Evaluation of a Nature-like Bypass for Non-Salmonids in the Sesan River
Abstract
:1. Introduction
2. Materials and Methods
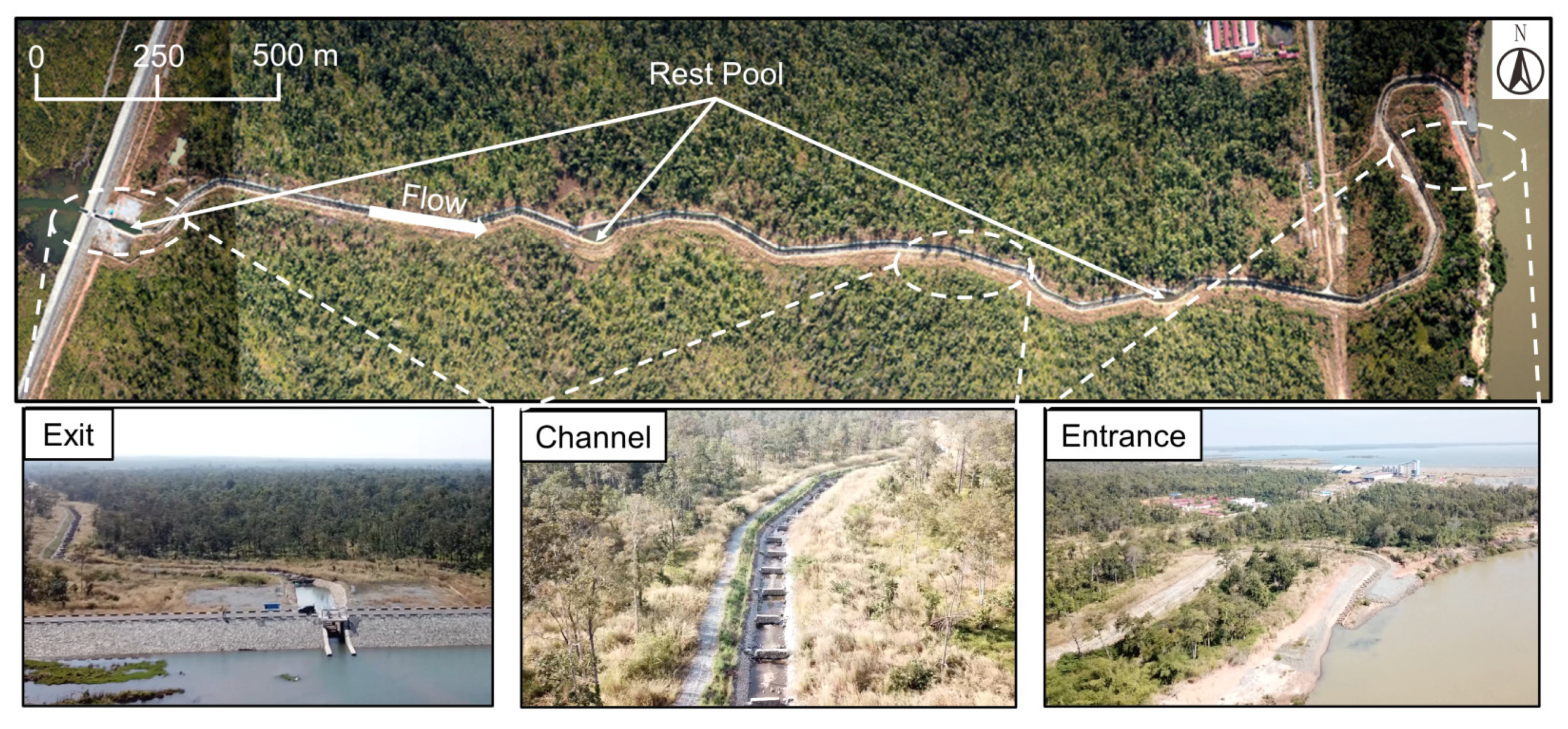
2.1. Study Site
2.2. Fishway Design
2.3. Fishway Monitoring
2.3.1. Fish Sampling
2.3.2. Video Monitoring
2.4. Data Analysis
3. Results
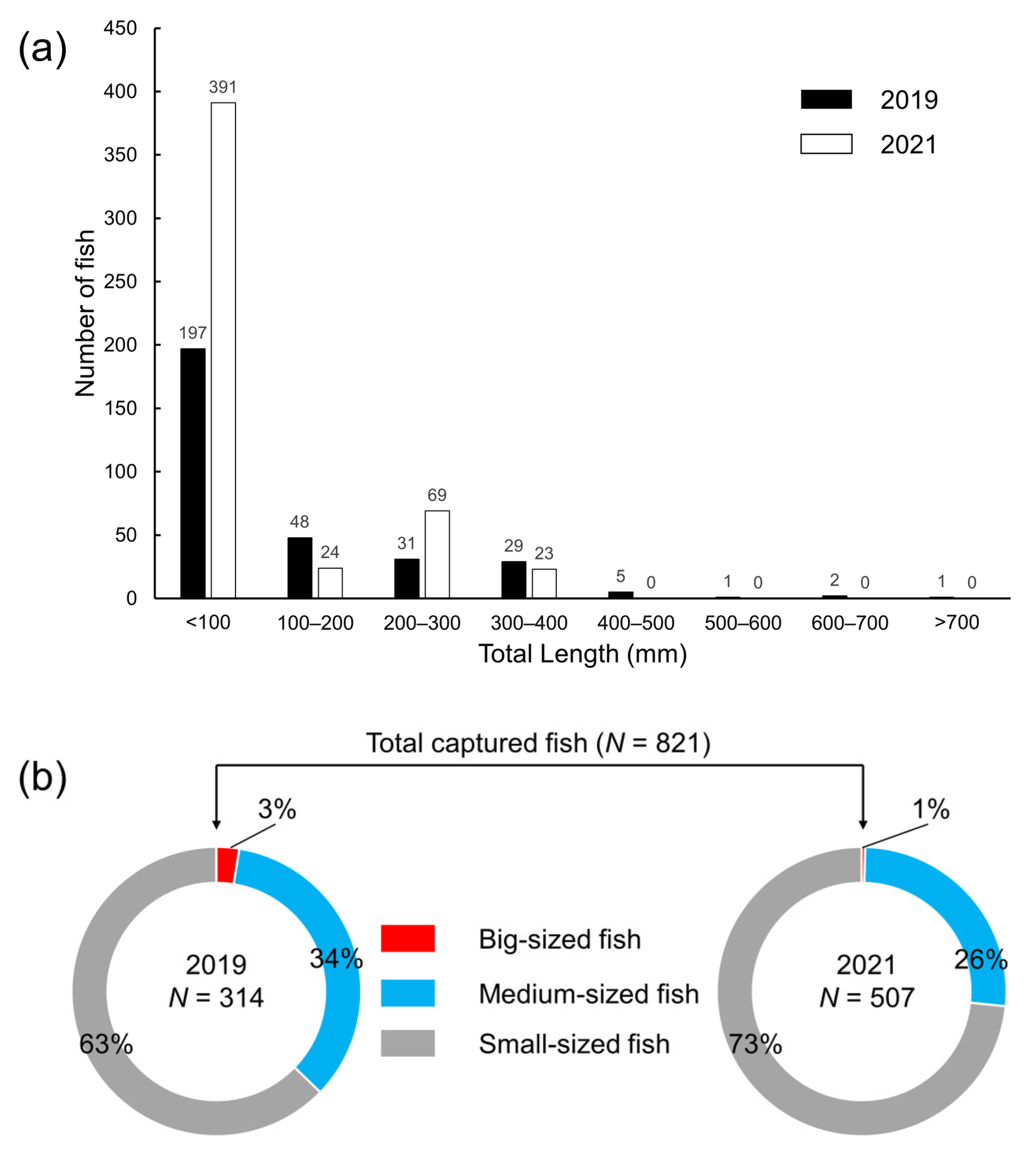
3.1. Species Composition and Biological Characteristics of the Collected Fish in the NLP
3.2. Monitoring of Migration Behaviors at the Exit of the NLP
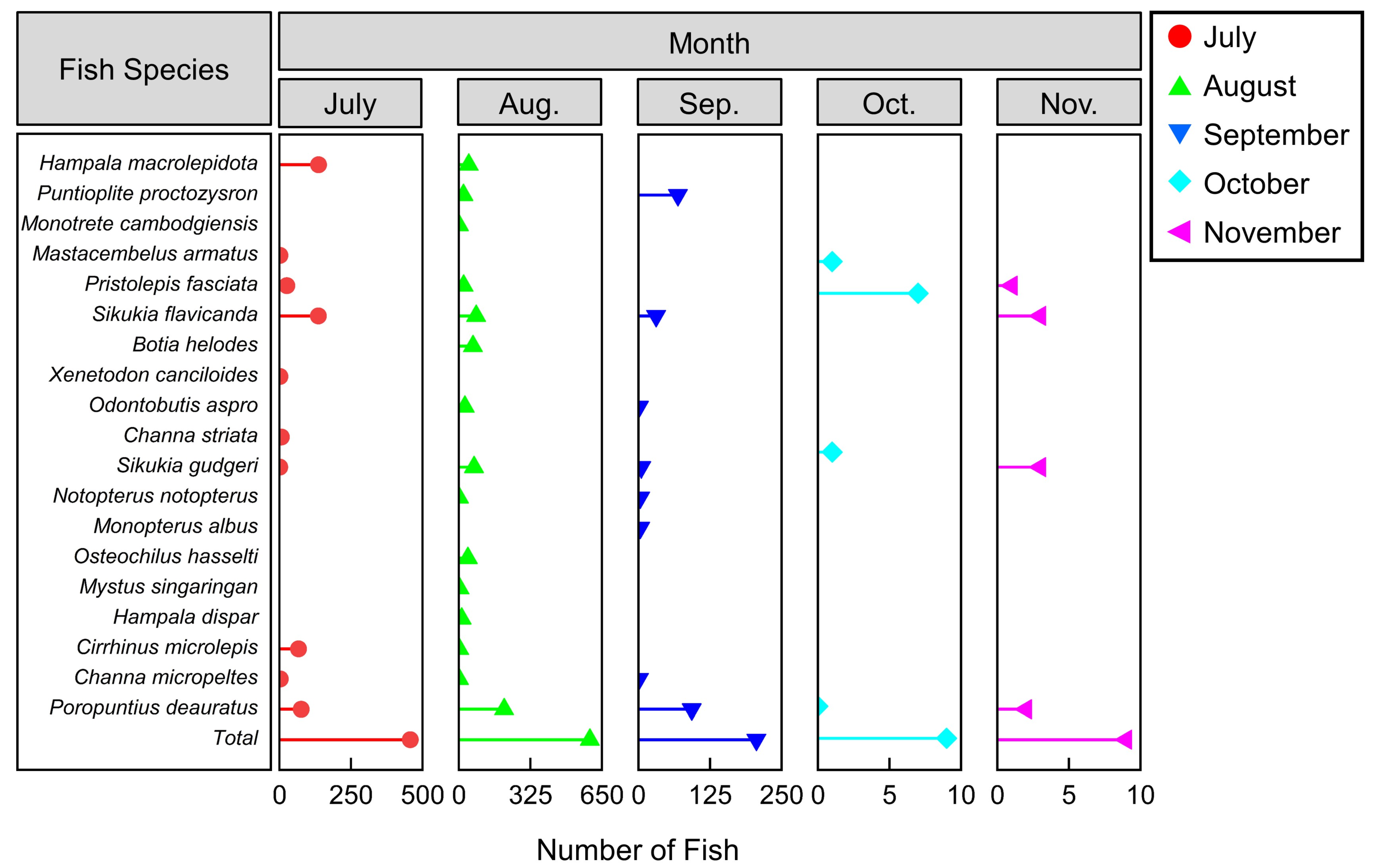
3.2.1. Upstream Migration Behaviors across Different Months
3.2.2. Circadian Rhythms of Upstream and Downstream Migration Behaviors
3.3. Influencing Factors of Effectiveness of the NLP
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cooke, S.J.; Midwood, J.D.; Thiem, J.D.; Klimley, P.; Lucas, M.C.; Thorstad, E.B.; Eiler, J.; Holbrook, C.; Ebner, B.C. Tracking animals in freshwater with electronic tags: Past, present and future. Anim. Biotelem. 2013, 1, 5. [Google Scholar] [CrossRef] [Green Version]
- Bao, J.H.; Li, W.W.; Zhang, C.S.; Mi, X.Y.; Li, H.T.; Zhao, X.J.; Cao, N.; Twardek, W.M.; Cooke, S.J.; Duan, M. Quantitative assessment of fish passage efficiency at a vertical-slot fishway on the Daduhe River in Southwest China. Ecol. Eng. 2019, 141, 105597. [Google Scholar] [CrossRef]
- Karppinen, P.; Hynninen, M.; Vehanen, T.; Vaha, J.P. Variations in migration behaviour and mortality of Atlantic salmon smolts in four different hydroelectric facilities. Fish. Manag. Ecol. Evol. 2021, 28, 253–267. [Google Scholar] [CrossRef]
- Frissell, C.A. Topology of extinction and endangerment of native fishes in the Pacific-Northwest and California (USA). Conserv. Biol. 1993, 7, 342–354. [Google Scholar] [CrossRef]
- Fahrig, L. Effects of habitat fragmentation on biodiversity. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 487–515. [Google Scholar] [CrossRef] [Green Version]
- Pasha, M.F.K.; Yeasmin, D.; Rentch, J.W. Dam-lake operation to optimize fish habitat. Environ. Process. 2015, 2, 631–645. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.T.; Lucas, M.C.; Castro-Santos, T.; Katopodis, C.; Baumgartner, L.J.; Thiem, J.D.; Aarestrup, K.; Pompeu, P.S.; O’Brien, G.C.; Braun, D.C.; et al. The future of fish passage science, engineering, and practice. Fish Fish. 2018, 19, 340–362. [Google Scholar] [CrossRef] [Green Version]
- Nogueira, J.G.; Teixeira, A.; Varandas, S.; Lopes-Lima, M.; Sousa, R. Assessment of a terrestrial protected area for the conservation of freshwater biodiversity. Aquat. Conserv. Mar. Freshw. Ecosyst. 2021, 31, 520–530. [Google Scholar] [CrossRef]
- Baras, E.; Lucas, M.C. Impacts of man’s modifications of river hydrology on the migration of freshwater fishes: A mechanistic perspective. Int. J. Ecohydrol. Hydrobiol. 2001, 1, 291–304. [Google Scholar]
- Schilt, C.R. Developing fish passage and protection at hydropower dams. Appl. Anim. Behav. Sci. 2007, 104, 295–325. [Google Scholar] [CrossRef]
- Forty, M.; Spees, J.; Lucas, M.C. Not just for adults! Evaluating the performance of multiple fish passage designs at low-head barriers for the upstream movement of juvenile and adult trout Salmo trutta. Ecol. Eng. 2016, 94, 214–224. [Google Scholar] [CrossRef]
- Clay, C.H. Design of Fishways and Other Fish Facilities; Lewis Publishers, CRC Press: Boca Raton, FL, USA, 1995; p. 256. [Google Scholar] [CrossRef]
- Bunt, C.M.; Katopodis, C.; McKinley, R.S. Attraction and passage efficiency of white suckers and smallmouth bass by two denil fishways. N. Am. J. Fish. Manag. 1999, 19, 793–803. [Google Scholar] [CrossRef]
- Noonan, M.J.; Grant, J.W.A.; Jackson, C.D. A quantitative assessment of fish passage efficiency. Fish Fish. 2012, 13, 450–464. [Google Scholar] [CrossRef]
- Mallen-Cooper, M.; Brand, D.A. Non-salmonids in a salmonid fishway: What do 50 years of data tell us about past and future fish passage? Fish. Manag. Ecol. 2007, 14, 319–332. [Google Scholar] [CrossRef]
- Bunt, C.M.; Castro-Santos, T.; Haro, A. Performance of fish passage structures at upstream barriers to migration. River Res. Appl. 2012, 28, 457–478. [Google Scholar] [CrossRef]
- Yoon, J.D.; Kim, J.H.; Yoon, J.; Baek, S.H.; Jang, M.H. Efficiency of a modified Ice Harbor-type fishway for Korean freshwater fishes passing a weir in South Korea. Aquat. Ecol. 2015, 49, 417–429. [Google Scholar] [CrossRef]
- Chen, K.Q.; Tao, J.; Chang, Z.N.; Cao, X.H.; Ge, H.F. Difficulties and prospects of fishways in China: An overview of the construction status and operation practice since 2000. Ecol. Eng. 2014, 70, 82–91. [Google Scholar] [CrossRef]
- Shi, X.T.; Kynard, B.; Liu, D.F.; Qiao, Y.; Chen, Q.W. Development of fish passage in China. Fisheries 2015, 40, 161–169. [Google Scholar] [CrossRef]
- Raabe, J.K.; Hightower, J.E.; Ellis, T.A.; Facendola, J.J. Evaluation of fish passage at a nature-like rock ramp fishway on a large coastal river. Trans. Am. Fish. Soc. 2019, 148, 798–816. [Google Scholar] [CrossRef]
- Brito-Santos, J.L.; Dias-Silva, K.; Brasil, L.S.; da Silva, J.B.; Santos, A.D.; de Sousa, L.M.; Vieira, T.B. Fishway in hydropower dams: A scientometric analysis. Environ. Monit. Assess. 2021, 193, 752. [Google Scholar] [CrossRef]
- Wang, X.Y.; Guo, J. Brief review on research and construction of fishways at home and abroad. J. China Inst. Water Resour. Hydropower Res. 2005, 3, 222–228. (In Chinese) [Google Scholar] [CrossRef]
- Castro-Santos, T.; Cotel, A.; Webb, P. Fishway evaluations for better bioengineering: An integrative approach. Am. Fish. Soc. Symp. 2009, 69, 557–575. [Google Scholar]
- Kemp, P.S. Meta-analyses, metrics and motivation: Mixed messages in the fish passage debate. River Res. Appl. 2016, 32, 2116–2124. [Google Scholar] [CrossRef]
- Franklin, A.E.; Haro, A.; Castro-Santos, T.; Noreika, J. Evaluation of nature-Like and technical fishways for the passage of alewives at two coastal streams in New England. Trans. Am. Fish. Soc. 2012, 141, 624–637. [Google Scholar] [CrossRef]
- Katopodis, C.; Williams, J.G. The development of fish passage research in a historical context. Ecol. Eng. 2012, 48, 8–18. [Google Scholar] [CrossRef]
- Katopodis, C.; Kells, J.A.; Acharya, M. Nature-like and conventional fishways: Alternative concepts? Can. Water Resour. J. 2001, 26, 211–232. [Google Scholar] [CrossRef] [Green Version]
- Breton, F.; Baki, A.B.M.; Link, O.; Zhu, D.Z.; Rajaratnam, N. Flow in nature-like fishway and its relation to fish behaviour. Can. J. Civ. Eng. 2013, 40, 567–573. [Google Scholar] [CrossRef]
- Steffensen, S.M.; Thiem, J.D.; Stamplecoskie, K.M.; Binder, T.R.; Hatry, C.; Langlois-Anderson, N.; Cooke, S.J. Biological effectiveness of an inexpensive nature-like fishway for passage of warmwater fish in a small Ontario stream. Ecol. Freshw. Fish 2013, 22, 374–383. [Google Scholar] [CrossRef]
- Baki, A.B.M.; Zhu, D.Z.; Rajaratnam, N. Mean Flow Characteristics in a Rock-Ramp-Type Fish Pass. J. Hydraul. Eng. 2014, 140, 156–168. [Google Scholar] [CrossRef]
- Richer, E.E.; Fetherman, E.R.; Krone, E.A.; Wright, F.B.; Kondratieff, M.C. Multispecies fish passage evaluation at a rock-ramp fishway in a Colorado Transition Zone Stream. N. Am. J. Fish. Manag. 2020, 40, 1510–1522. [Google Scholar] [CrossRef]
- Hershey, H. Updating the consensus on fishway efficiency: A meta-analysis. Fish Fish. 2021, 22, 735–748. [Google Scholar] [CrossRef]
- Kim, J.H.; Yoon, J.D.; Baek, S.H.; Park, S.H.; Lee, J.W.; Lee, J.A.; Jang, M.H. An efficiency analysis of a nature-like fishway for freshwater fish ascending a large Korean River. Water 2016, 8, 3. [Google Scholar] [CrossRef] [Green Version]
- Mallen-Cooper, M.; Stuart, I.G. Optimising denil fishways for passage of small and large fishes. Fish. Manag. Ecol. 2007, 14, 61–71. [Google Scholar] [CrossRef]
- Sun, S.K.; Zhang, G.Q. Environment-friendly fishway in close-to-nature types. J. China Inst. Water Resour. Hydropower Res. 2012, 10, 41–47. (In Chinese) [Google Scholar] [CrossRef]
- Bunt, C.; Jacobson, B. Rainbow Trout migration and use of a nature-like fishway at a great lakes tributary. N. Am. J. Fish. Manag. 2019, 39, 460–467. [Google Scholar] [CrossRef]
- Johnson, N.S.; Miehls, S. Guiding our-migrating juvenile sea lamprey (Petromyzon marinus) with pulsed direct current. River Res. Appl. 2014, 30, 1146–1156. [Google Scholar] [CrossRef]
- Nyqvist, D.; Nilsson, P.A.; Alenas, I.; Elghagen, J.; Hebrand, M.; Karlsson, S.; Klappe, S.; Calles, O. Upstream and downstream passage of migrating adult Atlantic salmon: Remedial measures improve passage performance at a hydropower dam. Ecol. Eng. 2017, 102, 331–343. [Google Scholar] [CrossRef]
- Sanz-Ronda, F.J.; Fuentes-Perez, J.F.; Garcia-Vega, A.; Bravo-Cordoba, F.J. Fishways as downstream routes in small hydropower plants: Experiences with a potamodromous cyprinid. Water 2021, 13, 1041. [Google Scholar] [CrossRef]
- Parasiewicz, P.; Wisniewolski, W.; Mokwa, M.; Ziola, S.; Prus, P.; Godlewska, M. A low-voltage electric fish guidance system NEPTUN. Fish. Res. 2016, 181, 25–33. [Google Scholar] [CrossRef]
- Zhou, Y.M.; Zhu, H.F.; Li, M.N.; Wang, L.; Shi, X.T.; Mo, W.J.; He, Z.J. Downstream migration of juvenile bighead carp through a hydraulic fish guidance system. J. Hydroecol. 2020, 43, 110–116. (In Chinese) [Google Scholar] [CrossRef]
- Algera, D.A.; Rytwinski, T.; Taylor, J.J.; Bennett, J.R.; Smokorowski, K.E.; Harrison, P.M.; Clarke, K.D.; Enders, E.C.; Power, M.; Bevelhimer, M.S.; et al. What are the relative risks of mortality and injury for fish during downstream passage at hydroelectric dams in temperate regions? A systematic review. Environ. Evid. 2020, 9, 3. [Google Scholar] [CrossRef]
- Putland, R.L.; Mensinger, A.F. Acoustic deterrents to manage fish populations. Rev. Fish Biol. Fish. 2019, 29, 789–807. [Google Scholar] [CrossRef]
- Zhou, Y.M. A brief discussion on facilities assisting downstream mirants over the dam. Inner Mongolia Water Resour. 2020, 3, 50–51. (In Chinese) [Google Scholar]
- Popper, A.N.; Hawkins, A.D.; Jacobs, F.; Jacobson, P.T.; Johnson, P.; Krebs, J. Use of sound to guide the movement of eels and other fishes within rivers: A critical review. Rev. Fish Biol. Fish. 2020, 30, 605–622. [Google Scholar] [CrossRef]
- Williams, J.M. Is three a crowd? River basin institutions and the governance of the Mekong River. Int. J. Water Resour. Dev. 2021, 37, 720–740. [Google Scholar] [CrossRef]
- Piman, T.; Cochrane, T.A.; Arias, M.E.; Green, A.; Dat, N.D. Assessment of Flow Changes from Hydropower Development and Operations in Sekong, Sesan, and Srepok Rivers of the Mekong Basin. J. Water Resour. Plan. Manag. 2013, 139, 723–732. [Google Scholar] [CrossRef]
- Null, S.E.; Farshid, A.; Goodrum, G.; Gray, C.A.; Lohani, S.; Morrisett, C.N.; Prudencio, L.; Sor, R. A Meta-Analysis of Environmental Tradeoffs of Hydropower Dams in the Sekong, Sesan, and Srepok (3S) Rivers of the Lower Mekong Basin. Water 2021, 13, 63. [Google Scholar] [CrossRef]
- Roscoe, D.W.; Hinch, S.G. Effectiveness monitoring of fish passage facilities: Historical trends, geographic patterns and future directions. Fish Fish. 2010, 11, 12–33. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. FishBase; Fisheries Centre, University of British Columbia: Vancouver, BC, Canada, 2010. [Google Scholar]
- Xie, Y. Progress and application of IUCN Red List of Threatened Species. Biodivers. Sci. 2022, 30, 66–83. (In Chinese) [Google Scholar] [CrossRef]
- Luo, S.J.; Wu, H.R.; Xiong, L.G.; Xu, H.; Li, Q. Design of a Sesan Ⅱ nature-like bypass at the right bank. Yunnan Water Power 2018, 34, 90–93. (In Chinese) [Google Scholar] [CrossRef]
- Hermann, A.; Chladek, J.; Stepputtis, D. iFO (infrared Fish Observation)—An open source low-cost infrared underwater video system. HardwareX 2020, 8, e00149. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.Z.; Zhang, Y.Y.; Yang, F.; Zhang, K.; He, Y. An efficiency analysis of the low-head gate Dam Fishway for freshwater fish ascending Liuxi River in South China. Ecol. Eng. 2020, 158, 106018. [Google Scholar] [CrossRef]
- Li, J.; Li, X.H.; Pan, F.; Li, Y.F.; Yang, M.Y.; Yu, F.; Tan, X.C.; Yang, J.P.; Shuai, F.M. Preliminary study on the operating effect of Xiniu fishway in Lianjiang River. J. Hydroecol. 2013, 34, 53–57. (In Chinese) [Google Scholar] [CrossRef]
- Tan, X.C.; Tao, J.P.; Huang, D.M.; Li, X.H. Fish population structure in the fishway of Changzhou hydro-junction. Chin. J. Appl. Ecol. 2013, 34, 58–62. (In Chinese) [Google Scholar] [CrossRef]
- Wongyai, N.; Jutagate, A.; Grudpan, C.; Jutagate, T. Condition Index, Reproduction and Feeding of Three Non-Obligatory Riverine Mekong Cyprinids in Different Environments. Trop. Life Sci. Res. 2020, 31, 159–173. [Google Scholar] [CrossRef]
- Sokheng, C.; Chhea, C.K.; Viravong, S.; Bouakhamvongsa, K.; Suntornratana, U.; Yoorong, N.; Tung, N.T.; Bao, T.Q.; Poulsen, A.F.; Jorgensen, J.V. Fish Migrations and Spawning Habits in the Mekong Mainstream: A Survey Using Local Knowledge (Basin-Wide); Assessment of Mekong Fisheries: Fish Migrations Spawning the Impact of Water Management Project—AMFP Report; Mekong River Commission For Sustainable Development: Vientiane, Laos, 1999. [Google Scholar]
- Naughton, G.P.; Caudill, C.C.; Keefer, M.L.; Bjornn, T.C.; Stuehrenberg, L.C.; Peery, C.A. Late-season mortality during migration of radio-tagged adult sockeye salmon (Oncorhynchus nerka) in the Columbia River. Can. J. Fish. Aqua. Sci. 2005, 62, 30–47. [Google Scholar] [CrossRef] [Green Version]
- Larinier, M. Fish passage experience at small-scale hydro-electric power plants in France. Hydrobiologia 2008, 609, 97–108. [Google Scholar] [CrossRef]
- Tao, J.P.; Wen, J.Y.; He, D.; Hou, Y.Q.; Hu, W.B.; Cao, X.H.; Chen, X.J.; Chen, K.L.; Chang, J.B. Review on Monitoring and Evaluating of Fish Passage Facilities for Upper Migration. Resour. Environ. Yangtze Basin 2018, 27, 2270–2282. (In Chinese) [Google Scholar]
- Ficke, A.D.; Myrick, C.A.; Jud, N. The Swimming and Jumping Ability of Three Small Great Plains Fishes: Implications for Fishway Design. Trans. Am. Fish. Soc. 2011, 140, 1521–1531. [Google Scholar] [CrossRef]
- Branco, P.; Santos, J.M.; Katopodis, C.; Pinheiro, A.; Ferreira, M.T. Pool-Type Fishways: Two Different Morpho-Ecological Cyprinid Species Facing Plunging and Streaming Flows. PLoS ONE 2013, 8, e65089. [Google Scholar] [CrossRef]
- Romao, F.; Quaresma, A.L.; Branco, P.; Santos, J.M.; Amaral, S.; Ferreira, M.T.; Katopodis, C.; Pinheiro, A.N. Passage performance of two cyprinids with different ecological traits in a fishway with distinct vertical slot configurations. Ecol. Eng. 2017, 105, 180–188. [Google Scholar] [CrossRef] [Green Version]
- Grieve, B.; Baumgartner, L.J.; Robinson, W.; Silva, L.G.M.; Pomorin, K.; Thorncraft, G.; Ning, N. Flexible and non-invasive passive integrated transponder (PIT) tagging protocols for tropical freshwater fish species. MethodsX 2018, 5, 299–303. [Google Scholar] [CrossRef] [PubMed]




| Fish Species | Ecological Habit | Migratory Month | States 1 | |
|---|---|---|---|---|
| Cypriniformes | ||||
| Cyprinidae | ||||
| 1 | Probarbus jullieni | Demersal, Omnivorous | Nov. to Feb. | CR |
| 2 | Poropuntius deauratus | Benthopelagic, Omnivorous | Unknown | EN |
| 3 | Cirrhinus molitorella | Benthopelagic, Herbivorous | May to June | NT |
| 4 | Hypsibarbus malcolmi | Benthopelagic, Carnivorous | May, Nov. to Dec. | LC |
| 5 | Henicorhynchus lobatus | Benthopelagic, Omnivorous | Nov. to Feb., May to July | LC |
| 6 | Cyclocheilichthys enoplos | Benthopelagic, Omnivorous | Nov. to Feb., May to Aug. | LC |
| Siluriformes | ||||
| Pangasiidae | ||||
| 7 | Pangasianodon hypophthalmus | Benthopelagic, Omnivorous | May to July | EN |
| 8 | Pangasius larnaudii | Benthopelagic, Omnivorous | May to July | LC |
| Clupeiformes | ||||
| Clupeidae | ||||
| 9 | Tenualosa thibaudeaui | Pelagic, Omnivorous | Jan. to Feb., June to July | VU |
| Perciformes | ||||
| Osphronemidae | ||||
| 10 | Osphronemus exodon | Pelagic, Omnivorous | Nov. to Dec. | VU |
| Order | Fish Species | N * | TL 1 (cm) | BW 1 (g) | May 2021 | Dec. 2019 |
|---|---|---|---|---|---|---|
| Range | Range | |||||
| Cypriniformes | ||||||
| Cyprinidae | ||||||
| 1 | Hampala dispar | 127, 25.1% (7, 2.2%) | 6–17 | 10–70 | + | + |
| 2 | Sikukia gudgeri | 54, 10.7% (12, 3.8%) | 10–50 | 20–1000 | + | + |
| 3 | Hampala macrolepidota | 24, 4.7% (26, 8.3%) | 9–43 | 50–1500 | + | + |
| 4 | Puntioplites proctozystron | 22, 4.3% (59, 18.8%) | 10–43 | 40–400 | + | + |
| 5 | Poropuntius deauratus | 8, 1.6% | 9–17 | 30–70 | + | |
| 6 | Sikukia flavicaudata | 4, 0.8% (57, 18.2%) | 9–24 | 10–50 | + | + |
| 7 | Osteochilus hasselti | (2, 0.6%) | / | / | + | |
| 8 | Cirrhinus microlepis | (1, 0.3%) | / | / | + | |
| Botiidae | ||||||
| 9 | Botia helodes | 2, 0.4% (9, 2.9%) | 5–8 | 10 | + | + |
| 10 | Botia modesta | 1, 0.2% (6, 1.9%) | 16 | 80 | + | + |
| Synbranchiformes | ||||||
| Mastacembelidae | ||||||
| 11 | Mastacembelus armatus | 169, 33.4% (21, 6.7%) | 8–37 | 20–250 | + | + |
| 12 | Macrognathus siamensis | (2, 0.6%) | / | / | + | |
| Synbranchidae | ||||||
| 13 | Monopterus albus | 31, 6.1% (3, 1.0%) | 7–37 | 10–180 | + | + |
| Anabantiformes | ||||||
| Pristolepididae | ||||||
| 14 | Pristolepis fasciata | 32, 6.3% (34, 10.8%) | 7–27 | 20–200 | + | + |
| Osteoglossiformes | ||||||
| Notopteridae | ||||||
| 15 | Notopterus notopterus | 12, 2.4% (16, 5.1%) | 10–27 | 20–300 | + | + |
| Gobiiformes | ||||||
| Odontobutidae | ||||||
| 16 | Odontobutis aspro | 8, 1.6% (9, 2.9%) | 9–15 | 10–50 | + | + |
| Siluriformes | ||||||
| Clariidae | ||||||
| 17 | Clarias fuscus | 6, 1.2% (8, 2.5%) | 23–33 | 50–100 | + | + |
| Bagridae | ||||||
| 18 | Mystus singaringan | 2, 0.4% (12, 3.8%) | 21–25 | 100–200 | + | + |
| Siluridae | ||||||
| 19 | Hemisilurus mekongensis | (2, 0.6%) | / | / | + | |
| Anabantiformes | ||||||
| Channidae | ||||||
| 20 | Channa gachua | 2, 0.4% (1, 0.3%) | 16–30 | 100 | + | + |
| 21 | Channa striata | 1, 0.2% (18, 5.7%) | 19 | 200 | + | + |
| 22 | Channa micropeltes | (1, 0.3%) | / | / | + | |
| Tetraodontiformes | ||||||
| Tetraodontidae | ||||||
| 23 | Monotrete cambodgiensis | 1, 0.2% (7, 2.2%) | 14 | 80 | + | + |
| Beloniformes | ||||||
| Belonidae | ||||||
| 24 | Xenentodon canciloides | (1, 0.3%) | / | / | + | |
| Total | 506, 100% (314, 100%) | 6–50 | 10–1500 | 18 | (23) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Yu, F.; Zhang, Q.; Luo, S.; Zhou, W.; Zhang, H.; Tan, J.; Shi, X.; Shen, Y.; Shi, J. Evaluation of a Nature-like Bypass for Non-Salmonids in the Sesan River. Water 2023, 15, 421. https://doi.org/10.3390/w15030421
Sun J, Yu F, Zhang Q, Luo S, Zhou W, Zhang H, Tan J, Shi X, Shen Y, Shi J. Evaluation of a Nature-like Bypass for Non-Salmonids in the Sesan River. Water. 2023; 15(3):421. https://doi.org/10.3390/w15030421
Chicago/Turabian StyleSun, Junjian, Fuqiang Yu, Qi Zhang, Shujing Luo, Wu Zhou, Hui Zhang, Junjun Tan, Xiaotao Shi, Yinting Shen, and Jiayue Shi. 2023. "Evaluation of a Nature-like Bypass for Non-Salmonids in the Sesan River" Water 15, no. 3: 421. https://doi.org/10.3390/w15030421





