Effects of Nutrients on the Performance of the Biological Sulfur Recovery Unit for Sulfur Removal from Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Medium Preparation
2.3. Cultivation
2.4. Preliminary Assessment
2.5. Immobilized Cells
2.6. Sample Analysis
2.6.1. Dry-Weight Analysis
2.6.2. Measurement of Particle Density
2.6.3. Determination of Sulfide Concentration
2.6.4. Determination of Sulfate Concentration
3. Results and Discussion
3.1. Primary Evaluation of Cell Growth
3.2. Influence of Nutrients on the Cell Growth
3.2.1. Evaluation of Turbidity in the Medium
3.2.2. Evaluation of Liquid Acidity
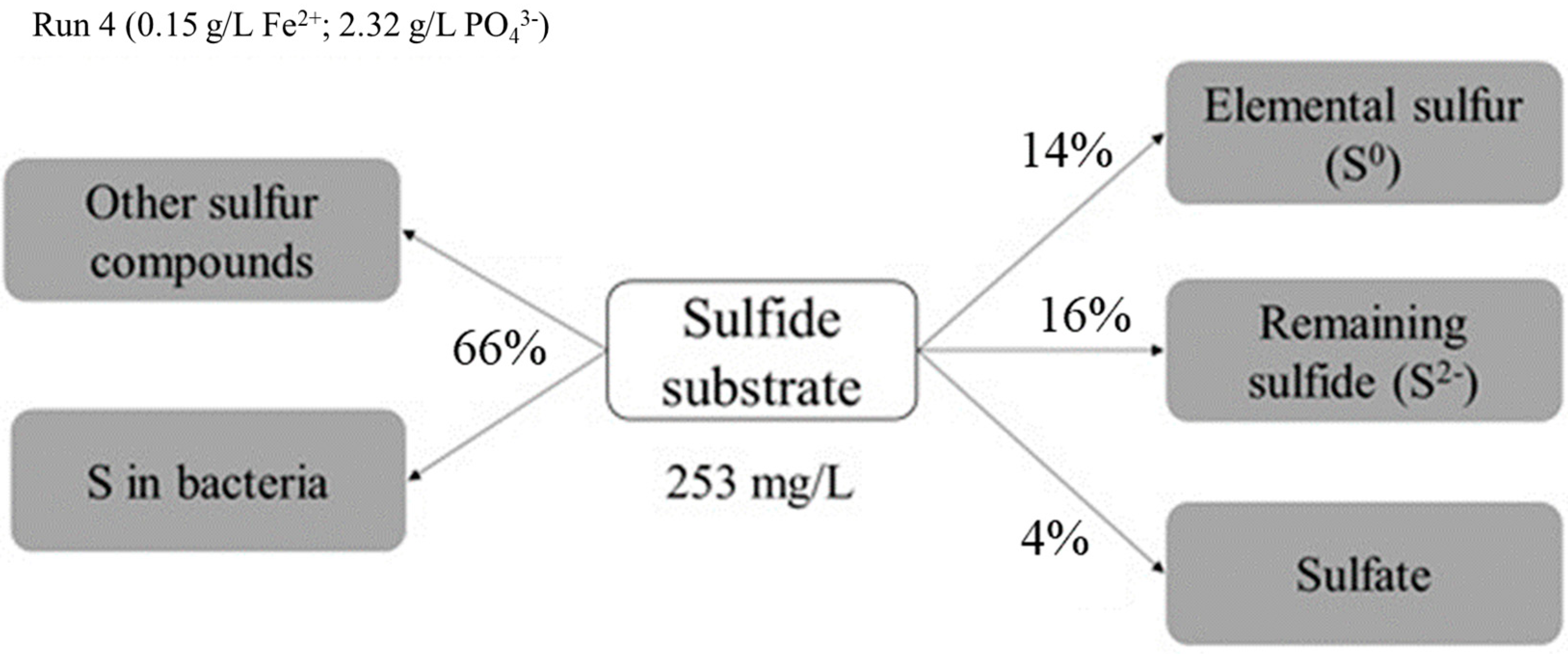
3.3. Sulfur and Sulfate Formation by Immobilized Cells
- Step 1: S2O32− + 0.25 O2 + 0.5 H2O → 0.5 S4O62− + OH− (enzymatic)
- Step 2: 2 S4O62− → S3O62− + S5O62− (nonbiological dismutation)
- Step 3: S5O62− → S4O62− + S0 (nonbiological decomposition)
- Step 4: S3O62− + H2O → S2O32− + SO42− + H+ (nonbiological hydrolysis)
- Step 5: S3O62− + 2 O2 + 2 H2O → 3 SO42− + 4 H+ (enzymatic)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sun, J.; Dai, X.; Liu, Y.; Peng, L.; Ni, B.-J. Sulfide Removal and Sulfur Production in a Membrane Aerated Biofilm Reactor: Model Evaluation. Chem. Eng. J. 2017, 309, 454–462. [Google Scholar] [CrossRef]
- Fisher, R.M.; Alvarez-Gaitan, J.P.; Stuetz, R.M.; Moore, S.J. Sulfur Flows and Biosolids Processing: Using Material Flux Analysis (MFA) Principles at Wastewater Treatment Plants. J. Environ. Manag. 2017, 198, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Wu, J. Review on Sulfur Compounds in Petroleum and Its Products: State-of-the-Art and Perspectives. Energy Fuels 2021, 35, 14445–14461. [Google Scholar] [CrossRef]
- Li, R.; Han, Z.; Shen, H.; Qi, F.; Sun, D. Volatile Sulfur Compound Emissions and Health Risk Assessment from an A2/O Wastewater Treatment Plant. Sci. Total Environ. 2021, 794, 148741. [Google Scholar] [CrossRef]
- Hou, J.; Guo, W.; Wen, Y. Effect of Sulfate Load on Sulfur Removal in Model Constructed Wetlands. IOP Conf. Ser. Earth Environ. Sci. 2018, 186, 012078. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Contaminant Candidate List Regulatory Determination Support Document for Sulfate; U.S. Environmental Protection Agency: Washington, DC, USA, 2003.
- Caliari, P.C.; Pacheco, M.J.; Ciríaco, L.; Lopes, A. Tannery Wastewater: Organic Load and Sulfide Removal Dynamics by Electrochemical Oxidation at Different Anode Materials. Environ. Technol. Innov. 2019, 14, 100345. [Google Scholar] [CrossRef]
- Pirieh, P.; Naeimpoor, F. Discrimination of Chemical and Biological Sulfide Oxidation in a Hybrid Two-Phase Process. J. Environ. Chem. Eng. 2019, 7, 103027. [Google Scholar] [CrossRef]
- Indarto, A.; Choi, J.W.; Lee, H.; Song, H.K. Decomposition of CCl4 and CHCl3 on gliding arc plasma. J. Environ. Sci. 2006, 18, 83–89. [Google Scholar] [CrossRef]
- Cai, J.; Zheng, P.; Qaisar, M.; Zhang, J. Elemental Sulfur Recovery of Biological Sulfide Removal Process from Wastewater: A Review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 2079–2099. [Google Scholar] [CrossRef]
- Sahinkaya, E.; Hasar, H.; Kaksonen, A.H.; Rittmann, B.E. Performance of a Sulfide-Oxidizing, Sulfur-Producing Membrane Biofilm Reactor Treating Sulfide-Containing Bioreactor Effluent. Environ. Sci. Technol. 2011, 45, 4080–4087. [Google Scholar] [CrossRef]
- Fan, F.; Xu, R.; Wang, D.; Meng, F. Application of Activated Sludge for Odor Control in Wastewater Treatment Plants: Approaches, Advances and Outlooks. Water Res. 2020, 181, 115915. [Google Scholar] [CrossRef]
- Pokorna, D.; Zabranska, J. Sulfur-Oxidizing Bacteria in Environmental Technology. Biotechnol. Adv. 2015, 33, 1246–1259. [Google Scholar] [CrossRef]
- Kidnay, A.J.; Parrish, W.R. Fundamentals of Natural Gas Processing, 1st ed.; CRC Press: Boca Raton, FL, USA, 2006; ISBN 9780429135644. [Google Scholar]
- Cline, C.; Hoksberg, A.; Abry, R.; Janssen, A. Biological Process for H~2S Removal from Gas Streams: The Shell-Paques/THIOPAQTM Gas Desulfurization Process. In Proceedings of the Laurance Reid Gas Conditioning Conference, Norman, OK, USA, 20–23 February 2003. [Google Scholar]
- Abdel-Monaem Zytoon, M.; Ahmad AlZahrani, A.; Hamed Noweir, M.; Ahmed El-Marakby, F. Bioconversion of High Concentrations of Hydrogen Sulfide to Elemental Sulfur in Airlift Bioreactor. Sci. World J. 2014, 2014, 675673. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, I. Mechanisms of Inorganic Oxidation and Energy Coupling. Annu. Rev. Microbiol. 1974, 28, 85–102. [Google Scholar] [CrossRef]
- Giro, M.E.A.; Garcia, O.; Zaiat, M. Immobilized Cells of Acidithiobacillus Ferrooxidans in PVC Strands and Sulfite Removal in a Pilot-Scale Bioreactor. Biochem. Eng. J. 2006, 28, 201–207. [Google Scholar] [CrossRef]
- Tariq, M.; Wang, J.; Malik, A.J.; Akhter, M.S.; Mahmood, Q. Effect of Substrate Ratios on the Simultaneous Carbon, Nitrogen, Sulfur and Phosphorous Conversions in Microbial Fuel Cells. Heliyon 2021, 7, e07338. [Google Scholar] [CrossRef]
- Wang, Q.; Wei, Z.; Yi, X.; Tang, J.; Feng, C.; Dang, Z. Biogenic Iron Mineralization of Polyferric Sulfate by Dissimilatory Iron Reducing Bacteria: Effects of Medium Composition and Electric Field Stimulation. Sci. Total Environ. 2019, 684, 466–475. [Google Scholar] [CrossRef]
- Dong, Y.; Chong, S.; Lin, H. Enhanced Effect of Biochar on Leaching Vanadium and Copper from Stone Coal Tailings by Thiobacillus Ferrooxidans. Environ. Sci. Pollut. Res. 2022, 29, 20398–20408. [Google Scholar] [CrossRef]
- Lin, C.Y.; Turchyn, A.V.; Steiner, Z.; Bots, P.; Lampronti, G.I.; Tosca, N.J. The Role of Microbial Sulfate Reduction in Calcium Carbonate Polymorph Selection. Geochim. Cosmochim. Acta 2018, 237, 184–204. [Google Scholar] [CrossRef] [Green Version]
- Lesia, H.; Lizama, H.M.; Isamu, S. Selective Inhibition of the Oxidation of Ferrous Iron or Sulfur in Thiobacillus Ferrooxidans. Appl. Environ. Microbiol. 2000, 66, 1031–1037. [Google Scholar] [CrossRef] [Green Version]
- Guo, G.; Wu, D.; Hao, T.; Mackey, H.R.; Wei, L.; Wang, H.; Chen, G. Functional Bacteria and Process Metabolism of the Denitrifying Sulfur Conversion-Associated Enhanced Biological Phosphorus Removal (DS-EBPR) System: An Investigation by Operating the System from Deterioration to Restoration. Water Res. 2016, 95, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Li, Z.; Chen, L.; Ling, Q.; Zan, F.; Isawi, H.; Hao, T.; Ma, J.; Wang, Z.; Chen, G.; et al. Advances in Elemental Sulfur-Driven Bioprocesses for Wastewater Treatment: From Metabolic Study to Application. Water Res. 2022, 213, 118143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhao, Y.; Zhou, C.; Duan, H.; Wang, G. Dynamic Sulfur–Iron Cycle Promoted Phosphorus Mobilization in Sediments Driven by the Algae Decomposition. Ecotoxicology 2021, 30, 1662–1671. [Google Scholar] [CrossRef] [PubMed]
- Matei, E.; Predescu, A.M.; Șăulean, A.A.; Râpă, M.; Sohaciu, M.G.; Coman, G.; Berbecaru, A.-C.; Predescu, C.; Vâju, D.; Vlad, G. Ferrous Industrial Wastes—Valuable Resources for Water and Wastewater Decontamination. Int. J. Environ. Res. Public Health 2022, 19, 13951. [Google Scholar] [CrossRef]
- Warwick, C.; Guerreiro, A.; Soares, A. Sensing and Analysis of Soluble Phosphates in Environmental Samples: A Review. Biosens. Bioelectron. 2013, 41, 1–11. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, Z.; Sklodowska, A.; Musialowski, M.; Bajda, T.; Yin, H.; Drewniak, L. Microbiological Sulfide Removal—From Microorganism Isolation to Treatment of Industrial Effluent. Microorganisms 2021, 9, 611. [Google Scholar] [CrossRef]
- Shuler, M.L.; Kargi, F. Bioprocess Engineering: Basic Concepts, 2nd ed.; Prentice Hall: Hoboken, NJ, USA, 2002. [Google Scholar]
- Zhu, Y. Immobilized Cell Fermentation for Production of Chemicals and Fuels. In Bioprocessing for Value-Added Products from Renewable Resources; Yang, S.-T., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 373–396. ISBN 978-0-444-52114-9. [Google Scholar]
- Nemati, M.; Webb, C. Immobilized Cell Bioreactors. In Comprehensive Biotechnology, 3rd ed.; Moo-Young, M., Ed.; Elsevier: Oxford, UK, 2011; pp. 489–504. ISBN 978-0-444-64047-5. [Google Scholar]
- Duarte, J.C.; Rodrigues, J.A.R.; Moran, P.J.S.; Valença, G.P.; Nunhez, J.R. Effect of Immobilized Cells in Calcium Alginate Beads in Alcoholic Fermentation. AMB Express 2013, 3, 31. [Google Scholar] [CrossRef] [Green Version]
- Rice, E.W.; Baird, R.B.; Eaton, A.D.; Clesceri, L.S. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2012; Volume 10. [Google Scholar]
- Kim, B.W.; Chang, H.N. Removal of Hydrogen Sulfide by Chlorobium Thiosulfatophilum in Immobilized-Cell and Sulfur-Settling Free-Cell Recycle Reactors. Biotechnol. Prog. 1991, 7, 495–500. [Google Scholar] [CrossRef]
- Pronk, J. Oxidation of Reduced Inorganic Sulphur Compounds by Acidophilic Thiobacilli. FEMS Microbiol. Lett. 1990, 75, 293–306. [Google Scholar] [CrossRef]
- Lacey, D.T.; Lawson, F. Kinetics of the Liquid-Phase Oxidation of Acid Ferrous Sulfate by the BacteriumThiobacillus Ferrooxidens. Biotechnol. Bioeng. 1970, 12, 29–50. [Google Scholar] [CrossRef]
- Yan, J.; Wen, H.; Li, Q.; Wang, S.; Xie, J.; Chen, M.; Li, J.; Chang, X.; Zhang, H.; Hong, Y. Enhanced Elemental Sulfur Recovery and Nitrogen Removal through Coupling of Sulfide-Dependent Denitrification and Anammox Processes during Ammonium- and Sulfide-Laden Waste Stream Treatment. Int. Biodeterior. Biodegrad. 2020, 155, 105086. [Google Scholar] [CrossRef]
- Drobner, E.; Huber, H.; Stetter, K.; Mikrobiologie, L.; Regensburg, U. Facultative Hydrogen Oxidizer. Microbiology 1990, 56, 2922–2923. [Google Scholar]
- Janssen, A.J.H.; Sleyster, R.; van der Kaa, C.; Jochemsen, A.; Bontsema, J.; Lettinga, G. Biological Sulphide Oxidation in a Fed-Batch Reactor. Biotechnol. Bioeng. 1995, 47, 327–333. [Google Scholar] [CrossRef]
- Nakamura, K.; Miki, H.; Amano, Y. Thiobacillus Thiooxidans S3 in a PH-Controlled Thiosulfate Medium. J. Gen. Appl. Microbiol. 1990, 36, 369–376. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, I.; Lee, D.; Mackay, B.; Harahuc, L.; Oh, J.K. Effect of Various Ions, PH, and Osmotic Pressure on Oxidation of Elemental Sulfur by Thiobacillus Thiooxidans. Appl. Environ. Microbiol. 1999, 65, 5163–5168. [Google Scholar] [CrossRef] [Green Version]
- Yin, R.; Fan, C.; Sun, J.; Shang, C. Oxidation of Iron Sulfide and Surface-Bound Iron to Regenerate Granular Ferric Hydroxide for in-Situ Hydrogen Sulfide Control by Persulfate, Chlorine and Peroxide. Chem. Eng. J. 2018, 336, 587–594. [Google Scholar] [CrossRef]
- Vishniac, W.; Santer, M. The Thiobacilli. Bacteriol. Rev. 1957, 21, 195–213. [Google Scholar] [CrossRef]
- Tamiya, H.; Haga, K.; Huzisige, H. Zur Physiologie Der Chemoautotrophen Schwefelbakterien. Acta Phytochem Tokyo 1941, 12, 173–225. [Google Scholar]
- Wentzel, M.C.; Ekama, G.A.; Loewenthal, R.E. Fundamentals of Biological Behaviour and Wastewater Strength Tests. In Handbook of Water and Wastewater Microbiology; Mara, D., Horan, N.J., Eds.; Elsevier: London, UK, 2003; pp. 145–173. ISBN 978-0-12-470100-7. [Google Scholar]
- Shchegolkova, N.M.; Krasnov, G.S.; Belova, A.A.; Dmitriev, A.A.; Kharitonov, S.L.; Klimina, K.M.; Melnikova, N.V.; Kudryavtseva, A.V. Microbial Community Structure of Activated Sludge in Treatment Plants with Different Wastewater Compositions. Front. Microbiol. 2016, 7, 90. [Google Scholar] [CrossRef] [Green Version]
- Cai, J.; Sun, Y.; Qaisar, M.; Wang, K.; Chen, B. Revealing the Effect of Multiple Nitrogen Sources on Sulfide Oxidation by Progressively Changing Nitrate to Nitrite. Sep. Purif. Technol. 2022, 283, 120226. [Google Scholar] [CrossRef]
- Lohwacharin, J.; Annachhatre, A.P. Biological Sulfide Oxidation in an Airlift Bioreactor. Bioresour. Technol. 2010, 101, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- García de Lomas, J.; Corzo, A.; Gonzalez, J.M.; Andrades, J.A.; Iglesias, E.; Montero, M.J. Nitrate Promotes Biological Oxidation of Sulfide in Wastewaters: Experiment at Plant-Scale. Biotechnol. Bioeng. 2006, 93, 801–811. [Google Scholar] [CrossRef] [PubMed]

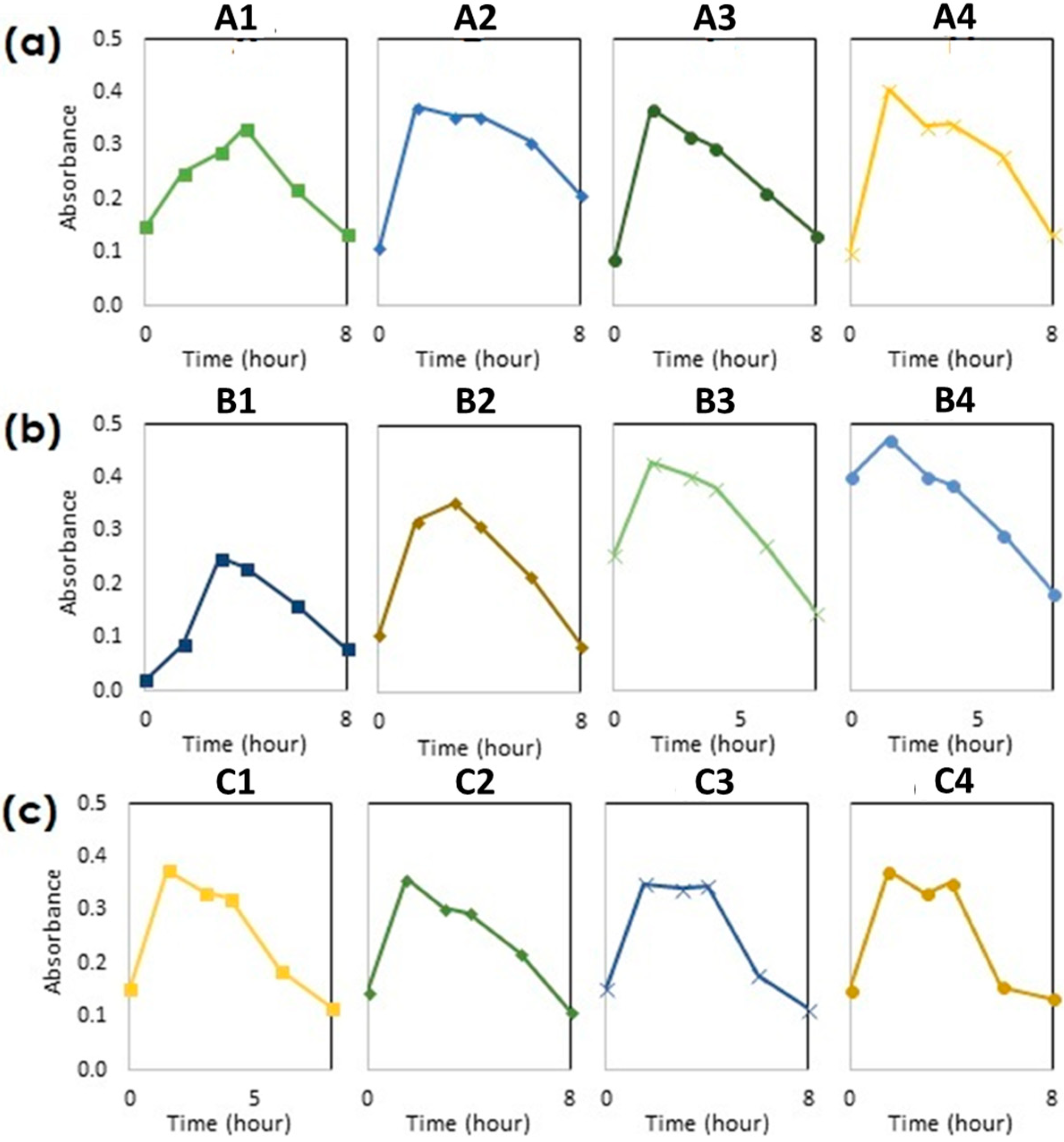


| Run | Nutrient (g/L) | |||
|---|---|---|---|---|
| PO43− | SO42− | Fe2+ | ||
| KH2PO4 | K2HPO4 | Na2SO4 | FeSO4·7H2O | |
| A1 | 0 | 0 | 0.09 | 0.05 |
| A2 | 1.70 | 1.36 | 0.09 | 0.05 |
| A3 | 3.40 | 2.72 | 0.09 | 0.05 |
| A4 | 5.10 | 4.08 | 0.09 | 0.05 |
| B1 | 1.70 | 1.36 | 0 | 0.05 |
| B2 | 1.70 | 1.36 | 0.09 | 0.05 |
| B3 | 1.70 | 1.36 | 0.18 | 0.05 |
| B4 | 1.70 | 1.36 | 0.27 | 0.05 |
| C1 | 1.70 | 1.36 | 0.09 | 0 |
| C2 | 1.70 | 1.36 | 0.09 | 0.05 |
| C3 | 1.70 | 1.36 | 0.09 | 0.10 |
| C4 | 1.70 | 1.36 | 0.09 | 0.15 |
| Run | Nutrient (g/L) | Alginate Beads * (Immobilized Cells) | |||
|---|---|---|---|---|---|
| KH2PO4 | K2HPO4 | FeSO4·7H2O | Na2S | ||
| 1 | 0 | 0 | 0.05 | 0.89 | 20 mL/150 mL |
| 2 | 1.70 | 1.36 | 0.05 | 0.89 | 20 mL/150 mL |
| 3 | 0 | 0 | 0.15 | 0.89 | 20 mL/150 mL |
| 4 | 1.70 | 1.36 | 0.15 | 0.89 | 20 mL/150 mL |
| Blank | 1.70 | 1.36 | 0.05 | 0.89 | - |
| Concentration | Units | Blank | Run 1 | Run 2 | Run 3 | Run 4 |
|---|---|---|---|---|---|---|
| Sulfide | ||||||
| Initial | mg/L | 114 | 180 | 212 | 135 | 135 |
| Final | mg/L | 49 | 45 | 47 | 51 | 41 |
| Conversion | mg/L | 65 | 135 | 165 | 84 | 94 |
| % | 57 | 75 | 78 | 62 | 69 | |
| Sulfate | ||||||
| Initial | mg/L | 27 | 105 | 111 | 116 | 116 |
| Final | mg/L | 232 | 52 | 256 | 158 | 145 |
| Formation | mg/L | 206 | n/a | 145 | 42 | 30 |
| % | 767 | n/a | 132 | 36 | 27 | |
| Sulfur | ||||||
| Final/generated | mg/L | 0.5 | 3.8 | 17.0 | 2.7 | 34.8 |
| Parameter | Units | Run 1 | Run 2 | Run 3 | Run 4 |
|---|---|---|---|---|---|
| Sulfur formation rate | - | 0.78 | 7.35 | 0.65 | 8.75 |
| Final sulfide | mg/L | 45 | 47 | 51 | 41 |
| Sulfide conversion | % | 82 | 80 | 81 | 84 |
| Sulfur-to-sulfate molar ratio | - | n/a | 1.2 | 0.06 | 3.5 |
| Consortium | Formed Sulfur (%) | Formed Sulfate (%) | Sulfide Conversion (%) | Ref. |
|---|---|---|---|---|
| Sludge * | 58.9–90.5 | - | 96.41 | [48] |
| Active sludge | 50.0–90.0 | 50–90 | 90.0 | [49] |
| Thiomirospia | - | - | 68.4–94.7 | [50] |
| Thiobacillus | 0.0–3.7 | 9.0–60.5 | 58.0–86.0 | [8] |
| Arcobacter | 60 | 17–19 | ~100 | [29] |
| Thiobacillus | 14.0 | 4.0 | 84.0 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Purwadi, R.; Ginting, D.A.E.B.; Anbibie, A.; Mohtar, W.H.M.W.; Ramli, Y.; Indarto, A. Effects of Nutrients on the Performance of the Biological Sulfur Recovery Unit for Sulfur Removal from Water. Water 2023, 15, 530. https://doi.org/10.3390/w15030530
Purwadi R, Ginting DAEB, Anbibie A, Mohtar WHMW, Ramli Y, Indarto A. Effects of Nutrients on the Performance of the Biological Sulfur Recovery Unit for Sulfur Removal from Water. Water. 2023; 15(3):530. https://doi.org/10.3390/w15030530
Chicago/Turabian StylePurwadi, Ronny, Dessi A. E. Br Ginting, Anbibie Anbibie, Wan Hanna Melini Wan Mohtar, Yusrin Ramli, and Antonius Indarto. 2023. "Effects of Nutrients on the Performance of the Biological Sulfur Recovery Unit for Sulfur Removal from Water" Water 15, no. 3: 530. https://doi.org/10.3390/w15030530






