Wastewater-Based Epidemiology: Assessing Illicit Drug Usage and Impact through an Innovative Approach
Abstract
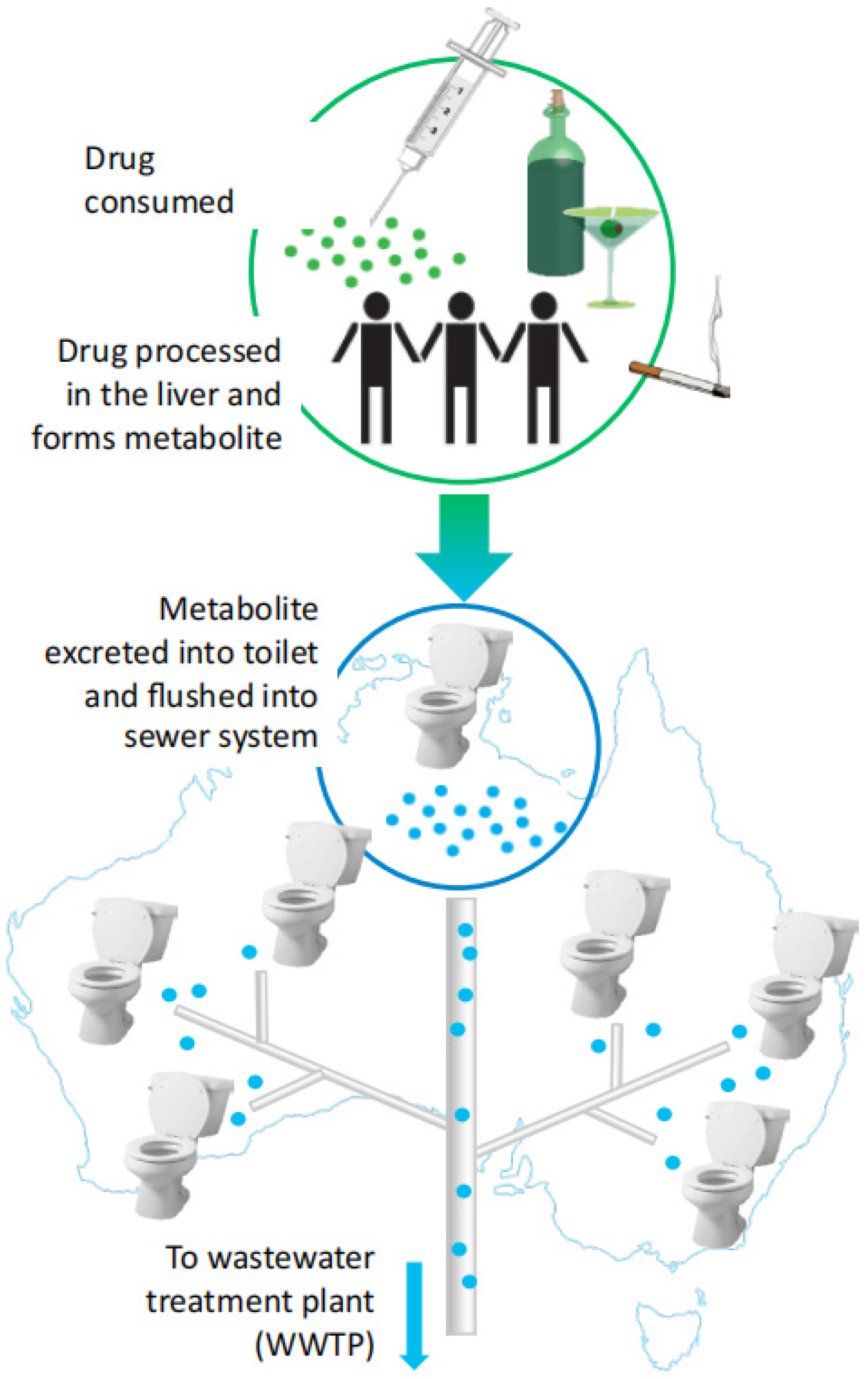
:1. Introduction
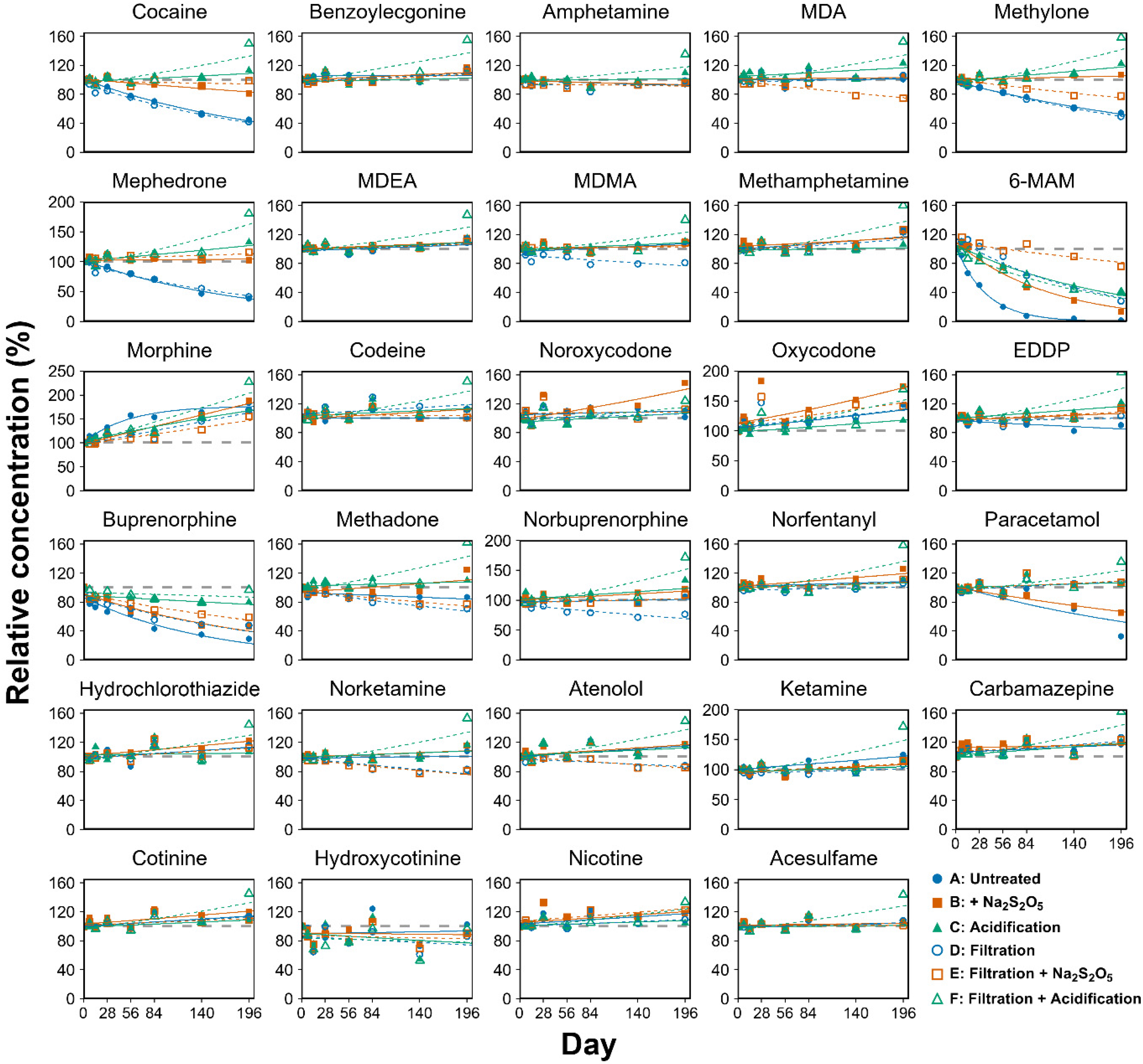
2. Biomarkers and Their Stability
| Drug Consumed | Biomarkers Measured | Stability | Reference |
|---|---|---|---|
| Methylamphetamine | Methylamphetamine | High | [20] |
| Amphetamine | Variable | ||
| Amphetamine | Amphetamine | Variable | [36] |
| MDMA | MDMA | High | [34] |
| MDA | High | ||
| Heroin | Morphine | High | [37] |
| Codeine | High | ||
| Cocaine | Cocaine | Low | [38] |
| Benzoylecgonine | Medium–high | ||
| THC | THC-COOH | Variable | [39] |
| THC-OH | |||
| Ketamine | Ketamine | High | [40] |
| Norketamine | High |
3. Analytical Techniques
3.1. Chromatography–Mass Spectrometry
3.2. Optical Methods
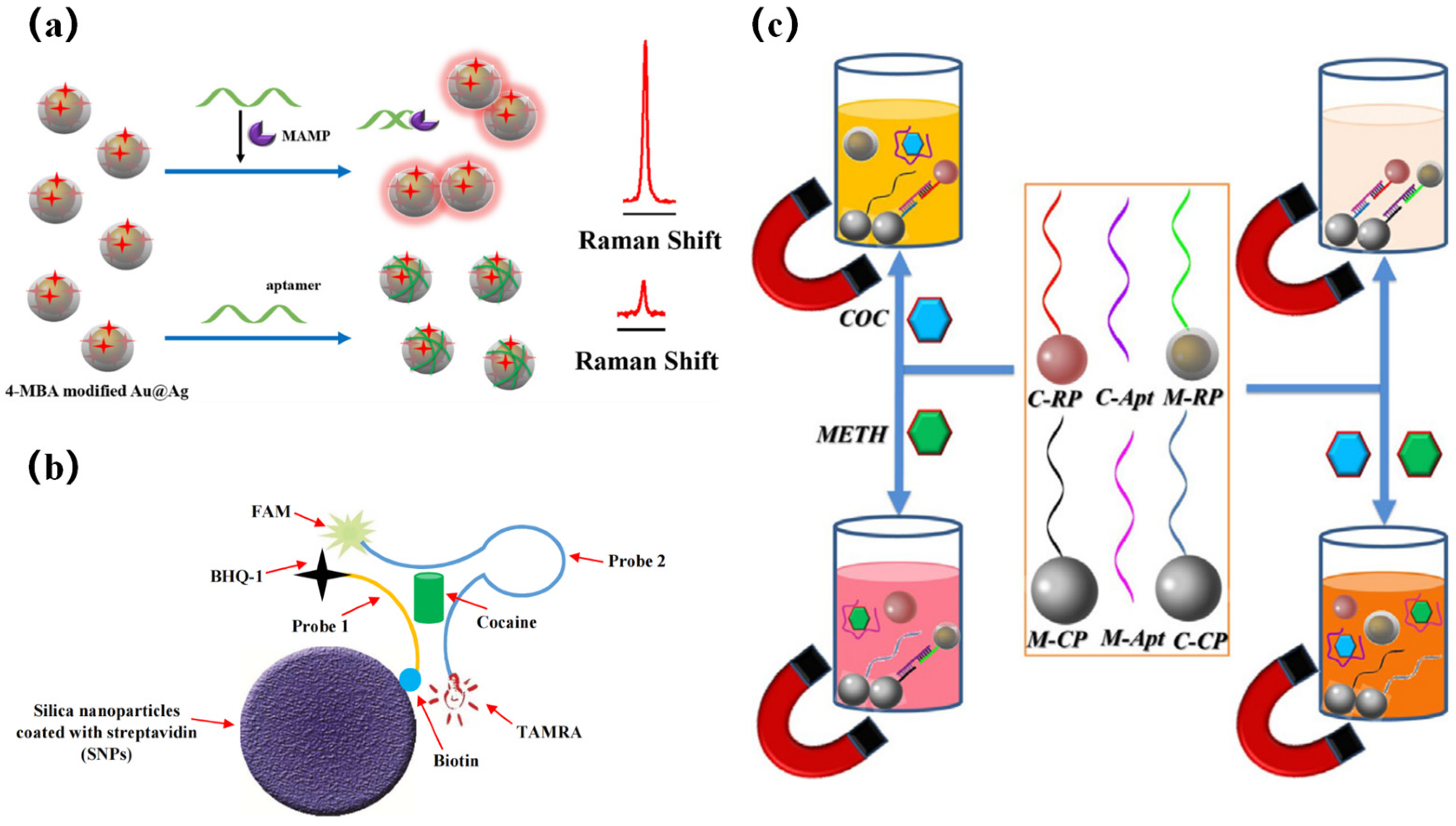
3.3. Electrochemical Strategies
4. Illicit Drug Abuse Assessment
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mounteney, J.; Griffiths, P.; Sedefov, R.; Noor, A.; Vicente, J.; Simon, R. The drug situation in Europe: An overview of data available on illicit drugs and new psychoactive substances from European monitoring in 2015. Addiction 2016, 111, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Nwogu, M.I.O. Drug Abuse and Crime–The Challenges to Nation Building. Am. J. Law 2022, 4, 57–65. [Google Scholar] [CrossRef]
- Daughton, C.G. Emerging pollutants, and communicating the science of environmental chemistry and mass spectrometry: Pharmaceuticals in the environment. J. Am. Soc. Mass Spectrom. 2001, 12, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Australian Criminal Intelligence Commission. National Wastewater Drug Monitoring Program: Report 1, March 2017; Australian Criminal Intelligence Commission: Canberra City, ACT, Australia, 2017.
- Boogaerts, T.; Jurgelaitiene, L.; Dumitrascu, C.; Kasprzyk-Hordern, B.; Kannan, A.; Been, F.; Emke, E.; de Voogt, P.; Covaci, A.; van Nuijs, A.L. Application of wastewater-based epidemiology to investigate stimulant drug, alcohol and tobacco use in Lithuanian communities. Sci. Total Environ. 2021, 777, 145914. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Tscharke, B.; O’Brien, J.; Gerber, C.; Mackie, R.; Gao, J.; Thai, P. Uncertainties in estimating alcohol and tobacco consumption by wastewater-based epidemiology. Curr. Opin. Environ. Sci. Health 2019, 9, 13–18. [Google Scholar] [CrossRef]
- Driver, E.M.; Gushgari, A.; Chen, J.; Halden, R.U. Alcohol, nicotine, and caffeine consumption on a public US university campus determined by wastewater-based epidemiology. Sci. Total Environ. 2020, 727, 138492. [Google Scholar] [CrossRef]
- Senta, I.; Rodríguez-Mozaz, S.; Corominas, L.; Petrovic, M. Wastewater-based epidemiology to assess human exposure to personal care and household products–A review of biomarkers, analytical methods, and applications. Trends Environ. Anal. Chem. 2020, 28, e00103. [Google Scholar] [CrossRef]
- Senta, I.; Rodríguez-Mozaz, S.; Corominas, L.; Covaci, A.; Petrovic, M. Applicability of an on-line solid-phase extraction liquid chromatography–Tandem mass spectrometry for the wastewater-based assessment of human exposure to chemicals from personal care and household products. Sci. Total Environ. 2022, 845, 157309. [Google Scholar] [CrossRef]
- Boogaerts, T.; Ahmed, F.; Choi, P.M.; Tscharke, B.; O’Brien, J.; De Loof, H.; Gao, J.; Thai, P.; Thomas, K.; Mueller, J.F. Current and future perspectives for wastewater-based epidemiology as a monitoring tool for pharmaceutical use. Sci. Total Environ. 2021, 789, 148047. [Google Scholar] [CrossRef]
- Kim, K.Y.; Oh, J.-E. Evaluation of pharmaceutical abuse and illicit drug use in South Korea by wastewater-based epidemiology. J. Hazard. Mater. 2020, 396, 122622. [Google Scholar] [CrossRef]
- Mackie, R.S.; Tscharke, B.J.; O’Brien, J.W.; Choi, P.M.; Gartner, C.E.; Thomas, K.V.; Mueller, J.F. Trends in nicotine consumption between 2010 and 2017 in an Australian city using the wastewater-based epidemiology approach. Environ. Int. 2019, 125, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Verovšek, T.; Heath, D.; Heath, E. Occurrence, fate and determination of tobacco (nicotine) and alcohol (ethanol) residues in waste-and environmental waters. Trends Environ. Anal. Chem. 2022, 34, e00164. [Google Scholar] [CrossRef]
- Burgard, D.A.; Williams, J.; Westerman, D.; Rushing, R.; Carpenter, R.; LaRock, A.; Sadetsky, J.; Clarke, J.; Fryhle, H.; Pellman, M. Using wastewater-based analysis to monitor the effects of legalized retail sales on cannabis consumption in Washington State, USA. Addiction 2019, 114, 1582–1590. [Google Scholar] [CrossRef] [PubMed]
- Choi, P.M.; Tscharke, B.; Samanipour, S.; Hall, W.D.; Gartner, C.E.; Mueller, J.F.; Thomas, K.V.; O’Brien, J.W. Social, demographic, and economic correlates of food and chemical consumption measured by wastewater-based epidemiology. Proc. Natl. Acad. Sci. USA 2019, 116, 21864–21873. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zheng, X.; Li, S.; Lam, N.S.; Wang, Y.; Chu, D.K.; Poon, L.L.; Tun, H.M.; Peiris, M.; Deng, Y. The first case study of wastewater-based epidemiology of COVID-19 in Hong Kong. Sci. Total Environ. 2021, 790, 148000. [Google Scholar] [CrossRef] [PubMed]
- Gagliano, E.; Biondi, D.; Roccaro, P. Wastewater-based epidemiology approach: The learning lessons from COVID-19 pandemic and the development of novel guidelines for future pandemics. Chemosphere 2023, 313, 137361. [Google Scholar] [CrossRef] [PubMed]
- Zuccato, E.; Chiabrando, C.; Castiglioni, S.; Bagnati, R.; Fanelli, R. Estimating community drug abuse by wastewater analysis. Environ. Health Perspect. 2008, 116, 1027–1032. [Google Scholar] [CrossRef]
- Choi, P.M.; Tscharke, B.J.; Donner, E.; O’Brien, J.W.; Grant, S.C.; Kaserzon, S.L.; Mackie, R.; O’Malley, E.; Crosbie, N.D.; Thomas, K.V. Wastewater-based epidemiology biomarkers: Past, present and future. TrAC Trends Anal. Chem. 2018, 105, 453–469. [Google Scholar] [CrossRef]
- Shao, X.-T.; Liu, Y.-S.; Tan, D.-Q.; Wang, Z.; Zheng, X.-Y.; Wang, D.-G. Methamphetamine use in typical Chinese cities evaluated by wastewater-based epidemiology. Environ. Sci. Pollut. Res. 2020, 27, 8157–8165. [Google Scholar] [CrossRef]
- González-Mariño, I.; Baz-Lomba, J.A.; Alygizakis, N.A.; Andrés-Costa, M.J.; Bade, R.; Bannwarth, A.; Barron, L.P.; Been, F.; Benaglia, L.; Berset, J.D. Spatio-temporal assessment of illicit drug use at large scale: Evidence from 7 years of international wastewater monitoring. Addiction 2020, 115, 109–120. [Google Scholar] [CrossRef]
- Skees, A.J.; Foppe, K.S.; Loganathan, B.; Subedi, B. Contamination profiles, mass loadings, and sewage epidemiology of neuropsychiatric and illicit drugs in wastewater and river waters from a community in the Midwestern United States. Sci. Total Environ. 2018, 631, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Rice, J.; Kannan, A.M.; Castrignanò, E.; Jagadeesan, K.; Kasprzyk-Hordern, B. Wastewater-based epidemiology combined with local prescription analysis as a tool for temporalmonitoring of drugs trends-A UK perspective. Sci. Total Environ. 2020, 735, 139433. [Google Scholar] [CrossRef] [PubMed]
- Bijlsma, L.; Botero-Coy, A.M.; Rincón, R.J.; Peñuela, G.A.; Hernández, F. Estimation of illicit drug use in the main cities of Colombia by means of urban wastewater analysis. Sci. Total Environ. 2016, 565, 984–993. [Google Scholar] [CrossRef]
- Metcalfe, C.; Tindale, K.; Li, H.; Rodayan, A.; Yargeau, V. Illicit drugs in Canadian municipal wastewater and estimates of community drug use. Environ. Pollut. 2010, 158, 3179–3185. [Google Scholar] [CrossRef] [PubMed]
- Ripanda, A.S.; Rwiza, M.J.; Nyanza, E.C.; Machunda, R.L.; Vuai, S.H. Contribution of illicit drug use to pharmaceutical load in the environment: A focus on Sub-Saharan Africa. J. Environ. Public Health 2022, 2022, 9056476. [Google Scholar] [CrossRef]
- Archer, E.; Volschenk, M.; Brocker, L.; Wolfaardt, G.M. A two-year study of emerging micro-pollutants and drugs of abuse in two Western Cape wastewater treatment works (South Africa). Chemosphere 2021, 285, 131460. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Tscharke, B.; O’Brien, J.; Mueller, J.F.; Wilkins, C.; Padhye, L.P. Assessment of drugs of abuse in a wastewater treatment plant with parallel secondary wastewater treatment train. Sci. Total Environ. 2019, 658, 947–957. [Google Scholar] [CrossRef]
- Ledgerwood, D.M.; Goldberger, B.A.; Risk, N.K.; Lewis, C.E.; Price, R.K. Comparison between self-report and hair analysis of illicit drug use in a community sample of middle-aged men. Addict. Behav. 2008, 33, 1131–1139. [Google Scholar] [CrossRef]
- Feng, L.-Y.; Yu, W.-J.; Chang, W.-T.; Han, E.; Chung, H.; Li, J.-H. Comparison of illegal drug use pattern in Taiwan and Korea from 2006 to 2014. Subst. Abus. Treat. Prev. Policy 2016, 11, 34. [Google Scholar] [CrossRef]
- Ti, L.; Ti, L. Leaving the hospital against medical advice among people who use illicit drugs: A systematic review. Am. J. Public Health 2015, 105, e53–e59. [Google Scholar] [CrossRef]
- Australian Criminal Intelligence Commission. National Wastewater Drug Monitoring Program: Report 14, October 2021; Australian Criminal Intelligence Commission: Canberra City, ACT, Australia, 2021.
- Cyranoski, D. Chinese cities scan sewers for signs of illegal drug use. Nature 2018, 559, 310–311. [Google Scholar] [CrossRef] [PubMed]
- González-Mariño, I.; Zuccato, E.; Santos, M.M.; Castiglioni, S. Monitoring MDMA metabolites in urban wastewater as novel biomarkers of consumption. Water Res. 2017, 115, 1–8. [Google Scholar] [CrossRef] [PubMed]
- McCall, A.-K.; Bade, R.; Kinyua, J.; Lai, F.Y.; Thai, P.K.; Covaci, A.; Bijlsma, L.; van Nuijs, A.L.; Ort, C. Critical review on the stability of illicit drugs in sewers and wastewater samples. Water Res. 2016, 88, 933–947. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Du, P.; Li, K.; Gao, T.; Wang, Z.; Fu, X.; Li, X. Tracing methamphetamine and amphetamine sources in wastewater and receiving waters via concentration and enantiomeric profiling. Sci. Total Environ. 2017, 601, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Thai, P.K.; Bai, Y.; Zhou, Z.; Xu, Z.; Zhang, X.; Wang, J.; Zhang, C.; Hao, F.; Li, X. Monitoring consumption of methadone and heroin in major Chinese cities by wastewater-based epidemiology. Drug Alcohol Depend. 2019, 205, 107532. [Google Scholar] [CrossRef]
- da Silva, K.M.; Quintana, J.B.; González-Mariño, I.; Rodil, R.; Gallassi, A.D.; Arantes, L.C.; Sodré, F.F. Assessing cocaine use patterns in the Brazilian Capital by wastewater-based epidemiology. Int. J. Environ. Anal. Chem. 2018, 98, 1370–1387. [Google Scholar] [CrossRef]
- Pandopulos, A.J.; Bade, R.; O’Brien, J.W.; Tscharke, B.J.; Mueller, J.F.; Thomas, K.; White, J.M.; Gerber, C. Towards an efficient method for the extraction and analysis of cannabinoids in wastewater. Talanta 2020, 217, 121034. [Google Scholar] [CrossRef]
- Liu, S.-Y.; Yu, W.-J.; Wang, Y.-R.; Shao, X.-T.; Wang, D.-G. Tracing consumption patterns of stimulants, opioids, and ketamine in China by wastewater-based epidemiology. Environ. Sci. Pollut. Res. 2021, 28, 16754–16766. [Google Scholar] [CrossRef]
- Castiglioni, S.; Bagnati, R.; Calamari, D.; Fanelli, R.; Zuccato, E. A multiresidue analytical method using solid-phase extraction and high-pressure liquid chromatography tandem mass spectrometry to measure pharmaceuticals of different therapeutic classes in urban wastewaters. J. Chromatogr. A 2005, 1092, 206–215. [Google Scholar] [CrossRef]
- Devault, D.A.; Lévi, Y.; Karolak, S. Applying sewage epidemiology approach to estimate illicit drug consumption in a tropical context: Bias related to sewage temperature and pH. Sci. Total Environ. 2017, 584, 252–258. [Google Scholar] [CrossRef]
- González-Mariño, I.; Quintana, J.B.; Rodríguez, I.; Cela, R. Determination of drugs of abuse in water by solid-phase extraction, derivatisation and gas chromatography–ion trap-tandem mass spectrometry. J. Chromatogr. A 2010, 1217, 1748–1760. [Google Scholar] [CrossRef] [PubMed]
- Heuett, N.V.; Ramirez, C.E.; Fernandez, A.; Gardinali, P.R. Analysis of drugs of abuse by online SPE-LC high resolution mass spectrometry: Communal assessment of consumption. Sci. Total Environ. 2015, 511, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Senta, I.; Krizman, I.; Ahel, M.; Terzic, S. Assessment of stability of drug biomarkers in municipal wastewater as a factor influencing the estimation of drug consumption using sewage epidemiology. Sci. Total Environ. 2014, 487, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Thai, P.K.; Jiang, G.; Gernjak, W.; Yuan, Z.; Lai, F.Y.; Mueller, J.F. Effects of sewer conditions on the degradation of selected illicit drug residues in wastewater. Water Res. 2014, 48, 538–547. [Google Scholar] [CrossRef]
- Lin, X.; Choi, P.M.; Thompson, J.; Reeks, T.; Verhagen, R.; Tscharke, B.J.; O’Malley, E.; Shimko, K.M.; Guo, X.; Thomas, K.V. Systematic evaluation of the in-sample stability of selected pharmaceuticals, illicit drugs, and their metabolites in wastewater. Environ. Sci. Technol. 2021, 55, 7418–7429. [Google Scholar] [CrossRef]
- Kumar, N.; Rana, M.; Geiwitz, M.; Khan, N.I.; Catalano, M.; Ortiz-Marquez, J.C.; Kitadai, H.; Weber, A.; Dweik, B.; Ling, X. Rapid, multianalyte detection of opioid metabolites in wastewater. ACS Nano 2022, 16, 3704–3714. [Google Scholar] [CrossRef]
- Mao, K.; Zhang, H.; Pan, Y.; Zhang, K.; Cao, H.; Li, X.; Yang, Z. Nanomaterial-based aptamer sensors for analysis of illicit drugs and evaluation of drugs consumption for wastewater-based epidemiology. TrAC Trends Anal. Chem. 2020, 130, 115975. [Google Scholar] [CrossRef]
- Bisceglia, K.J.; Kroening, G.; Subedi, B. GC-MS Methods for Monitoring Illicit Drug Biomarkers in Wastewater: A Critical Review. In Wastewater-Based Epidemiology: Estimation of Community Consumption of Drugs and Diets; ACS Publications: Washington, DC, USA, 2019; pp. 51–77. [Google Scholar]
- Chen, X.; Liu, S.; Jiang, R.; Luan, T.; Ouyang, G. Rapid detection and speciation of illicit drugs via a thin-film microextraction approach for wastewater-based epidemiology study. Sci. Total Environ. 2022, 842, 156888. [Google Scholar] [CrossRef]
- Chio, W.-I.K.; Xie, H.; Zhang, Y.; Lan, Y.; Lee, T.-C. SERS biosensors based on cucurbituril-mediated nanoaggregates for wastewater-based epidemiology. TrAC Trends Anal. Chem. 2022, 146, 116485. [Google Scholar] [CrossRef]
- Wang, Z.; Shao, X.-T.; Tan, D.-Q.; Yan, J.-H.; Xiao, Y.; Zheng, Q.-D.; Pei, W.; Wang, Z.; Wang, D.-G. Reduction in methamphetamine consumption trends from 2015 to 2018 detected by wastewater-based epidemiology in Dalian, China. Drug Alcohol Depend. 2019, 194, 302–309. [Google Scholar] [CrossRef]
- Hernández, F.; Castiglioni, S.; Covaci, A.; de Voogt, P.; Emke, E.; Kasprzyk-Hordern, B.; Ort, C.; Reid, M.; Sancho, J.V.; Thomas, K.V. Mass spectrometric strategies for the investigation of biomarkers of illicit drug use in wastewater. Mass Spectrom. Rev. 2018, 37, 258–280. [Google Scholar] [CrossRef] [PubMed]
- Kachhawaha, A.S.; Nagarnaik, P.M.; Labhasetwar, P.; Banerjee, K. A review of recently developed LC–MS/MS methods for the analysis of pharmaceuticals and personal care products in water. J. AOAC Int. 2020, 103, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Christophoridis, C.; Veloutsou, S.; Mitsika, E.; Zacharis, C.K.; Christia, C.; Raikos, N.; Fytianos, K. Determination of illicit drugs and psychoactive pharmaceuticals in wastewater from the area of Thessaloniki (Greece) using LC–MS/MS: Estimation of drug consumption. Environ. Monit. Assess. 2021, 193, 249. [Google Scholar] [CrossRef] [PubMed]
- Daglioglu, N.; Guzel, E.Y.; Kilercioglu, S. Assessment of illicit drugs in wastewater and estimation of drugs of abuse in Adana Province, Turkey. Forensic Sci. Int. 2019, 294, 132–139. [Google Scholar] [CrossRef]
- Wu, S.; Dong, Y.; Lin, B.; Shen, X.; Xiang, P.; Huang, C. Sensitive determination of illicit drugs in wastewater using enrichment bag-based liquid-phase microextraction and liquid-chromatography tandem mass spectrometry. J. Chromatogr. A 2022, 1661, 462684. [Google Scholar] [CrossRef]
- Asimakopoulos, A.G.; Kannan, P.; Higgins, S.; Kannan, K. Determination of 89 drugs and other micropollutants in unfiltered wastewater and freshwater by LC-MS/MS: An alternative sample preparation approach. Anal. Bioanal. Chem. 2017, 409, 6205–6225. [Google Scholar] [CrossRef]
- Hernández, F.; Ibáñez, M.; Botero-Coy, A.-M.; Bade, R.; Bustos-López, M.C.; Rincón, J.; Moncayo, A.; Bijlsma, L. LC-QTOF MS screening of more than 1,000 licit and illicit drugs and their metabolites in wastewater and surface waters from the area of Bogotá, Colombia. Anal. Bioanal. Chem. 2015, 407, 6405–6416. [Google Scholar] [CrossRef]
- Ort, C.; Lawrence, M.G.; Reungoat, J.; Mueller, J.F. Sampling for PPCPs in wastewater systems: Comparison of different sampling modes and optimization strategies. Environ. Sci. Technol. 2010, 44, 6289–6296. [Google Scholar] [CrossRef]
- Rongnan, Y.; Yan, W. Research progress on surface-enhanced Raman spectroscopy technique for the detection of microRNA. Acta Chim. Sin. 2021, 79, 694. [Google Scholar]
- Zhang, B.; Hou, X.; Zhen, C.; Wang, A.X. Sub-part-per-billion level sensing of fentanyl residues from wastewater using portable Surface-enhanced Raman scattering sensing. Biosensors 2021, 11, 370. [Google Scholar] [CrossRef]
- Mao, K.; Zhou, Z.; Han, S.; Zhou, X.; Hu, J.; Li, X.; Yang, Z. A novel biosensor based on Au@ Ag core-shell nanoparticles for sensitive detection of methylamphetamine with surface enhanced Raman scattering. Talanta 2018, 190, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Abnous, K.; Danesh, N.M.; Ramezani, M.; Taghdisi, S.M.; Emrani, A.S. A novel amplified double-quenching aptasensor for cocaine detection based on split aptamer and silica nanoparticles. Anal. Methods 2018, 10, 3232–3236. [Google Scholar] [CrossRef]
- Mao, K.; Ma, J.; Li, X.; Yang, Z. Rapid duplexed detection of illicit drugs in wastewater using gold nanoparticle conjugated aptamer sensors. Sci. Total Environ. 2019, 688, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Zanfrognini, B.; Pigani, L.; Zanardi, C. Recent advances in the direct electrochemical detection of drugs of abuse. J. Solid State Electrochem. 2020, 24, 2603–2616. [Google Scholar] [CrossRef]
- Hashemi, P.; Bagheri, H.; Afkhami, A.; Ardakani, Y.H.; Madrakian, T. Fabrication of a novel aptasensor based on three-dimensional reduced graphene oxide/polyaniline/gold nanoparticle composite as a novel platform for high sensitive and specific cocaine detection. Anal. Chim. Acta 2017, 996, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Zuccato, E.; Chiabrando, C.; Castiglioni, S.; Calamari, D.; Bagnati, R.; Schiarea, S.; Fanelli, R. Cocaine in surface waters: A new evidence-based tool to monitor community drug abuse. Environ. Health 2005, 4, 14. [Google Scholar] [CrossRef]
- Ambre, J. The urinary excretion of cocaine and metabolites in humans: A kinetic analysis of published data. J. Anal. Toxicol. 1985, 9, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Damien, D.A.; Thomas, N.; Hélène, P.; Sara, K.; Yves, L. First evaluation of illicit and licit drug consumption based on wastewater analysis in Fort de France urban area (Martinique, Caribbean), a transit area for drug smuggling. Sci. Total Environ. 2014, 490, 970–978. [Google Scholar] [CrossRef]
- Van Nuijs, A.L.; Mougel, J.-F.; Tarcomnicu, I.; Bervoets, L.; Blust, R.; Jorens, P.G.; Neels, H.; Covaci, A. Sewage epidemiology—A real-time approach to estimate the consumption of illicit drugs in Brussels, Belgium. Environ. Int. 2011, 37, 612–621. [Google Scholar] [CrossRef]
- Sodré, F.F.; Feitosa, R.S.; Jardim, W.F.; Maldaner, A.O. Wastewater-based epidemiology of cocaine in the Brazilian Federal District: Spatial distribution, weekly variation and sample preservation strategies. J. Braz. Chem. Soc. 2018, 29, 2287–2298. [Google Scholar] [CrossRef]
- Gao, J.; Burgard, D.A.; Tscharke, B.J.; Lai, F.Y.; O’Brien, J.W.; Nguyen, H.D.; Zheng, Q.; Li, J.; Du, P.; Li, X. Refining the estimation of amphetamine consumption by wastewater-based epidemiology. Water Res. 2022, 225, 119182. [Google Scholar] [CrossRef] [PubMed]
- Foppe, K.S.; Hammond-Weinberger, D.R.; Subedi, B. Estimation of the consumption of illicit drugs during special events in two communities in Western Kentucky, USA using sewage epidemiology. Sci. Total Environ. 2018, 633, 249–256. [Google Scholar] [CrossRef] [PubMed]
- González-Mariño, I.; Gracia-Lor, E.; Rousis, N.I.; Castrignanò, E.; Thomas, K.V.; Quintana, J.B.; Kasprzyk-Hordern, B.; Zuccato, E.; Castiglioni, S. Wastewater-based epidemiology to monitor synthetic cathinones use in different European countries. Environ. Sci. Technol. 2016, 50, 10089–10096. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.V.; Amador, A.; Baz-Lomba, J.A.; Reid, M. Use of mobile device data to better estimate dynamic population size for wastewater-based epidemiology. Environ. Sci. Technol. 2017, 51, 11363–11370. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Li, K.; Li, J.; Xu, Z.; Fu, X.; Yang, J.; Zhang, H.; Li, X. Methamphetamine and ketamine use in major Chinese cities, a nationwide reconnaissance through sewage-based epidemiology. Water Res. 2015, 84, 76–84. [Google Scholar] [CrossRef]
- Bijlsma, L.; Picó, Y.; Andreu, V.; Celma, A.; Estévez-Danta, A.; González-Mariño, I.; Hernández, F.; de Alda, M.L.; López-García, E.; Marcé, R.M. The embodiment of wastewater data for the estimation of illicit drug consumption in Spain. Sci. Total Environ. 2021, 772, 144794. [Google Scholar] [CrossRef]
- Castrignanò, E.; Yang, Z.; Bade, R.; Baz-Lomba, J.A.; Castiglioni, S.; Causanilles, A.; Covaci, A.; Gracia-Lor, E.; Hernandez, F.; Kinyua, J. Enantiomeric profiling of chiral illicit drugs in a pan-European study. Water Res. 2018, 130, 151–160. [Google Scholar] [CrossRef]
| Biomarkers | Chromatographic Column | Mobile Phase | Ion Source and Mass Detector | Limit of Detection (LOD, ng/L) | Reference |
|---|---|---|---|---|---|
| Multiclass illicit drugs (cocaines, opiates, amphetamines, and cannabinoids) | Acquity UPLC, BEH C18 (100 × 2.1 mm, 1.7 μm) column (Waters, UK) | Mobile phase A (0.1% v/v aqueous formic acid solution) and mobile phase B (0.1% v/v methanolic formic acid solution) | ESI+, QqQ MS/MS detector (Waters, UK) | 0.8–9.4 | [56] |
| 10 kinds of illicit drugs including cocaine, MDMA, THC, etc. | Shimadzu CBM-20A UPLC, Allure Pentafluorophenylpropyl (PFPP) column (50 mm × 2.1 mm, 5 µm) | 10 mM ammonium formate in water (solution A) and methanol (solution B) | ESI+, QqQ Mass Spectrometry (Shimadzu, Japan) | 0.04–38 | [57] |
| 6 kinds of illicit drugs including fentanyl, codeine, MDMA, etc. | Ultimate 3000 UHPLC, Hypersil GOLD PFP column (2.1 mm × 100 mm, 3 μm, Thermo Scientific, USA) | Mobile phase A was 0.1% of a formic acid solution containing 20 mmol/L ammonium acetate | ESI+, QqQ MS/MS detector (Thermo Scientific, USA) | 0.3–8.7 | [58] |
| 89 drugs including lysergic acid diethylamide (LSD), morphine, MDMA, etc. | HPLC, Zorbax SB-Aq column (150 mm × 2.1 mm, 3.5 μm; Santa Clara, CA, USA) serially connected to a Javelin guard column (Betasil C18, 2.1 mm × 20 mm, 5 μm; Thermo Electron Corp.) | Methanol (A) and water containing 0.1% (v/v) formic acid (B). | ESI+, QqQ MS/MS detector (Applied Biosystems, USA) | 0.11–202 | [59] |
| More than 1000 licit and illicit drugs | Acquity UPLC, BEH C18 (100 × 2.1 mm, 1.7 μm) column (Waters, UK) | Mobile phase A was water with 0.01% formic acid and mobile phase B was methanol with 0.01% formic acid | ESI in both positive and negative, Q-TOF mass spectrometer (Waters, UK) | - | [60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, R.; Zeng, T.; Chen, J.; Liu, D.; Yang, X.; Zhao, M.; Zhou, Z. Wastewater-Based Epidemiology: Assessing Illicit Drug Usage and Impact through an Innovative Approach. Water 2023, 15, 4192. https://doi.org/10.3390/w15234192
Yi R, Zeng T, Chen J, Liu D, Yang X, Zhao M, Zhou Z. Wastewater-Based Epidemiology: Assessing Illicit Drug Usage and Impact through an Innovative Approach. Water. 2023; 15(23):4192. https://doi.org/10.3390/w15234192
Chicago/Turabian StyleYi, Rongnan, Taotao Zeng, Junhao Chen, Dongxian Liu, Xiaojing Yang, Mingming Zhao, and Zeyan Zhou. 2023. "Wastewater-Based Epidemiology: Assessing Illicit Drug Usage and Impact through an Innovative Approach" Water 15, no. 23: 4192. https://doi.org/10.3390/w15234192
APA StyleYi, R., Zeng, T., Chen, J., Liu, D., Yang, X., Zhao, M., & Zhou, Z. (2023). Wastewater-Based Epidemiology: Assessing Illicit Drug Usage and Impact through an Innovative Approach. Water, 15(23), 4192. https://doi.org/10.3390/w15234192






