Influence of the Combined Magnetic Field and High Dilution Technology on the Intrinsic Emission of Aqueous Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Experimental Setup for Magnetic Field Treatment
2.3. Non-Contact Effect
2.4. Emission Spectra Recording
2.5. Data Analysis and Statistics
- (1)
- For each sample, a normality assumption, a mean, and a standard deviation of the distribution of repeated measurements for the given wavelength were assessed.
- (2)
- The assumption of the repeated measurements being distributed normally was not violated. This allows performing the generation of new spectra points at each wavelength, using the normal distribution with parameters obtained at the first step. By generating a curve point-by-point, a whole synthetic spectrum was obtained for each sample.
- (3)
- The generated batch of samples’ spectra were also processed with PCA and clustered by the K-means as the original spectra. For each pair of samples, their co-occurrence in the same cluster was recorded.
- (4)
- Steps 2 and 3 were repeated 1000 times.
- (5)
- For each pair of samples, the ratio of co-occurrence in one cluster [49,51] among the 1000 simulations was considered as a bootstrapped p-value. The pairwise comparison p-values were adjusted using Holm’s procedure for multiplicity of comparisons. Differences were considered statistically significant at p < 0.05.
3. Results
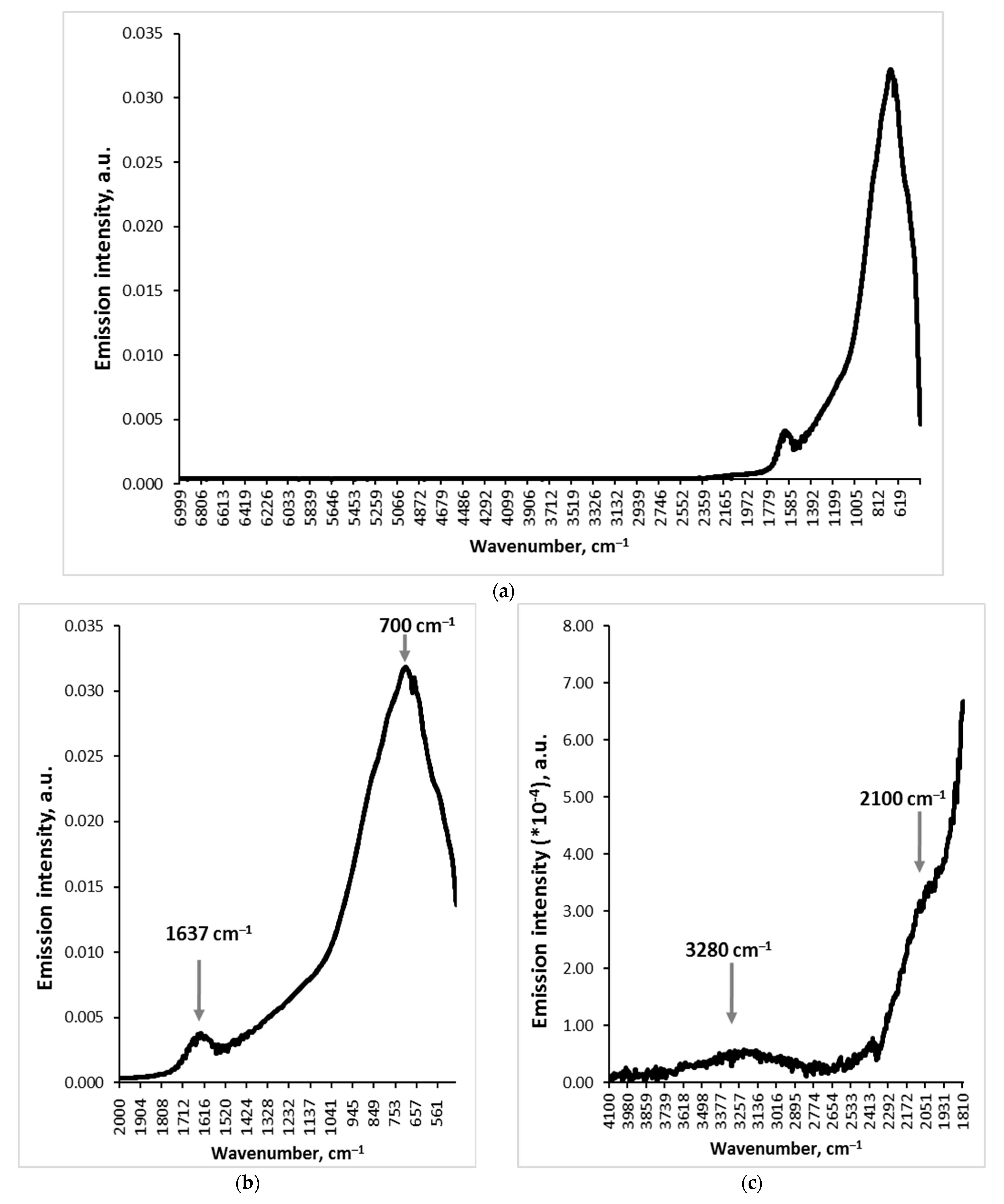
3.1. Emission Spectrum of Water in the Infrared Range
3.2. Impact of a Magnetic Field on Samples
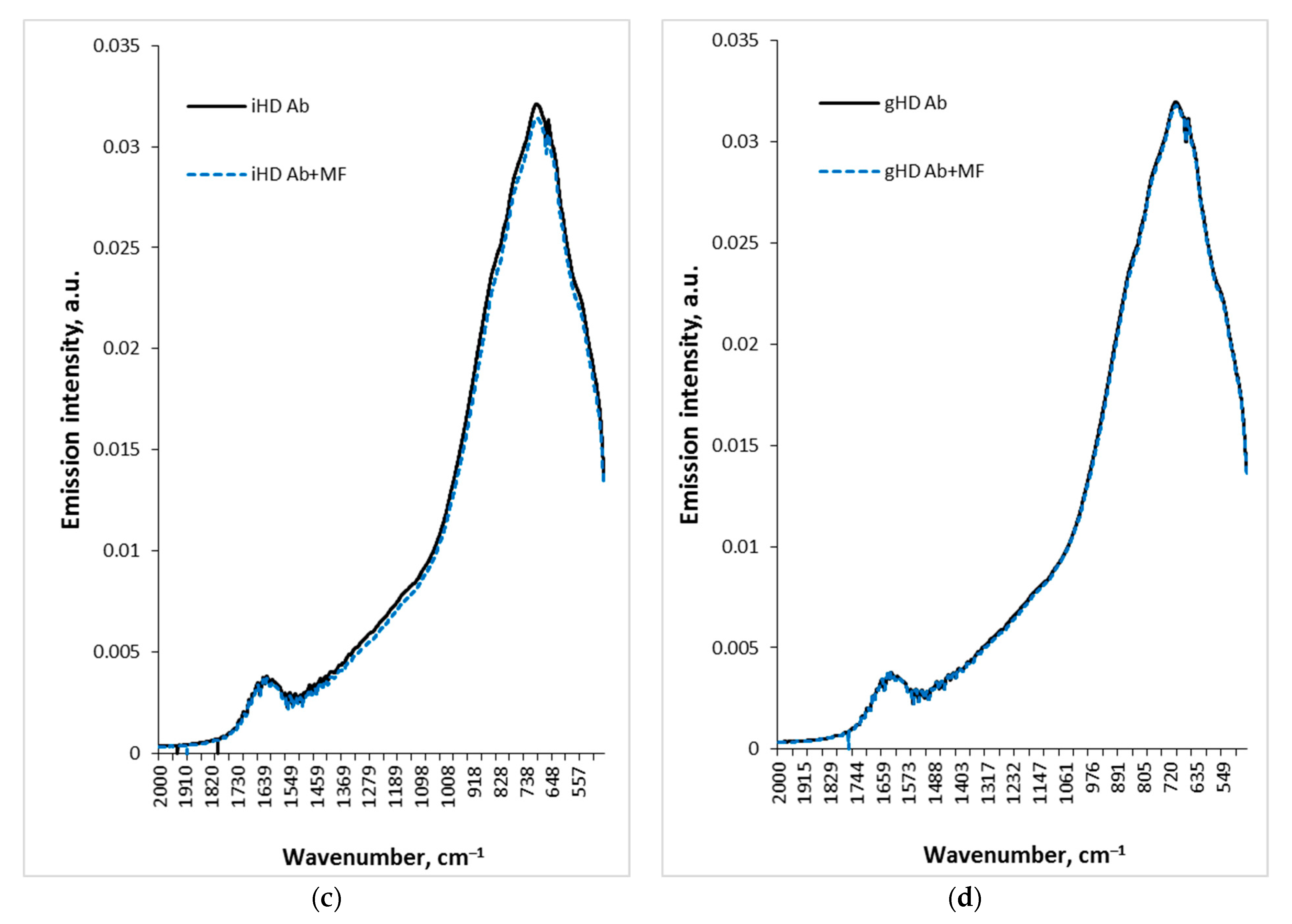
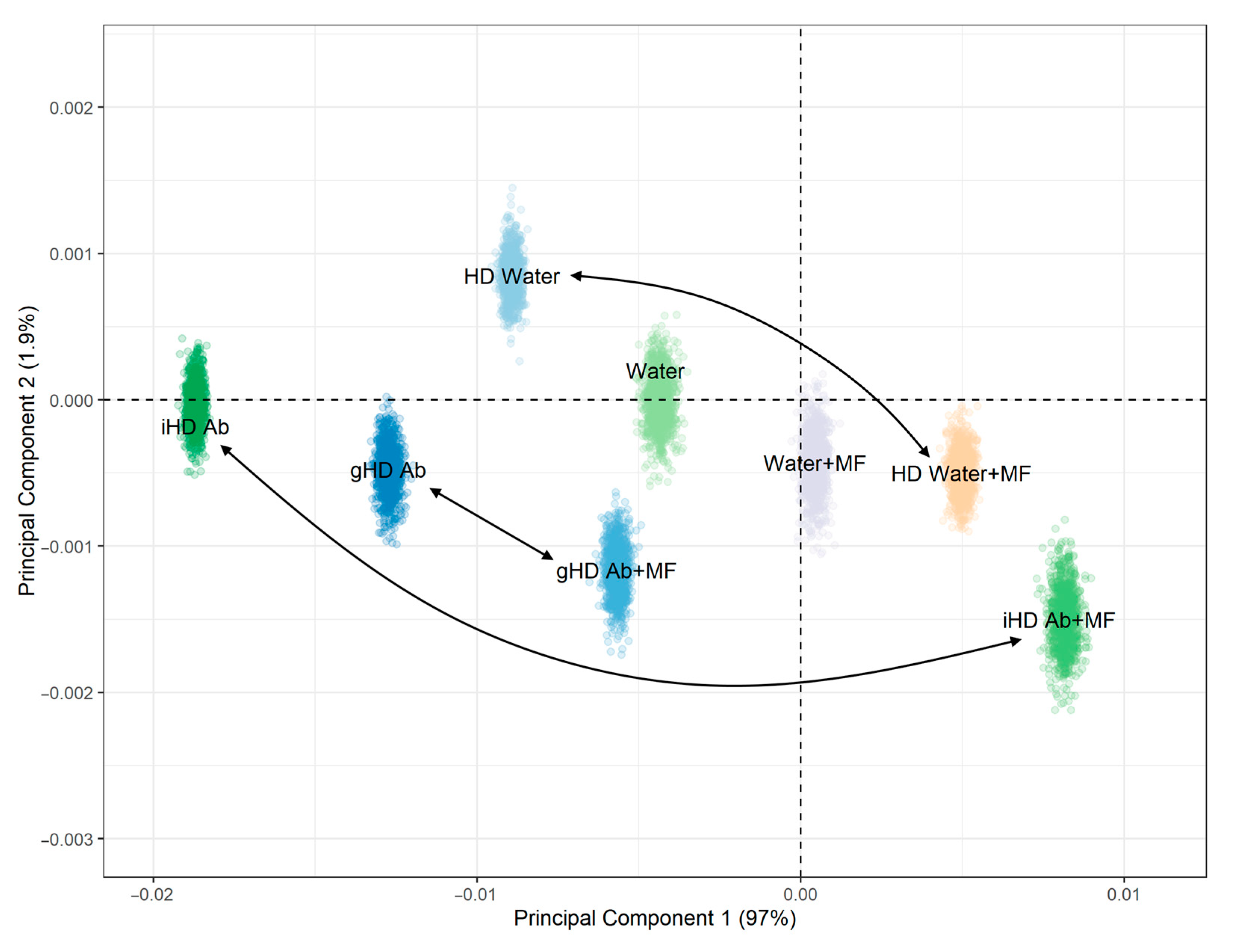
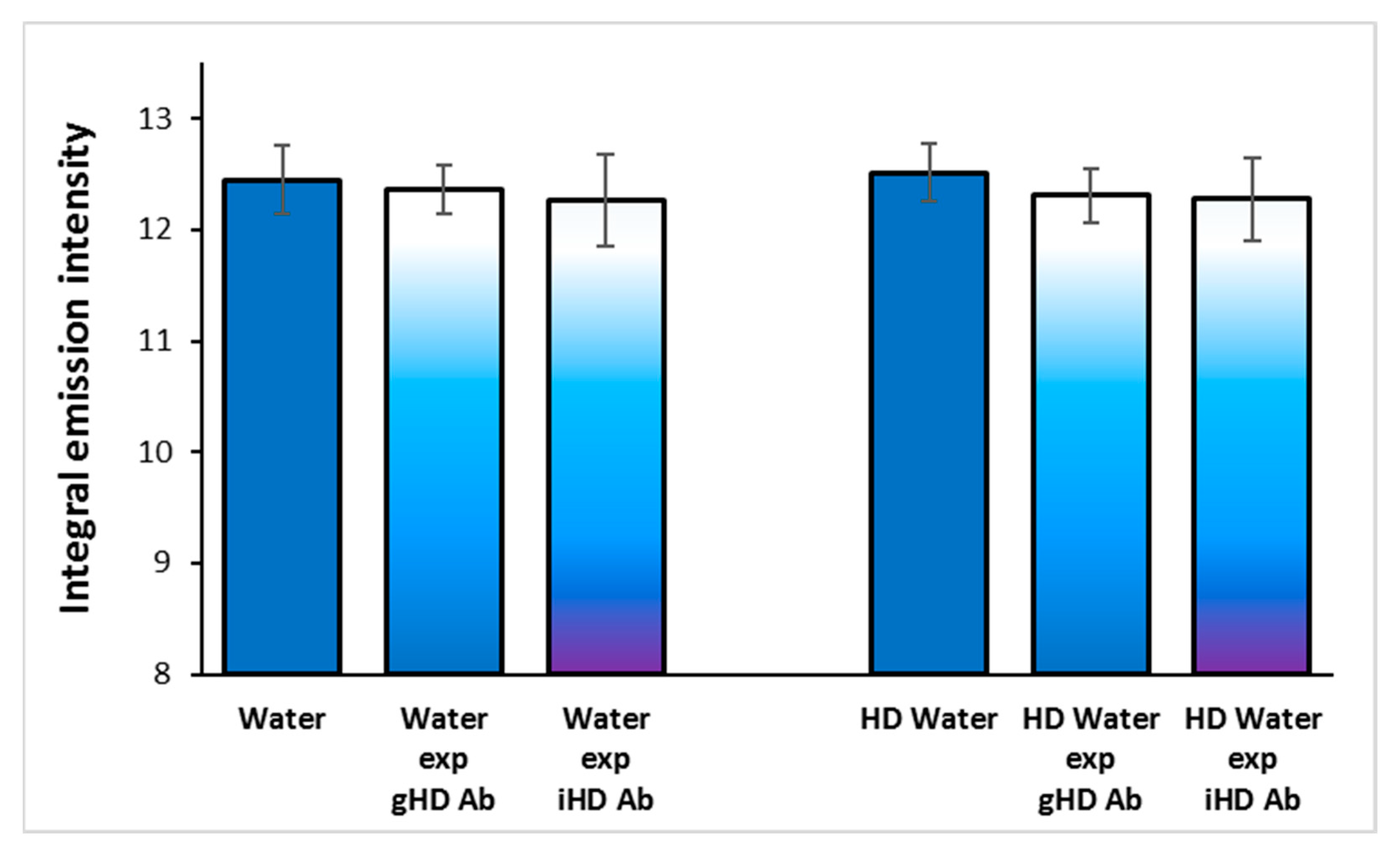
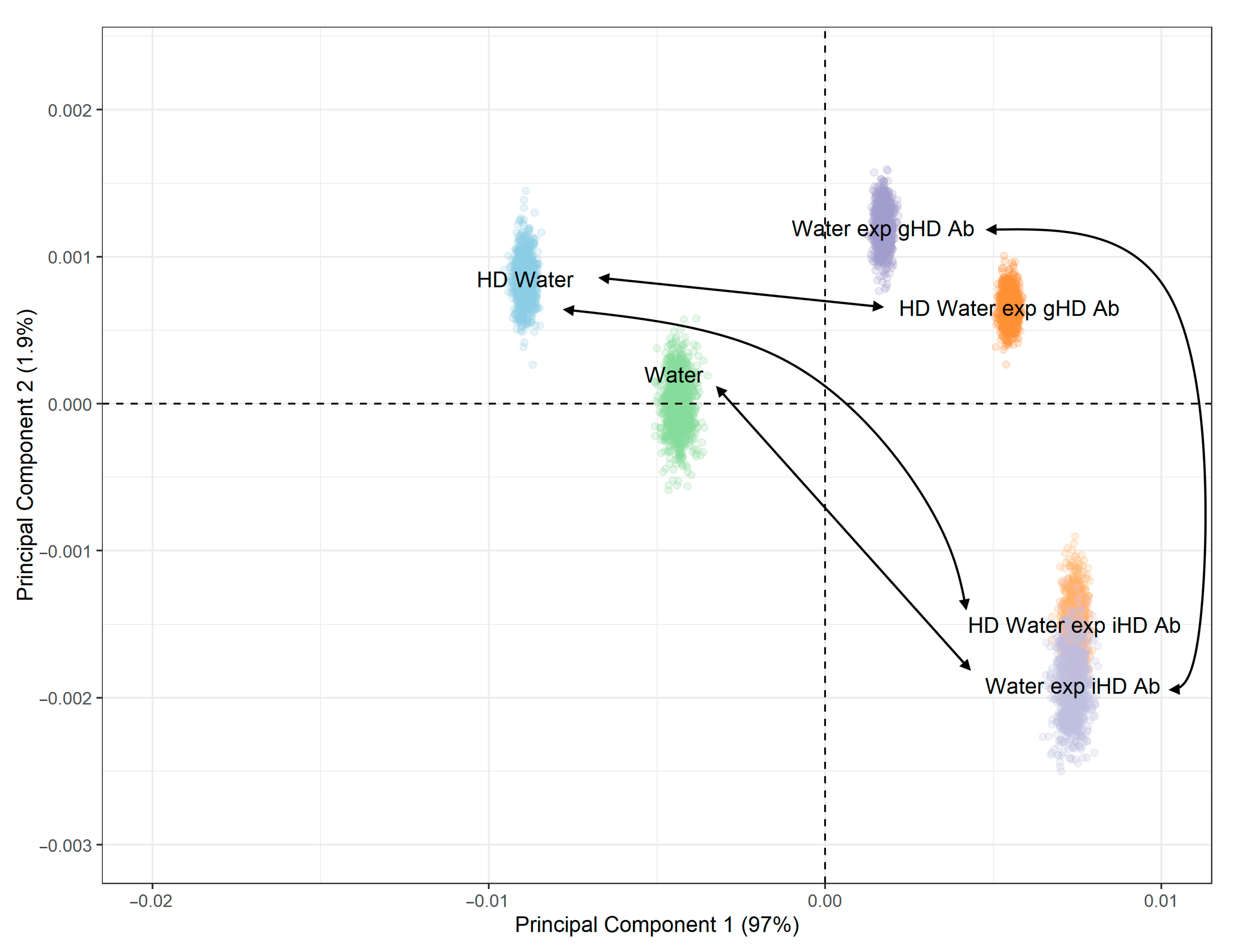
3.3. Non-Contact Effect of iHD Ab and gHD Ab on Water Samples and HD Water
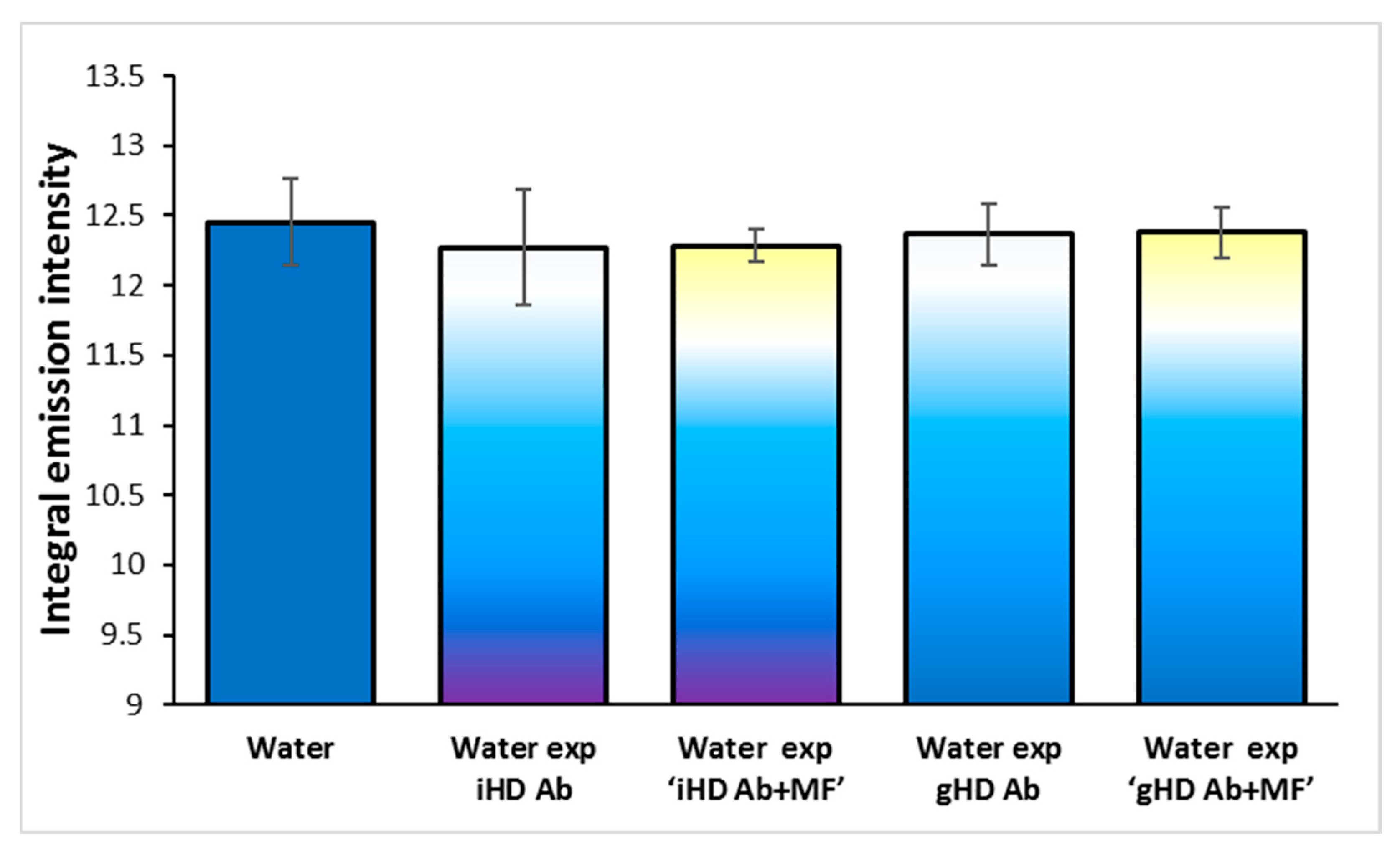
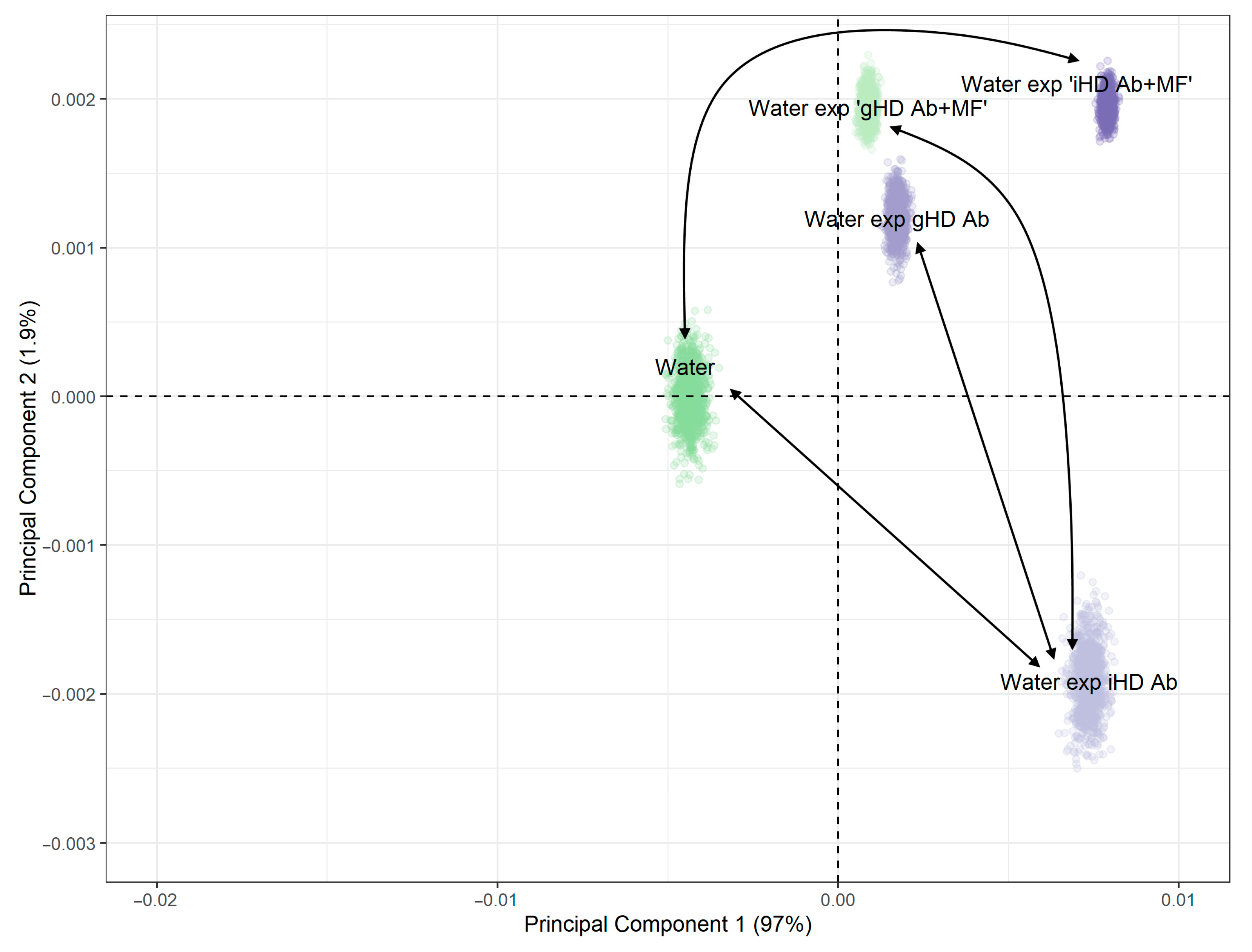
3.4. Non-Contact Effect of HD Ab Treated with a Magnetic Field on Water
4. Discussion
5. Conclusions
- An MF with the cyclotron resonance frequency of H9O4+ changes the emission properties of highly diluted aqueous solutions.
- Exposure to an MF and non-contact exposure to HD solution effectors similarly change the emission properties of solution sensors.
- The manifestation of the non-contact effect of HD solutions depends on the technology of preparation of both the effector and sensor solutions. To implement the non-contact effect, at least one of the HD solutions must be prepared using intense shaking.
- Pre-treatment of HD solution effectors with an MF does not affect the presence and magnitude of their non-contact effect on the IR-emission properties of water.
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nilsson, A.; Pettersson, L.G. The structural origin of anomalous properties of liquid water. Nat. Commun. 2015, 6, 8998. [Google Scholar] [CrossRef]
- Zhao, L.; Ma, K.; Yang, Z. Changes of water hydrogen bond network with different externalities. Int. J. Mol. Sci. 2015, 16, 8454–8489. [Google Scholar] [CrossRef]
- Fesenko, E.E.; Gluvstein, A.Y. Changes in the state of water, induced by radiofrequency electromagnetic fields. FEBS Lett. 1995, 367, 53–55. [Google Scholar] [CrossRef]
- Chibowski, E.; Szcześ, A.; Hołysz, L. Influence of magnetic field on evaporation rate and surface tension of water. Colloids Interfaces 2018, 2, 68. [Google Scholar] [CrossRef]
- Ghaani, M.R.; Kusalik, P.G.; English, N.J. Massive generation of metastable bulk nanobubbles in water by external electric fields. Sci. Adv. 2020, 6, eaaz0094. [Google Scholar] [CrossRef]
- Sronsri, C.; U-yen, K.; Sittipol, W. Analyses of vibrational spectroscopy, thermal property and salt solubility of magnetized water. J. Mol. Liq. 2021, 323, 114613. [Google Scholar] [CrossRef]
- Wu, T.; Brant, J.A. Magnetic field effects on pH and electrical conductivity: Implications for water and wastewater treatment. Environ. Eng. Sci. 2020, 37, 717–727. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, H.; Li, Z. Effect of magnetic field on the physical properties of water. Results Phys. 2018, 8, 262–267. [Google Scholar] [CrossRef]
- Sankin, G.N.; Teslenko, V.S. Slow variation of the electrical conductivity of water under weak permanent magnetic fields. Tech. Phys. 2000, 45, 351–352. [Google Scholar]
- Shcherbakov, I.A.; Baimler, I.V.; Gudkov, S.V.; Lyakhov, G.A.; Mikhailova, G.N.; Pustovoy, V.I.; Sarimov, R.M.; Simakin, A.V.; Troitsky, A.V. Influence of a constant magnetic field on some properties of water solutions. Dokl. Phys. 2020, 65, 273–275. [Google Scholar]
- Colic, M.; Morse, D. Mechanism of the long-term effects of electromagnetic radiation on solutions and suspended colloids. Langmuir 1998, 14, 783–787. [Google Scholar] [CrossRef]
- Dosar, A.A.; Srivastava, V. Improvement of hard water characteristics and scale formation under the effect of pulsating electromagnetic field. In Advances in Construction Materials and Sustainable Environment; Springer: Singapore, 2022; pp. 259–269. [Google Scholar]
- Liboff, A.R. The charge-to-mass icr signature in weak elf bioelectromagnetic effects. In Advances in Electromagnetic Fields in Living Systems; Springer: Boston, MA, USA, 2005; Volume 4, pp. 189–218. [Google Scholar]
- Halle, B. On the cyclotron resonance mechanism for magnetic field effects on transmembrane ion conductivity. Bioelectromagnetics 1988, 9, 381–385. [Google Scholar] [CrossRef]
- D’Emilia, E.; Ledda, M.; Foletti, A.; Lisi, A.; Giuliani, L.; Grimaldi, S.; Liboff, A.R. Weak-field H3O+ ion cyclotron resonance alters water refractive index. Electromagn. Biol. Med. 2017, 36, 55–62. [Google Scholar] [CrossRef]
- Colic, M.; Morse, D. The elusive mechanism of the magnetic ‘memory’ of water. Colloids Surf. A Physicochem. Eng. Asp. 1999, 154, 167–174. [Google Scholar] [CrossRef]
- Zhadin, M.N.; Novikov, V.V.; Barnes, F.S.; Pergola, N.F. Combined action of static and alternating magnetic fields on ionic current in aqueous glutamic acid solution. Bioelectromagnetics 1998, 19, 41–45. [Google Scholar] [CrossRef]
- Novikov, V.V.; Karnaukhov, A.V. Mechanism of action of weak electromagnetic field on ionic currents in aqueous solutions of amino acids. Bioelectromagnetics 1997, 18, 25–27. [Google Scholar] [CrossRef]
- Fesenko, E.E.; Geletyuk, V.I.; Kazachenko, V.N.; Chemeris, N.K. Preliminary microwave irradiation of water solutions changes their channel-modifying activity. FEBS Lett. 1995, 366, 49–52. [Google Scholar] [CrossRef]
- Grundler, W.; Kaiser, F.; Keilmann, F.; Walleczek, J. Mechanisms of electromagnetic interaction with cellular systems. Naturwissenschaften 1992, 79, 551–559. [Google Scholar] [CrossRef]
- Walleczek, J. Electromagnetic field effects on cells of the immune system: The role of calcium signaling. FASEB J. 1992, 6, 3177–3185. [Google Scholar] [CrossRef]
- Zieliński, M.; Zielińska, M.; Cydzik-Kwiatkowska, A.; Rusanowska, P.; Dębowski, M. Effect of static magnetic field on microbial community during anaerobic digestion. Bioresour. Technol. 2021, 323, 124600. [Google Scholar] [CrossRef]
- Yadollahpour, A.; Rashidi, S.; Fatemeh, K. Applications of magnetic water technology in farming and agriculture development: A review of recent advances. Curr. World Environ. 2014, 9, 695–703. [Google Scholar]
- Al-Hilali, A.H. Effect of magnetically treated water on physiological and biochemical blood parameters of japanese quail. Int. J. Poult. Sci. 2018, 17, 78–84. [Google Scholar] [CrossRef]
- Carbonell, M.V.; Martínez, E.; Díaz, J.E.; Amaya, J.M.; Flórez, M. Influence of magnetically treated water on germination of signalgrass seeds. Seed Sci. Technol. 2004, 32, 617–619. [Google Scholar] [CrossRef]
- Hozayn, M.; Elaoud, A.; El-Monem, A.A.A.; Salah, N.B. Effect of magnetic field on growth and yield of barley treated with different salinity levels. Arab. J. Geosci. 2021, 14, 701. [Google Scholar] [CrossRef]
- Elhindi, K.M.; Al-Mana, F.A.; Algahtani, A.M.; Alotaibi, M.A. Effect of irrigation with saline magnetized water and different soil amendments on growth and flower production of calendula officinalis L. plants. Saudi J. Biol. Sci. 2020, 27, 3072–3078. [Google Scholar] [CrossRef]
- Bashasha, J.A.; El-Mugrbi, W.S.; Imryed, Y.F. Effect of magnetic treatment in improve growth of three wheat cultivars irrigated with seawater. Multidiscip. Sci. Adv. Technol. 2021, 1, 24–32. [Google Scholar]
- El Sabry, M.I.; Abdelfattah, M.H.; Abdellatif, H.A.; Aggrey, S.E.; Elnesr, S.S. Physicochemical properties of magnetic water and its effect on egg production traits in hens at late laying period. J. Anim. Plant Sci. 2021, 31, 317–321. [Google Scholar]
- Lee, H.J.; Kang, M.H. Effect of the magnetized water supplementation on blood glucose, lymphocyte DNA damage, antioxidant status, and lipid profiles in stz-induced rats. Nutr. Res. Pr. 2013, 7, 34–42. [Google Scholar] [CrossRef]
- Al-Saffar, S.F.; Amer, N.; Zaki, L.S.; Faris, A.S.; Imran, N.J.; Nadhim, Z.F.; Jaafar, R.I. Effect of magnetized water on histological structure of heart, lung and spleen of albino rats. J. Al-Nahrain Univ. 2020, 16, 152–160. [Google Scholar] [CrossRef]
- Ahmed, N.H.; El-Hamed, N.N.B.A. Impact of magnetic water treatment technology on water parameters, growth performance and blood parameters of the nile tilapia (Oreochromis niloticus). Egypt. J. Aquat. Biol. Fish. 2020, 24, 645–655. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Penkov, N.V.; Baimler, I.V.; Lyakhov, G.A.; Pustovoy, V.I.; Simakin, A.V.; Sarimov, R.M.; Scherbakov, I.A. Effect of mechanical shaking on the physicochemical properties of aqueous solutions. Int. J. Mol. Sci. 2020, 21, 8033. [Google Scholar] [CrossRef] [PubMed]
- Gudkov, S.V.; Baimler, I.V.; Uvarov, O.V.; Smirnova, V.V.; Volkov, M.Y.; Semenova, A.A.; Lisitsyn, A.B. Influence of the concentration of Fe and Cu nanoparticles on the dynamics of the size distribution of nanoparticles. Front. Phys. 2020, 8, 622551. [Google Scholar] [CrossRef]
- Ryzhkina, I.S.; Murtazina, L.I.; Kiseleva, Y.V.; Konovalov, A.I. Self-organization and physicochemical properties of aqueous solutions of the antibodies to interferon gamma at ultrahigh dilution. Dokl. Phys. Chem. 2015, 462, 110–114. [Google Scholar] [CrossRef]
- Don, E.; Farafonova, O.; Pokhil, S.; Barykina, D.; Nikiforova, M.; Shulga, D.; Borshcheva, A.; Tarasov, S.; Ermolaeva, T.; Epstein, O. Use of piezoelectric immunosensors for detection of interferon-gamma interaction with specific antibodies in the presence of released-active forms of antibodies to interferon-gamma. Sensors 2016, 16, 96. [Google Scholar] [CrossRef]
- Kardash, E.V.; Ertuzun, I.A.; Khakimova, G.R.; Kolyadin, A.N.; Tarasov, S.A.; Wagner, S.; Andriambeloson, E.; Ivashkin, V.T.; Epstein, O.I. Dose-response effect of antibodies to S100 protein and cannabinoid receptor type 1 in released-active form in the light-dark test in mice. Dose Response 2018, 16, 1559325818779752. [Google Scholar] [CrossRef]
- Castagne, V.; Lemaire, M.; Kheyfets, I.; Dugina, J.L.; Sergeeva, S.A.; Epstein, O.I. Antibodies to S100 proteins have anxiolytic-like activity at ultra-low doses in the adult rat. J. Pharm. Pharmacol. 2008, 60, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Pschenitza, M.; Gavrilova, E.S.; Tarasov, S.; Knopp, D.; Niessner, R.; Epstein, O.I. Application of a heterogeneous immunoassay for the quality control testing of release-active forms of diclofenac. Int. Immunopharmacol. 2014, 21, 225–230. [Google Scholar] [CrossRef]
- Spitsin, A.I.; Bush, A.A.; Kamentsev, K.E. Piezoelectric and dielectric properties of Bi3TiNbO9 prepared by hot pressing from powders activated using the serial dilution method. Sci. Rep. 2020, 10, 22198. [Google Scholar] [CrossRef]
- Kamentsev, K.E.; Bush, A.A. The superconducting properties of YBa2Cu3Oy ceramics fabricated using ultrahigh dilution technology. Ceram. Int. 2022, 48, 32196–32204. [Google Scholar] [CrossRef]
- Penkov, N. Antibodies processed using high dilution technology distantly change structural properties of IFNγ aqueous solution. Pharmaceutics 2021, 13, 1864. [Google Scholar] [CrossRef]
- Penkov, N.; Penkova, N. Analysis of emission infrared spectra of protein solutions in low concentrations. Front. Phys. 2020, 8, 624779. [Google Scholar] [CrossRef]
- Novikov, V.V.; Yablokova, E.V.; Fesenko, E.E. The role of water in the effect of weak combined magnetic fields on production of reactive oxygen species (ROS) by neutrophils. Appl. Sci. 2020, 10, 3326. [Google Scholar] [CrossRef]
- Tarasov, S.A.; Gorbunov, E.A.; Don, E.S.; Emelyanova, A.G.; Kovalchuk, A.L.; Yanamala, N.; Schleker, A.S.S.; Klein-Seetharaman, J.; Groenestein, R.; Tafani, J.P.; et al. Insights into the mechanism of action of highly diluted biologics. J. Immunol. 2020, 205, 1345–1354. [Google Scholar] [CrossRef]
- Penkov, N.V.; Penkova, N.A. Infrared emission spectroscopy for investigation of biological molecules in aqueous solutions. Phys. Wave Phenom. 2021, 29, 164–168. [Google Scholar] [CrossRef]
- Penkov, N.; Penkova, N. Measurement of the emission spectra of protein solutions in the infrared range. Description of the method and testing using solution of human interferon gamma as an example. Front. Phys. 2020, 8, 615917. [Google Scholar] [CrossRef]
- Qin, B.; Li, Z.; Luo, Z.; Li, Y.; Zhang, H. Terahertz time-domain spectroscopy combined with PCA-CFSFDP applied for pesticide detection. Opt. Quantum Electron. 2017, 49, 244. [Google Scholar] [CrossRef]
- Zhang, N.; Leatham, K.; Xiong, J.; Zhong, J. PCA-K-means based clustering algorithm for high dimensional and overlapping spectra signals. In Proceedings of the 2018 Ninth International Conference on Intelligent Control and Information Processing (ICICIP), Wanzhou, China, 9–11 November 2018; pp. 349–354. [Google Scholar]
- Lima, R.P.D.; Marfurt, K.J. Principal component analysis and k-means analysis of airborne gamma-ray spectrometry surveys. In Seg Technical Program Expanded Abstracts 2018; Society of Exploration Geophysicists: Houston, TX, USA, 2018; pp. 2277–2281. [Google Scholar]
- Kamath, S.D.; Mahato, K.K. Optical pathology using oral tissue fluorescence spectra: Classification by principal component analysis and k-means nearest neighbor analysis. J. Biomed. Opt. 2007, 12, 014028. [Google Scholar] [CrossRef]
- Yukhnevich, G.V. Infrakrasnaya Spektroskopiya Vody (Infrared Spectroscopy of Water); Nauka: Moscow, Russia, 1973; p. 208. [Google Scholar]
- Mkrtumyan, A.; Ametov, A.; Demidova, T.; Volkova, A.; Dudinskaya, E.; Vertkin, A.; Vorobiev, S. A new approach to overcome insulin resistance in patients with impaired glucose tolerance: The results of a multicenter, double-blind, placebo-controlled, randomized clinical trial of efficacy and safety of subetta. J. Clin. Med. 2022, 11, 1390. [Google Scholar] [CrossRef]
- Geppe, N.A.; Blokhin, B.M.; Shamsheva, O.V.; Abdrakhmanova, S.T.; Alikhanova, K.A.; Myrzabekova, G.T. Efficacy and safety of Ergoferon in children from 6 months to 6 years old with acute respiratory viral infections in contemporary outpatient practice: A multicenter, double-blind, placebo-controlled randomized trial. Can. Respir. J. 2021, 2021, 5570178. [Google Scholar] [CrossRef]
- Chatterjee, A.; Paul, B.K.; Kar, S.; Das, S.; Basu, R.; Bhar, D.S.; Nandy, P.S. Effect of ultrahigh diluted homeopathic medicines on the electrical properties of PVDF-HFP. Int. J. High Dilution Res. 2016, 5, 10–17. [Google Scholar] [CrossRef]
- Lobyshev, V.I. Non-monotonous impedance patterns of diclofenac centesimal potentiated solutions and their evolution. Water 2022. [Google Scholar] [CrossRef]
- Bunkin, N.F.; Bolotskova, P.N.; Bondarchuk, E.V.; Gryaznov, V.G.; Gudkov, S.V.; Kozlov, V.A.; Okuneva, M.A.; Ovchinnikov, O.V.; Smoliy, O.P.; Turkanov, I.F. Long-term effect of low-frequency electromagnetic irradiation in water and isotonic aqueous solutions as studied by photoluminescence from polymer membrane. Polymers 2021, 13, 1443. [Google Scholar] [CrossRef]
- Szcześ, A.; Chibowski, E.; Hołysz, L.; Rafalski, P. Effects of static magnetic field on water at kinetic condition. Chem. Eng. Process. Process Intensif. 2011, 50, 124–127. [Google Scholar] [CrossRef]
- Coey, J.M.D.; Cass, S. Magnetic water treatment. J. Magn. Magn. Mater. 2000, 209, 71–74. [Google Scholar] [CrossRef]
- Gorlenko, N.P.; Safronov, V.N.; Abzaev, Y.A.; Sarkisov, Y.S.; Kugaevskaya, S.N.; Ermilova, T.A. Magnetic field as factor of control for structure and properties of cement systems. Part 1. Theoretical prerequisites for magnetic effect on physicochemical processes. Vestn. Tomsk. State Univ. Archit. Build. 2015, 3, 134–150. [Google Scholar]
- Penkov, V.N. Temporal dynamics of the scattering properties of deionized water. Phys. Wave Phenom. 2020, 28, 135–139. [Google Scholar] [CrossRef]
- Novikov, V.V.; Yablokova, E.V. Interaction between highly diluted samples, protein solutions and water in a controlled magnetic field. Appl. Sci. 2022, 12, 5185. [Google Scholar] [CrossRef]
- Huang, C.; Wikfeldt, K.T.; Tokushima, T.; Nordlund, D.; Harada, Y.; Bergmann, U.; Niebuhr, M.; Weiss, T.M.; Horikawa, Y.; Leetmaa, M.; et al. The inhomogeneous structure of water at ambient conditions. Proc. Natl. Acad. Sci. USA 2009, 106, 15214–15218. [Google Scholar] [CrossRef]
- Marechal, Y. The molecular structure of liquid water delivered by absorption spectroscopy in the whole IR region completed with thermodynamics data. J. Mol. Struct. 2011, 1004, 146–155. [Google Scholar] [CrossRef]
- Holten, V.; Anisimov, M.A. Entropy-driven liquid-liquid separation in supercooled water. Sci. Rep. 2012, 2, 713. [Google Scholar] [CrossRef]
- Russo, J.; Tanaka, H. Understanding water’s anomalies with locally favoured structures. Nat. Commun. 2014, 5, 3556. [Google Scholar] [CrossRef]
- Rad, I.; Pollack, G.H. Cooling of pure water at room temperature by weak electric currents. J. Phys. Chem. B 2018, 122, 7711–7717. [Google Scholar] [CrossRef] [PubMed]
- Toledo, E.J.L.; Ramalho, T.C.; Magriotis, Z.M. Influence of magnetic field on physical–chemical properties of the liquid water: Insights from experimental and theoretical models. J. Mol. Struct. 2008, 888, 409–415. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Penkov, N.V. Influence of the Combined Magnetic Field and High Dilution Technology on the Intrinsic Emission of Aqueous Solutions. Water 2023, 15, 599. https://doi.org/10.3390/w15030599
Penkov NV. Influence of the Combined Magnetic Field and High Dilution Technology on the Intrinsic Emission of Aqueous Solutions. Water. 2023; 15(3):599. https://doi.org/10.3390/w15030599
Chicago/Turabian StylePenkov, Nikita V. 2023. "Influence of the Combined Magnetic Field and High Dilution Technology on the Intrinsic Emission of Aqueous Solutions" Water 15, no. 3: 599. https://doi.org/10.3390/w15030599
APA StylePenkov, N. V. (2023). Influence of the Combined Magnetic Field and High Dilution Technology on the Intrinsic Emission of Aqueous Solutions. Water, 15(3), 599. https://doi.org/10.3390/w15030599




