Detection of Pesticides in Water through an Electronic Tongue and Data Processing Methods
Abstract
:1. Introduction
2. Materials and Methods
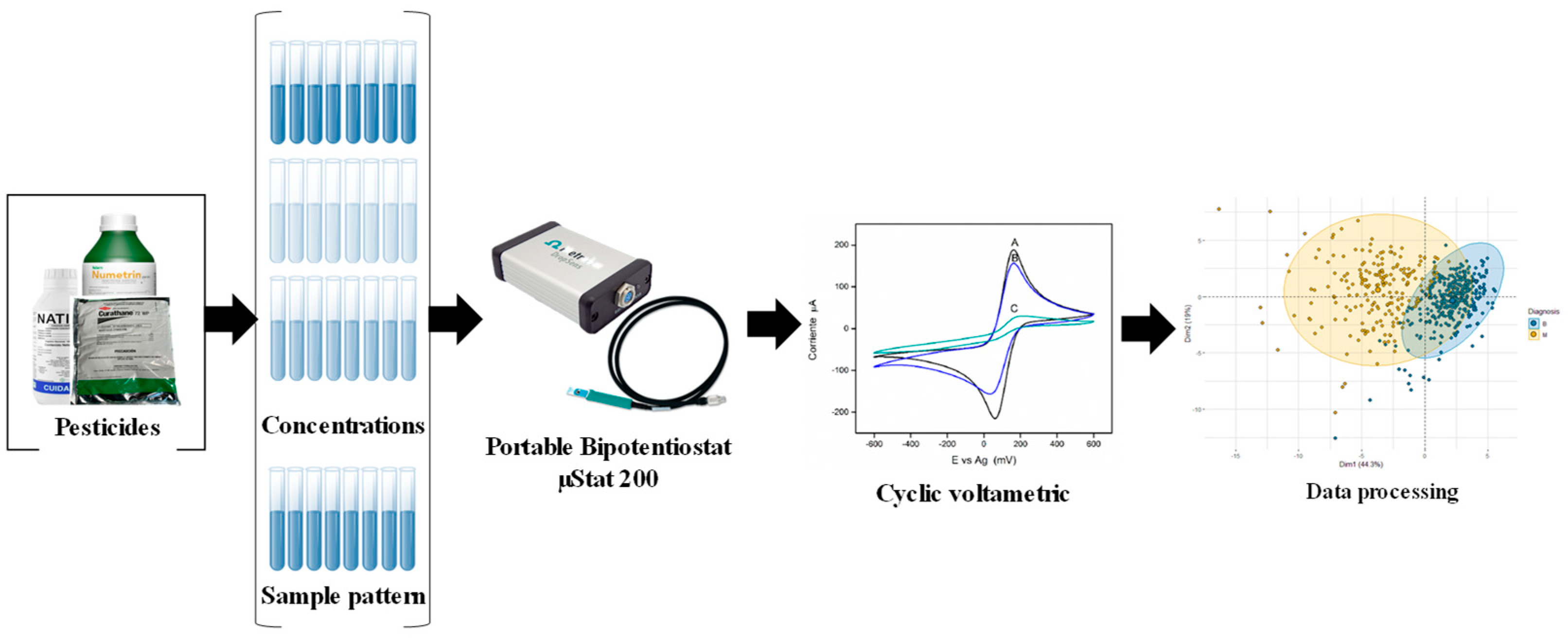
2.1. Sample Preparation
2.2. Electronic Tongue
2.3. Data Processing
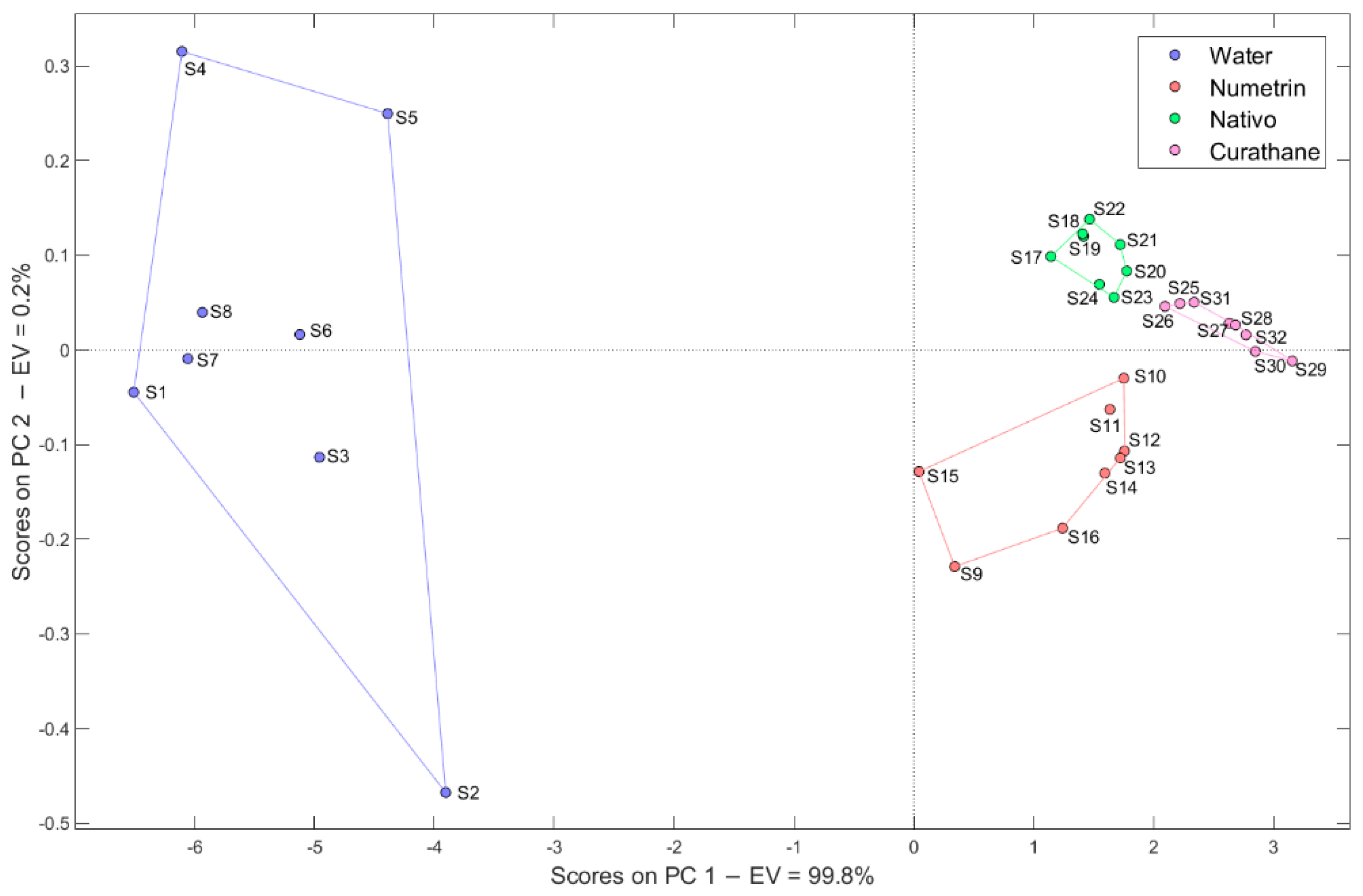
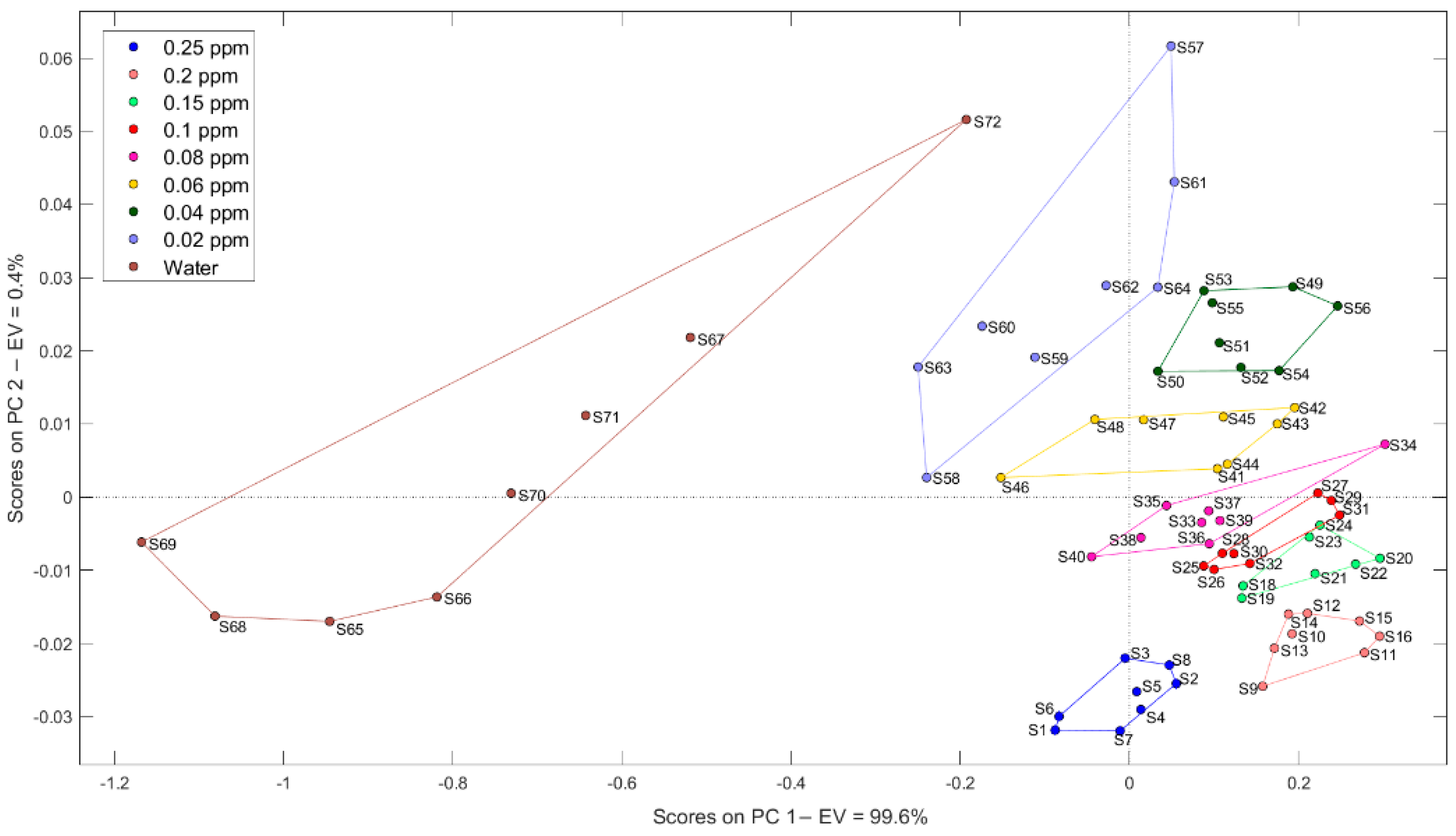
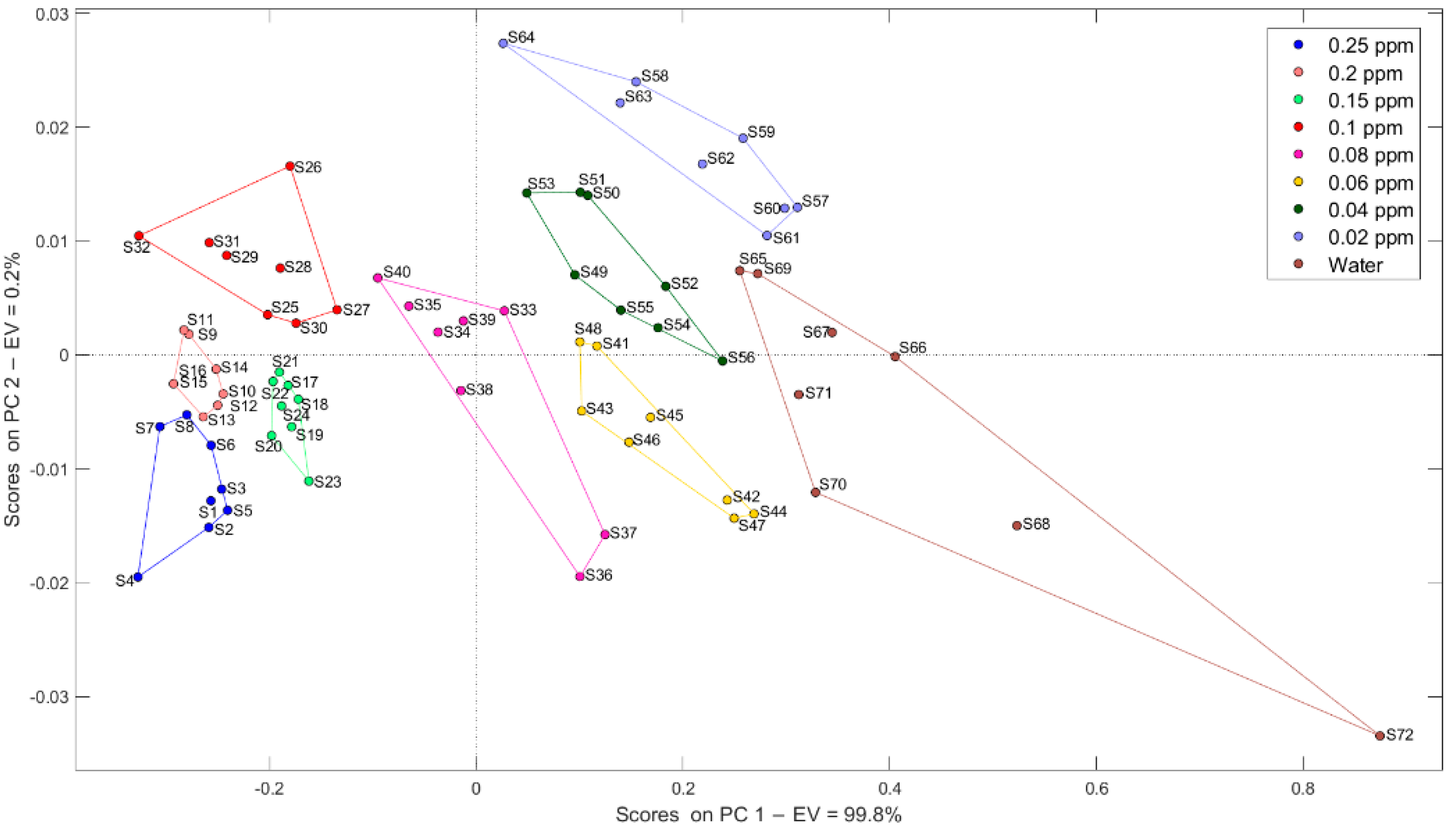
2.3.1. Principal Component Analysis (PCA)
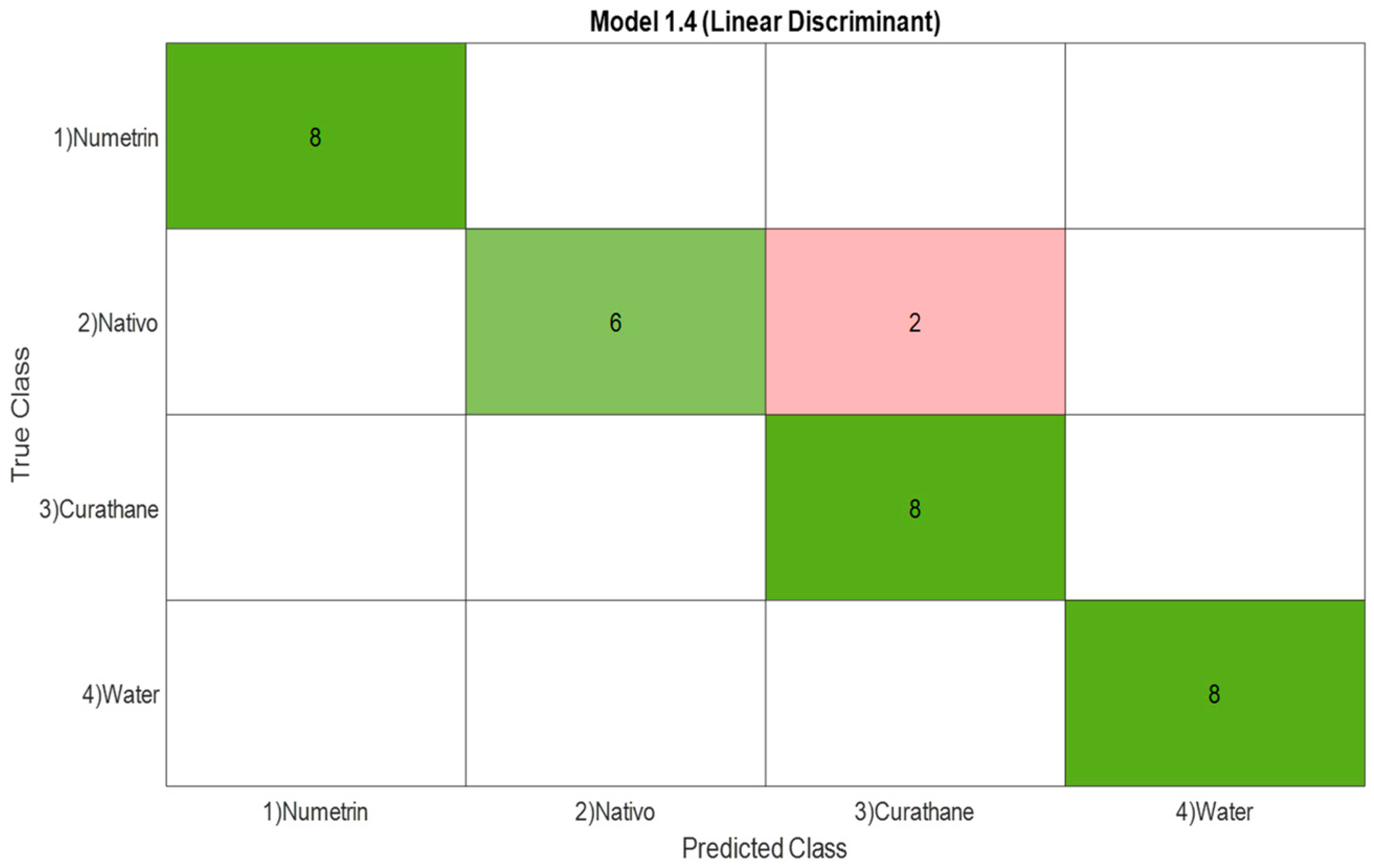
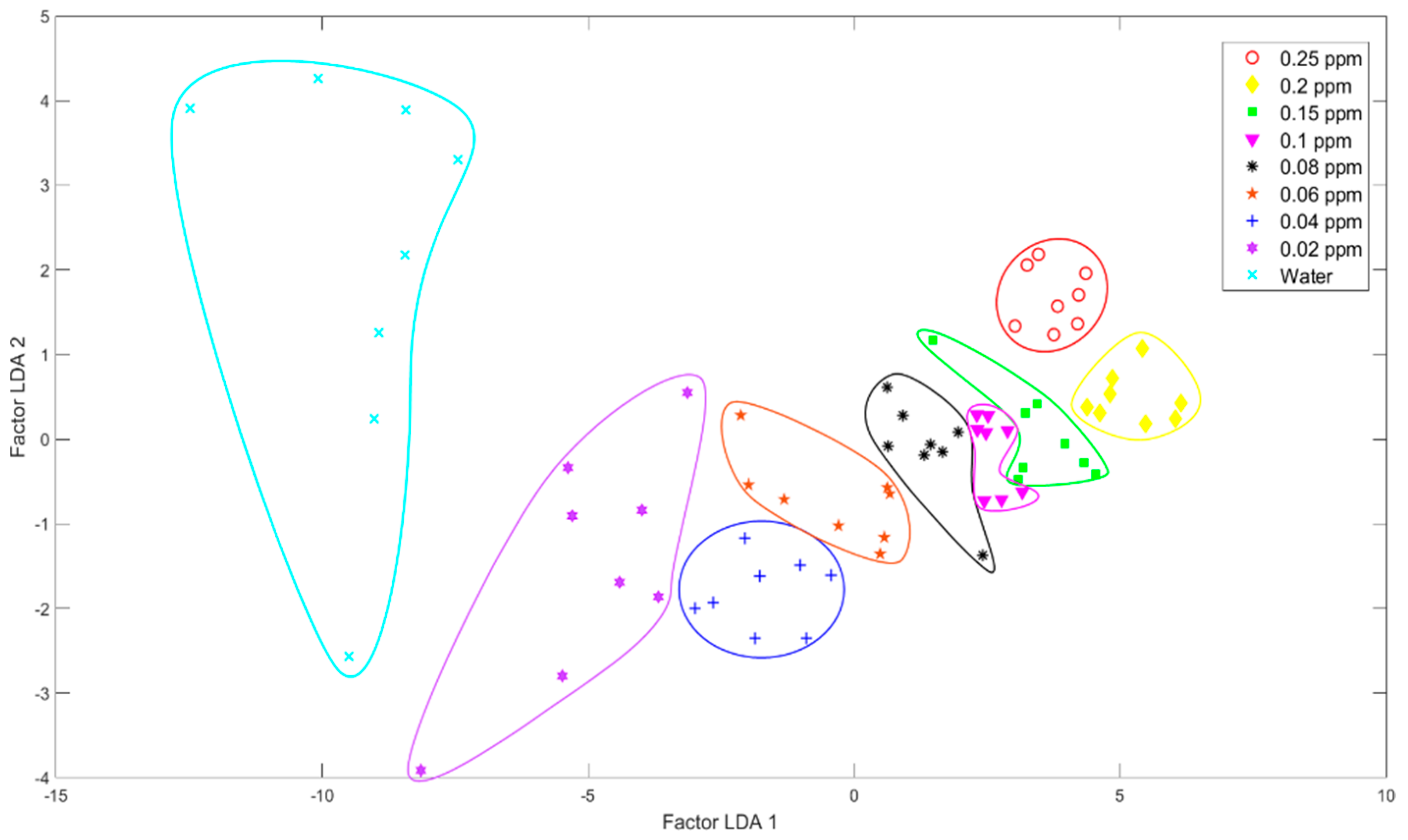
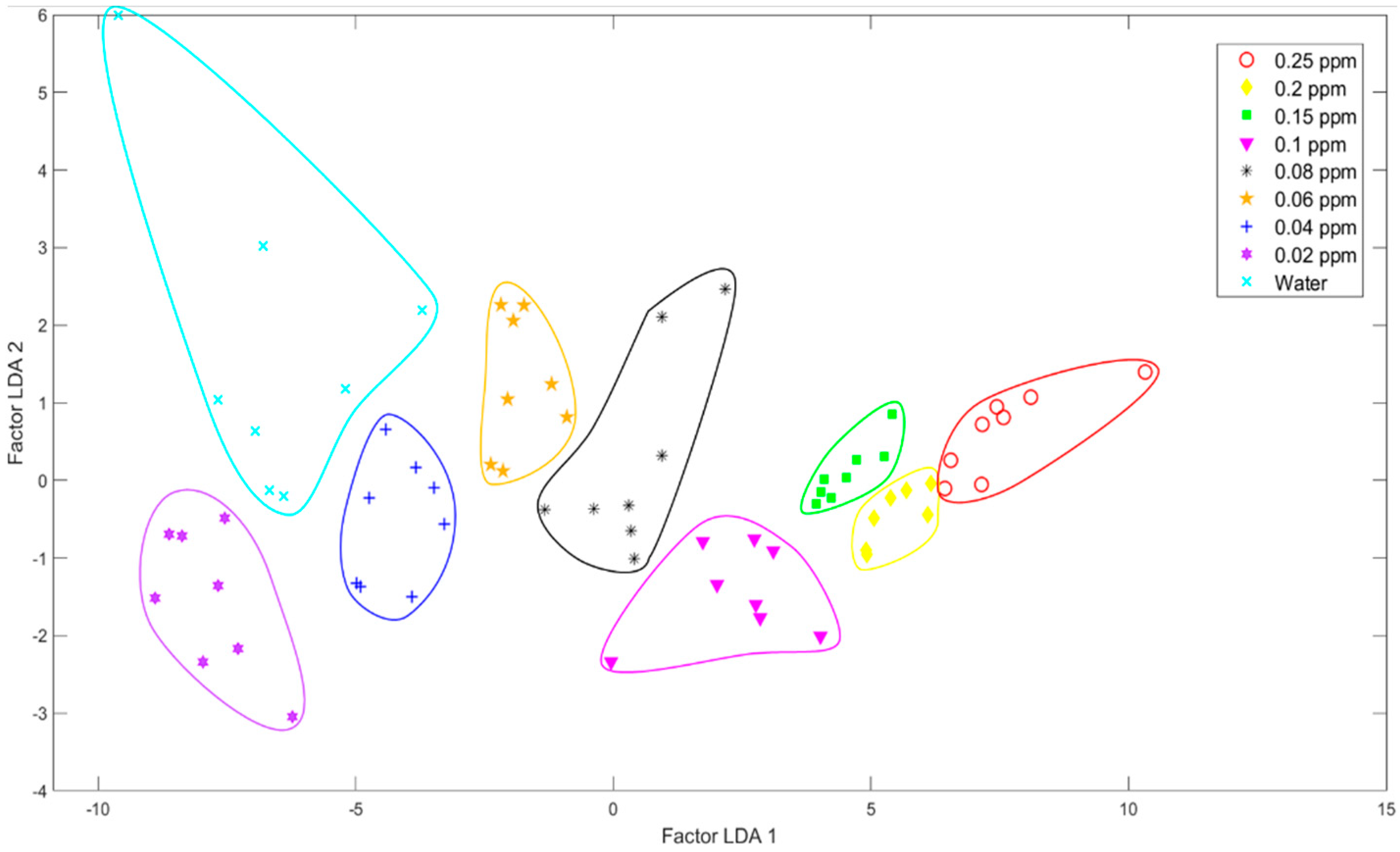
2.3.2. Linear Discriminant Analysis (LDA)
2.3.3. Naïve Bayes (Naïve Bayesian Classifier)
2.3.4. Support Vector Machines (SVMs)
2.3.5. k-Nearest Neighbors (kNN)
3. Results and Discussion
3.1. Categories (Pesticides) and Distilled Water
3.1.1. Pattern Recognition Methods
3.1.2. Machine Learning
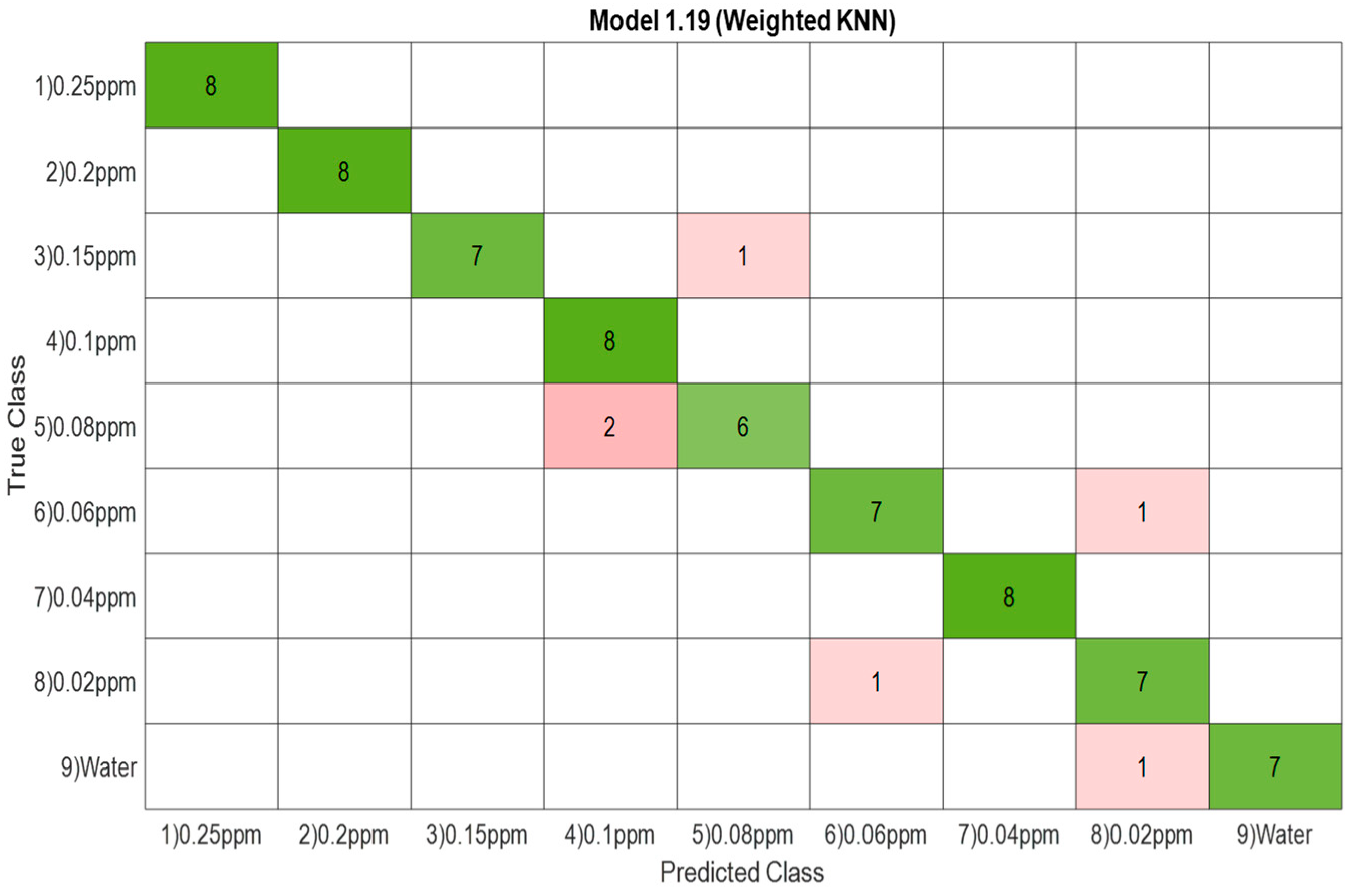
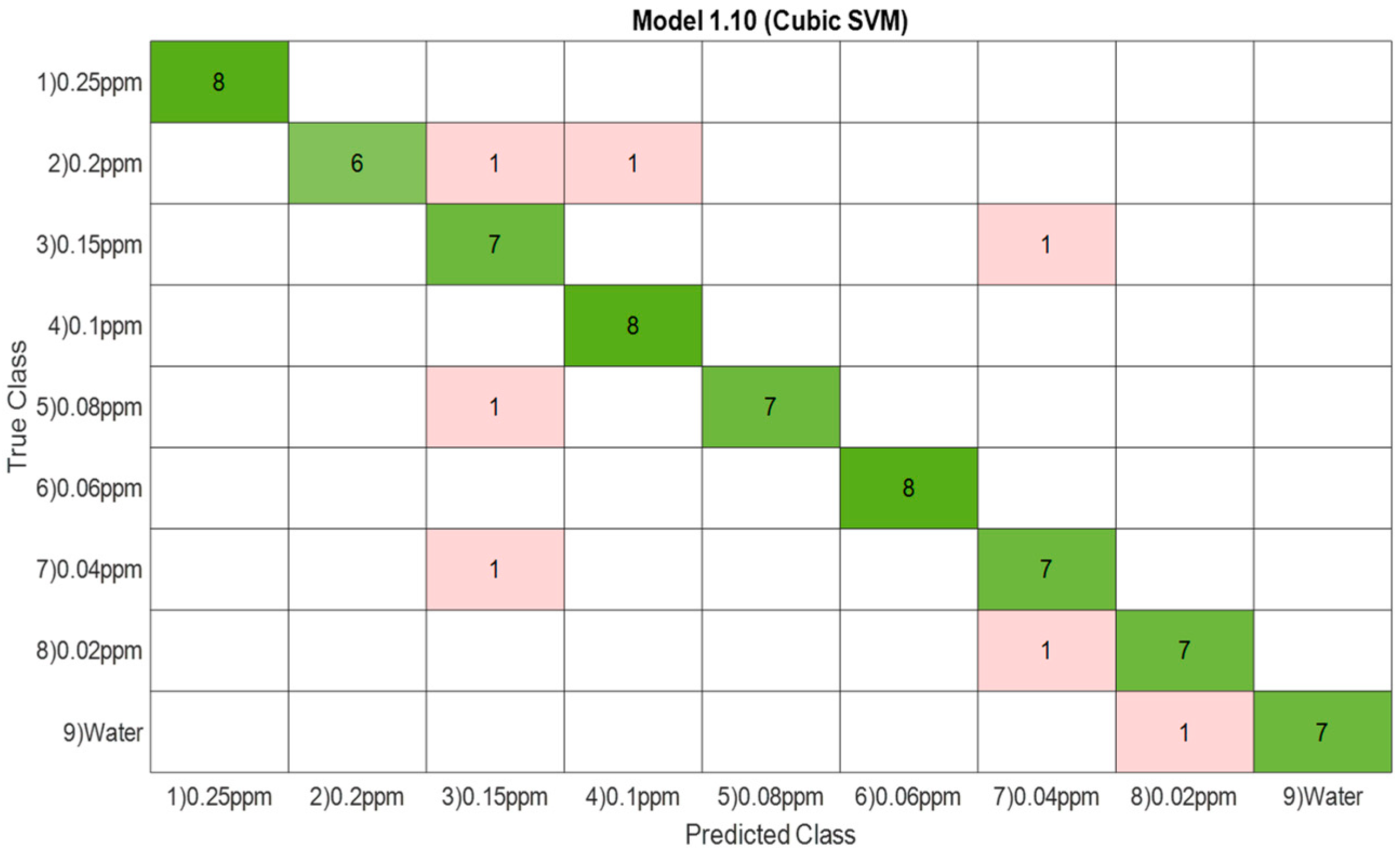
3.1.3. Confusion Matrix
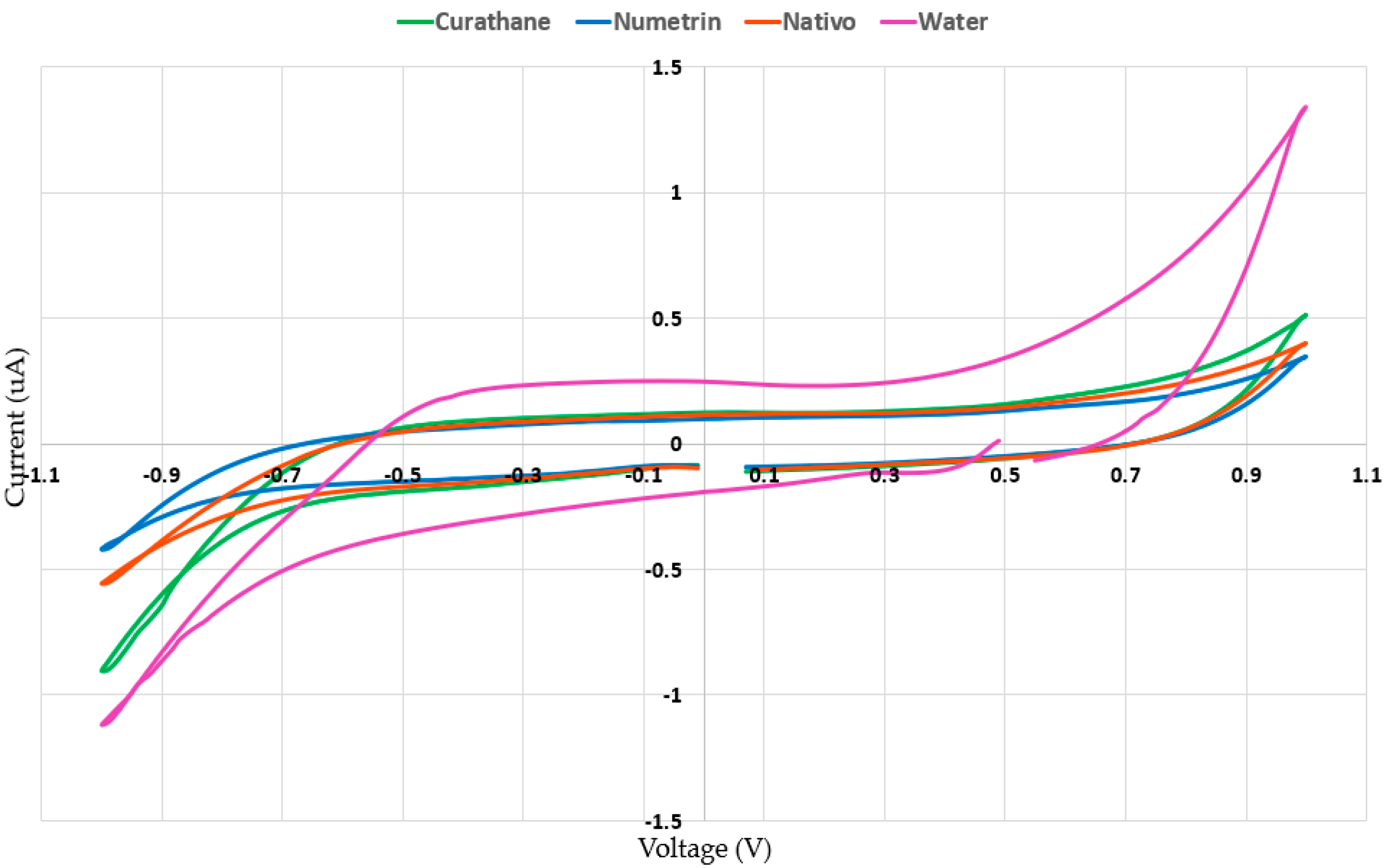
3.2. Concentrations of Pesticides and Water
3.2.1. Curathane and Water Concentrations
3.2.2. Nativo and Water Concentrations
3.2.3. Numetrin and Water Concentrations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Skendžić, S.; Zovko, M.; Živković, I.P.; Lešić, V.; Lemić, D. The impact of climate change on agricultural insects pests. Insects 2021, 12, 440. [Google Scholar] [CrossRef]
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global food demand and the sustainable intensification of agriculture. Int. Econ. J. 2022, 36, 126–145. [Google Scholar] [CrossRef] [PubMed]
- Popp, J.; Pető, K.; Nagy, J. Pesticide productivity and food security. A review. Agron. Sustain. Dev. 2013, 33, 243–255. [Google Scholar] [CrossRef]
- Damalas, C.A. Understanding benefits and risks of pesticide use. Sci. Res. Essays 2009, 4, 945–949. [Google Scholar]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical Pesticides and Human Health: The Urgent Need for a New Concept in Agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef]
- National Research Council. Pesticides in the Diets of Infants and Children; National Research Council: Washington, DC, USA, 1993. [Google Scholar]
- Agrawal, A.; Pandey, R.S.; Sharma, B. Water Pollution with Special Reference to Pesticide Contamination in India. J. Water Resour. Prot. 2010, 2, 432–448. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D.; et al. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef]
- Syafrudin, M.; Kristanti, R.A.; Yuniarto, A.; Hadibarata, T.; Rhee, J.; Al-Onazi, W.A.; Algarni, T.S.; Almarri, A.H.; Al-Mohaimeed, A.M. Pesticides in Drinking Water—A Review. Int. J. Environ. Res. Public Health 2021, 18, 468. [Google Scholar] [CrossRef]
- Hassaan, M.A.; El Nemr, A. Pesticides pollution: Classifications, human health impact, extraction and treatment techniques. Egypt. J. Aquat. Res. 2020, 46, 207–220. [Google Scholar] [CrossRef]
- Eurostat. Agri-Environmental Indicator—Consumption of Pesticides—Statistics Explained. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Agri-environmental_indicator_-_consumption_of_pesticides (accessed on 2 January 2023).
- Tudi, M.; Ruan, H.D.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D. Agriculture Development, Pesticide Application and Its Impact on the Environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef]
- Manosathiyadevan, M.; Bhuvaneshwari, V.; Latha, R. Impact of Insects and Pests in loss of Crop Production: A Review. Sustain. Agric. Food Secur. 2017, 57–67. [Google Scholar] [CrossRef]
- Aktar, W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef]
- Oliveira, C.; Auad, A.; Mendes, S.; Frizzas, M. Crop losses and the economic impact of insect pests on Brazilian agriculture. Crop Prot. 2014, 56, 50–54. [Google Scholar] [CrossRef]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Culliney, T. Crop losses to arthropods. In Integrated Pest Management: Pesticide Problems; Springer: Dordrecht, The Netherlands, 2014; Volume 3, pp. 201–225. [Google Scholar] [CrossRef]
- Pradhan, S.S.; Gowda, G.B.; Adak, T.; Guru-Pirasanna-Pandi, G.; Patil, N.B.; Annamalai, M.; Rath, P.C. Pesticides Occurrence in Water Sources and Decontamination Techniques. In Pesticides-Updates on Toxicity, Efficacy and Risk Assessment; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Sharma, B.M.; Bharat, G.K.; Tayal, S.; Nizzetto, L.; Čupr, P.; Larssen, T. Environment and human exposure to persistent organic pollutants (POPs) in India: A systematic review of recent and historical data. Environ. Int. 2014, 66, 48–64. [Google Scholar] [CrossRef]
- Tobergte, D.R.; Curtis, S. Convenio de Estocolmo sobre Contaminantes Orgánicos Persistentes. 2013. Available online: https://observatoriop10.cepal.org/es/tratados/convenio-estocolmo-contaminantes-organicos-persistentes (accessed on 3 January 2023).
- Spiewak, R. Pesticides as a cause of occupational skin diseases in farmers. Soc. Work Educ. 2002, 21, 117–123. [Google Scholar] [CrossRef]
- Namulanda, G.; Monti, M.M.; Mulay, P.; Higgins, S.; Lackovic, M.; Schwartz, A.; Prado, J.B.; Waltz, J.; Mitchell, Y.; Calvert, G.M. Acute Nonoccupational Pesticide-Related Illness and Injury—United States, 2007–2011. MMWR. Morb. Mortal. Wkly. Rep. 2019, 63, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Reygner, J.; Condette, C.J.; Bruneau, A.; Delanaud, S.; Rhazi, L.; Depeint, F.; Abdennebi-Najar, L.; Bach, V.; Mayeur, C.; Khorsi-Cauet, H. Changes in Composition and Function of Human Intestinal Microbiota Exposed to Chlorpyrifos in Oil as Assessed by the SHIME® Model. Int. J. Environ. Res. Public Health 2016, 13, 1088. [Google Scholar] [CrossRef]
- Khan, D.A.; Hashmi, I.; Mahjabeen, W.; Naqvi, T.A. Monitoring health implications of pesticide exposure in factory workers in Pakistan. Environ. Monit. Assess. 2010, 168, 231–240. [Google Scholar] [CrossRef]
- Betarbet, R.; Sherer, T.B.; MacKenzie, G.; Garcia-Osuna, M.; Panov, A.V.; Greenamyre, J.T. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat. Neurosci. 2000, 3, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Fitzmaurice, A.G.; Rhodes, S.L.; Lulla, A.; Murphy, N.P.; Lam, H.A.; O’Donnell, K.C.; Barnhill, L.; Casida, J.E.; Cockburn, M.; Sagasti, A.; et al. Aldehyde dehydrogenase inhibition as a pathogenic mechanism in Parkinson disease. Proc. Natl. Acad. Sci. USA 2013, 110, 636–641. [Google Scholar] [CrossRef] [PubMed]
- González-Alzaga, B.; Lacasaña, M.; Aguilar-Garduño, C.; Rodríguez-Barranco, M.; Ballester, F.; Rebagliato, M.; Hernández, A. A systematic review of neurodevelopmental effects of prenatal and postnatal organophosphate pesticide exposure. Toxicol. Lett. 2014, 230, 104–121. [Google Scholar] [CrossRef] [PubMed]
- Pedroso, T.M.A.; Benvindo-Souza, M.; Nascimento, F.D.A.; Woch, J.; dos Reis, F.G.; Silva, D.D.M.E. Cancer and occupational exposure to pesticides: A bibliometric study of the past 10 years. Environ. Sci. Pollut. Res. 2022, 29, 17464–17475. [Google Scholar] [CrossRef] [PubMed]
- Bassil, K.L.; Vakil, C.; Sanborn, M.; Cole, D.C.; Kaur, J.S.; Kerr, K.J. Cancer health effects of pesticides: Systematic review. Can. Fam. Physician 2007, 53, 1704–1711. [Google Scholar] [PubMed]
- Huang, W.; He, Y.; Xiao, J.; Huang, Y.; Li, A.; He, M.; Wu, K. Risk of breast cancer and adipose tissue concentrations of polychlorinated biphenyls and organochlorine pesticides: A hospital-based case-control study in Chinese women. Environ. Sci. Pollut. Res. 2019, 26, 32128–32136. [Google Scholar] [CrossRef]
- Ye, M.; Beach, J.; Martin, J.W.; Senthilselvan, A. Occupational Pesticide Exposures and Respiratory Health. Int. J. Environ. Res. Public Health 2013, 10, 6442–6471. [Google Scholar] [CrossRef]
- Salameh, P.; Waked, M.; Baldi, I.; Brochard, P.; Saleh, B.A. Respiratory diseases and pesticide exposure: A case-control study in Lebanon. J. Epidemiol. Community Health 2006, 60, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Rani, L.; Thapa, K.; Kanojia, N.; Sharma, N.; Singh, S.; Grewal, A.S.; Srivastav, A.L.; Kaushal, J. An extensive review on the consequences of chemical pesticides on human health and environment. J. Clean. Prod. 2021, 283, 124–657. [Google Scholar] [CrossRef]
- Buralli, R.J.; Ribeiro, H.; Mauad, T.; Amato-Lourenço, L.F.; Salge, J.M.; Diaz-Quijano, F.A.; Leão, R.S.; Marques, R.C.; Silva, D.S.; Guimarães, J.R.D. Respiratory Condition of Family Farmers Exposed to Pesticides in the State of Rio de Janeiro, Brazil. Int. J. Environ. Res. Public Health 2018, 15, 1203. [Google Scholar] [CrossRef] [Green Version]
- Arbuckle, T.E.; Lin, Z.; Mery, L.S. An exploratory analysis of the effect of pesticide exposure on the risk of spontaneous abortion in an Ontario farm population. Environ. Health Perspect. 2001, 109, 851–857. [Google Scholar] [CrossRef]
- Lauretta, R.; Sansone, A.; Sansone, M.; Romanelli, F.; Appetecchia, M. Endocrine Disrupting Chemicals: Effects on Endocrine Glands. Front. Endocrinol. 2019, 10, 178. [Google Scholar] [CrossRef]
- Leemans, M.; Couderq, S.; Demeneix, B.; Fini, J.-B. Pesticides With Potential Thyroid Hormone-Disrupting Effects: A Review of Recent Data. Front. Endocrinol. 2019, 10, 743. [Google Scholar] [CrossRef]
- De Coster, S.; van Larebeke, N. Endocrine-Disrupting Chemicals: Associated Disorders and Mechanisms of Action. J. Environ. Public Health 2012, 2012, 713696. [Google Scholar] [CrossRef] [PubMed]
- Mnif, W.; Hassine, A.I.H.; Bouaziz, A.; Bartegi, A.; Thomas, O.; Roig, B. Effect of Endocrine Disruptor Pesticides: A Review. Int. J. Environ. Res. Public Health 2011, 8, 2265–2303. [Google Scholar] [CrossRef]
- Khot, R.; Joshi, P.; Pandharipande, M.; Nagpure, K.; Thakur, D. Glyphosate poisoning with acute pulmonary edema. Toxicol. Int. 2014, 21, 328–330. [Google Scholar] [CrossRef]
- Reeves, W.R.; McGuire, M.K.; Stokes, M.; Vicini, J.L. Assessing the Safety of Pesticides in Food: How Current Regulations Protect Human Health. Adv. Nutr. Int. Rev. J. 2019, 10, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, U.; Sandhu, K.S. Effect of handling and processing on pesticide residues in food—A review. J. Food Sci. Technol. 2014, 51, 201–220. [Google Scholar] [CrossRef]
- Schleiffer, M.; Speiser, B. Presence of pesticides in the environment, transition into organic food, and implications for quality assurance along the European organic food chain—A review. Environ. Pollut. 2022, 313, 120116. [Google Scholar] [CrossRef] [PubMed]
- Schwanz, T.G.; Carpilovsky, C.K.; Weis, G.C.C.; Costabeber, I.H. Validation of a multi-residue method and estimation of measurement uncertainty of pesticides in drinking water using gas chromatography–mass spectrometry and liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2019, 1585, 10–18. [Google Scholar] [CrossRef]
- Bernardes, M.F.F.; Pazin, M.; Pereira, L.C.; Dorta, D.J. Impact of Pesticides on Environmental and Human Health. In Toxicology Studies—Cells, Drugs and Environment; IntechOpen: London, UK, 2015. [Google Scholar]
- Pelaez, V.; Da Silva, L.R.; Araújo, E.B. Regulation of pesticides: A comparative analysis. Sci. Public Policy 2013, 40, 644–656. [Google Scholar] [CrossRef]
- Hamilton, D.J.; Ambrus, A.; Dieterle, R.M.; Felsot, A.S.; Harris, C.A.; Holland, P.T.; Katayama, A.; Kurihara, N.; Linders, J.; Unsworth, J.; et al. Regulatory limits for pesticide residues in water (IUPAC Technical Report). Pure Appl. Chem. 2003, 75, 1123–1155. [Google Scholar] [CrossRef]
- Parlamento Europeo y Consejo. Directiva 2006/118/EC del 12 de diciembre de 2006 sobre la protección de las aguas subterráneas contra la contaminación y el deterioro. D. Of. Unión Eur. 2006, 372, 19–31. [Google Scholar]
- Parlamento Europeo y Consejo. Directiva del Consejo 98/83/EC del 3 de noviembre de 1998 sobre la calidad de agua destinada al consumo humano. D. Of. Unión Eur. 1998, 330, 32–54. [Google Scholar]
- Parlamento Europeo y Consejo. Directiva 2000/60/EC del 23 de octubre de 2000 por la que se establece un marco para la acción comunitaria en el campo de la política del agua. D. Of. Unión Eur. 2000, 327, 1–73. [Google Scholar]
- Parlamento Europeo y Consejo. Directiva de la Unión Europea 2008/105/EC del Parlamento Europeo y del Consejo sobre estándares de calidad ambiental en el campo de la política del agua. D. Of. Unión Eur. 2008, 348, 84–97. [Google Scholar]
- Parlamento Europeo y Consejo. Directiva 2013/39/UE del 12 de agosto de 2013 por la que se modifican las Directivas 2000/60/CE y 2008/105/CE en lo que respecta a las sustancias prioritarias en el ámbito de la política de aguas. D. Of. Unión Eur. 2013, 226, 1. [Google Scholar]
- Parlamento Europeo y Consejo. Directiva (UE) 2020/2184 del 16 de diciembre de 2020 sobre la calidad de las aguas destinadas al consumo humano. D. Of. Unión Eur. 2020, 435, 1–62. [Google Scholar]
- Normas de Certificación para los Aplicadores de Pesticidas | US EPA. Available online: https://espanol.epa.gov/seguridad-laboral-al-usar-pesticidas/normas-de-certificacion-para-los-aplicadores-de-pesticidas (accessed on 9 January 2023).
- DECRETO 1843 1991—Colpensiones—Administradora Colombiana de Pensione. Available online: https://normativa.colpensiones.gov.co/colpens/docs/decreto_1843_1991.htm (accessed on 7 January 2023).
- Saleh, I.A.; Zouari, N.; Al-Ghouti, M.A. Removal of pesticides from water and wastewater: Chemical, physical and biological treatment approaches. Environ. Technol. Innov. 2020, 19, 101026. [Google Scholar] [CrossRef]
- Chow, R.; Scheidegger, R.; Doppler, T.; Dietzel, A.; Fenicia, F.; Stamm, C. A review of long-term pesticide monitoring studies to assess surface water quality trends. Water Res. X 2020, 9, 100064. [Google Scholar] [CrossRef]
- Aulakh, J.S.; Malik, A.K.; Kaur, V.; Schmitt-Kopplin, P. A Review on Solid Phase Micro Extraction—High Performance Liquid Chromatography (SPME-HPLC) Analysis of Pesticides. Crit. Rev. Anal. Chem. 2005, 35, 71–85. [Google Scholar] [CrossRef]
- Harshit, D.; Charmy, K.; Nrupesh, P. Organophosphorus pesticides determination by novel HPLC and spectrophotometric method. Food Chem. 2017, 230, 448–453. [Google Scholar] [CrossRef]
- Chiron, S.; Barceló, D. Determination of pesticides in drinking water by on-line solid-phase disk extraction followed by various liquid chromatographic systems. J. Chromatogr. A 1993, 645, 125–134. [Google Scholar] [CrossRef]
- Ballesteros, E.; Parrado, M. Continuous solid-phase extraction and gas chromatographic determination of organophosphorus pesticides in natural and drinking waters. J. Chromatogr. A 2004, 1029, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Khetagoudar, M.C.; Chetti, M.B.; Bilehal, D.C. Gas Chromatographic-Mass Spectrometric Detection of Pesticide Residues in Grapes. In Gas Chromatography-Derivatization, Sample Preparation, Application; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Campanale, C.; Massarelli, C.; Losacco, D.; Bisaccia, D.; Triozzi, M.; Uricchio, V.F. The monitoring of pesticides in water matrices and the analytical criticalities: A review. TrAC Trends Anal. Chem. 2021, 144, 116423. [Google Scholar] [CrossRef]
- Menezes, H.C.; Paulo, B.P.; Paiva, M.J.N.; Cardeal, Z.L. A Simple and Quick Method for the Determination of Pesticides in Environmental Water by HF-LPME-GC/MS. J. Anal. Methods Chem. 2016, 2016, 1–11. [Google Scholar] [CrossRef]
- Cetó, X.; del Valle, M. Electronic tongue applications for wastewater and soil analysis. IScience 2022, 25, 104304. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, F.; Braunger, M.; Riul, A. Heavy Metal/Toxins Detection Using Electronic Tongues. Chemosensors 2019, 7, 36. [Google Scholar] [CrossRef]
- Nowshad, F.; Khan, M.S. Electronic Tongue for Food Safety and Quality Assessment. Tech. Meas. Food Saf. Qual. 2021, 229–247. [Google Scholar] [CrossRef]
- Titova, T.; Nachev, V. “Electronic tongue” in the Food Industry. Food Sci. Appl. Biotechnol. 2020, 3, 71–76. [Google Scholar] [CrossRef]
- Pérez-Ràfols, C.; Serrano, N.; Ariño, C.; Esteban, M.; Díaz-Cruz, J.M. Voltammetric Electronic Tongues in Food Analysis. Sensors 2019, 19, 4261. [Google Scholar] [CrossRef]
- Tan, J.; Xu, J. Applications of electronic nose (e-nose) and electronic tongue (e-tongue) in food quality-related properties determination: A review. Artif. Intell. Agric. 2020, 4, 104–115. [Google Scholar] [CrossRef]
- Calvini, R.; Pigani, L. Toward the Development of Combined Artificial Sensing Systems for Food Quality Evaluation: A Review on the Application of Data Fusion of Electronic Noses, Electronic Tongues and Electronic Eyes. Sensors 2022, 22, 577. [Google Scholar] [CrossRef]
- Herrera-Chacón, A.; Torabi, F.; Faridbod, F.; Ghasemi, J.B.; González-Calabuig, A.; del Valle, M. Voltammetric Electronic Tongue for the Simultaneous Determination of Three Benzodiazepines. Sensors 2019, 19, 5002. [Google Scholar] [CrossRef]
- Vamathevan, J.; Clark, D.; Czodrowski, P.; Dunham, I.; Ferran, E.; Lee, G.; Li, B.; Madabhushi, A.; Shah, P.; Spitzer, M.; et al. Applications of machine learning in drug discovery and development. Nat. Rev. Drug Discov. 2019, 18, 463–477. [Google Scholar] [CrossRef]
- Łabańska, M.; Ciosek-Skibińska, P.; Wróblewski, W. Critical Evaluation of Laboratory Potentiometric Electronic Tongues for Pharmaceutical Analysis—An Overview. Sensors 2019, 19, 5376. [Google Scholar] [CrossRef] [PubMed]
- Rudnitskaya, A.; Legin, A. Sensor systems, electronic tongues and electronic noses, for the monitoring of biotechnological processes. J. Ind. Microbiol. Biotechnol. 2008, 35, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Podrażka, M.; Bączyńska, E.; Kundys, M.; Jeleń, P.S.; Witkowska Nery, E. Electronic Tongue—A Tool for All Tastes? Biosensors 2018, 8, 3. [Google Scholar] [CrossRef]
- Latha, R.S.; Lakshmi, P.K. Electronic tongue: An analytical gustatory tool. J. Adv. Pharm. Technol. Res. 2012, 3, 3–8. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, M.; Bhandari, B.; Adhikari, B. Application of electronic tongue for fresh foods quality evaluation: A review. Food Rev. Int. 2018, 34, 746–769. [Google Scholar] [CrossRef]
- Carrillo-Gómez, J.K.; Acevedo, C.M.D.; García-Rico, R.O. Detection of the bacteria concentration level in pasteurized milk by using two different artificial multisensory methods. Sens. Bio-Sensing Res. 2021, 33, 100428. [Google Scholar] [CrossRef]
- Krantz-Rülcker, C.; Stenberg, M.; Winquist, F.; Lundström, I. Electronic tongues for environmental monitoring based on sensor arrays and pattern recognition: A review. Anal. Chim. Acta 2001, 426, 217–226. [Google Scholar] [CrossRef]
- Kirsanov, D.; Mukherjee, S.; Pal, S.; Ghosh, K.; Bhattacharyya, N.; Bandyopadhyay, R.; Jendrlin, M.; Radu, A.; Zholobenko, V.; Dehabadi, M.; et al. A Pencil-Drawn Electronic Tongue for Environmental Applications. Sensors 2021, 21, 4471. [Google Scholar] [CrossRef] [PubMed]
- Magro, C.; Mateus, E.P.; Raposo, M.; Ribeiro, A. Overview of electronic tongue sensing in environmental aqueous matrices: Potential for monitoring emerging organic contaminants. Environ. Rev. 2019, 27, 202–214. [Google Scholar] [CrossRef]
- Galindo-Miranda, J.M.; Guízar-González, C.; Becerril-Bravo, E.J.; Moeller-Chávez, G.; León-Becerril, E.; Vallejo-Rodríguez, R. Occurrence of emerging contaminants in environmental surface waters and their analytical methodology—A review. Water Supply 2019, 19, 1871–1884. [Google Scholar] [CrossRef]
- Kirsanov, D.; Correa, D.S.; Gaal, G.; Riul, A.; Braunger, M.L.; Shimizu, F.M.; Oliveira, O.N.; Liang, T.; Wan, H.; Wang, P.; et al. Electronic Tongues for Inedible Media. Sensors 2019, 19, 5113. [Google Scholar] [CrossRef] [PubMed]
- Agüera, A.; Martínez Bueno, M.J.; Fernández-Alba, A.R. New trends in the analytical determination of emerging contaminants and their transformation products in environmental waters. Environ. Sci. Pollut. Res. Int. 2013, 20, 3496–3515. [Google Scholar] [CrossRef]
- Gutés, A.; Ibáñez, A.B.; Céspedes, F.; Alegret, S.; Del Valle, M. Simultaneous determination of phenolic compounds by means of an automated voltammetric “electronic tongue”. Anal. Bioanal. Chem. 2005, 382, 471–476. [Google Scholar] [CrossRef]
- González-Calabuig, A.; Cetó, X.; del Valle, M. A Voltammetric Electronic Tongue for the Resolution of Ternary Nitrophenol Mixtures. Sensors 2018, 18, 216. [Google Scholar] [CrossRef]
- Cortina, M.; del Valle, M.; Marty, J.-L. Electronic Tongue Using an Enzyme Inhibition Biosensor Array for the Resolution of Pesticide Mixtures. Electroanalysis 2008, 20, 54–60. [Google Scholar] [CrossRef]
- Valdés-Ramírez, G.; Gutiérrez-Capitán, M.; del Valle, M.; Ramírez-Silva, M.; Fournier, D.; Marty, J.-L. Automated resolution of dichlorvos and methylparaoxon pesticide mixtures employing a Flow Injection system with an inhibition electronic tongue. Biosens. Bioelectron. 2009, 24, 1103–1108. [Google Scholar] [CrossRef]
- Alonso-Silverio, G.A.; Muñoz, R.; Marty, J.-L. Automatic Electronic Tongue for On-Line Detection and Quantification of Organophosphorus and Carbamate Pesticides Using Enzymatic Screen Printed Biosensors. Anal. Lett. 2013, 46, 1743–1757. [Google Scholar] [CrossRef]
- Facure, M.H.; Mercante, L.A.; Mattoso, L.H.; Correa, D.S. Detection of trace levels of organophosphate pesticides using an electronic tongue based on graphene hybrid nanocomposites. Talanta 2017, 167, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Tahara, Y.; Toko, K. Electronic Tongues—A Review. IEEE Sensors J. 2013, 13, 3001–3011. [Google Scholar] [CrossRef]
- Kadam, U.S.; Hong, J.C. Advances in aptameric biosensors designed to detect toxic contaminants from food, water, human fluids, and the environment. Trends Environ. Anal. Chem. 2020, 36, 00184. [Google Scholar] [CrossRef]
- Inam, A.K.M.S.; Angeli, M.A.C.; Douaki, A.; Shkodra, B.; Lugli, P.; Petti, L. An Aptasensor Based on a Flexible Screen-Printed Silver Electrode for the Rapid Detection of Chlorpyrifos. Sensors 2022, 22, 2754. [Google Scholar] [CrossRef]
- Xu, Y.; Cheng, N.; Luo, Y.; Huang, K.; Chang, Q.; Pang, G.; Xu, W. An Exo III-assisted catalytic hairpin assembly-based self-fluorescence aptasensor for pesticide detection. Sens. Actuators B Chem. 2022, 358, 131441. [Google Scholar] [CrossRef]
- Dropsens. Available online: https://polco.com.co/dropsens/ (accessed on 3 January 2023).
- Corradi, E.; Agostini, M.; Greco, G.; Massidda, D.; Santi, M.; Calderisi, M.; Signore, G.; Cecchini, M. An objective, principal-component-analysis (PCA) based, method which improves the quartz-crystal-microbalance (QCM) sensing performance. Sens. Actuators A Phys. 2020, 315, 112323. [Google Scholar] [CrossRef]
- Asante-Okyere, S.; Shen, C.; Ziggah, Y.Y.; Rulegeya, M.M.; Zhu, X. Principal component analysis (PCA) based hybrid models for the accurate estimation of reservoir water saturation. Comput. Geosci. 2020, 145, 104555. [Google Scholar] [CrossRef]
- Vaibhaw; Sarraf, J.; Pattnaik, P.K. Brain-computer interfaces and their applications. Ind. IoT Approach Pharm. Ind. Growth 2020, 2, 31–54. [Google Scholar] [CrossRef]
- Martinez, A.; Kak, A. PCA versus LDA. IEEE Trans. Pattern Anal. Mach. Intell. 2021, 23, 228–233. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, P.; Ren, J.-X.; Li, X.-B.; Wang, H.-L.; Ding, L.; Kong, W.-B. Development of novel prediction model for drug-induced mitochondrial toxicity by using naïve Bayes classifier method. Food Chem. Toxicol. 2017, 110, 122–129. [Google Scholar] [CrossRef]
- Chen, S.; Webb, G.I.; Liu, L.; Ma, X. A novel selective naïve Bayes algorithm. Knowl. Based Syst. 2020, 192, 105361. [Google Scholar] [CrossRef]
- Pisner, D.A.; Schnyer, D.M. Support vector machine. Mach. Learn. Methods Appl. Brain Disord. 2020, 101–121. [Google Scholar] [CrossRef]
- Zhang, S.; Li, X.; Zong, M.; Zhu, X.; Cheng, D. Learning k for kNN Classification. ACM Trans. Intell. Syst. Technol. 2017, 8, 3. [Google Scholar] [CrossRef] [Green Version]
- Wilson, A.D. Diverse Applications of Electronic-Nose Technologies in Agriculture and Forestry. Sensors 2013, 13, 2295–2348. [Google Scholar] [CrossRef]
- Braz, D.C.; Neto, M.P.; Shimizu, F.M.; Sá, A.C.; Lima, R.S.; Gobbi, A.L.; Melendez, M.E.; Arantes, L.M.B.; Carvalho, A.L.; Paulovich, F.V.; et al. Using machine learning and an electronic tongue for discriminating saliva samples from oral cavity cancer patients and healthy individuals. Talanta 2022, 243. [Google Scholar] [CrossRef] [PubMed]
- Pascual, L.; Campos, I.; Vivancos, J.-L.; Quintás, G.; Loras, A.; Martínez-Bisbal, M.C.; Martínez-Máñez, R.; Boronat, F.; Ruiz-Cerdà, J.L. Detection of prostate cancer using a voltammetric electronic tongue. Analyst 2023, 141, 4562–4567. [Google Scholar] [CrossRef] [PubMed]
- Zniber, M.; Vahdatiyekta, P.; Huynh, T.-P. Analysis of urine using electronic tongue towards non-invasive cancer diagnosis. Biosens. Bioelectron. 2023, 219, 114810. [Google Scholar] [CrossRef]
- Lvova, L. Electronic Tongue Principles and Applications in the Food Industry. In Electronic Noses and Tongues in Food Science; Academic Press: Cambridge, MA, USA, 2016; pp. 151–160. [Google Scholar] [CrossRef]
- Wadehra, A.; Patil, P.S. Application of electronic tongues in food processing. Anal. Methods 2016, 8, 474–480. [Google Scholar] [CrossRef]
- Park, H.; Kim, G.; Seo, Y.; Yoon, Y.; Min, J.; Park, C.; Lee, T. Improving Biosensors by the Use of Different Nanomaterials: Case Study with Microcystins as Target Analytes. Biosensors 2021, 11, 525. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Lin, X.; Peng, Z.; Xu, S.; Jin, L.; Zheng, X.; Luo, H. Materials and Methods of Biosensor Interfaces With Stability. Front. Mater. 2021, 7, 438. [Google Scholar] [CrossRef]
- Naresh, V.; Lee, N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.O.; Singh, B. Electrochemical Biosensors for Detection of Pesticides and Heavy Metal Toxicants in Water: Recent Trends and Progress. ACS ES&T Water 2021, 1, 462–478. [Google Scholar] [CrossRef]
- Bhavadharini, B.; Kavimughil, M.; Malini, B.; Vallath, A.; Prajapati, H.K.; Sunil, C.K. Recent Advances in Biosensors for Detection of Chemical Contaminants in Food—A Review. Food Anal. Methods 2021, 15, 1545–1564. [Google Scholar] [CrossRef]

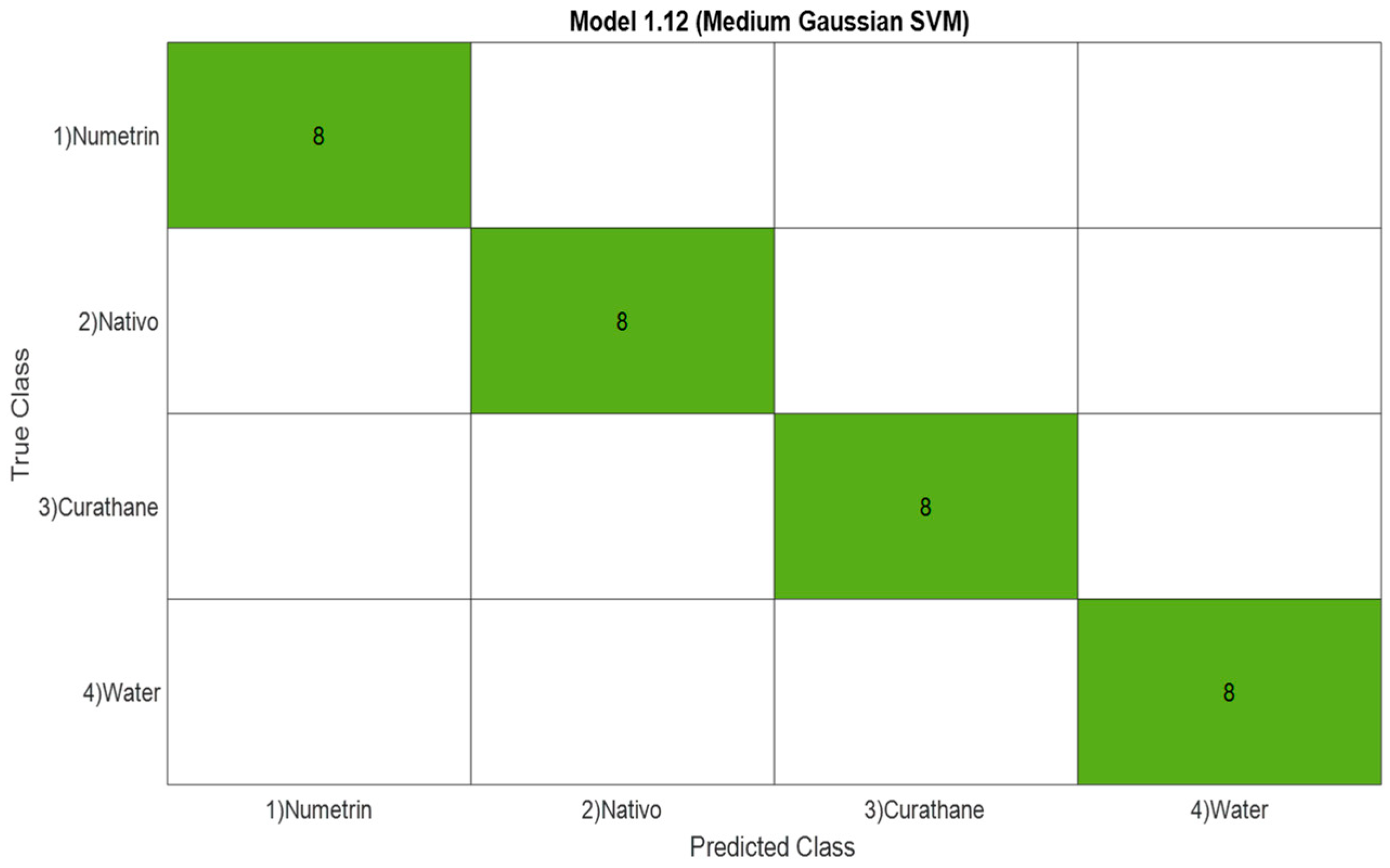
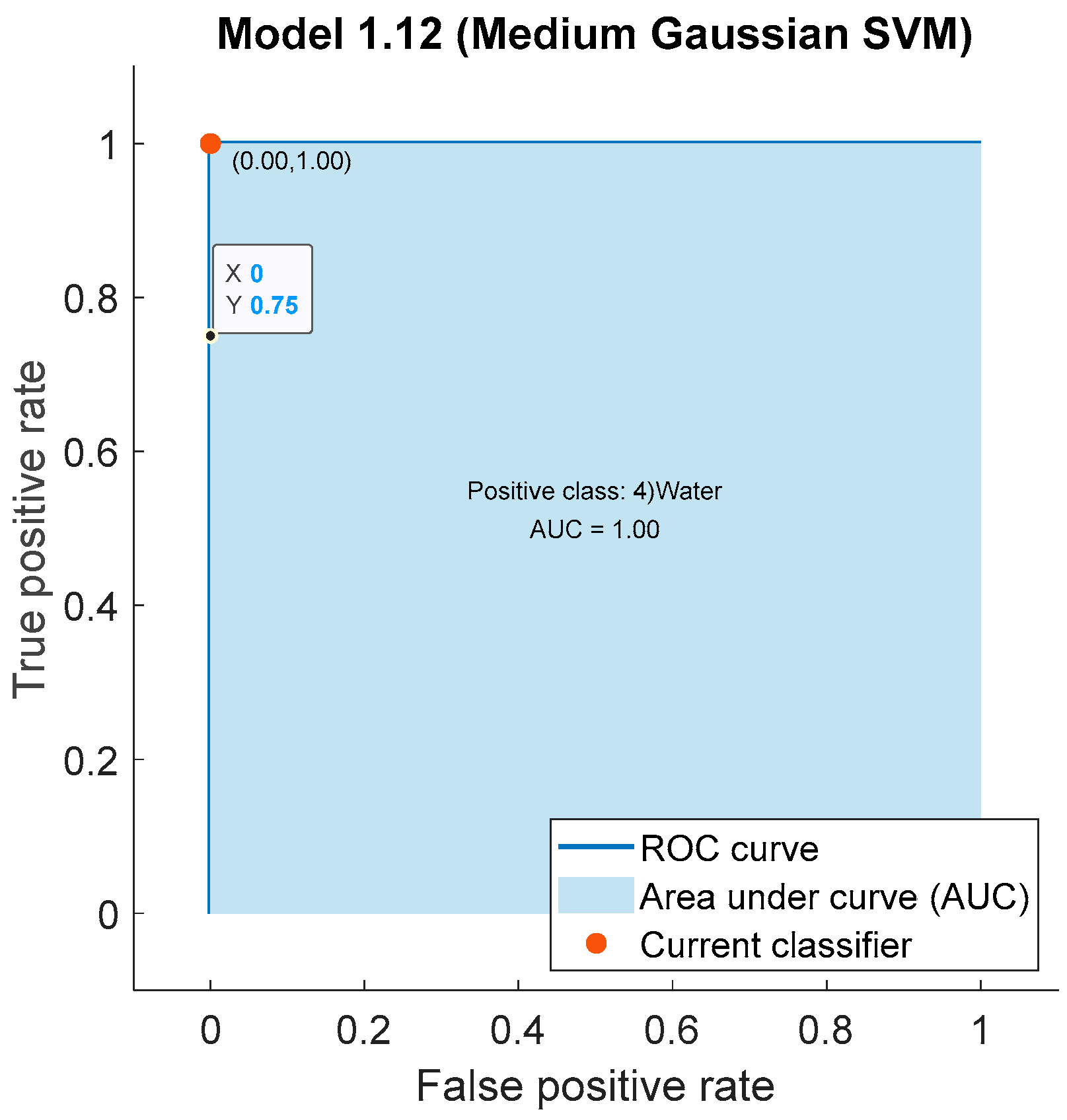



| N° | Model | Accuracy |
|---|---|---|
| 1 | Linear Discriminant | 93.8% |
| 2 | Naïve Bayes | 100% |
| 3 | SVM | 100% |
| 4 | kNN | 100% |
| Model | Accuracy | ||
|---|---|---|---|
| Curathane + H2O | Nativo + H2O | Numetrin + H2O | |
| Linear Discriminant | 76.4% | 83.3% | 83.3% |
| Naïve Bayes | 81.9% | 77.8% | 90.3% |
| SVM | 86.1% | 93.1% | 90.3% |
| kNN | 91.7% | 93.1% | 68.1% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez, J.K.C.; Puentes, Y.A.N.; Niño, D.D.C.; Acevedo, C.M.D. Detection of Pesticides in Water through an Electronic Tongue and Data Processing Methods. Water 2023, 15, 624. https://doi.org/10.3390/w15040624
Gómez JKC, Puentes YAN, Niño DDC, Acevedo CMD. Detection of Pesticides in Water through an Electronic Tongue and Data Processing Methods. Water. 2023; 15(4):624. https://doi.org/10.3390/w15040624
Chicago/Turabian StyleGómez, Jeniffer Katerine Carrillo, Yuliana Alexandra Nieto Puentes, Dayan Diomedes Cárdenas Niño, and Cristhian Manuel Durán Acevedo. 2023. "Detection of Pesticides in Water through an Electronic Tongue and Data Processing Methods" Water 15, no. 4: 624. https://doi.org/10.3390/w15040624
APA StyleGómez, J. K. C., Puentes, Y. A. N., Niño, D. D. C., & Acevedo, C. M. D. (2023). Detection of Pesticides in Water through an Electronic Tongue and Data Processing Methods. Water, 15(4), 624. https://doi.org/10.3390/w15040624








