Removal of Contaminants of Emerging Concern from Wastewater Using an Integrated Column System Containing Zero Valent Iron Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. R-nFe Synthesis
2.3. Wastewater Source and Characteristics
2.4. Experimental Set-Up
2.4.1. Batch Experiments
2.4.2. Column Experiments
2.5. Elaboration of Data
2.6. Sampling Procedure
2.7. Analytical Methods
3. Results and Discussion
3.1. Effect of Target Compounds Initial Concentration
3.2. Effect of Contact Time
3.3. Effect of pH Adjustment and Contact Time
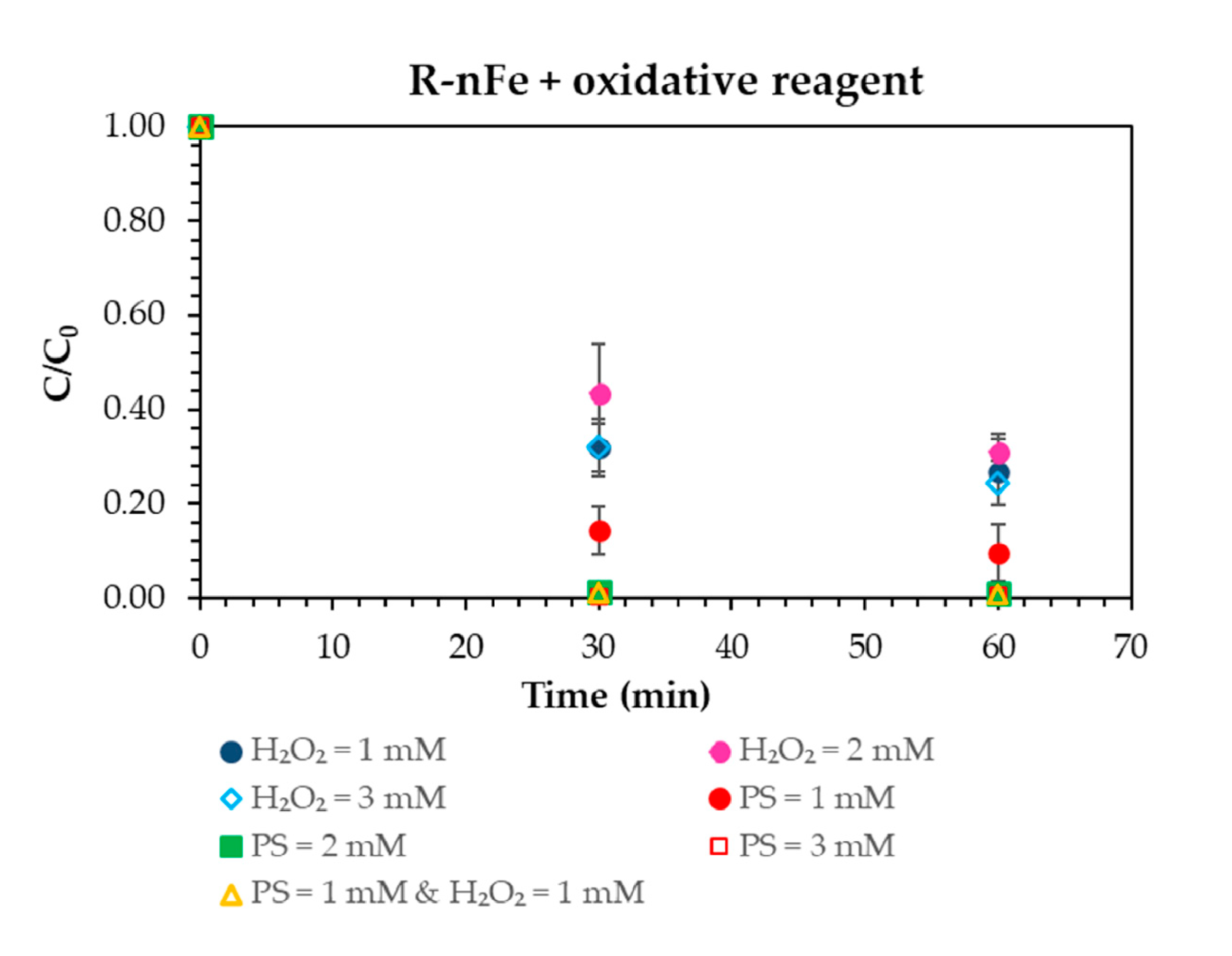
3.4. Oxidative Reagent Selection
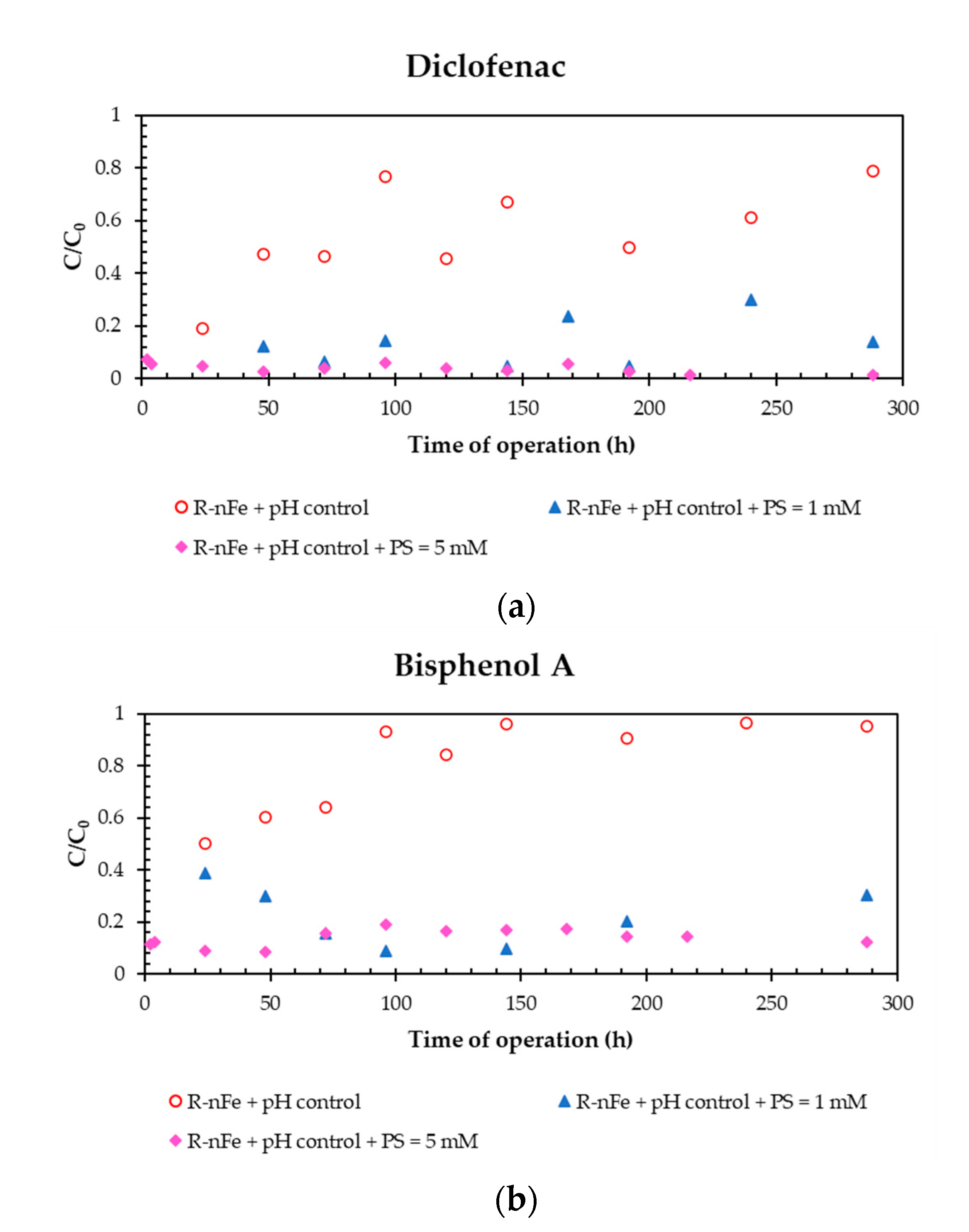
3.5. Effect of PS Dose at Controlled pH
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geissen, V.; Mol, H.; Klumpp, E.; Umlauf, G.; Nadal, M.; van der Ploeg, M.; van de Zee, S.E.A.T.M.; Ritsema, C.J. Emerging Pollutants in the Environment: A Challenge for Water Resource Management. Int. Soil Water Conserv. Res. 2015, 3, 57–65. [Google Scholar] [CrossRef]
- Abdulrazaq, Y.; Abdulsalam, A.; Larayetan Rotimi, A.; Aliyu Abdulbasit, A.; Clifford, O.; Abdulazeez Abdulsalam, O.; Nayo Racheal, O.; Akor Joy, A.; Omale Victor, F.; Mbese Johannes, Z.; et al. Classification, Potential Routes and Risk of Emerging Pollutants/Contaminant. In Emerging Contaminants; Nuro, A., Ed.; IntechOpen: London, UK, 2021; ISBN 978-1-83962-418-6. [Google Scholar]
- Arman, N.Z.; Salmiati, S.; Aris, A.; Salim, M.R.; Nazifa, T.H.; Muhamad, M.S.; Marpongahtun, M. A Review on Emerging Pollutants in the Water Environment: Existences, Health Effects and Treatment Processes. Water 2021, 13, 3258. [Google Scholar] [CrossRef]
- Sivaranjanee, R.; Kumar, P.S. A Review on Remedial Measures for Effective Separation of Emerging Contaminants from Wastewater. Environ. Technol. Innov. 2021, 23, 101741. [Google Scholar] [CrossRef]
- López-Pacheco, I.Y.; Silva-Núñez, A.; Salinas-Salazar, C.; Arévalo-Gallegos, A.; Lizarazo-Holguin, L.A.; Barceló, D.; Iqbal, H.M.N.; Parra-Saldívar, R. Anthropogenic Contaminants of High Concern: Existence in Water Resources and Their Adverse Effects. Sci. Total Environ. 2019, 690, 1068–1088. [Google Scholar] [CrossRef] [PubMed]
- la Farré, M.; Pérez, S.; Kantiani, L.; Barceló, D. Fate and Toxicity of Emerging Pollutants, Their Metabolites and Transformation Products in the Aquatic Environment. TrAC Trends Anal. Chem. 2008, 27, 991–1007. [Google Scholar] [CrossRef]
- Caliman, F.A.; Gavrilescu, M. Pharmaceuticals, Personal Care Products and Endocrine Disrupting Agents in the Environment—A Review. Clean Soil Air Water 2009, 37, 277–303. [Google Scholar] [CrossRef]
- Świacka, K.; Michnowska, A.; Maculewicz, J.; Caban, M.; Smolarz, K. Toxic Effects of NSAIDs in Non-Target Species: A Review from the Perspective of the Aquatic Environment. Environ. Pollut. 2021, 273, 115891. [Google Scholar] [CrossRef]
- Gao, X.; Kang, S.; Xiong, R.; Chen, M. Environment-Friendly Removal Methods for Endocrine Disrupting Chemicals. Sustainability 2020, 12, 7615. [Google Scholar] [CrossRef]
- Gross-Sorokin, M.Y.; Roast, S.D.; Brighty, G.C. Assessment of Feminization of Male Fish in English Rivers by the Environment Agency of England and Wales. Environ. Health Perspect. 2006, 114, 147–151. [Google Scholar] [CrossRef]
- Hayes, T.B.; Anderson, L.L.; Beasley, V.R.; de Solla, S.R.; Iguchi, T.; Ingraham, H.; Kestemont, P.; Kniewald, J.; Kniewald, Z.; Langlois, V.S.; et al. Demasculinization and Feminization of Male Gonads by Atrazine: Consistent Effects across Vertebrate Classes. J. Steroid Biochem. Mol. Biol. 2011, 127, 64–73. [Google Scholar] [CrossRef] [Green Version]
- Zha, J.; Sun, L.; Spear, P.A.; Wang, Z. Comparison of Ethinylestradiol and Nonylphenol Effects on Reproduction of Chinese Rare Minnows (Gobiocypris Rarus). Ecotoxicol. Environ. Saf. 2008, 71, 390–399. [Google Scholar] [CrossRef]
- Ji, H.; Song, N.; Ren, J.; Li, W.; Xu, B.; Li, H.; Shen, G. Metabonomics Reveals Bisphenol A Affects Fatty Acid and Glucose Metabolism through Activation of LXR in the Liver of Male Mice. Sci. Total Environ. 2020, 703, 134681. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Chen, Y.; Lin, L.; Liu, Y.; Chi, Y.; Lin, Y.; Ye, G.; Zhu, H.; Dong, S. Different Effects of Bisphenol a and Its Halogenated Derivatives on the Reproduction and Development of Oryzias Melastigma under Environmentally Relevant Doses. Sci. Total Environ. 2017, 595, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Marcial, H.S.; Hagiwara, A.; Snell, T.W. Estrogenic Compounds Affect Development of Harpacticoid Copepod Tigriopus Japonicus. Environ. Toxicol Chem 2003, 22, 3025. [Google Scholar] [CrossRef]
- O’Connor, J.C.; Chapin, R.E. Critical Evaluation of Observed Adverse Effects of Endocrine Active Substances on Reproduction and Development, the Immune System, and the Nervous System. Pure Appl. Chem. 2003, 75, 2099–2123. [Google Scholar] [CrossRef]
- Priyanka; Trivedi, A.; Maske, P.; Mote, C.; Dighe, V. Gestational and Lactational Exposure to Triclosan Causes Impaired Fertility of F1 Male Offspring and Developmental Defects in F2 Generation. Environ. Pollut. 2020, 257, 113617. [Google Scholar] [CrossRef] [PubMed]
- Tabari, S.A.; Esfahani, M.L.; Hosseini, S.M.; Rahimi, A. Neurobehavioral Toxicity of Triclosan in Mice. Food Chem. Toxicol. 2019, 130, 154–160. [Google Scholar] [CrossRef]
- da Silva, T.L.; Costa, C.S.D.; da Silva, M.G.C.; Vieira, M.G.A. Overview of Non-Steroidal Anti-Inflammatory Drugs Degradation by Advanced Oxidation Processes. J. Clean. Prod. 2022, 346, 131226. [Google Scholar] [CrossRef]
- Azizi, D.; Arif, A.; Blair, D.; Dionne, J.; Filion, Y.; Ouarda, Y.; Pazmino, A.G.; Pulicharla, R.; Rilstone, V.; Tiwari, B.; et al. A Comprehensive Review on Current Technologies for Removal of Endocrine Disrupting Chemicals from Wastewaters. Environ. Res. 2022, 207, 112196. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, A.; Tiwari, M.K.; Ghangrekar, M.M. A Review on Environmental Occurrence, Toxicity and Microbial Degradation of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs). J. Environ. Manag. 2021, 300, 113694. [Google Scholar] [CrossRef]
- de Andrade, J.R.; Oliveira, M.F.; Canevesi, R.L.S.; Landers, R.; da Silva, M.G.C.; Vieira, M.G.A. Comparative Adsorption of Diclofenac Sodium and Losartan Potassium in Organophilic Clay-Packed Fixed-Bed: X-Ray Photoelectron Spectroscopy Characterization, Experimental Tests and Theoretical Study on DFT-Based Chemical Descriptors. J. Mol. Liq. 2020, 312, 113427. [Google Scholar] [CrossRef]
- Park, Y.; Ayoko, G.A.; Frost, R.L. Application of Organoclays for the Adsorption of Recalcitrant Organic Molecules from Aqueous Media. J. Colloid Interface Sci. 2011, 354, 292–305. [Google Scholar] [CrossRef] [PubMed]
- Jedynak, K.; Szczepanik, B.; Rędzia, N.; Słomkiewicz, P.; Kolbus, A.; Rogala, P. Ordered Mesoporous Carbons for Adsorption of Paracetamol and Non-Steroidal Anti-Inflammatory Drugs: Ibuprofen and Naproxen from Aqueous Solutions. Water 2019, 11, 1099. [Google Scholar] [CrossRef]
- Streit, A.F.M.; Collazzo, G.C.; Druzian, S.P.; Verdi, R.S.; Foletto, E.L.; Oliveira, L.F.S.; Dotto, G.L. Adsorption of Ibuprofen, Ketoprofen, and Paracetamol onto Activated Carbon Prepared from Effluent Treatment Plant Sludge of the Beverage Industry. Chemosphere 2021, 262, 128322. [Google Scholar] [CrossRef]
- Sui, Q.; Huang, J.; Liu, Y.; Chang, X.; Ji, G.; Deng, S.; Xie, T.; Yu, G. Rapid Removal of Bisphenol A on Highly Ordered Mesoporous Carbon. J. Environ. Sci. 2011, 23, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Yi, H.; Lai, C.; Liu, X.; Huo, X.; An, Z.; Li, L.; Fu, Y.; Li, B.; Zhang, M.; et al. Critical Review of Advanced Oxidation Processes in Organic Wastewater Treatment. Chemosphere 2021, 275, 130104. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Activation of Persulfate (PS) and Peroxymonosulfate (PMS) and Application for the Degradation of Emerging Contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Hussain, I.; Li, M.; Zhang, Y.; Li, Y.; Huang, S.; Du, X.; Liu, G.; Hayat, W.; Anwar, N. Insights into the Mechanism of Persulfate Activation with NZVI/BC Nanocomposite for the Degradation of Nonylphenol. Chem. Eng. J. 2017, 311, 163–172. [Google Scholar] [CrossRef]
- Gomes, J.; Costa, R.; Quinta-Ferreira, R.M.; Martins, R.C. Application of Ozonation for Pharmaceuticals and Personal Care Products Removal from Water. Sci. Total Environ. 2017, 586, 265–283. [Google Scholar] [CrossRef]
- Ning, B.; Graham, N.; Zhang, Y.; Nakonechny, M.; Gamal El-Din, M. Degradation of Endocrine Disrupting Chemicals by Ozone/AOPs. Ozone Sci. Eng. 2007, 29, 153–176. [Google Scholar] [CrossRef]
- Wang, J.; Chen, H. Catalytic Ozonation for Water and Wastewater Treatment: Recent Advances and Perspective. Sci. Total Environ. 2020, 704, 135249. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Sutton, N.B.; Rijnaarts, H.H.H.; Langenhoff, A.A.M. Degradation of Pharmaceuticals in Wastewater Using Immobilized TiO2 Photocatalysis under Simulated Solar Irradiation. Appl. Catal. B Environ. 2016, 182, 132–141. [Google Scholar] [CrossRef]
- Kaur, A.; Umar, A.; Kansal, S.K. Sunlight-Driven Photocatalytic Degradation of Non-Steroidal Anti-Inflammatory Drug Based on TiO2 Quantum Dots. J. Colloid Interface Sci. 2015, 459, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Vela, N.; Calín, M.; Yáñez-Gascón, M.J.; Garrido, I.; Pérez-Lucas, G.; Fenoll, J.; Navarro, S. Photocatalytic Oxidation of Six Pesticides Listed as Endocrine Disruptor Chemicals from Wastewater Using Two Different TiO2 Samples at Pilot Plant Scale under Sunlight Irradiation. J. Photochem. Photobiol. A Chem. 2018, 353, 271–278. [Google Scholar] [CrossRef]
- Villanueva-Rodríguez, M.; Bello-Mendoza, R.; Hernández-Ramírez, A.; Ruiz-Ruiz, E.J. Degradation of Anti-Inflammatory Drugs in Municipal Wastewater by Heterogeneous Photocatalysis and Electro-Fenton Process. Environ. Technol. 2019, 40, 2436–2445. [Google Scholar] [CrossRef]
- Zatloukalová, K.; Obalová, L.; Koči, K.; Čapek, L.; Matěj, Z.; Šnajdhaufová, H.; Ryczkowski, J.; Słowik, G. Photocatalytic Degradation of Endocrine Disruptor Compounds in Water over Immobilized TiO2 Photocatalysts. Iran. J. Chem. Chem. Eng. 2017, 36, 29–38. [Google Scholar] [CrossRef]
- Augsburger, N.; Zaouri, N.; Cheng, H.; Hong, P.-Y. The Use of UV/H2O2 to Facilitate Removal of Emerging Contaminants in Anaerobic Membrane Bioreactor Effluents. Environ. Res. 2021, 198, 110479. [Google Scholar] [CrossRef]
- Wang, P.; Bu, L.; Wu, Y.; Ma, W.; Zhu, S.; Zhou, S. Mechanistic Insight into the Degradation of Ibuprofen in UV/H2O2 Process via a Combined Experimental and DFT Study. Chemosphere 2021, 267, 128883. [Google Scholar] [CrossRef]
- Jain, B.; Singh, A.K.; Kim, H.; Lichtfouse, E.; Sharma, V.K. Treatment of Organic Pollutants by Homogeneous and Heterogeneous Fenton Reaction Processes. Environ. Chem. Lett. 2018, 16, 947–967. [Google Scholar] [CrossRef]
- Adityosulindro, S.; Barthe, L.; González-Labrada, K.; Jáuregui Haza, U.J.; Delmas, H.; Julcour, C. Sonolysis and Sono-Fenton Oxidation for Removal of Ibuprofen in (Waste)Water. Ultrason. Sonochem. 2017, 39, 889–896. [Google Scholar] [CrossRef] [Green Version]
- Camargo-Perea, A.L.; Rubio-Clemente, A.; Peñuela, G.A. Use of Ultrasound as an Advanced Oxidation Process for the Degradation of Emerging Pollutants in Water. Water 2020, 12, 1068. [Google Scholar] [CrossRef]
- Peng, J.; Wang, Z.; Wang, S.; Liu, J.; Zhang, Y.; Wang, B.; Gong, Z.; Wang, M.; Dong, H.; Shi, J.; et al. Enhanced Removal of Methylparaben Mediated by Cobalt/Carbon Nanotubes (Co/CNTs) Activated Peroxymonosulfate in Chloride-Containing Water: Reaction Kinetics, Mechanisms and Pathways. Chem. Eng. J. 2021, 409, 128176. [Google Scholar] [CrossRef]
- Dai, Y.; Cao, H.; Qi, C.; Zhao, Y.; Wen, Y.; Xu, C.; Zhong, Q.; Sun, D.; Zhou, S.; Yang, B.; et al. L-Cysteine Boosted Fe(III)-Activated Peracetic Acid System for Sulfamethoxazole Degradation: Role of L-Cysteine and Mechanism. Chem. Eng. J. 2023, 451, 138588. [Google Scholar] [CrossRef]
- Dai, Y.; Qi, C.; Cao, H.; Wen, Y.; Zhao, Y.; Xu, C.; Yang, S.; He, H. Enhanced Degradation of Sulfamethoxazole by Microwave-Activated Peracetic Acid under Alkaline Condition: Influencing Factors and Mechanism. Sep. Purif. Technol. 2022, 288, 120716. [Google Scholar] [CrossRef]
- Baldermann, A.; Kaufhold, S.; Dohrmann, R.; Baldermann, C.; Letofsky-Papst, I.; Dietzel, M. A Novel NZVI–Bentonite Nanocomposite to Remove Trichloroethene (TCE) from Solution. Chemosphere 2021, 282, 131018. [Google Scholar] [CrossRef]
- Gu, M.; Farooq, U.; Lu, S.; Zhang, X.; Qiu, Z.; Sui, Q. Degradation of Trichloroethylene in Aqueous Solution by RGO Supported NZVI Catalyst under Several Oxic Environments. J. Hazard. Mater. 2018, 349, 35–44. [Google Scholar] [CrossRef]
- Huang, L.; Weng, X.; Chen, Z.; Megharaj, M.; Naidu, R. Green Synthesis of Iron Nanoparticles by Various Tea Extracts: Comparative Study of the Reactivity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 130, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Raman, C.D.; Kanmani, S. Textile Dye Degradation Using Nano Zero Valent Iron: A Review. J. Environ. Manag. 2016, 177, 341–355. [Google Scholar] [CrossRef]
- Fazlzadeh, M.; Rahmani, K.; Zarei, A.; Abdoallahzadeh, H.; Nasiri, F.; Khosravi, R. A Novel Green Synthesis of Zero Valent Iron Nanoparticles (NZVI) Using Three Plant Extracts and Their Efficient Application for Removal of Cr(VI) from Aqueous Solutions. Adv. Powder Technol. 2017, 28, 122–130. [Google Scholar] [CrossRef]
- Selvan, B.K.; Thiyagarajan, K.; Das, S.; Jaya, N.; Jabasingh, S.A.; Saravanan, P.; Rajasimman, M.; Vasseghian, Y. Synthesis and Characterization of Nano Zerovalent Iron-Kaolin Clay (NZVI-Kaol) Composite Polyethersulfone (PES) Membrane for the Efficacious As2O3 Removal from Potable Water Samples. Chemosphere 2022, 288, 132405. [Google Scholar] [CrossRef]
- Toli, A.; Varouxaki, A.; Mystrioti, C.; Xenidis, A.; Papassiopi, N. Green Synthesis of Resin Supported Nanoiron and Evaluation of Efficiency for the Remediation of Cr(VI) Contaminated Groundwater by Batch Tests. Bull. Environ. Contam. Toxicol. 2018, 101, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Bose, P. Degradation Kinetics of Endosulfan Isomers by Micron- and Nano-Sized Zero Valent Iron Particles (MZVI and NZVI): Degradation Kinetics of Endosulfan Isomers. J. Chem. Technol. Biotechnol. 2016, 91, 2313–2321. [Google Scholar] [CrossRef]
- Thompson, J.M.; Chisholm, B.J.; Bezbaruah, A.N. Reductive Dechlorination of Chloroacetanilide Herbicide (Alachlor) Using Zero-Valent Iron Nanoparticles. Environ. Eng. Sci. 2010, 27, 227–232. [Google Scholar] [CrossRef]
- Wang, X.; Deng, R.; Shen, W.; Huang, J.; Li, Q.; Zhao, Y.; Wan, J.; Zhou, Y.; Long, T.; Zhang, S. Rapid Degradation of Nitrochlorobenzene by Activated Persulfate Oxidation With Biochar Supported Nanoscaled Zero Valent Iron. Front. Chem. 2021, 9, 615694. [Google Scholar] [CrossRef] [PubMed]
- Haneef, T.; Ul Mustafa, M.R.; Rasool, K.; Ho, Y.C.; Mohamed Kutty, S.R. Removal of Polycyclic Aromatic Hydrocarbons in a Heterogeneous Fenton Like Oxidation System Using Nanoscale Zero-Valent Iron as a Catalyst. Water 2020, 12, 2430. [Google Scholar] [CrossRef]
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: A Review of Synthesis Methods, Properties, Recent Progress, and Challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Khatir, N.M.; Sabbagh, F. Green Facile Synthesis of Silver-Doped Zinc Oxide Nanoparticles and Evaluation of Their Effect on Drug Release. Materials 2022, 15, 5536. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi Khatir, N.; Abdul-Malek, Z.; Zak, A.K.; Akbari, A.; Sabbagh, F. Sol–Gel Grown Fe-Doped ZnO Nanoparticles: Antibacterial and Structural Behaviors. J. Sol-Gel Sci. Technol. 2016, 78, 91–98. [Google Scholar] [CrossRef]
- Bruns, J.; Gheorghiu, F.; Borda, M.; Bosch, J. Experiences from Pilot- and Large-Scale Demonstration Sites from Across the Globe Including Combined Remedies with NZVI. In Nanoscale Zerovalent Iron Particles for Environmental Restoration; Phenrat, T., Lowry, G.V., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 335–357. ISBN 978-3-319-95338-0. [Google Scholar]
- Chanthapon, N.; Sarkar, S.; Kidkhunthod, P.; Padungthon, S. Lead Removal by a Reusable Gel Cation Exchange Resin Containing Nano-Scale Zero Valent Iron. Chem. Eng. J. 2018, 331, 545–555. [Google Scholar] [CrossRef]
- Li, S.; Wang, W.; Liu, Y.; Zhang, W. Zero-Valent Iron Nanoparticles (NZVI) for the Treatment of Smelting Wastewater: A Pilot-Scale Demonstration. Chem. Eng. J. 2014, 254, 115–123. [Google Scholar] [CrossRef]
- Němeček, J.; Lhotský, O.; Cajthaml, T. Nanoscale Zero-Valent Iron Application for in Situ Reduction of Hexavalent Chromium and Its Effects on Indigenous Microorganism Populations. Sci. Total Environ. 2014, 485–486, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Ponder, S.M.; Darab, J.G.; Mallouk, T.E. Remediation of Cr(VI) and Pb(II) Aqueous Solutions Using Supported, Nanoscale Zero-Valent Iron. Environ. Sci. Technol. 2000, 34, 2564–2569. [Google Scholar] [CrossRef]
- Tarekegn, M.M.; Hiruy, A.M.; Dekebo, A.H. Nano Zero Valent Iron (NZVI) Particles for the Removal of Heavy Metals (Cd2+, Cu2+ and Pb2+) from Aqueous Solutions. RSC Adv. 2021, 11, 18539–18551. [Google Scholar] [CrossRef]
- Toli, A.; Mystrioti, C.; Avgoustidis, I.; Papassiopi, N. Fixed-Bed Flow Experiments with Supported Green NZVI for the Remediation of Contaminated Waters: Effect of PH and Background Solution Composition. Chemosphere 2021, 279, 130472. [Google Scholar] [CrossRef]
- Sheu, Y.T.; Lien, P.J.; Chen, K.F.; Ou, J.H.; Kao, C.M. Application of NZVI-Contained Emulsified Substrate to Bioremediate PCE-Contaminated Groundwater—A Pilot-Scale Study. Chem. Eng. J. 2016, 304, 714–727. [Google Scholar] [CrossRef]
- Song, Y.; Fang, G.; Zhu, C.; Zhu, F.; Wu, S.; Chen, N.; Wu, T.; Wang, Y.; Gao, J.; Zhou, D. Zero-Valent Iron Activated Persulfate Remediation of Polycyclic Aromatic Hydrocarbon-Contaminated Soils: An in Situ Pilot-Scale Study. Chem. Eng. J. 2019, 355, 65–75. [Google Scholar] [CrossRef]
- Mdlovu, N.V.; Lin, K.-S.; Hsien, M.-J.; Chang, C.-J.; Kunene, S.C. Synthesis, Characterization, and Application of Zero-Valent Iron Nanoparticles for TNT, RDX, and HMX Explosives Decontamination in Wastewater. J. Taiwan Inst. Chem. Eng. 2020, 114, 186–198. [Google Scholar] [CrossRef]
- Jerold, M.; Joseph, D.; Patra, N.; Sivasubramanian, V. Fixed-Bed Column Studies for the Removal of Hazardous Malachite Green Dye from Aqueous Solution Using Novel Nano Zerovalent Iron Algal Biocomposite. Nanotechnol. Environ. Eng. 2016, 1, 8. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, J.; Tai, C.; Zhou, Q.; Hu, J.; Jiang, G. Rapid Decolorization of Water Soluble Azo-Dyes by Nanosized Zero-Valent Iron Immobilized on the Exchange Resin. Sci. China Ser. B-Chem. 2008, 51, 186–192. [Google Scholar] [CrossRef]
- Makhathini, T.P.; Mulopo, J.; Bakare, B.F. Enriched Co-Treatment of Pharmaceutical and Acidic Metal-Containing Wastewater with Nano Zero-Valent Iron. Minerals 2021, 11, 220. [Google Scholar] [CrossRef]
- Sulaiman, S.M.; Al-Jabari, M.H. Enhanced Adsorptive Removal of Diclofenac Sodium from Aqueous Solution by Bentonite-Supported Nanoscale Zero-Valent Iron. Arab J. Basic Appl. Sci. 2021, 28, 51–63. [Google Scholar] [CrossRef]
- Esmaeili Bidhendi, M.; Parandi, E.; Mahmoudi Meymand, M.; Sereshti, H.; Rashidi Nodeh, H.; Joo, S.-W.; Vasseghian, Y.; Mahmoudi Khatir, N.; Rezania, S. Removal of Lead Ions from Wastewater Using Magnesium Sulfide Nanoparticles Caged Alginate Microbeads. Environ. Res. 2023, 216, 114416. [Google Scholar] [CrossRef]
- Girit, B.; Dursun, D.; Olmez-Hanci, T.; Arslan-Alaton, I. Treatment of Aqueous Bisphenol A Using Nano-Sized Zero-Valent Iron in the Presence of Hydrogen Peroxide and Persulfate Oxidants. Water Sci. Technol. 2015, 71, 1859–1868. [Google Scholar] [CrossRef] [PubMed]
- Tyumina, E.A.; Bazhutin, G.A.; Cartagena Gómez, A.d.P.; Ivshina, I.B. Nonsteroidal Anti-Inflammatory Drugs as Emerging Contaminants. Microbiology 2020, 89, 148–163. [Google Scholar] [CrossRef]
- Panagou, I.; Noutsopoulos, C.; Mystrioti, C.; Barka, E.; Koumaki, E.; Kalli, M.; Malamis, S.; Papassiopi, N.; Mamais, D. Assessing the Performance of Environmentally Friendly-Produced Zerovalent Iron Nanoparticles to Remove Pharmaceuticals from Water. Sustainability 2021, 13, 12708. [Google Scholar] [CrossRef]
- Tran, N.H.; Reinhard, M.; Gin, K.Y.-H. Occurrence and Fate of Emerging Contaminants in Municipal Wastewater Treatment Plants from Different Geographical Regions-a Review. Water Res. 2018, 133, 182–207. [Google Scholar] [CrossRef] [PubMed]
- Flint, S.; Markle, T.; Thompson, S.; Wallace, E. Bisphenol A Exposure, Effects, and Policy: A Wildlife Perspective. J. Environ. Manag. 2012, 104, 19–34. [Google Scholar] [CrossRef]
- Gao, F.; Li, Y.; Xiang, B. Degradation of Bisphenol A through Transition Metals Activating Persulfate Process. Ecotoxicol. Environ. Saf. 2018, 158, 239–247. [Google Scholar] [CrossRef]
- Wu, J.; Wang, B.; Cagnetta, G.; Huang, J.; Wang, Y.; Deng, S.; Yu, G. Nanoscale Zero Valent Iron-Activated Persulfate Coupled with Fenton Oxidation Process for Typical Pharmaceuticals and Personal Care Products Degradation. Sep. Purif. Technol. 2020, 239, 116534. [Google Scholar] [CrossRef]
- Gao, Y.; Gao, N.; Wang, W.; Kang, S.; Xu, J.; Xiang, H.; Yin, D. Ultrasound-Assisted Heterogeneous Activation of Persulfate by Nano Zero-Valent Iron (NZVI) for the Propranolol Degradation in Water. Ultrason. Sonochem. 2018, 49, 33–40. [Google Scholar] [CrossRef]
- Gao, J.; Han, D.; Xu, Y.; Liu, Y.; Shang, J. Persulfate Activation by Sulfide-Modified Nanoscale Iron Supported by Biochar (S-NZVI/BC) for Degradation of Ciprofloxacin. Sep. Purif. Technol. 2020, 235, 116202. [Google Scholar] [CrossRef]
- Samaras, V.G.; Thomaidis, N.S.; Stasinakis, A.S.; Lekkas, T.D. An Analytical Method for the Simultaneous Trace Determination of Acidic Pharmaceuticals and Phenolic Endocrine Disrupting Chemicals in Wastewater and Sewage Sludge by Gas Chromatography-Mass Spectrometry. Anal. Bioanal. Chem. 2011, 399, 2549–2561. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Afshinb, S.; Poureshgh, Y.; Azari, A.; Rashtbari, Y.; Feizizadeh, A.; Hamzezadeh, A.; Fazlzadeh, M. Green Preparation of Activated Carbon from Pomegranate Peel Coated with Zero-Valent Iron Nanoparticles (NZVI) and Isotherm and Kinetic Studies of Amoxicillin Removal in Water. Environ. Sci. Pollut. Res. 2020, 27, 36732–36743. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, M.H.; Karri, R.R.; Alimohammadi, M.; Nazmara, S.; Zarei, A.; Saeedi, Z. Insights into Endocrine-Disrupting Bisphenol-A Adsorption from Pharmaceutical Effluent by Chitosan Immobilized Nanoscale Zero-Valent Iron Nanoparticles. J. Mol. Liq. 2020, 311, 113317. [Google Scholar] [CrossRef]
- Soares, S.F.; Trindade, T.; Daniel-da-Silva, A.L. Enhanced Removal of Non-Steroidal Inflammatory Drugs from Water by Quaternary Chitosan-Based Magnetic Nanosorbents. Coatings 2021, 11, 964. [Google Scholar] [CrossRef]
- Senin, R.; Ion, I.; Ion, A. A Sorption Study of Bisphenol A in AqueousSolutions on Pristine and Oxidized Multi-WalledCarbon Nanotubes. Pol. J. Environ. Stud. 2018, 27, 2245–2257. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gao, B.; Li, Y.; Creamer, A.E.; He, F. Adsorptive Removal of Arsenate from Aqueous Solutions by Biochar Supported Zero-Valent Iron Nanocomposite: Batch and Continuous Flow Tests. J. Hazard. Mater. 2017, 322, 172–181. [Google Scholar] [CrossRef]
- Eljamal, O. Continuous-Flow of Nanoscale Zero Valent Iron Based System for Phosphorus Removal. 2021. [Google Scholar]
- Rezaei, F.; Vione, D. Effect of PH on Zero Valent Iron Performance in Heterogeneous Fenton and Fenton-Like Processes: A Review. Molecules 2018, 23, 3127. [Google Scholar] [CrossRef]
- Liu, X.; Cao, Z.; Yuan, Z.; Zhang, J.; Guo, X.; Yang, Y.; He, F.; Zhao, Y.; Xu, J. Insight into the Kinetics and Mechanism of Removal of Aqueous Chlorinated Nitroaromatic Antibiotic Chloramphenicol by Nanoscale Zero-Valent Iron. Chem. Eng. J. 2018, 334, 508–518. [Google Scholar] [CrossRef]
- Bao, T.; Damtie, M.M.; Hosseinzadeh, A.; Wei, W.; Jin, J.; Phong Vo, H.N.; Ye, J.S.; Liu, Y.; Wang, X.F.; Yu, Z.M.; et al. Bentonite-Supported Nano Zero-Valent Iron Composite as a Green Catalyst for Bisphenol A Degradation: Preparation, Performance, and Mechanism of Action. J. Environ. Manag. 2020, 260, 110105. [Google Scholar] [CrossRef]
- Gao, J.-F.; Wu, Z.-L.; Duan, W.-J.; Zhang, W.-Z. Simultaneous Adsorption and Degradation of Triclosan by Ginkgo Biloba L. Stabilized Fe/Co Bimetallic Nanoparticles. Sci. Total Environ. 2019, 662, 978–989. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Zhu, N.; Huang, X.; Kang, N.; Wu, P.; Dang, Z. Efficient Degradation of Sodium Diclofenac via Heterogeneous Fenton Reaction Boosted by Pd/Fe@Fe3O4 Nanoparticles Derived from Bio-Recovered Palladium. J. Environ. Manag. 2020, 260, 110072. [Google Scholar] [CrossRef] [PubMed]
- Ziylan, A.; Koltypin, Y.; Gedanken, A.; Ince, N.H. More on Sonolytic and Sonocatalytic Decomposition of Diclofenac Using Zero-Valent Iron. Ultrason. Sonochem. 2013, 20, 580–586. [Google Scholar] [CrossRef]
- Daneshkhah, M.; Hossaini, H.; Malakootian, M. Removal of Metoprolol from Water by Sepiolite-Supported Nanoscale Zero-Valent Iron. J. Environ. Chem. Eng. 2017, 5, 3490–3499. [Google Scholar] [CrossRef]
- Mondal, S.K.; Saha, A.K.; Sinha, A. Removal of Ciprofloxacin Using Modified Advanced Oxidation Processes: Kinetics, Pathways and Process Optimization. J. Clean. Prod. 2018, 171, 1203–1214. [Google Scholar] [CrossRef]
- Dogan, M.; Ozturk, T.; Olmez-Hanci, T.; Arslan-Alaton, I. Persulfate and Hydrogen Peroxide-Activated Degradation of Bisphenol A with Nano-Scale Zero-Valent Iron and Aluminum. J. Adv. Oxid. Technol. 2016, 19, 266–275. [Google Scholar] [CrossRef]
- Choi, J.; Cui, M.; Lee, Y.; Kim, J.; Son, Y.; Khim, J. Hydrodynamic Cavitation and Activated Persulfate Oxidation for Degradation of Bisphenol A: Kinetics and Mechanism. Chem. Eng. J. 2018, 338, 323–332. [Google Scholar] [CrossRef]
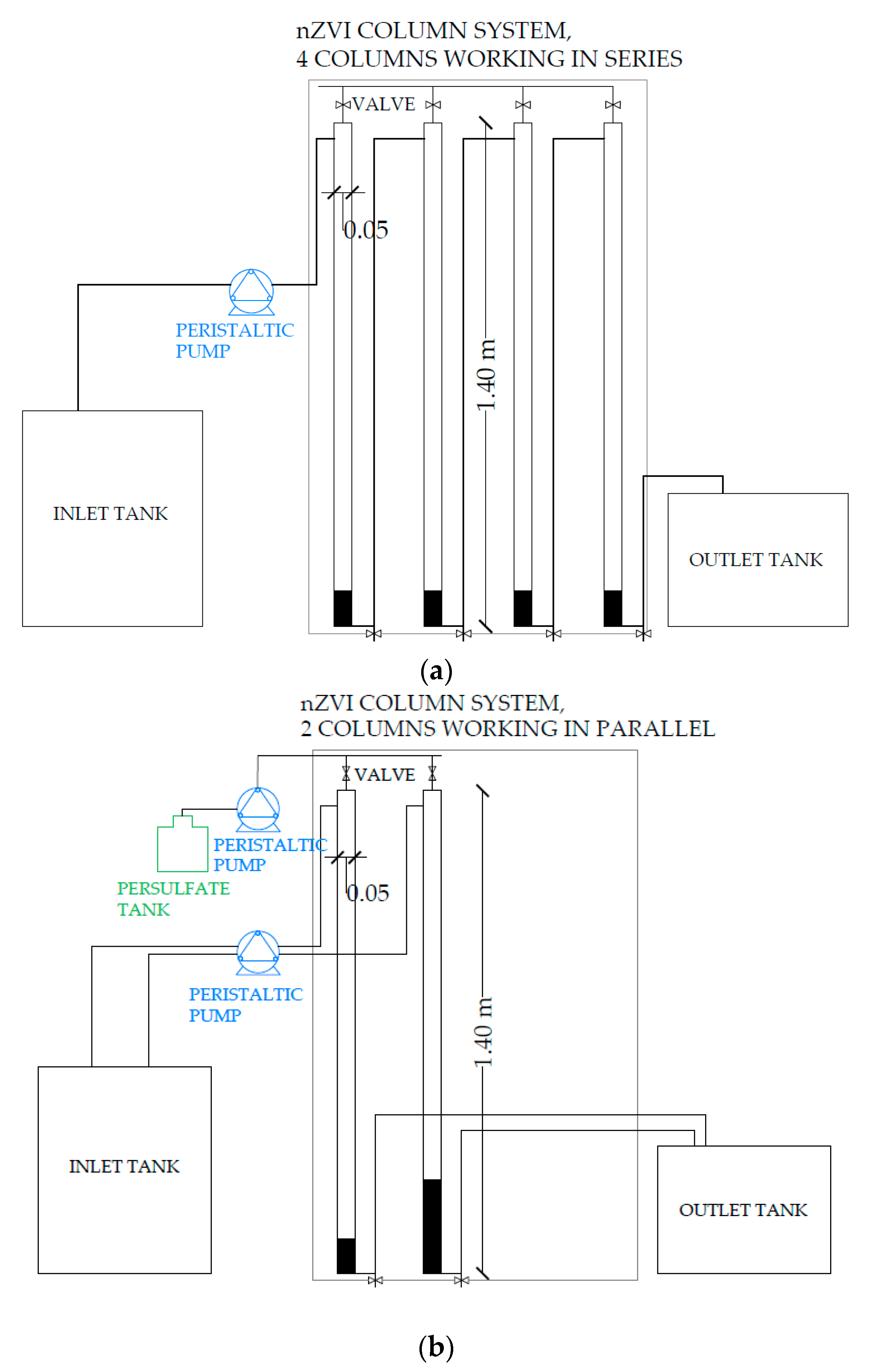
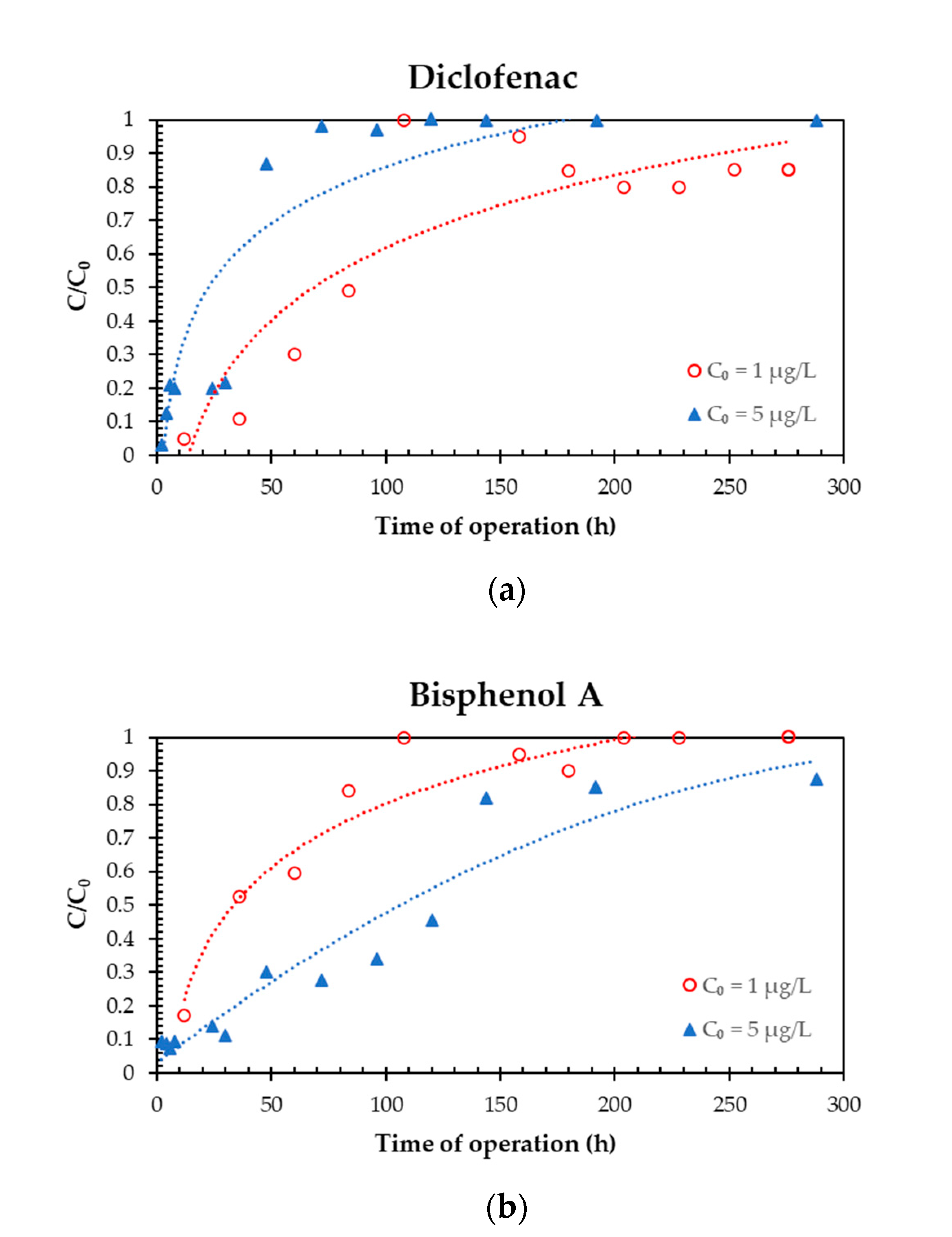
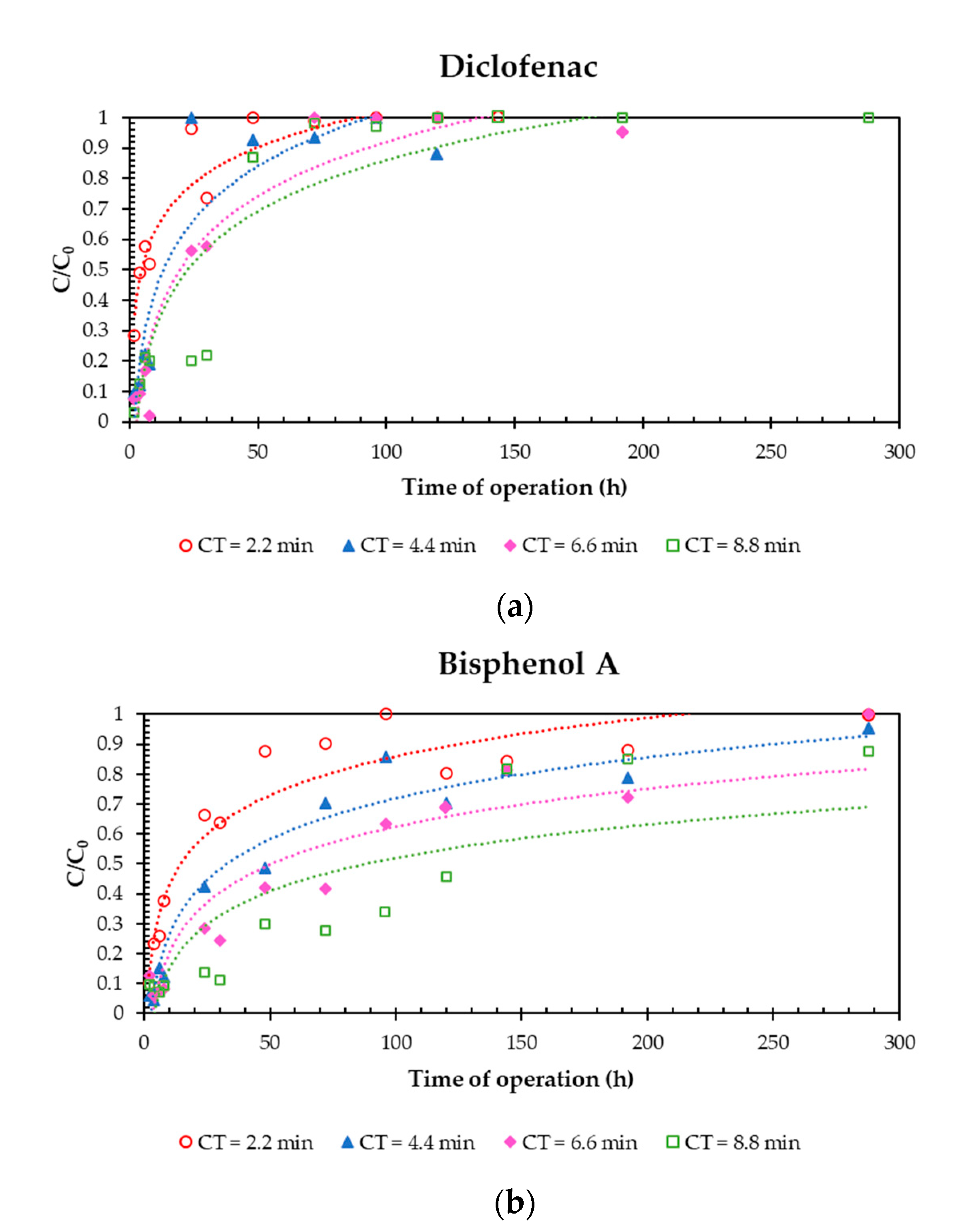

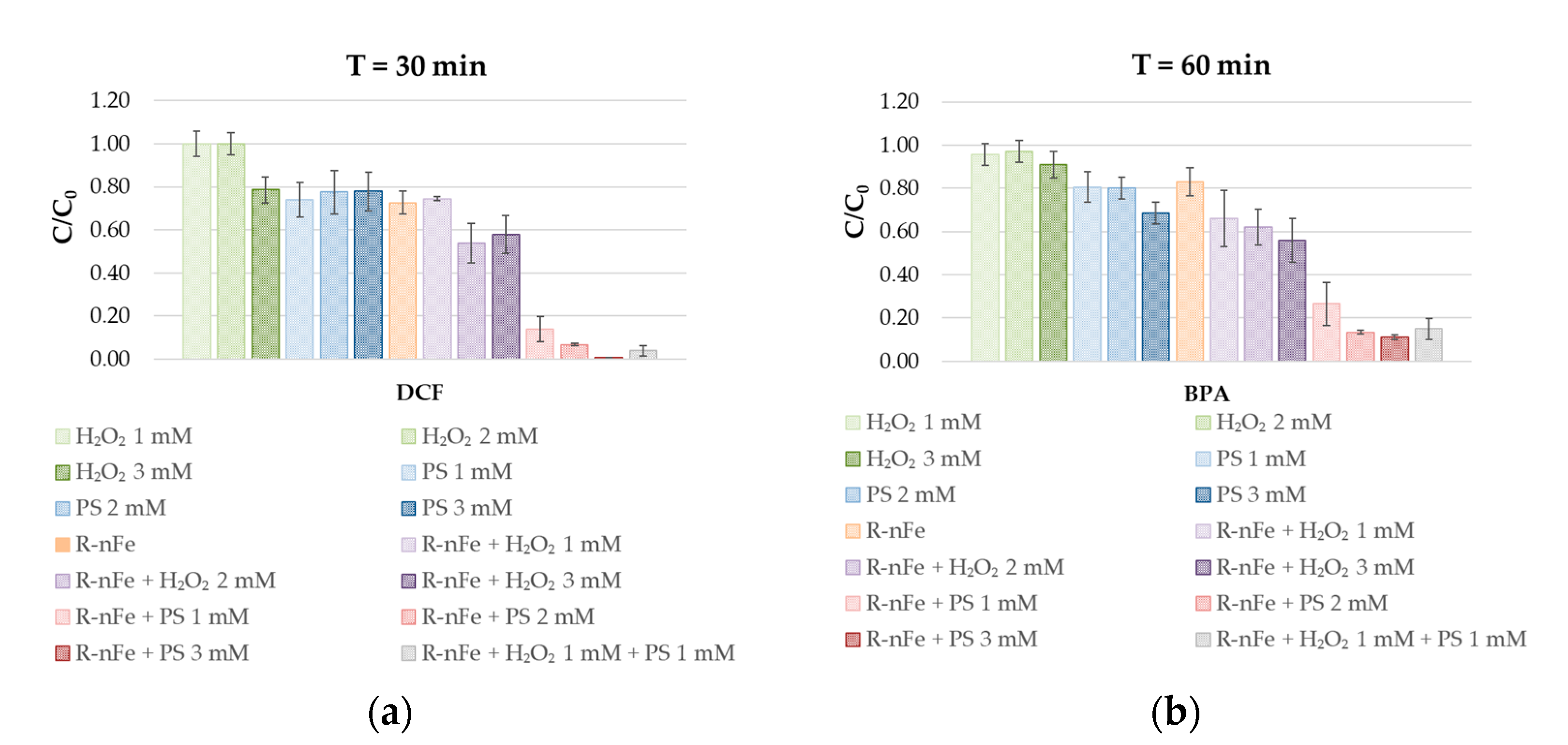
| Parameter | Tertiary Effluent (Average ± Stdev) | Units |
|---|---|---|
| pH | 7.30 ± 0.1 | |
| Alkalinity | 160 ± 23 | mg CaCO3/L |
| Electric conductivity | 1354 ± 50 | μS/cm |
| Total organic carbon (TOC) | 12 ± 3 | mg/L |
| Total chemical oxygen demand (CODt) | 32.4 ± 1.20 | mg/L |
| Total suspended solids (TSS) | 0.64 ± 0.17 | mg/L |
| Nitrate | 7.13 ± 0.11 | mg/L |
| Sulfate anions | 94 ± 11 | mg/L |
| Bisphenol-A (BPA) | 192 ± 51 | ng/L |
| Ibuprofen (IBU) | <LOQ 1 | ng/L |
| Naproxen (NPX) | 155 ± 4 | ng/L |
| Diclofenac (DCF) | 585 ± 60 | ng/L |
| Ketoprofen (KTP) | 457 ± 50 | ng/L |
| Experimental Conditions | Batch Test Name | CECs | R-nFe | PS | H2O2 | pH |
|---|---|---|---|---|---|---|
| μg/L | g/L | mM | mM | |||
| Effect of R-nFe | R-nFe | 10 | 15 | - | - | 3–3.5 |
| Effect of H2O2 | H2O2 1mM | 10 | - | - | 1 | 3–3.5 |
| H2O2 2mM | - | - | 2 | |||
| H2O2 3mM | - | - | 3 | |||
| R-nFe + H2O2 1mM | 15 | - | 1 | |||
| R-nFe + H2O2 2mM | 15 | - | 2 | |||
| R-nFe + H2O2 3mM | 15 | - | 3 | |||
| Effect of PS | PS 1mM | 10 | - | 1 | - | 3–3.5 |
| PS 2mM | - | 2 | - | |||
| PS 3mM | - | 3 | - | |||
| R-nFe + PS 1mM | 15 | 1 | - | |||
| R-nFe + PS 2mM | 15 | 2 | - | |||
| R-nFe + PS 3mM | 15 | 3 | - | |||
| Effect of R-nFe, H2O2, and PS | R-nFe + H2O2 1mM + PS 1mM | 10 | 15 | 1 | 1 | 3–3.5 |
| Column Experiment | CECs | Contact Time | PS | pH |
|---|---|---|---|---|
| μg/L | Minutes | mM | ||
| Effect of CECs’ initial concentration | 1 and 5 | 8.8 | - | 7 |
| Effect of contact time | 5 | 2.2, 4.4, 6.6, and 8.8 | - | 7 |
| Effect of pH adjustment and contact time | 5 | 2.2 and 4.4 | - | 3.5 and 7 |
| Effect of oxidative reagent dose | 5 | 2.2 | 1 and 5 | 3.5 |
| Target Compound | Mass of Pollutant Removed per Mass of R-nFe (μg/g) at the End of Each Experiment (CT = 8.8 min) | |
|---|---|---|
| C0 = 1 μg/L | C0 = 5 μg/L | |
| IBU | 0.05 | 0.19 |
| NPX | 0.08 | 0.27 |
| KFN | 0.08 | 0.18 |
| DCF | 0.11 | 0.19 |
| BPA | 0.05 | 0.74 |
| Target Compound | Mass of Pollutant Removed per Mass of R-nFe (μg/g) at the End of Each Experiment (C0 = 5 μg/L) | |||
|---|---|---|---|---|
| CT = 2.2 min | CT = 4.4 min | CT = 6.6 min | CT = 8.8 min | |
| IBU | 0.90 | 0.24 | 0.29 | 0.19 |
| NPX | 0.49 | 0.51 | 0.34 | 0.27 |
| KFN | 0.27 | 0.28 | 0.39 | 0.18 |
| DCF | 0.18 | 0.18 | 0.18 | 0.19 |
| BPA | 0.89 | 0.89 | 0.74 | 0.74 |
| Target Compound | Mass of Pollutant Removed per Mass of R-nFe (μg/g) at the End of Each Experiment (C0 = 5 μg/L) | |||
|---|---|---|---|---|
| CT = 2.2 min, pH = 7 | CT = 2.2 min, pH = 3.5 | CT = 4.4 min, pH = 7 | CT = 4.4 min, pH = 3.5 | |
| IBU | 0.90 | 2.04 | 0.24 | 1.39 |
| NPX | 0.49 | 3.08 | 0.51 | 2.38 |
| KFN | 0.27 | 2.78 | 0.28 | 2.15 |
| DCF | 0.18 | 3.38 | 0.18 | 2.39 |
| BPA | 0.89 | 1.21 | 0.89 | 1.39 |
| Target Compound | Mass of Pollutant Removed per Mass of R-nFe (μg/g) at the End of Each Experiment (C0 = 5 μg/L, CT = 2.2 min) | |
|---|---|---|
| PS = 1 mM | PS = 5 mM | |
| IBU | 3.87 | 6.74 |
| NPX | 7.14 | 7.32 |
| KFN | 2.72 | 5.02 |
| DCF | 6.70 | 7.56 |
| BPA | 6.01 | 6.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barka, E.; Noutsopoulos, C.; Galani, A.; Panagou, I.; Kalli, M.; Koumaki, E.; Malamis, S.; Mamais, D. Removal of Contaminants of Emerging Concern from Wastewater Using an Integrated Column System Containing Zero Valent Iron Nanoparticles. Water 2023, 15, 598. https://doi.org/10.3390/w15030598
Barka E, Noutsopoulos C, Galani A, Panagou I, Kalli M, Koumaki E, Malamis S, Mamais D. Removal of Contaminants of Emerging Concern from Wastewater Using an Integrated Column System Containing Zero Valent Iron Nanoparticles. Water. 2023; 15(3):598. https://doi.org/10.3390/w15030598
Chicago/Turabian StyleBarka, Evridiki, Constantinos Noutsopoulos, Andriani Galani, Iliana Panagou, Maria Kalli, Elena Koumaki, Simos Malamis, and Daniel Mamais. 2023. "Removal of Contaminants of Emerging Concern from Wastewater Using an Integrated Column System Containing Zero Valent Iron Nanoparticles" Water 15, no. 3: 598. https://doi.org/10.3390/w15030598










