From Renewable Biomass to Water Purification Systems: Oil Palm Empty Fruit Bunch as Bio-Adsorbent for Domestic Wastewater Remediation and Methylene Blue Removal
Abstract
1. Introduction
2. Materials and Methods
2.1. Wastewater Characterization
2.2. Oil Palm Empty Fruit Bunch Wastes Conditioning
2.3. Experimental Model
2.3.1. Biofilters Operation
2.3.2. Batch Adsorption Tests Using Post Adsorbents
2.4. Analytical Methods
2.4.1. Influent/Effluent Monitoring
2.4.2. Raw/Post OPEFBF Characterization
2.4.3. MB Quantification before/after the Adsorption Batch Tests
2.5. Data Analysis
Isotherm and Kinetic Models
2.6. Statistical Analysis
2.6.1. Biofilters
2.6.2. Batch Adsorption Tests
3. Results
3.1. Raw/Post OPEFBF Characterization
3.2. Biofilter Performance
3.3. Batch Adsorption Tests
3.3.1. Optimal Dose and Contact Time
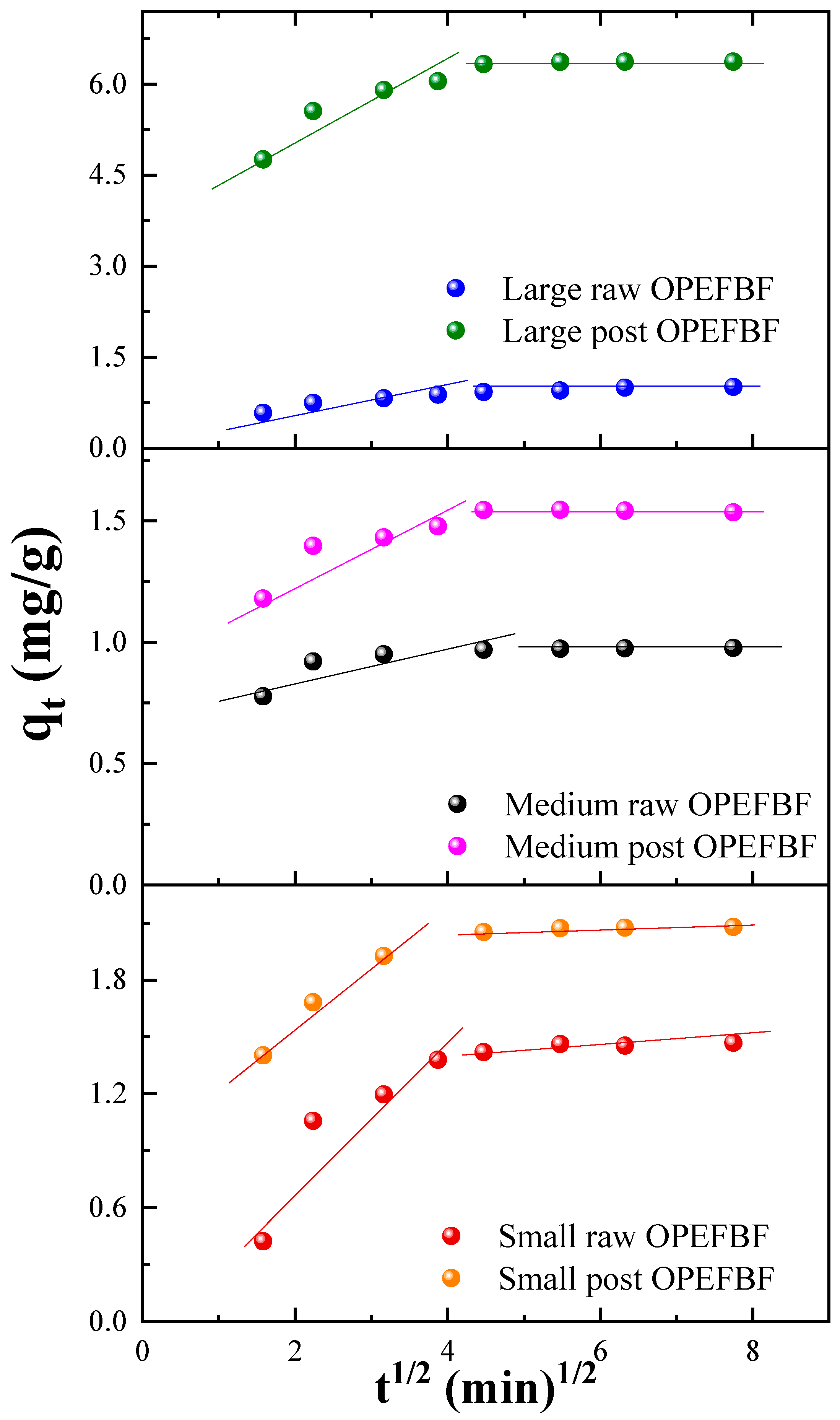
3.3.2. Adsorption Kinetics
3.3.3. Adsorption Isotherms
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Water Assessment Programme (United Nations). Wastewater: The Untapped Resource: The United Nations World Water Development Report 2017; UNESCO: Paris, France, 2018; ISBN 9789231002014. [Google Scholar]
- UNESCO; World Water Assessment Programme (United Nations); UN-Water. Water and Jobs; UNESCO: Paris, France, 2016; ISBN 9789231001468. [Google Scholar]
- WWAP. Informe Mundial de Las Naciones Unidas sobre el Desarrollo de los Recursos Hídricos 2017: Aguas Residuales: El Recurso Desaprovechado; UNESCO: Paris, France, 2017. [Google Scholar]
- Gao, T.; Xiao, K.; Zhang, J.; Xue, W.; Wei, C.; Zhang, X.; Liang, S.; Wang, X.; Huang, X. Techno-Economic Characteristics of Wastewater Treatment Plants Retrofitted from the Conventional Activated Sludge Process to the Membrane Bioreactor Process. Front. Environ Sci. Eng. 2021, 16, 49. [Google Scholar] [CrossRef]
- Capodaglio, A.G.; Callegari, A.; Cecconet, D.; Molognoni, D. Sustainability of Decentralized Wastewater Treatment Technologies. Water Pract. Technol. 2017, 12, 463–477. [Google Scholar] [CrossRef]
- Syafiuddin, A.; Boopathy, R.; Mehmood, M.A. Recent Advances on Bacterial Quorum Quenching as an Effective Strategy to Control Biofouling in Membrane Bioreactors. Bioresour. Technol. Rep. 2021, 15, 100745. [Google Scholar] [CrossRef]
- Tarpani, R.R.Z.; Azapagic, A. Life Cycle Costs of Advanced Treatment Techniques for Wastewater Reuse and Resource Recovery from Sewage Sludge. J. Clean. Prod. 2018, 204, 832–847. [Google Scholar] [CrossRef]
- Loh, Z.Z.; Zaidi, N.S.; Syafiuddin, A.; Yong, E.L.; Boopathy, R.; Kueh, A.B.H.; Prastyo, D.D. Shifting from Conventional to Organic Filter Media in Wastewater Biofiltration Treatment: A Review. Appl. Sci. 2021, 11, 8650. [Google Scholar] [CrossRef]
- Singh, R.; Das, R.; Sangwan, S.; Rohatgi, B.; Khanam, R.; Peera, S.K.P.G.; Das, S.; Lyngdoh, Y.A.; Langyan, S.; Shukla, A.; et al. Utilisation of Agro-Industrial Waste for Sustainable Green Production: A Review. Environ. Sustain. 2021, 4, 619–636. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Lazaridis, N.K.; Mitropoulos, A.C. Removal of Dyes from Aqueous Solutions with Untreated Coffee Residues as Potential Low-Cost Adsorbents: Equilibrium, Reuse and Thermodynamic Approach. Chem. Eng. J. 2012, 189–190, 148–159. [Google Scholar] [CrossRef]
- Saliling, W.J.B.; Westerman, P.W.; Losordo, T.M. Wood Chips and Wheat Straw as Alternative Biofilter Media for Denitrification Reactors Treating Aquaculture and Other Wastewaters with High Nitrate Concentrations. Aquac. Eng. 2007, 37, 222–233. [Google Scholar] [CrossRef]
- Tejedor, J.; Cóndor, V.; Almeida-Naranjo, C.E.; Guerrero, V.H.; Villamar, C.A. Performance of Wood Chips/Peanut Shells Biofilters Used to Remove Organic Matter from Domestic Wastewater. Sci. Total Environ. 2020, 738, 39589. [Google Scholar] [CrossRef]
- Almeida-Naranjo, C.E.; Valle, V.; Aguilar, A.; Cadena, F.; Kreiker, J.; Raggiotti, B. Water Absorption Behavior of Oil Palm Empty Fruit Bunch (OPEFB) and Oil Palm Kernel Shell (OPKS) as Fillers in Acrylic Thermoplastic Composites. Materials 2022, 15, 5015. [Google Scholar] [CrossRef]
- Vigueras-Cortés, J.M.; Villanueva-Fierro, I.; Garzón-Zúñiga, M.A.; De Jesús Návar-Cháidez, J.; Chaires-Hernández, I.; Hernández-Rodríguez, C. Performance of a Biofilter System with Agave Fiber Filter Media for Municipal Wastewater Treatment. Water Sci. Technol. 2013, 68, 599–607. [Google Scholar] [CrossRef]
- Danish, M.; Ahmad, T.; Hashim, R.; Hafiz, M.R.; Ghazali, A.; Sulaiman, O.; Hiziroglu, S. Characterization and Adsorption Kinetic Study of Surfactant Treated Oil Palm (Elaeis guineensis) Empty Fruit Bunches. Desalin. Water Treat. 2016, 57, 9474–9487. [Google Scholar] [CrossRef]
- Setiabudi, H.D.; Jusoh, R.; Suhaimi, S.F.R.M.; Masrur, S.F. Adsorption of Methylene Blue onto Oil Palm (Elaeis guineensis) Leaves: Process Optimization, Isotherm, Kinetics and Thermodynamic Studies. J. Taiwan Inst. Chem. Eng. 2016, 63, 363–370. [Google Scholar] [CrossRef]
- Indexmundi Palm Oil Production by Country in 1000 MT. Available online: https://www.indexmundi.com/agriculture/?commodity=palm-oil (accessed on 7 November 2023).
- IRENA. Renewable Power Generation Costs 2020; IRENA: Abu Dhabi, United Arab Emirates, 2021; ISBN 978-92-9260-348-9. [Google Scholar]
- May, J.; Thoe, L.; Surugau, N.; Lye, H.; Chong, H. Application of Oil Palm Empty Fruit Bunch as Adsorbent: A Review. Trans. Sci. Technol. 2019, 6, 9–26. [Google Scholar]
- Joseph, C.G.; Daud, W.M.A.W.; Shane, Q.K.; Sanmugam, K. Parametric and Adsorption Kinetic Studies of Reactive Black 5 Removal from Textile Simulated Wastewater Using Oil Palm (Elais guineensis) Empty Fruit Bunch. J. Appl. Sci. 2015, 15, 1103–1111. [Google Scholar] [CrossRef][Green Version]
- Almeida-Naranjo, C.E.; Frutos, M.; Tejedor, J.; Cuestas, J.; Valenzuela, F.; Rivadeneira, M.I.; Villamar, C.A.; Guerrero, V.H. Caffeine Adsorptive Performance and Compatibility Characteristics (Eisenia foetida Savigny) of Agro-Industrial Residues Potentially Suitable for Vermifilter Beds. Sci. Total Environ. 2021, 801, 149666. [Google Scholar] [CrossRef]
- Bolong, N.; Saad, I.; Makinda, J.; Yaser, A.Z.; Abdullah, M.H.; Ismail, A.F. Influence of oil palm empty fruit bunch (OPEFB) agro-waste properties as filtration medium to improve urban stormwater. J. Teknol. 2016, 78, 5357. [Google Scholar] [CrossRef]
- Oriausifo, E. Evaluating the Use of Granular Dual-Media Deep-Bed Filters for Production of Potable Water. J. Eng. Appl. Sci. 2022, 9, 1–10. [Google Scholar]
- Rial, J.B.; Ferreira, M.L. Potential Applications of Spent Adsorbents and Catalysts: Re-Valorization of Waste. Sci. Total Environ. 2022, 823, 153370. [Google Scholar] [CrossRef]
- Yadav, A.; Bagotia, N.; Sharma, A.K.; Kumar, S. Advances in Decontamination of Wastewater Using Biomass-Basedcomposites: A Critical Review. Sci. Total Environ. 2021, 784, 147108. [Google Scholar] [CrossRef]
- Baskar, A.V.; Bolan, N.; Hoang, S.A.; Sooriyakumar, P.; Kumar, M.; Singh, L.; Jasemizad, T.; Padhye, L.P.; Singh, G.; Vinu, A.; et al. Recovery, Regeneration and Sustainable Management of Spent Adsorbents from Wastewater Treatment Streams: A Review. Sci. Total Environ. 2022, 822, 153555. [Google Scholar] [CrossRef] [PubMed]
- Omorogie, M.O.; Babalola, J.O.; Unuabonah, E.I. Regeneration Strategies for Spent Solid Matrices Used in Adsorption of Organic Pollutants from Surface Water: A Critical Review. Desalin. Water Treat. 2016, 57, 518–544. [Google Scholar] [CrossRef]
- Liu, M.; Li, X.; Du, Y.; Han, R. Adsorption of Methyl Blue from Solution Using Walnut Shell and Reuse in a Secondary Adsorption for Congo Red. Bioresour. Technol. Rep. 2019, 5, 238–242. [Google Scholar] [CrossRef]
- Almeida-Naranjo, C.E.; Espinoza-Montero, P.J.; Muñoz-Rodríguez, M.I.; Villamar-Ayala, C.A. Hydraulic Retention Time Influence on Improving Flocculation in the Activated Sludge Processes Through Polyelectrolytes. Water Air Soil Pollut. 2017, 228, 1–9. [Google Scholar] [CrossRef]
- Villamar, C.A.; Vera-Puerto, I.; Rivera, D.; De la Hoz, F.D. Reuse and Recycling of Livestock and Municipal Wastewater in Chilean Agriculture: A Preliminary Assessment. Water 2018, 10, 817. [Google Scholar] [CrossRef]
- Valle, V.; Baquero, P.; Rico, P.; Kreiker, J.; Raggiotti, B.; Cadena, F. Mechanical Characteristics of Composites Based on Oil Palm Empty Fruit Bunch, Modified Oca Starch and Polyvinyl Alcohol. J. Compos. Mater. 2021, 55, 2459–2468. [Google Scholar] [CrossRef]
- Aguilar, A.D.; Valle, V.; Almeida-Naranjo, C.E.; Naranjo, Á.; Cadena, F.; Kreiker, J.; Raggiotti, B. Characterization Dataset of Oil Palm Empty Fruit Bunch (OPEFB) Fibers—Natural Reinforcement/Filler for Materials Development. Data Brief 2022, 45, 108618. [Google Scholar] [CrossRef]
- Rice, E.W.; Baird, R.B.; Eaton, A.D. (Eds.) Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017; ISBN 9780123821652. [Google Scholar]
- ASTM D4442-2020; Standard Test Methods for Direct Moisture Content Measurement of Wood and Wood-Based Materials. American Society for Testing Materials: West Conshohocken, PA, USA, 2020.
- ASTM D 1107-2021; Standard Test Method for Ethanol-Toluene Solubility of Wood. American Society for Testing Materials: West Conshohocken, PA, USA, 2021.
- ASTM-D1109–84(2021); Standard Test Method for 1% Sodium Hydroxide Solubility of Wood. American Society for Testing Materials: West Conshohocken, PA, USA, 2021.
- ASTM D 1106-2021; Standard Test Method for Acid-Insoluble Lignin in Wood. American Society for Testing Materials: West Conshohocken, PA, USA, 2021.
- Gil, A.; Santamaría, L.; Korili, S.A. Removal of Ca Ff Eine and Diclofenac from Aqueous Solution by Adsorption on Multiwalled Carbon Nanotubes. Colloid Interface Sci. Commun. 2018, 22, 25–28. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Van, H.T.; Chu, T.H.H.; Nguyen, T.H.V.; Nguyen, T.D.; Hoang, L.P.; Hoang, V.H. Paper Waste Sludge-Derived Hydrochar Modified by Iron (III) Chloride for Enhancement of Ammonium Adsorption: An Adsorption Mechanism Study. Environ. Technol. Innov. 2021, 21, 101223. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Katsouromalli, A.; Pashalidis, I. Oxidized Biochar Obtained from Pine Needles as a Novel Adsorbent to Remove Caffeine from Aqueous Solutions. J. Mol. Liq. 2020, 304, 112661. [Google Scholar] [CrossRef]
- Jiang, F.; Dinh, D.M.; Hsieh, Y. Lo Adsorption and Desorption of Cationic Malachite Green Dye on Cellulose Nanofibril Aerogels. Carbohydr. Polym. 2017, 173, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Lubbers, R.J.M.; Dilokpimol, A.; Visser, J.; De Vries, R.P. Aspergillus Niger Uses the Peroxisomal CoA-Dependent β-Oxidative Genes to Degrade the Hydroxycinnamic Acids Caffeic Acid, Ferulic Acid, and p-Coumaric Acid. Appl. Genet. Mol. Biotechnol. 2021, 105, 4199–4211. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Ahluwalia, V.; Saran, S.; Kumar, J.; Patel, A.K.; Singhania, R.R. Recent Developments on Solid-State Fermentation for Production of Microbial Secondary Metabolites: Challenges and Solutions. Bioresour. Technol. 2021, 323, 124566. [Google Scholar] [CrossRef]
- Katarínašuchová, K.; Fehér, C.; Ravn, J.L.; Bed, O.B.S.; Biely, P.; Geijer, C. Cellulose-and Xylan-Degrading Yeasts: Enzymes, Applications and Biotechnological Potential. Biotechnol. Adv. 2022, 59, 107981. [Google Scholar] [CrossRef]
- Li, Q.; Zhu, F.; Shi, F.; Zhang, N.; Zhang, H. Pyrolysis Characteristics and Kinetics of Prunus avium L. Leaves Using Thermogravimetric Analysis. Bioresourse 2023, 18, 7320–7332. [Google Scholar] [CrossRef]
- Zhang, L.; Ren, M.H.; Lin, Y.; Fu, Z. Physicochemical Properties of Arenga pinnata (Wurmb.) Merr Starch: Effect of High-Speed Jet Treatment. Int. J. Food Prop. 2019, 22, 477–486. [Google Scholar] [CrossRef]
- Pawlak-Kruczek, H.; Krochmalny, K.; Wnukowski, M.; Niedzwiecki, L. Slow Pyrolysis of the Sewage Sludge with Additives: Calcium Oxide and Lignite. J. Energy Resour. Technol. Trans. ASME 2018, 140, 062206. [Google Scholar] [CrossRef]
- Sze, Y.S.; Aris, A.; Zaidi, N.S.; Bahrodin, M.B. Performance of Sand Filtration System with Different Sand Bed Depth for Polishing Wastewater Treatment. J. Environ. Treat. Tech. 2021, 9, 456. [Google Scholar] [CrossRef]
- Wahab, M.A.; Jellali, S.; Jedidi, N. Ammonium Biosorption onto Sawdust: FTIR Analysis, Kinetics and Adsorption Isotherms Modeling. Bioresour. Technol. 2010, 101, 5070–5075. [Google Scholar] [CrossRef]
- Rahman, N.S.A.; Yhaya, M.F.; Azahari, B.; Ismail, W.R. Utilisation of Natural Cellulose Fibres in Wastewater Treatment. Cellulose 2018, 25, 4887–4903. [Google Scholar] [CrossRef]
- Satoh, H.; Ono, H.; Rulin, B.; Kamo, J.; Okabe, S.; Fukushi, K.I. Macroscale and Microscale Analyses of Nitrification and Denitrification in Biofilms Attached on Membrane Aerated Biofilm Reactors. Water Res. 2004, 38, 1633–1641. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.O.; Boe-Hansen, R.; Musovic, S.; Smets, B.; Albrechtsen, H.J.; Binning, P. Effects of Dynamic Operating Conditions on Nitrification in Biological Rapid Sand Filters for Drinking Water Treatment. Water Res. 2014, 64, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Kastrinos, N.; Weber, K.M. Sustainable Development Goals in the Research and Innovation Policy of the European Union. Technol. Forecast. Soc. Chang. 2020, 157, 120056. [Google Scholar] [CrossRef]
- Kalaimurugan, D.; Durairaj, K.; Kumar, A.J.; Senthilkumar, P.; Venkatesan, S. Novel Preparation of Fungal Conidiophores Biomass as Adsorbent for Removal of Phosphorus from Aqueous Solution. Environ. Sci. Pollut. Res. 2020, 27, 20757–20769. [Google Scholar] [CrossRef]
- Tejada-Tovar, C.; Villabona-Ortiz, A.; Gonzalez-Delgado, A.D.; Marrugo-Cantillo, E.; Pajaro-Montero, M. Effect of Bed Height and Biomass Array on Removal of an Anion Surfactant Using a Continuous Rapid-Mixed Biofilter. Contemp. Eng. Sci. 2018, 11, 297–305. [Google Scholar] [CrossRef]
- Khosravihaftkhany, S.; Morad, N.; Teng, T.T.; Abdullah, A.Z.; Norli, I. Biosorption of Pb(II) and Fe(III) from Aqueous Solutions Using Oil Palm Biomasses as Adsorbents. Water Air Soil Pollut. 2013, 224, 1455. [Google Scholar] [CrossRef]
- Ouadi, B.; Bendraoua, A.; Boualla, N.; Adjdir, M. Efficiency of Pozzolan and Sawdust as Biofilter in the Treatment of Wastewater. Appl. Water Sci. 2020, 10, 143. [Google Scholar] [CrossRef]
- Osorio, F.; Hontoria, E. Optimization of Bed Material Height in a Submerged Biological Aerated Filter. J. Environ. Eng. 2001, 127, 974–978. [Google Scholar] [CrossRef]
- Li, H.; Liu, L.; Cui, J.; Cui, J.; Wang, F.; Zhang, F. High-Efficiency Adsorption and Regeneration of Methylene Blue and Aniline onto Activated Carbon from Waste Edible Fungus Residue and Its Possible Mechanism. RSC Adv. 2020, 10, 14262–14273. [Google Scholar] [CrossRef]
- Hashem, A.H.; Saied, E.; Sayed Hasanin, M. Green and Ecofriendly Bio-Removal of Methylene Blue Dye from Aqueous Solution Using Biologically Activated Banana Peel Waste. Sustain. Chem. Pharm. 2020, 18, 100333. [Google Scholar] [CrossRef]
- Jasper, E.E.; Ajibola, V.O.; Onwuka, J.C. Nonlinear Regression Analysis of the Sorption of Crystal Violet and Methylene Blue from Aqueous Solutions onto an Agro-Waste Derived Activated Carbon. Appl. Water Sci. 2020, 10, 132. [Google Scholar] [CrossRef]
- Wei, F.; Jin, S.; Yao, C.; Wang, T.; Zhu, S.; Ma, Y.; Qiao, H.; Shan, L.; Wang, R.; Lian, X.; et al. Revealing the Combined Effect of Active Sites and Intra-Particle Diffusion on Adsorption Mechanism of Methylene Blue on Activated Red-Pulp Pomelo Peel Biochar. Molecules 2023, 28, 4426. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Zahoor, M.; Din, W.U.; Muhammad, M.; Khan, F.A.; Sohail, A.; Ullah, R.; Ali, E.A.; Murthy, H.C.A. Removal of Methylene Blue from Aqueous Solution Using Black Tea Wastes: Used as Efficient Adsorbent. Adsorpt. Sci. Technol. 2022, 2022, 5713077. [Google Scholar] [CrossRef]
- Jawad, A.H.; Rashid, R.A.; Mahmuod, R.M.A.; Ishak, M.A.M.; Kasim, N.N.; Ismail, K. Adsorption of Methylene Blue onto Coconut (Cocos nucifera) Leaf: Optimization, Isotherm and Kinetic Studies. Desalin. Water Treat. 2016, 57, 8839–8853. [Google Scholar] [CrossRef]
- Shakoor, S.; Nasar, A. Removal of Methylene Blue Dye from Artificially Contaminated Water Using Citrus Limetta Peel Waste as a Very Low Cost Adsorbent. J. Taiwan Inst. Chem. Eng. 2016, 66, 154–163. [Google Scholar] [CrossRef]
- Hevira, L.; Zilfa; Rahmayeni; Ighalo, J.O.; Aziz, H.; Zein, R. Terminalia Catappa Shell as Low-Cost Biosorbent for the Removal of Methylene Blue from Aqueous Solutions. J. Ind. Eng. Chem. 2021, 97, 188–199. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.; Hosseini-bandegharaei, A.; Chao, H. Mistakes and Inconsistencies Regarding Adsorption of Contaminants from Aqueous Solutions: A Critical Review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef]
- Hasani, N.; Selimi, T.; Mele, A.; Thaçi, V.; Halili, J.; Berisha, A.; Sadiku, M. Theoretical, Equilibrium, Kinetics and Thermodynamic Investigations of Methylene Blue Adsorption onto Lignite Coal. Molecules 2022, 27, 1856. [Google Scholar] [CrossRef]
- Sakr, F.; Alahiane, S.; Sennaoui, A.; Dinne, M.; Bakas, I.; Assabbane, A. Removal of Cationic Dye (Methylene Blue) from Aqueous Solution by Adsorption on Two Type of Biomaterial of South Morocco. Mater. Today Proc. 2020, 22, 93–96. [Google Scholar] [CrossRef]
- Alver, E.; Metin, A.Ü.; Brouers, F. Methylene Blue Adsorption on Magnetic Alginate/Rice Husk Bio-Composite. Int. J. Biol. Macromol. 2020, 154, 104–113. [Google Scholar] [CrossRef]
- Reza, R.A.; Ahmaruzzaman, M.; Sil, A.K.; Gupta, V.K. Comparative Adsorption Behavior of Ibuprofen and Clofibric Acid onto Microwave Assisted Activated Bamboo Waste. Ind. Eng. Chem. Res. 2014, 53, 9331–9339. [Google Scholar] [CrossRef]

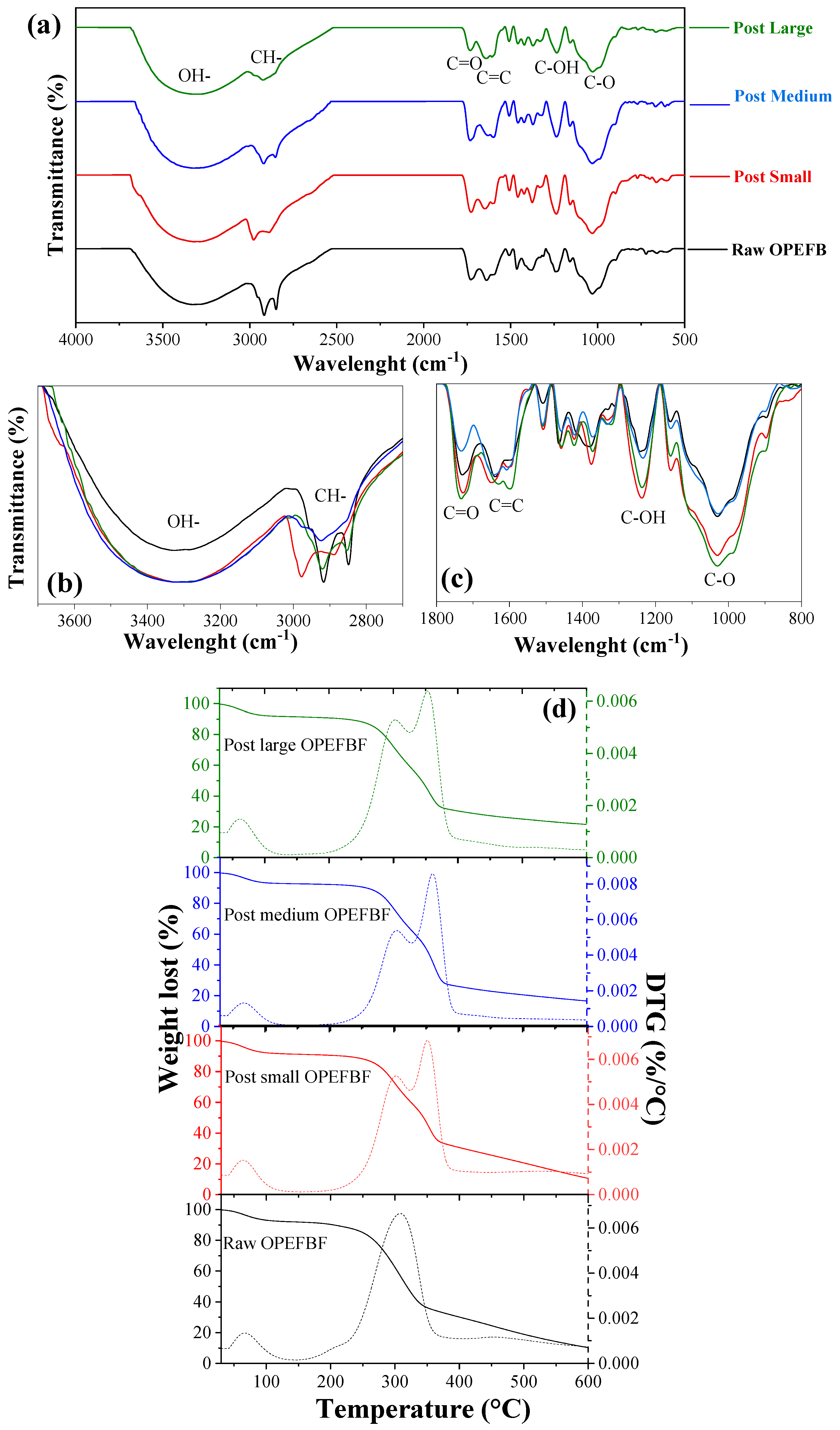

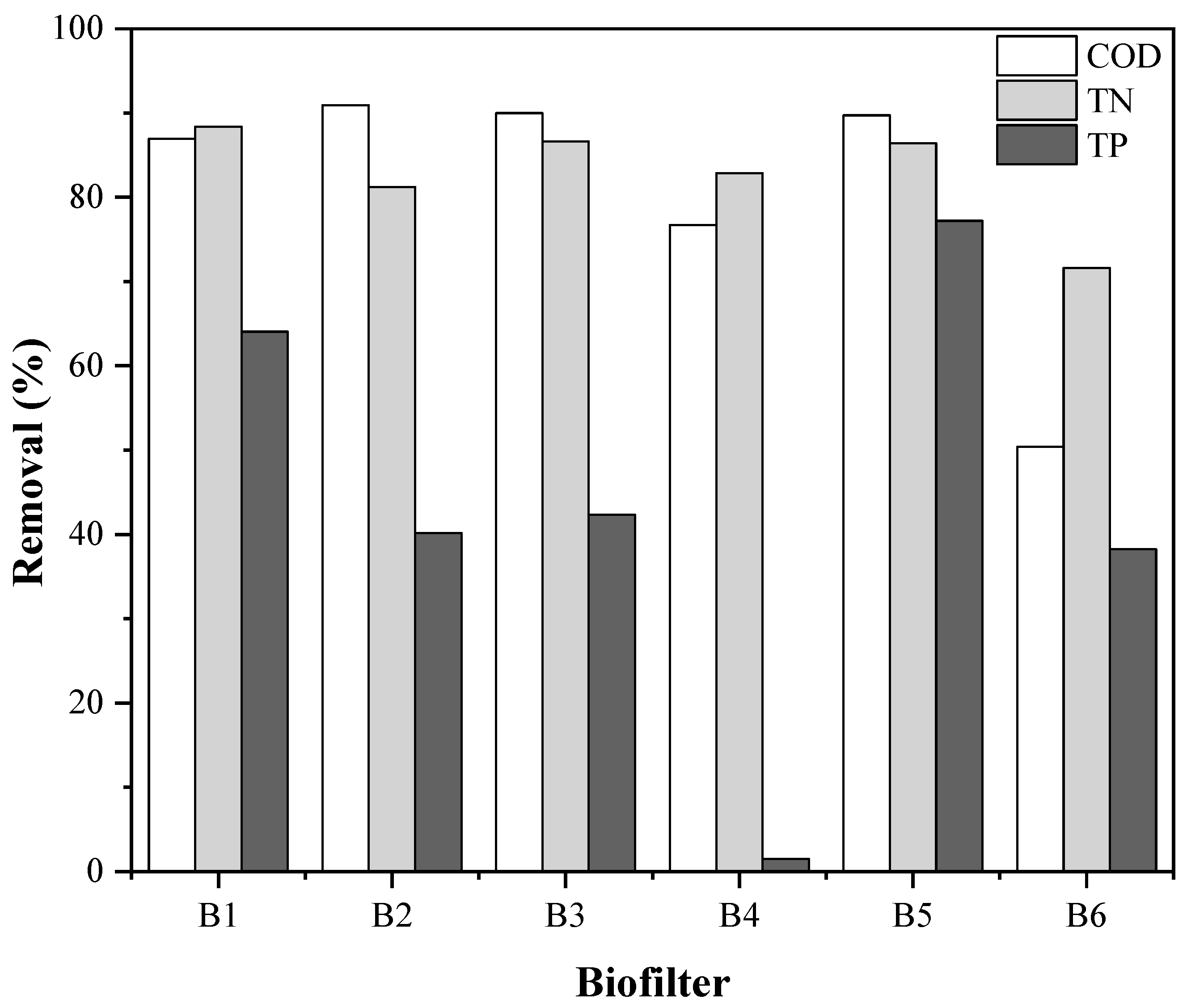
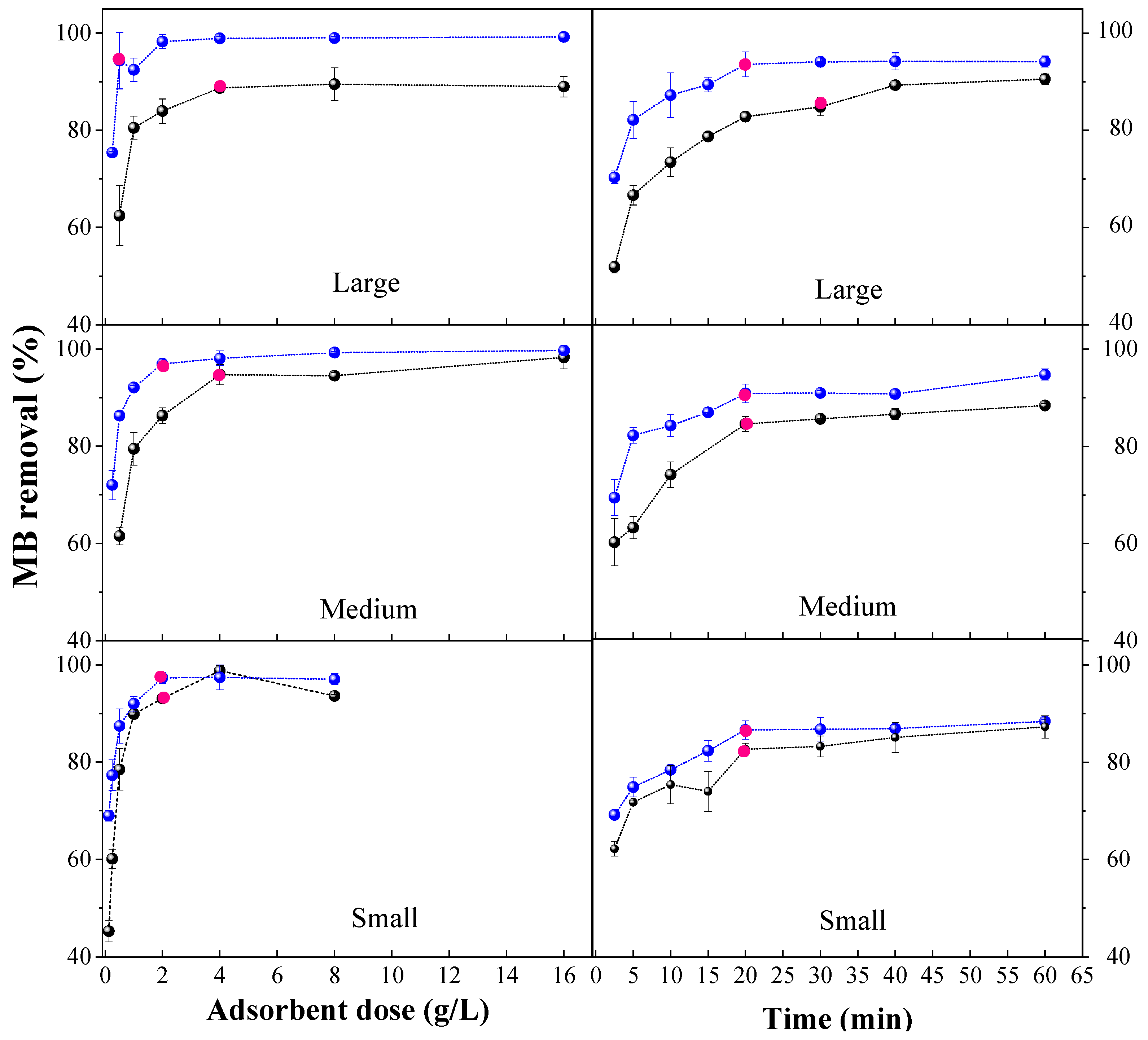
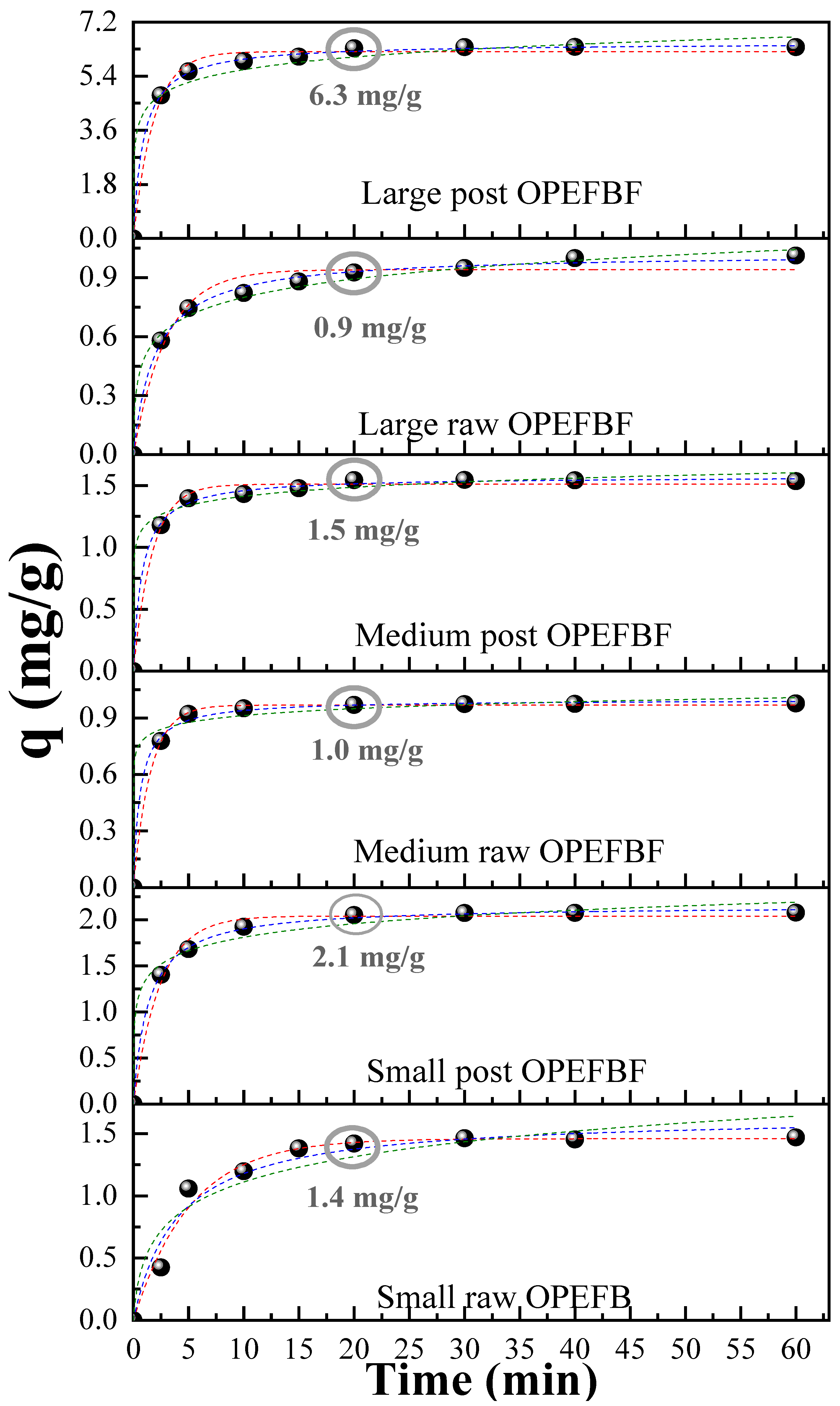

| Parameter | Unit | Average Value | Standard Deviation |
|---|---|---|---|
| Chemical Oxygen Demand | mg/L | 589.71 | 46.54 |
| Total Nitrogen | mg/L | 21.60 | 0.48 |
| Total Phosphorous | mg/L | 12.14 | 0.37 |
| Volatile Solids | mg/L | 517.20 | 39.46 |
| Total Solids | mg/L | 111.05 | 40.14 |
| Temperature | °C | 17.40 | 0.61 |
| pH | - | 6.48 | 0.35 |
| Dissolved Oxygen | mg/L | 6.75 | 0.22 |
| Model Type | Parameter (Units) | Small OPEFBF | Medium OPEFBF | Large OPEFBF | |||
|---|---|---|---|---|---|---|---|
| Raw | Post | Raw | Post | Raw | Post | ||
| Pseudo-first order | qe exp. (mg/g) | 1.420 | 2.052 | 0.969 | 1.544 | 0.926 | 6.329 |
| qe (mg/g) | 1.460 | 2.041 | 0.968 | 1.511 | 0.941 | 6.215 | |
| k1 (min−1) | 0.194 | 0.414 | 0.641 | 0.017 | 0.333 | 0.542 | |
| R2 | 0.980 | 0.992 | 0.999 | 0.994 | 0.973 | 0.992 | |
| χ2 | 0.006 | 0.005 | 8.001 × 10−5 | 0.002 | 0.003 | 0.039 | |
| SSE | 0.045 | 0.028 | 4.801 × 10−4 | 0.012 | 0.022 | 0.275 | |
| Pseudo-second order | qe (mg/g) | 1.650 | 2.157 | 0.998 | 1.574 | 1.024 | 6.510 |
| k2 (g/(mg min)) | 0.152 | 0.347 | 1.616 | 0.804 | 0.487 | 0.168 | |
| R2 | 0.964 | 0.999 | 0.998 | 0.998 | 0.996 | 0.999 | |
| χ2 | 0.012 | 5.018 × 10−4 | 3.225 × 10−4 | 5.896 × 10−4 | 4.334 × 10−4 | 0.004 | |
| SSE | 0.081 | 0.003 | 0.002 | 0.004 | 0.003 | 0.030 | |
| Elovich | α (mg/(g min)) | 1.201 | 108.673 | 163.297 | 651.832 | 5.710 | 556.341 |
| β (mg/g) | 3.345 | 0.472 | 0.189 | 0.943 | 7.493 | 0.202 | |
| R2 | 0.918 | 0.986 | 0.989 | 0.990 | 0.994 | 0.992 | |
| χ2 | 0.026 | 0.008 | 0.001 | 0.003 | 4.334 × 10−4 | 0.041 | |
| SSE | 0.184 | 0.050 | 0.009 | 0.002 | 0.005 | 0.284 | |
| Intra particle diffusion | kp1 (mg/(g min1/2)) | 0.312 | 0.625 | 0.314 | 0.373 | 0.226 | 1.532 |
| C1 (mg/g) | 0.141 | 0.162 | 0.114 | 0.289 | 0.115 | 1.126 | |
| R2 | 0.821 | 0.932 | 0.873 | 0.797 | 0.897 | 0.814 | |
| SSE | 0.112 | 0.150 | 0.076 | 0.316 | 0.053 | 4.772 | |
| kp2 (mg/(g min1/2)) | 0.013 | 0.007 | 0.002 | -0.003 | 0.028 | 0.011 | |
| C2 (mg/g) | 1.375 | 2.024 | 0.961 | 1.559 | 0.803 | 6.293 | |
| R2 | 0.659 | 0.732 | 0.887 | 0.797 | 0.909 | 0.576 | |
| SSE | 4.831 × 10−4 | 1.187 × 10−4 | 3.174 × 10−6 | 1.189 × 10−5 | 4.565 × 10−4 | 4.996 × 10−4 | |
| Model Type |
Parameter (Units) | Small OPEFBF | Medium OPEFBF | Large OPEFBF | |||
|---|---|---|---|---|---|---|---|
| Raw | Post | Raw | Post | Raw | Post | ||
| Langmuir | qe exp. (mg/g) | 3.008 | 3.787 | 2.938 | 3.590 | 1.655 | 9.590 |
| qm (mg/g) | 3.969 | 5.611 | 2.721 | 5.535 | 2.746 | 12.022 | |
| KL [L/mg] | 0.841 | 0.537 | 0.881 | 0.421 | 1.227 | 0.305 | |
| R2 | 0.956 | 0.979 | 0.933 | 0.970 | 0.965 | 0.980 | |
| χ2 | 0.084 | 0.074 | 0.068 | 0.089 | 0.958 | 0.375 | |
| SSE | 1.265 | 0.987 | 1.002 | 0.997 | 1.871 | 0.985 | |
| Freundlich | KF [(mg/g)1−1/n] | 1.737 | 2.003 | 1.4206 | 1.725 | 1.239 | 3.487 |
| n | 2.404 | 2.283 | 2.375 | 2.087 | 2.263 | 2.503 | |
| R2 | 0.962 | 0.994 | 0.982 | 0.994 | 0.992 | 0.996 | |
| χ2 | 0.071 | 0.019 | 0.018 | 0.019 | 0.007 | 0.068 | |
| SSE | 1.018 | 0.754 | 0.982 | 0.864 | 0.598 | 0.698 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida-Naranjo, C.E.; Gallegos, E.; Domínguez, E.; Gutiérrez, P.; Valle, V.; Aguilar, A.D.; Debut, A.; Vasco, C. From Renewable Biomass to Water Purification Systems: Oil Palm Empty Fruit Bunch as Bio-Adsorbent for Domestic Wastewater Remediation and Methylene Blue Removal. Water 2023, 15, 4116. https://doi.org/10.3390/w15234116
Almeida-Naranjo CE, Gallegos E, Domínguez E, Gutiérrez P, Valle V, Aguilar AD, Debut A, Vasco C. From Renewable Biomass to Water Purification Systems: Oil Palm Empty Fruit Bunch as Bio-Adsorbent for Domestic Wastewater Remediation and Methylene Blue Removal. Water. 2023; 15(23):4116. https://doi.org/10.3390/w15234116
Chicago/Turabian StyleAlmeida-Naranjo, Cristina E., Elvia Gallegos, Elizabeth Domínguez, Paola Gutiérrez, Vladimir Valle, Alex Darío Aguilar, Alexis Debut, and Catalina Vasco. 2023. "From Renewable Biomass to Water Purification Systems: Oil Palm Empty Fruit Bunch as Bio-Adsorbent for Domestic Wastewater Remediation and Methylene Blue Removal" Water 15, no. 23: 4116. https://doi.org/10.3390/w15234116
APA StyleAlmeida-Naranjo, C. E., Gallegos, E., Domínguez, E., Gutiérrez, P., Valle, V., Aguilar, A. D., Debut, A., & Vasco, C. (2023). From Renewable Biomass to Water Purification Systems: Oil Palm Empty Fruit Bunch as Bio-Adsorbent for Domestic Wastewater Remediation and Methylene Blue Removal. Water, 15(23), 4116. https://doi.org/10.3390/w15234116










