; Li, X.; Deng, J. Oxygen Vacancies-Rich A@Δ-MnO2 Mediated Activation of Peroxymonosulfate for the Degradation of Cip: The Role of Electron Transfer Process on the Surface. Chem. Eng. J. 2023, 458, 141415. [Google Scholar]
- Lin, X.; Ma, Y.; Wan, J.; Wang, Y.; Li, Y. Efficient degradation of Orange G with persulfate activated by recyclable FeMoO4. Chemosphere 2019, 214, 642–650. [Google Scholar] [CrossRef]
- Meng, F.; Song, M.; Song, B.; Wei, Y.; Cao, Q.; Cao, Y. Enhanced degradation of Rhodamine B via α-Fe2O3 microspheres induced persulfate to generate reactive oxidizing species. Chemosphere 2019, 243, 125322. [Google Scholar] [CrossRef]
- Moutusi, D.; Bhattacharyya, K.G. Oxidation of Rhodamine B in Aqueous Medium in Ambient Conditions with Raw and Acid-Activated MnO2, Nio, Zno as Catalysts. J. Mol. Catal. A Chem. 2014, 391, 121–129. [Google Scholar]
- Huixuan, Z.; Wang, J.; Zhang, X.; Li, B.; Cheng, X. Enhanced Removal of Lomefloxacin Based on Peroxymonosulfate Activation by Co3O4/Δ-Feooh Composite. Chem. Eng. J. 2019, 369, 834–844. [Google Scholar]
- Zhongjuan, W.; Nengzi, L.-C.; Zhang, X.; Zhao, Z.; Cheng, X. Novel NiCo2S4/Cs Membranes as Efficient Catalysts for Activating Persulfate and Its High Activity for Degradation of Nimesulide. Chem. Eng. J. 2020, 381, 122517. [Google Scholar]
- Huixuan, Z.; Nengzi, L.-C.; Li, X.; Wang, Z.; Li, B.; Liu, L.; Cheng, X. Construction of CuBi2O4/MnO2 Composite as Z-Scheme Photoactivator of Peroxymonosulfate for Degradation of Antibiotics. Chem. Eng. J. 2020, 386, 124011. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Gan, X.; Cao, S.; Shang, J.; Cheng, X. Efficient Removal of Rhodamine B in Wastewater via Activation of Persulfate by MnO2 with Different Morphologies. Water 2023, 15, 735. https://doi.org/10.3390/w15040735
Zhang X, Gan X, Cao S, Shang J, Cheng X. Efficient Removal of Rhodamine B in Wastewater via Activation of Persulfate by MnO2 with Different Morphologies. Water. 2023; 15(4):735. https://doi.org/10.3390/w15040735
Chicago/Turabian StyleZhang, Xinyi, Xinrui Gan, Shihu Cao, Jiangwei Shang, and Xiuwen Cheng. 2023. "Efficient Removal of Rhodamine B in Wastewater via Activation of Persulfate by MnO2 with Different Morphologies" Water 15, no. 4: 735. https://doi.org/10.3390/w15040735







