Vallisneria spiralis Promotes P and Fe Retention via Radial Oxygen Loss in Contaminated Sediments
Abstract
:1. Introduction
2. Materials and Methods
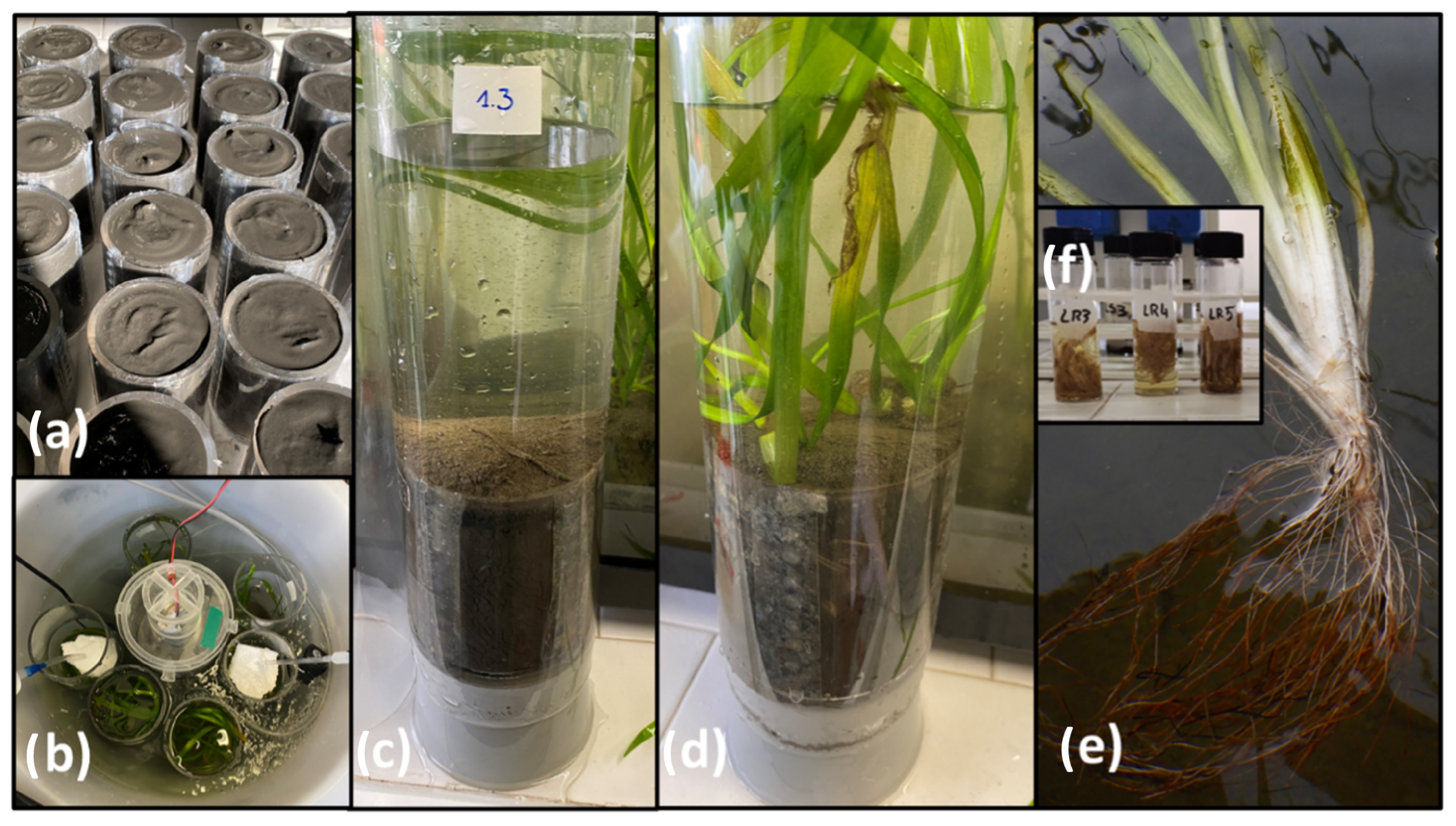
2.1. Sediment, Plants and Water Sampling and Microcosms Setup
2.2. Light-Dark Incubations
2.3. Sediment and Pore Water Feature Analyses
2.4. Root Analyses
2.5. Statistical Analyses
3. Results and Discussion
3.1. Sedimentary Features and Macrophytes Plasticity
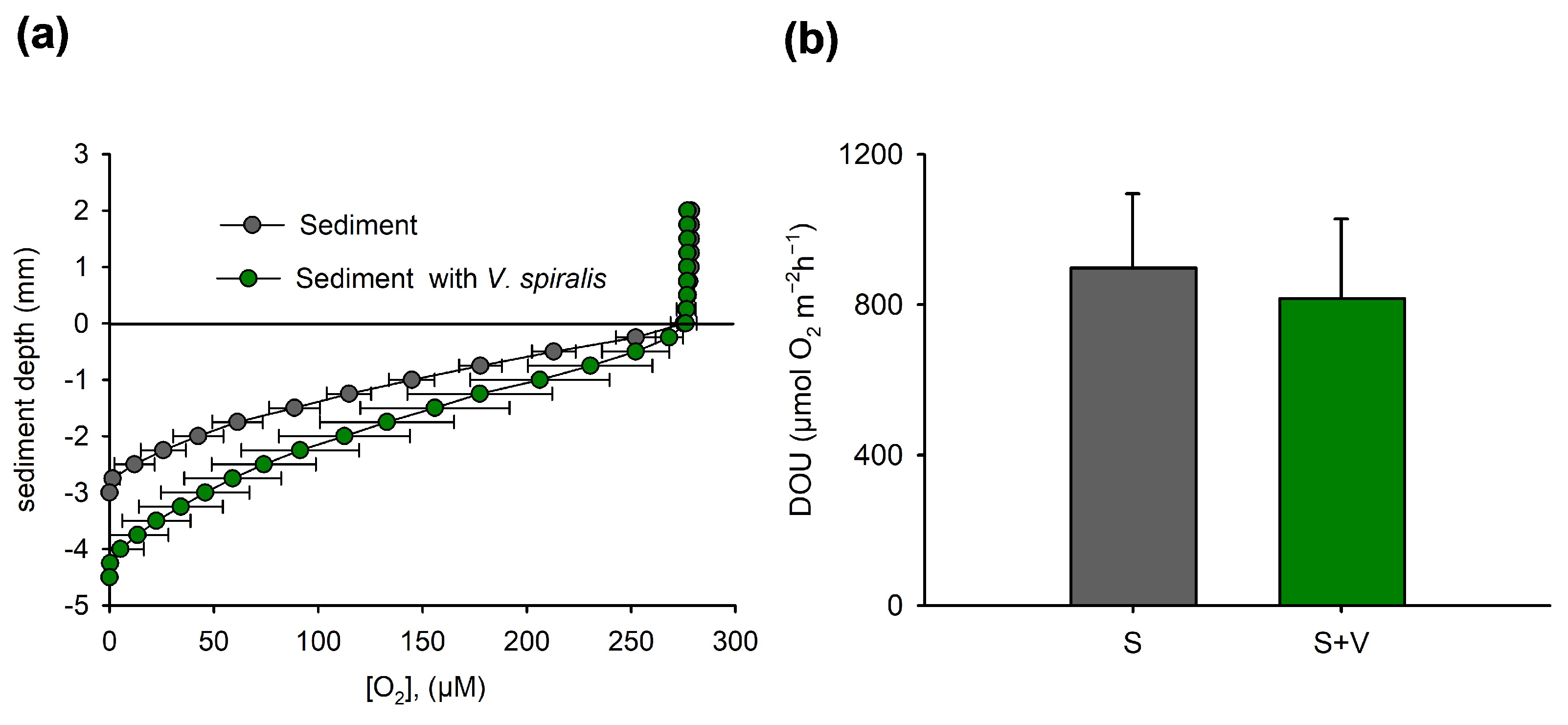
3.2. Oxygen Profiles and Diffusive O2 Uptake in Bare and Vegetated Sediments
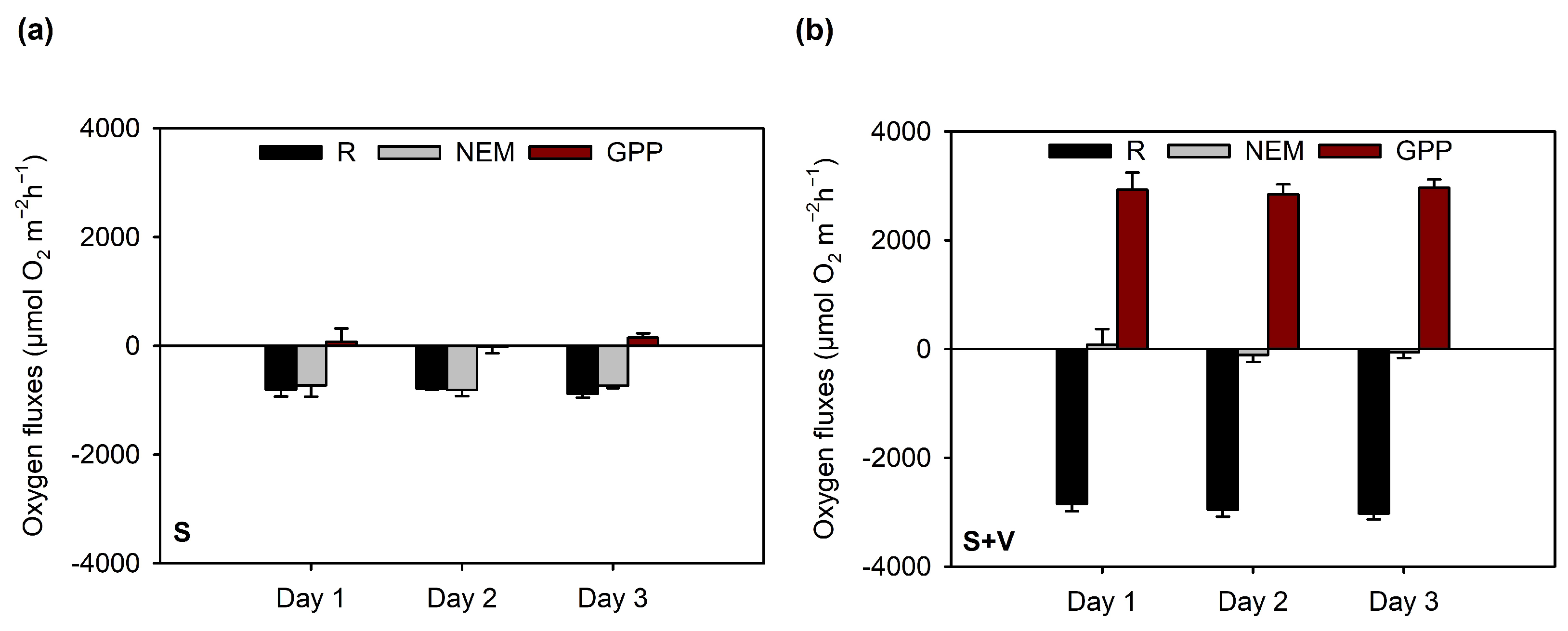
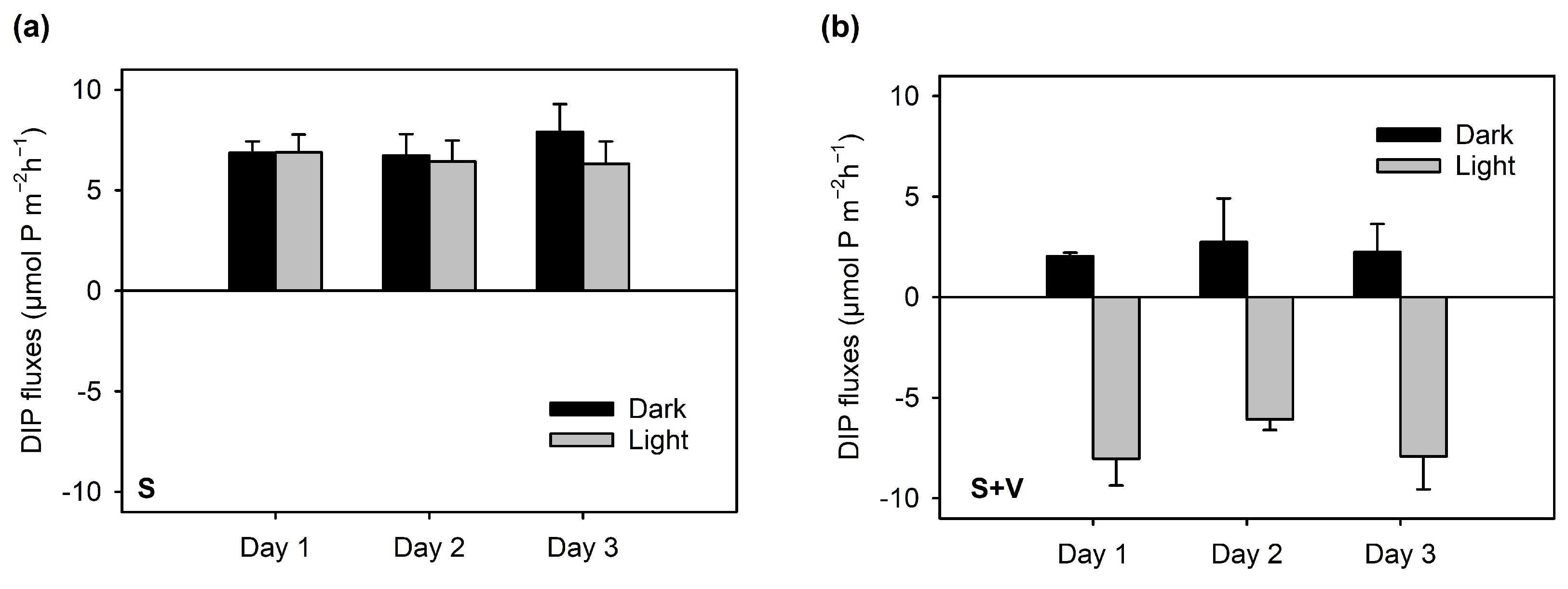
3.3. Net O2 and DIP Fluxes Measured in the Light and the Dark
3.4. Pore Water Features in Bare and Vegetated Sediments
3.5. Total P, Fe, and Mn within and on Roots
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DIP | Dissolved Inorganic Phosphorus |
| DOU | Diffusive Oxygen Uptake |
| GPP | Gross Primary Production |
| NEM | Net Ecosystem Metabolism |
| NPP | Net Primary Production |
| OPD | Oxygen Penetration Depth |
| ROL | Radial Oxygen Loss |
References
- Nixon, S.W. Coastal marine eutrophication: A definition, social causes, and future concerns. Ophelia 1995, 41, 199–219. [Google Scholar] [CrossRef]
- Nixon, S.W. Eutrophication and the macroscope. Hydrobiologia 2009, 629, 5–19. [Google Scholar] [CrossRef]
- Fiskal, A.; Deng, L.; Michel, A.; Eickenbusch, P.; Han, X.; Lagostina, L.; Zhu, R.; Sander, M.; Schroth, M.H.; Bernasconi, S.M.; et al. Effects of eutrophication on sedimentary organic carbon cycling in five temperate lakes. Biogeosciences 2019, 16, 3725–3746. [Google Scholar] [CrossRef]
- D’Angelo, E.M.; Reddy, K.R. Diagenesis of organic matter in a wetland receiving hypereutrophic Lake water: I. distribution of dissolved nutrients in the soil and water column. J. Environ. Qual. 1994, 23, 928–936. [Google Scholar] [CrossRef]
- Phillips, G.; Bramwell’, A.; Pitt, J.; Stansfield, J.; Perrow, M. Practical application of 25 years’ research into the management of shallow lakes. Hydrobiologia 1999, 395, 61–76. [Google Scholar] [CrossRef]
- Sas, H. Lake Restoration by Reduction of Nutrient Loading: Expectations, Experiences, Extrapolations; Academia Verlag: Berlin, Germany, 1989. [Google Scholar]
- Conley, D.J.; Quigley, M.A.; Schelske, C.L. Silica and phosphorus flux from sediments: Importance of internal recycling in Lake Michigan. Can. J. Fish. Aquat. Sci. 1988, 45, 1030–1035. [Google Scholar] [CrossRef]
- Tay, C.J.; Koh, H.L.; Mohd, M.H.; Teh, S.Y. Assessing the role of internal phosphorus recycling on eutrophication in four lakes in China and Malaysia. Ecol. Inform. 2022, 72, 101830. [Google Scholar] [CrossRef]
- Anderson, H.S.; Johengen, T.H.; Miller, R.; Godwin, C.M. Accelerated sediment phosphorus release in Lake Erie’s central basin during seasonal anoxia. Limnol. Oceanogr. 2021, 66, 3582–3595. [Google Scholar] [CrossRef]
- Mucci, M.; Maliaka, V.; Noyma, N.P.; Marinho, M.M.; Lürling, M. Assessment of possible solid-phase phosphate sorbents to mitigate eutrophication: Influence of pH and anoxia. Sci. Total Environ. 2018, 619–620, 1431–1440. [Google Scholar] [CrossRef]
- Wang, D.; Gan, X.; Wang, Z.; Jiang, S.; Zheng, X.; Zhao, M.; Zhang, Y.; Fan, C.; Wu, S.; Du, L. Research status on remediation of eutrophic water by submerged macrophytes: A review. Process. Saf. Environ. Prot. 2023, 169, 671–684. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Yan, Z.; Chao, C.; Yu, H.; Yu, D.; Liu, C. Effectiveness of dredging on internal phosphorus loading in a typical aquacultural lake. Sci. Total Environ. 2020, 744, 140883. [Google Scholar] [CrossRef]
- Zamparas, M.; Zacharias, I. Restoration of eutrophic freshwater by managing internal nutrient loads. A review. Sci. Total Environ. 2014, 496, 551–562. [Google Scholar] [CrossRef]
- Knopik, J.M.; Newman, R.M. Transplanting aquatic macrophytes to restore the littoral community of a eutrophic lake after the removal of common carp. Lake Reserv. Manag. 2018, 34, 365–375. [Google Scholar] [CrossRef]
- Barko, J.W.; Smart, R.M. Sediment-Related Mechanisms of Growth Limitation in Submersed Macrophytes. Ecology 1986, 67, 1328–1340. [Google Scholar] [CrossRef]
- Egertson, C.J.; Kopaska, J.A.; Downing, J.A. A century of change in macrophyte abundance and composition in response to agricultural eutrophication. Hydrobiologia 2004, 524, 145–156. [Google Scholar] [CrossRef]
- Geurts, J.J.; Sarneel, J.M.; Willers, B.J.; Roelofs, J.G.; Verhoeven, J.T.; Lamers, L.P. Interacting effects of sulphate pollution, sulphide toxicity and eutrophication on vegetation development in fens: A mesocosm experiment. Environ. Pollut. 2009, 157, 2072–2081. [Google Scholar] [CrossRef] [PubMed]
- Terrados, J.; Duarte, C.; Kamp-Nielsen, L.; Agawin, N.; Gacia, E.; Lacap, D.; Borum, J.; Lubanski, M.; Greve, T. Are seagrass growth and survival constrained by the reducing conditions of the sediment? Aquat. Bot. 1999, 65, 175–197. [Google Scholar] [CrossRef]
- Sand-Jensen, K.; Borum, J.; Binzer, T. Oxygen stress and reduced growth of Lobelia dortmanna in sandy lake sediments subject to organic enrichment. Freshw. Biol. 2005, 50, 1034–1048. [Google Scholar] [CrossRef]
- Preiner, S.; Dai, Y.; Pucher, M.; Reitsema, R.E.; Schoelynck, J.; Meire, P.; Hein, T. Effects of macrophytes on ecosystem metabolism and net nutrient uptake in a groundwater fed lowland river. Sci. Total Environ. 2020, 721, 137620. [Google Scholar] [CrossRef]
- Wu, L.; Kellogg, L.; Devol, A.H.; Tiedje, J.M.; Zhou, J. Microarray-based characterization of microbial community functional structure and heterogeneity in marine sediments from the Gulf of Mexico. Appl. Environ. Microbiol. 2008, 74, 4516–4529. [Google Scholar] [CrossRef]
- Xue, L.; Ren, H.; Li, S.; Leng, X.; Yao, X. Soil bacterial community structure and co-occurrence pattern during vegetation restoration in karst rocky desertification area. Front. Microbiol. 2017, 8, 2377. [Google Scholar] [CrossRef]
- Lemoine, D.G.; Mermillod-Blondin, F.; Barrat-Segretain, M.H.; Massé, C.; Malet, E. The ability of aquatic macrophytes to increase root porosity and radial oxygen loss determines their resistance to sediment anoxia. Aquat. Ecol. 2012, 46, 191–200. [Google Scholar] [CrossRef]
- Fritioff, A.; Kautsky, L.; Greger, M. Influence of temperature and salinity on heavy metal uptake by submersed plants. Environ. Pollut. 2005, 133, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.J. Paradigms of metal accumulation in rooted aquatic vascular plants. Sci. Total Environ. 1998, 219, 223–231. [Google Scholar] [CrossRef]
- Marchand, L.; Mench, M.; Jacob, D.L.; Otte, M.L. Metal and metalloid removal in constructed wetlands, with emphasis on the importance of plants and standardized measurements: A review. Environ. Pollut. 2010, 158, 3447–3461. [Google Scholar] [CrossRef] [PubMed]
- Brix, H. Do macrophytes play a role in constructed treatment wetlands? Water Sci. Technol. 1997, 35, 11–17. [Google Scholar] [CrossRef]
- Human, L.R.; Snow, G.C.; Adams, J.B.; Bate, G.C.; Yang, S.C. The role of submerged macrophytes and macroalgae in nutrient cycling: A budget approach. Estuar. Coast. Shelf Sci. 2015, 154, 169–178. [Google Scholar] [CrossRef]
- Malec, P.; Mysliwa-Kurdziel, B.; Prasad, M.N.V.; Waloszek, A.; Strzałka, K. Role of aquatic macrophytes in biogeochemical cycling of heavy metals, relevance to soil-sediment continuum detoxification and ecosystem health. In Detoxification of Heavy Metals; Springer: Berlin/Heidelberg, Germany, 2011; pp. 345–368. [Google Scholar] [CrossRef]
- Cronk, J.K.; Fennessy, M.S. Wetland Plants: Biology and Ecology; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Wigand, C.; Wehr, J.; Limburg, K.; Gorham, B.; Longergan, S.; Findlay, S. Effect of Vallisneria americana (L.) on community structure and ecosystem function in lake mesocosms. Hydrobiologia 2000, 418, 137–146. [Google Scholar] [CrossRef]
- Les, D.H.; Jacobs, S.W.L.; Tippery, N.P.; Chen, L.; Moody, M.L.; Wilstermann-Hildebrand, M. Systematics of Vallisneria (Hydrocharitaceae). Syst. Bot. 2008, 33, 49–65. [Google Scholar] [CrossRef]
- Soana, E.; Naldi, M.; Bonaglia, S.; Racchetti, E.; Castaldelli, G.; Brüchert, V.; Viaroli, P.; Bartoli, M. Benthic nitrogen metabolism in a macrophyte meadow (Vallisneria spiralis L.) under increasing sedimentary organic matter loads. Biogeochemistry 2015, 124, 387–404. [Google Scholar] [CrossRef]
- Wang, J.; Yu, D. Influence of sediment fertility on morphological variability of Vallisneria spiralis L. Aquat. Bot. 2007, 87, 127–133. [Google Scholar] [CrossRef]
- Xie, Y.; An, S.; Yao, X.; Xiao, K.; Zhang, C. Short-time response in root morphology of Vallisneria natans to sediment type and water-column nutrient. Aquat. Bot. 2005, 81, 85–96. [Google Scholar] [CrossRef]
- Yan, Z.S.; Guo, H.Y.; Song, T.S.; Hu, Y.; Jiang, H.L. Tolerance and remedial function of rooted submersed macrophyte Vallisneria spiralis to phenanthrene in freshwater sediments. Ecol. Eng. 2011, 37, 123–127. [Google Scholar] [CrossRef]
- Marzocchi, U.; Benelli, S.; Larsen, M.; Bartoli, M.; Glud, R.N. Spatial heterogeneity and short-term oxygen dynamics in the rhizosphere of Vallisneria spiralis: Implications for nutrient cycling. Freshw. Biol. 2019, 64, 532–543. [Google Scholar] [CrossRef]
- Soana, E.; Bartoli, M. Seasonal variation of radial oxygen loss in Vallisneria spiralis L.: An adaptive response to sediment redox? Aquat. Bot. 2013, 104, 228–232. [Google Scholar] [CrossRef]
- Pinardi, M.; Bartoli, M.; Longhi, D.; Marzocchi, U.; Laini, A.; Ribaudo, C.; Viaroli, P. Benthic metabolism and denitrification in a river reach: A comparison between vegetated and bare sediments. J. Limnol. 2009, 68, 133–145. [Google Scholar] [CrossRef]
- Racchetti, E.; Longhi, D.; Ribaudo, C.; Soana, E.; Bartoli, M. Nitrogen uptake and coupled nitrification-denitrification in riverine sediments with benthic microalgae and rooted macrophytes. Aquat. Sci. 2017, 79, 487–505. [Google Scholar] [CrossRef]
- Hupfer, M.; Dollan, A. Immobilisation of phosphorus by iron-coated roots of submerged macrophytes. Hydrobiologia 2003, 506–509, 635–640. [Google Scholar] [CrossRef]
- Han, C.; Ren, J.; Wang, Z.; Yang, S.; Ke, F.; Xu, D.; Xie, X. Characterization of phosphorus availability in response to radial oxygen losses in the rhizosphere of Vallisneria spiralis. Chemosphere 2018, 208, 740–748. [Google Scholar] [CrossRef]
- Stocum, E.T.; Plante, C.J. The effect of artificial defaunation on bacterial assemblages of intertidal sediments. J. Exp. Mar. Biol. Ecol. 2006, 337, 147–158. [Google Scholar] [CrossRef]
- Dalsgaard, T.; Nielsen, L.P.; Brotas, V.; Viaroli, P.; Underwood, G.J.C.; Nedwell, D.B.; Sundback, K.; Rysgaard, S.; Miles, A.; Bartoli, M.; et al. Protocol Handbook for NICE-Nitrogen Cycling in Estuaries: A Project under the EU Research Programme: Marine Science and Technology (MAST III); Department of Lake and Estuarine Ecology, Ministry of Environment and Energy National Environmental Research Institute: Silkeborg, Denmark, 2000. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewaters, 18th ed.; APHA: Washington, DC, USA; New York, NY, USA, 1992. [Google Scholar]
- Valderrama, J.C. Methods Used by the Hydrographic Department of the National Board of Fisheries; Grasshoff, K., Ed.; Interim Commission for the Protection of the Environment of the Baltic Sea: Goteborg, Sweden, 1977. [Google Scholar]
- Bartoli, M.; Longhi, D.; Nizzoli, D.; Como, S.; Magni, P.; Viaroli, P. Short term effects of hypoxia and bioturbation on solute fluxes, denitrification and buffering capacity in a shallow dystrophic pond. J. Exp. Mar. Biol. Ecol. 2009, 381, 105–113. [Google Scholar] [CrossRef]
- Rasmussen, H.; Jørgensen, B.B. Microelectrode studies of seasonal oxygen uptake in a coastal sediment: Role of molecular. Mar. Ecol. Prog. Ser. 1992, 81, 289–303. [Google Scholar] [CrossRef]
- Nielsen, L.P.; Christensen, P.B.; Revsbech, N.P.; Sørensen, J. Denitrification and oxygen respiration in biofilms studied with a microsensor for nitrous oxide and oxygen. Microb. Ecol. 1990, 19, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Ruttenberg, C. Development of a sequential extraction method for different forms of phosphorus in marine sediments. Limnol. Oceanogr. 1992, 37, 1460–1482. [Google Scholar] [CrossRef]
- Aspila, K.I.; Agemian, H.; Chau, A.S. A semi-automated method for the determination of inorganic, organic and total phosphate in sediments. Analyst 1976, 101, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Ribaudo, C.; Bartoli, M.; Racchetti, E.; Longhi, D.; Viaroli, P. Seasonal fluxes of O2, DIC and CH4 in sediments with Vallisneria spiralis: Indications for radial oxygen loss. Aquat. Bot. 2011, 94, 134–142. [Google Scholar] [CrossRef]
- Caffrey, J.M.; Kemp, W.M. Seasonal and spatial patterns of oxygen production, respiration and root-rhizome release in Potamogeton perfoliatus L. and Zostera marina L. Aquat. Bot. 1991, 40, 109–128. [Google Scholar] [CrossRef]
- Colmer, T.D. Long-distance transport of gases in plants: A perspective on internal aeration and radial oxygen loss from roots. Plant Cell Environ. 2003, 26, 17–36. [Google Scholar] [CrossRef]
- Pezeshki, S.R. Wetland plant responses to soil flooding. Environ. Exp. Bot. 2001, 46, 299–312. [Google Scholar] [CrossRef]
- Aldridge, K.T.; Ganf, G.G. Modification of sediment redox potential by three contrasting macrophytes: Implications for phosphorus adsorption/desorption. Mar. Freshw. Res. 2003, 54, 87–94. [Google Scholar] [CrossRef]
- Delgard, M.L.; Deflandre, B.; Bernard, G.; Richard, M.; Kochoni, E.; Charbonnier, C.; Cesbron, F.; Metzger, E.; Grémare, A.; Anschutz, P. Benthic oxygen exchange over a heterogeneous Zostera noltei meadow in a temperate coastal ecosystem. Mar. Ecol. Prog. Ser. 2016, 543, 55–71. [Google Scholar] [CrossRef]
- Barko, J.W.; Michael, R. Mobilization of sediment phosphorus by submersed freshwater macrophytes. Freshw. Biol. 1980, 10, 229–238. [Google Scholar] [CrossRef]
- Carignan, R.; Kalff, J. Phosphorus sources for aquatic weeds: Water or sediments? Science 1980, 29, 987–989. [Google Scholar] [CrossRef] [PubMed]
- Rattray, M.R.; Howard-Williams, C.; Brown, J.M.A. Sediment and water as sources of nitrogen and phosphorus for submerged rooted aquatic macrophytes. Aquat. Bot. 1991, 40, 225–237. [Google Scholar] [CrossRef]
- Carignan, R. An empirical model to estimate the relative importance of roots in phosphorus uptake by aquatic macrophytes. Can. J. Fish. Aquat. Sci. 1982, 39, 243–247. [Google Scholar] [CrossRef]
- Gao, Y.N.; Liu, B.Y.; Xu, D.; Zhou, Q.H.; Hu, C.Y.; Ge, F.J.; Zhang, L.P.; Wu, Z.B. Introduction phenolic compounds exuded from two submerged freshwater macrophytes and their allelopathic effects on microcystis aeruginosa. Pol. J. Environ. Stud. 2011, 20, 1153–1159. [Google Scholar]
- Soana, E.; Naldi, M.; Bartoli, M. Effects of increasing organic matter loads on pore water features of vegetated (Vallisneria spiralis L.) and plant-free sediments. Ecol. Eng. 2012, 47, 141–145. [Google Scholar] [CrossRef]
- Wigand, C.; Finn, M.; Findlay, S.; Fischer, D. Submersed macrophyte effects on nutrient exchanges in riverine sediments. Estuaries 2001, 24, 398–406. [Google Scholar] [CrossRef]
- Laskov, C.; Herzog, C.; Lewandowski, J.; Hupfer, M. Miniaturized photometrical methods for the rapid analysis of phosphate, ammonium, ferrous iron, and sulfate in pore water of freshwater sediments. Limnol. Oceanogr. Methods 2007, 4, 63–71. [Google Scholar] [CrossRef]
- Batty, L.C.; Baker, A.J.; Wheeler, B.D. Aluminium and phosphate uptake by Phragmites australis: The role of Fe, Mn and Al root plaques. Ann. Bot. 2002, 89, 443–449. [Google Scholar] [CrossRef]
- Jiang, F.Y.; Chen, X.; Luo, A.C. Iron plaque formation on wetland plants and its influence on phosphorus, calcium and metal uptake. Aquat. Ecol. 2009, 43, 879–890. [Google Scholar] [CrossRef]
- Zandi, P.; Yang, J.; Darma, A.; Bloem, E.; Xia, X.; Wang, Y.; Li, Q.; Schnug, E. Iron plaque formation, characteristics, and its role as a barrier and/or facilitator to heavy metal uptake in hydrophyte rice (Oryza sativa L.). Environ. Geochem. Health 2023, 45, 525–559. [Google Scholar] [CrossRef] [PubMed]
- Brodersen, K.E.; Koren, K.; Moßhammer, M.; Ralph, P.J.; Kühl, M.; Santner, J. Seagrass-mediated phosphorus and iron solubilization in tropical sediments. Environ. Sci. Technol. 2017, 51, 14155–14163. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.; Ding, S.; Liu, L.; Chen, M.; Yan, W.; Zhao, L.; Zhang, C. Direct evidence for the enhanced acquisition of phosphorus in the rhizosphere of aquatic plants: A case study on Vallisneria natans. Sci. Total Environ. 2018, 616–617, 386–396. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Chao, C.; Yu, H.; Yu, D.; Liu, C. Submerged macrophytes successfully restored a subtropical aquacultural lake by controlling its internal phosphorus loading. Environ. Pollut. 2021, 268, 115949. [Google Scholar] [CrossRef]
- Ozimek, T.; Kowalczewski, A. Long-term changes of the submerged macrophytes in eutrophic Lake Mikolajskie (Poland). Aquat. Bot. 1984, 19, 1–11. [Google Scholar] [CrossRef]



| Parameter | Source of Variation | DF | SS | MS | F | p |
|---|---|---|---|---|---|---|
| O2 fluxes | Treatment (S vs. S + V) | 1 | 8,625,871.36 | 8,625,871.36 | 72.83 | <0.001 |
| Condition (D vs. L) | 1 | 40,040,426.36 | 40,040,426.36 | 338.06 | <0.001 | |
| Time (D1 vs. D2 vs. D3) | 2 | 144,181.34 | 72,090.67 | 0.61 | 0.547 | |
| Treat. × Condit. | 1 | 36,375,693.71 | 36,375,693.71 | 307.12 | <0.001 | |
| Treat. × Time | 2 | 54,762.21 | 27,381.10 | 0.23 | 0.794 | |
| Condit. × Time | 2 | 63,928.11 | 31,964.05 | 0.27 | 0.764 | |
| Treat. × Condit. × Time | 2 | 1722.28 | 861.14 | 0.007 | 0.993 | |
| Residual | 60 | 7,106,348.76 | 118,439.14 | |||
| Total | 71 | 92,412,934.15 | 1,301,590.62 | |||
| DIP fluxes | Treatment (S vs. S + V) | 1 | 1633.11 | 1633.11 | 182.96 | <0.001 |
| Condition (D vs. L) | 1 | 509.97 | 509.97 | 57.13 | <0.001 | |
| Time (D1 vs. D2 vs. D3) | 2 | 3.19 | 1.59 | 0.18 | 0.837 | |
| Treat. × Condit. | 1 | 342.94 | 342.94 | 38.42 | <0.001 | |
| Treat. × Time | 2 | 8.12 | 4.06 | 0.45 | 0.637 | |
| Condit. × Time | 2 | 16.70 | 8.35 | 0.93 | 0.398 | |
| Treat. × Condit. × Time | 2 | 7.21 | 3.60 | 0.40 | 0.669 | |
| Residual | 60 | 535.54 | 8.92 | |||
| Total | 71 | 3056.81 | 43.05 |
| Parameter | Source of Variation | DF | SS | MS | F | p |
|---|---|---|---|---|---|---|
| pH | Between groups | 1 | 0.210 | 0.210 | 46.722 | <0.001 |
| Residual | 8 | 0.0360 | 0.0045 | |||
| Total | 9 | 0.246 | ||||
| ORP | Between groups | 1 | 27,878.4 | 27,878.4 | 198.776 | <0.001 |
| Residual | 8 | 1122.0 | 140.25 | |||
| Total | 9 | 29,000.4 | ||||
| DIP | Between groups | 1 | 2969.69 | 2968.69 | 637.736 | <0.001 |
| Residual | 8 | 37.253 | 4.657 | |||
| Total | 9 | 3006.943 | ||||
| Fe2+ | Between groups | 1 | 5326.652 | 5326.652 | 113.655 | <0.001 |
| Residual | 8 | 374.936 | 46.867 | |||
| Total | 9 | 5701.587 | ||||
| Mn2+ | Between groups | 1 | 29.872 | 29.872 | 126.319 | <0.001 |
| Residual | 8 | 1.892 | 0.236 | |||
| Total | 9 | 31.764 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magri, M.; Benelli, S.; Bartoli, M. Vallisneria spiralis Promotes P and Fe Retention via Radial Oxygen Loss in Contaminated Sediments. Water 2023, 15, 4222. https://doi.org/10.3390/w15244222
Magri M, Benelli S, Bartoli M. Vallisneria spiralis Promotes P and Fe Retention via Radial Oxygen Loss in Contaminated Sediments. Water. 2023; 15(24):4222. https://doi.org/10.3390/w15244222
Chicago/Turabian StyleMagri, Monia, Sara Benelli, and Marco Bartoli. 2023. "Vallisneria spiralis Promotes P and Fe Retention via Radial Oxygen Loss in Contaminated Sediments" Water 15, no. 24: 4222. https://doi.org/10.3390/w15244222





