Groundwater Hydrogeochemical Processes and Potential Threats to Human Health in Fengfeng Coal Mining Area, China
Abstract
1. Introduction
2. Materials and Methods
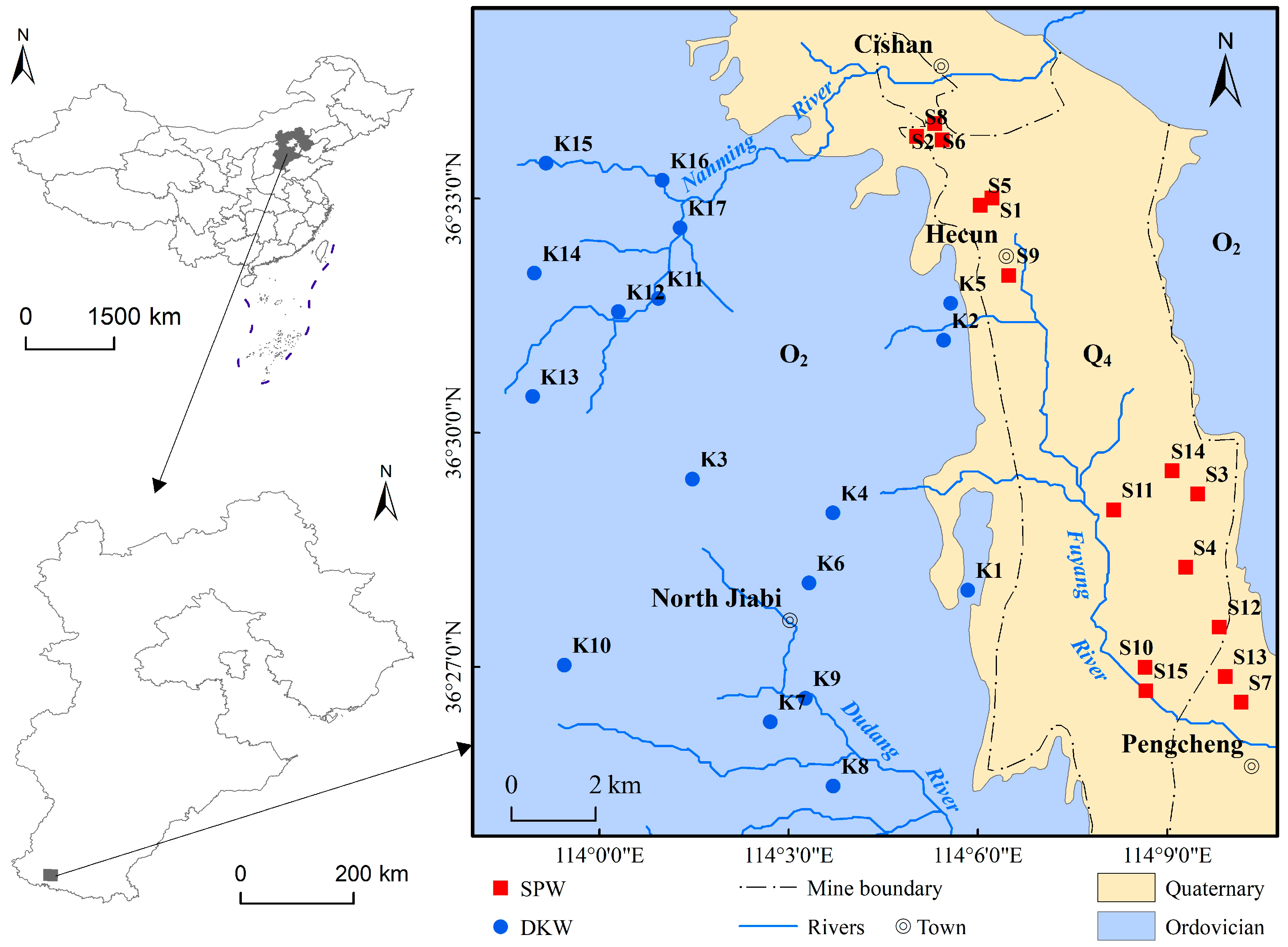
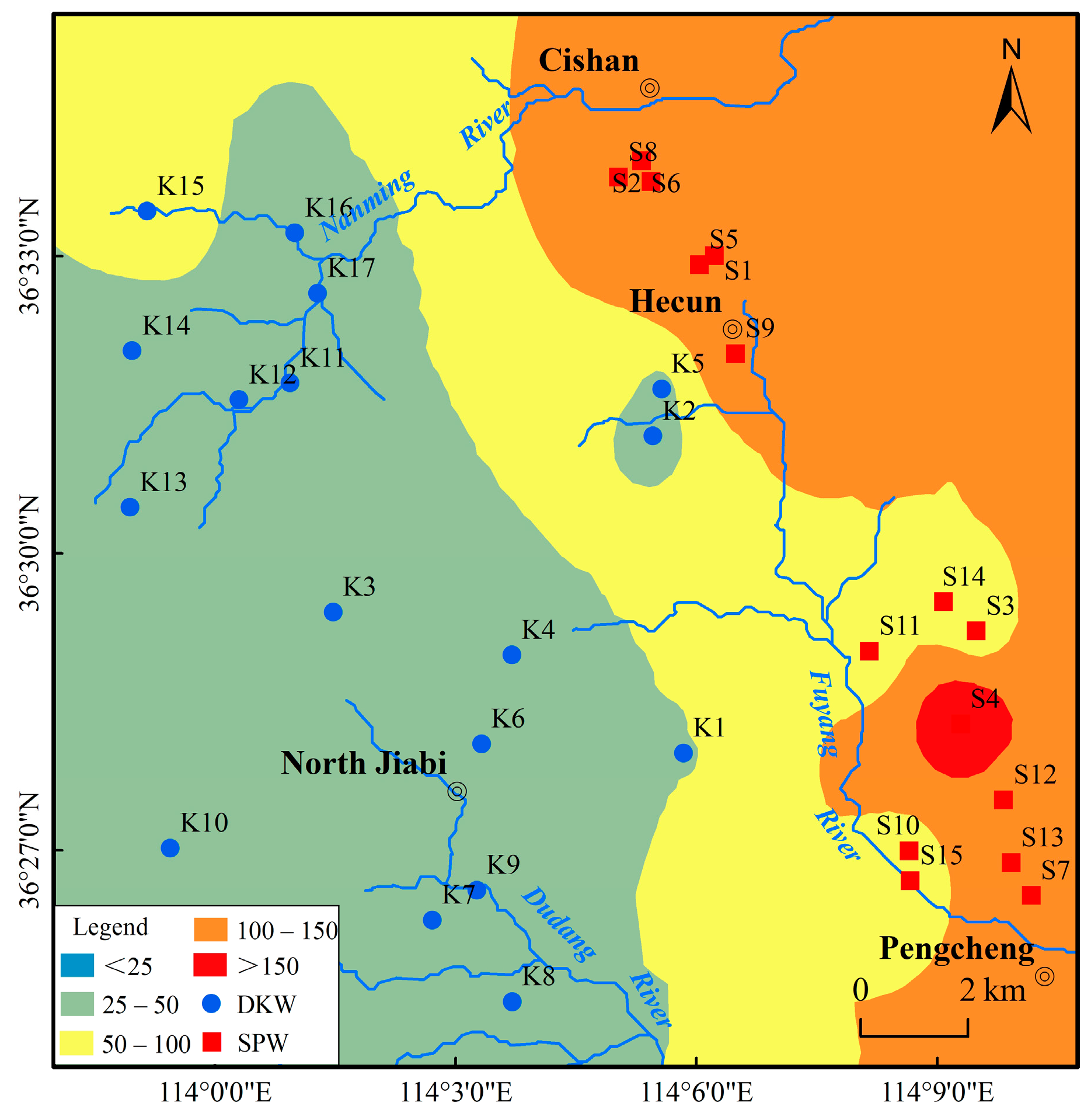
2.1. Study Area
2.2. Sampling and Analytical Techniques
2.3. Objective Combined Weight Water Quality Index (OCWQI)
2.4. Human Health Risk Assessment (HHRA) Model
3. Results
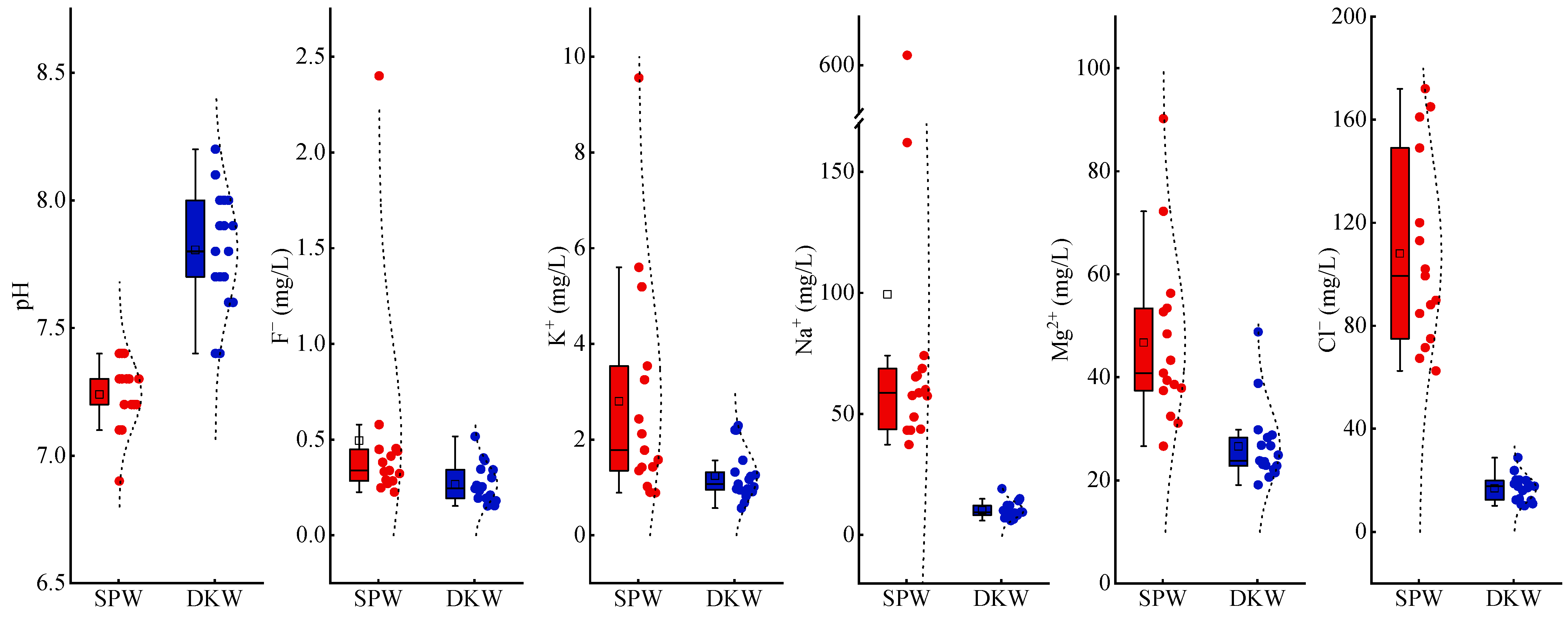
3.1. Descriptive Statistics
3.2. Hydrochemical Types
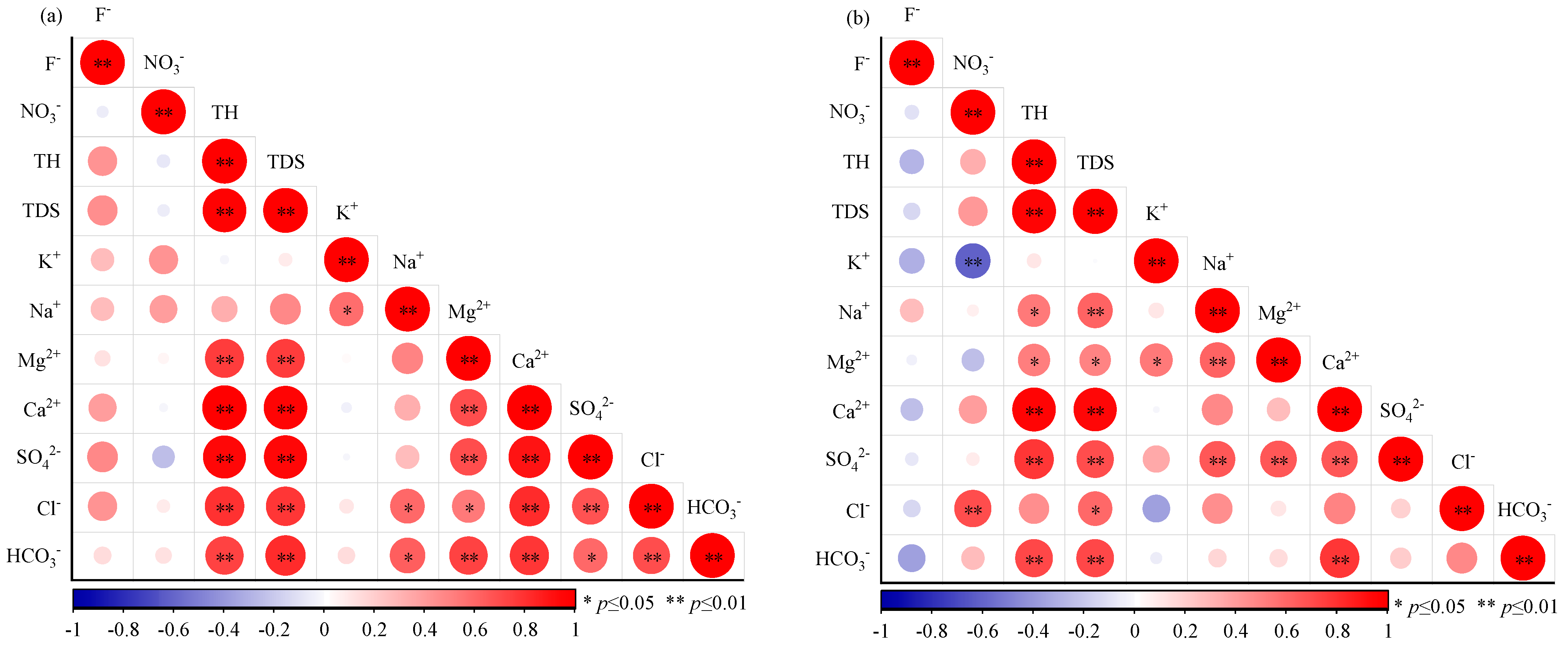
3.3. Correlation Analysis
4. Discussion
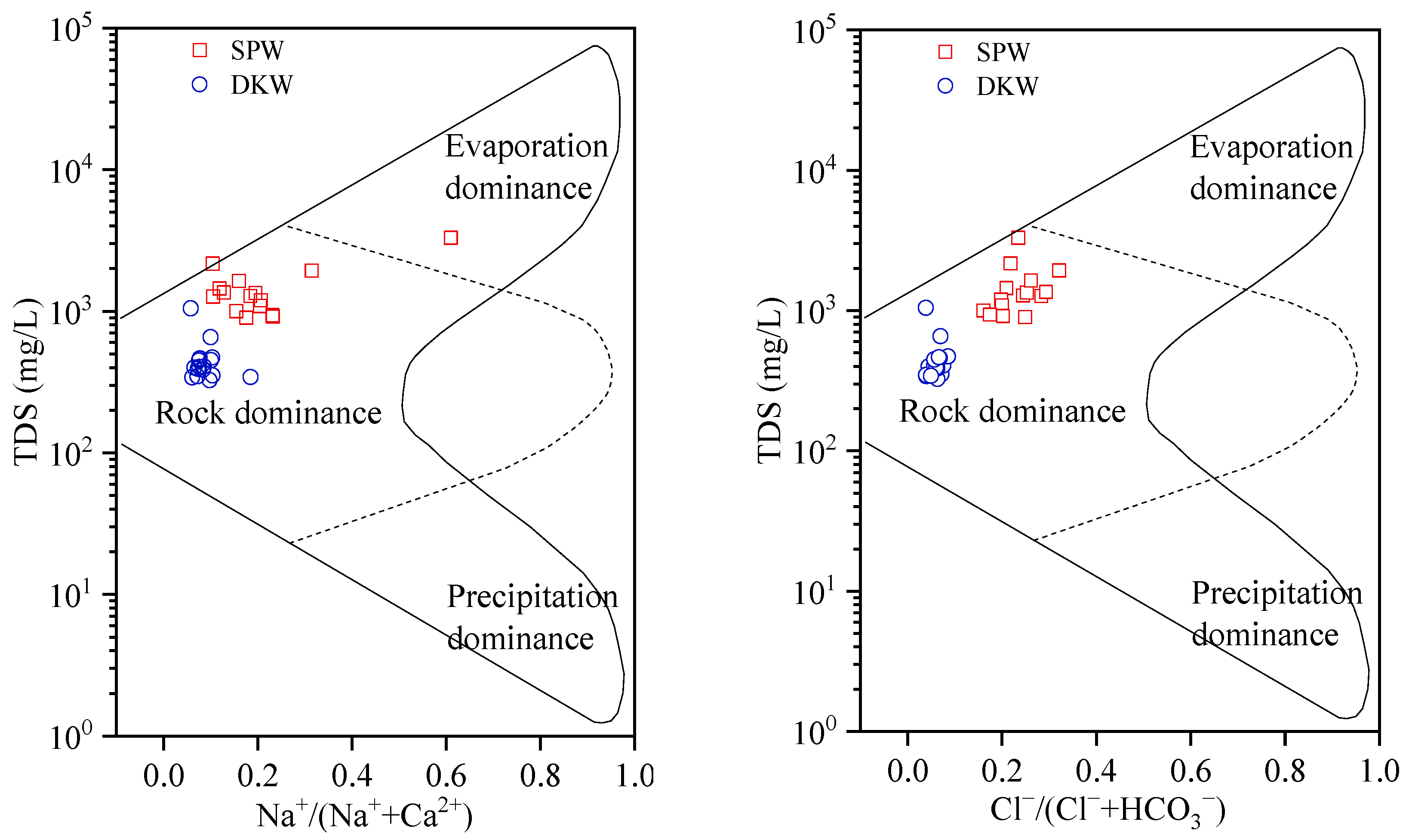
4.1. Hydrogeochemical Processes Identification
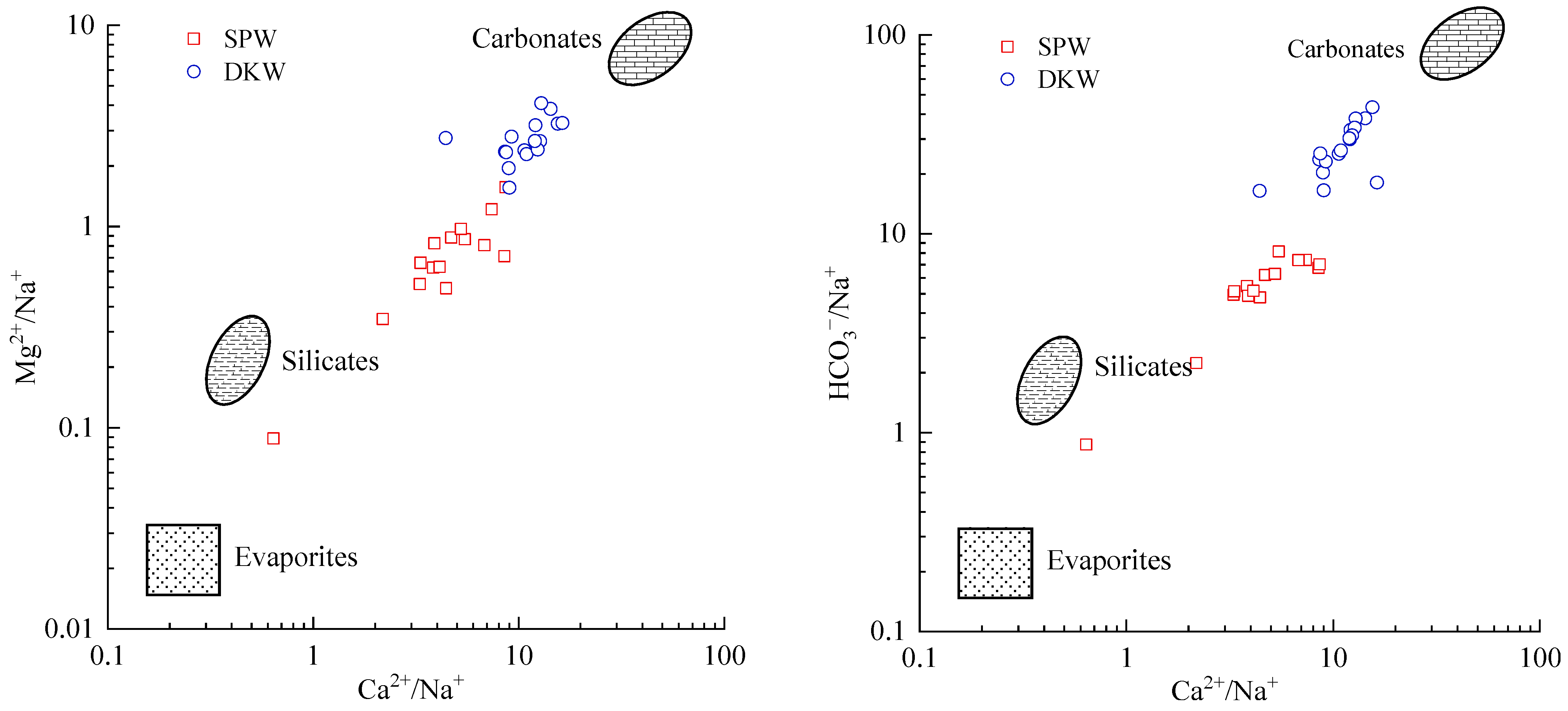
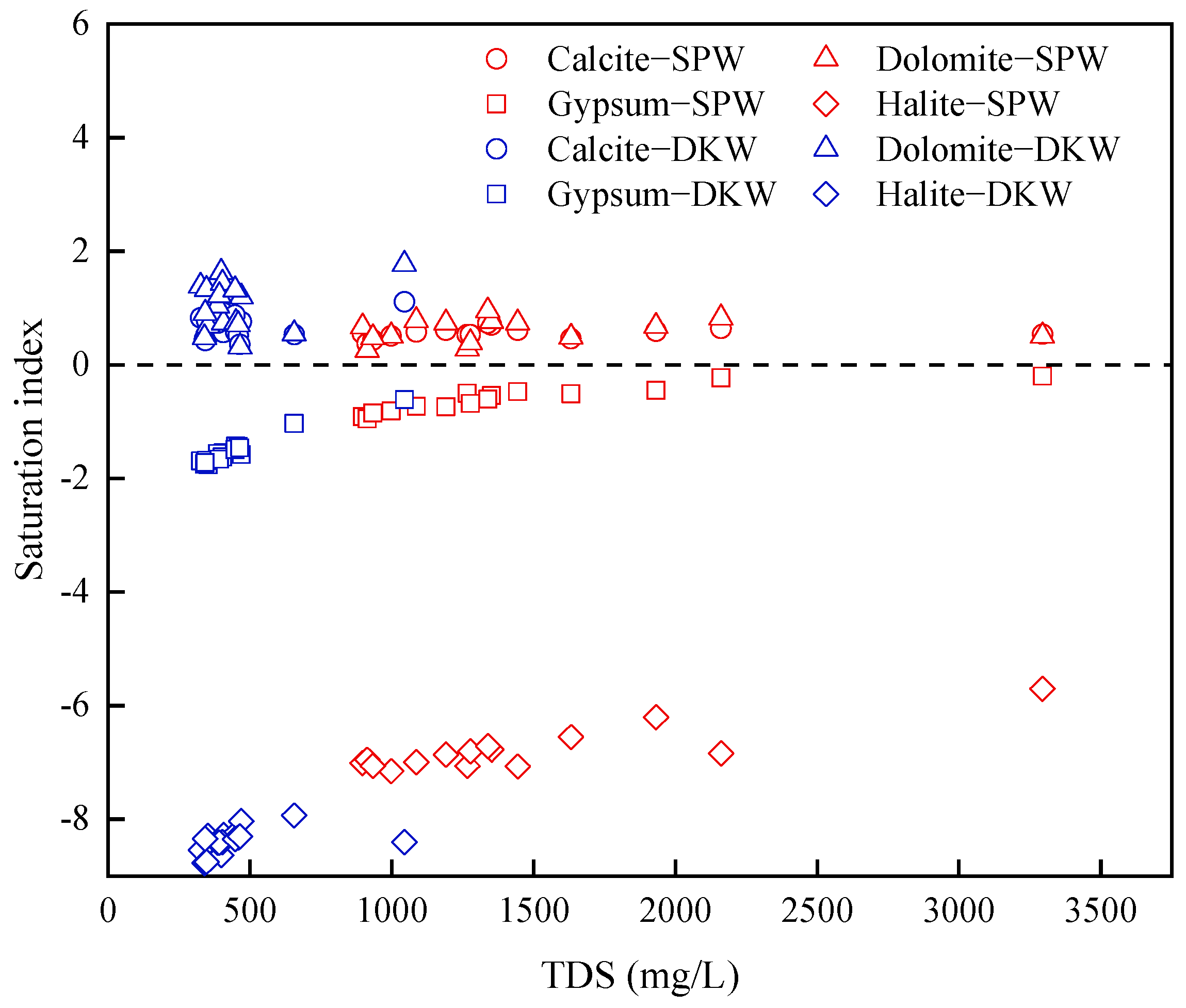
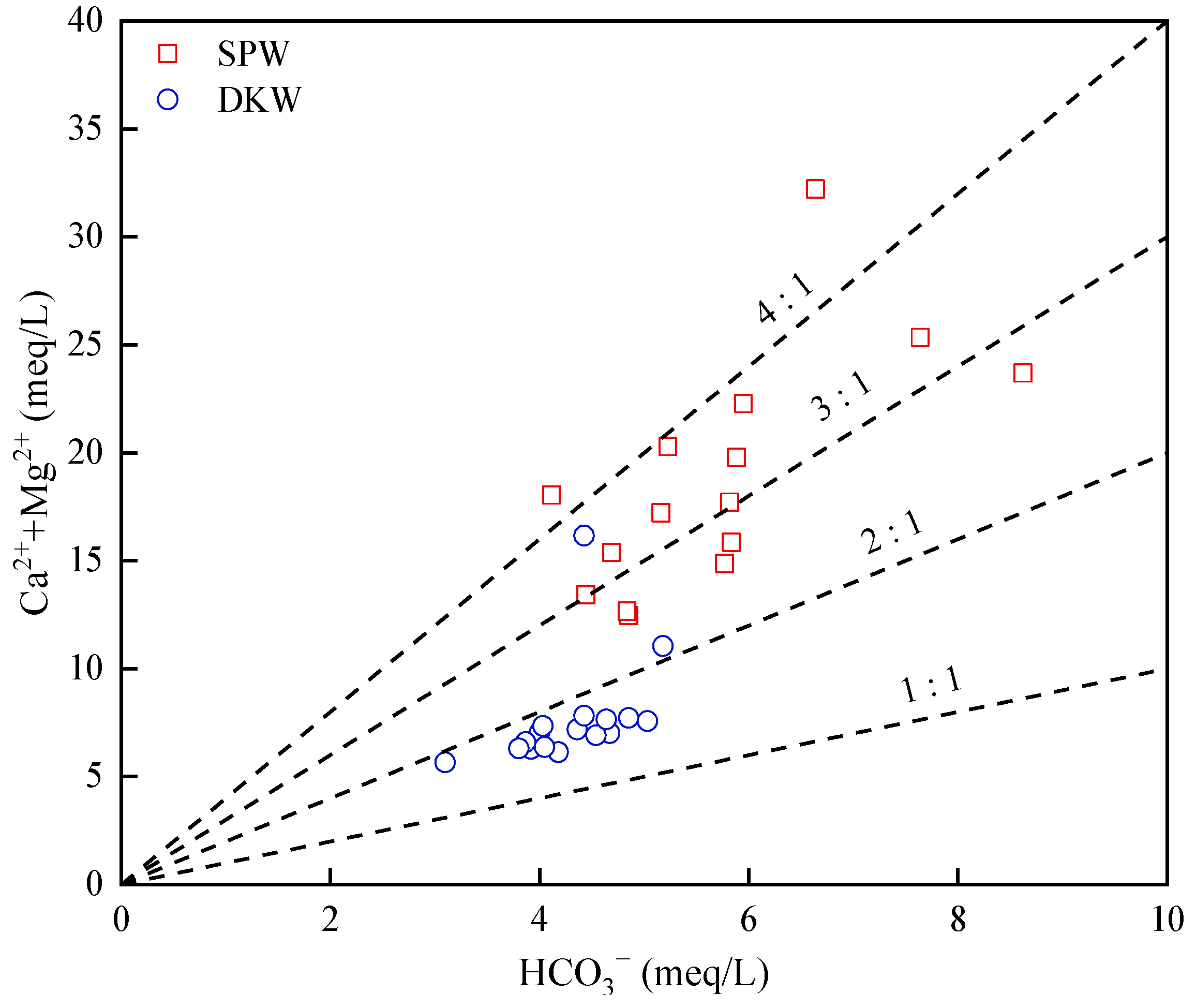
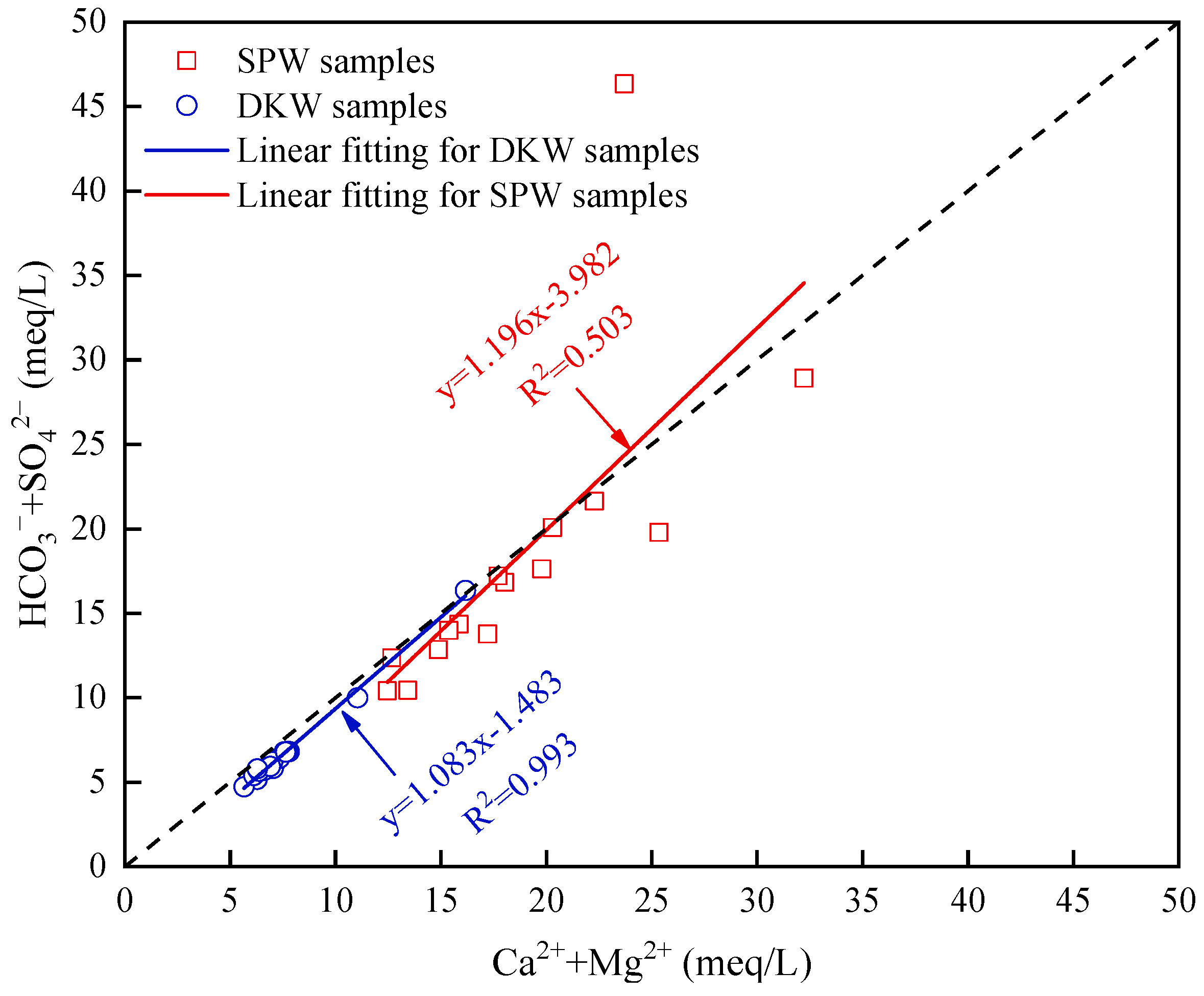
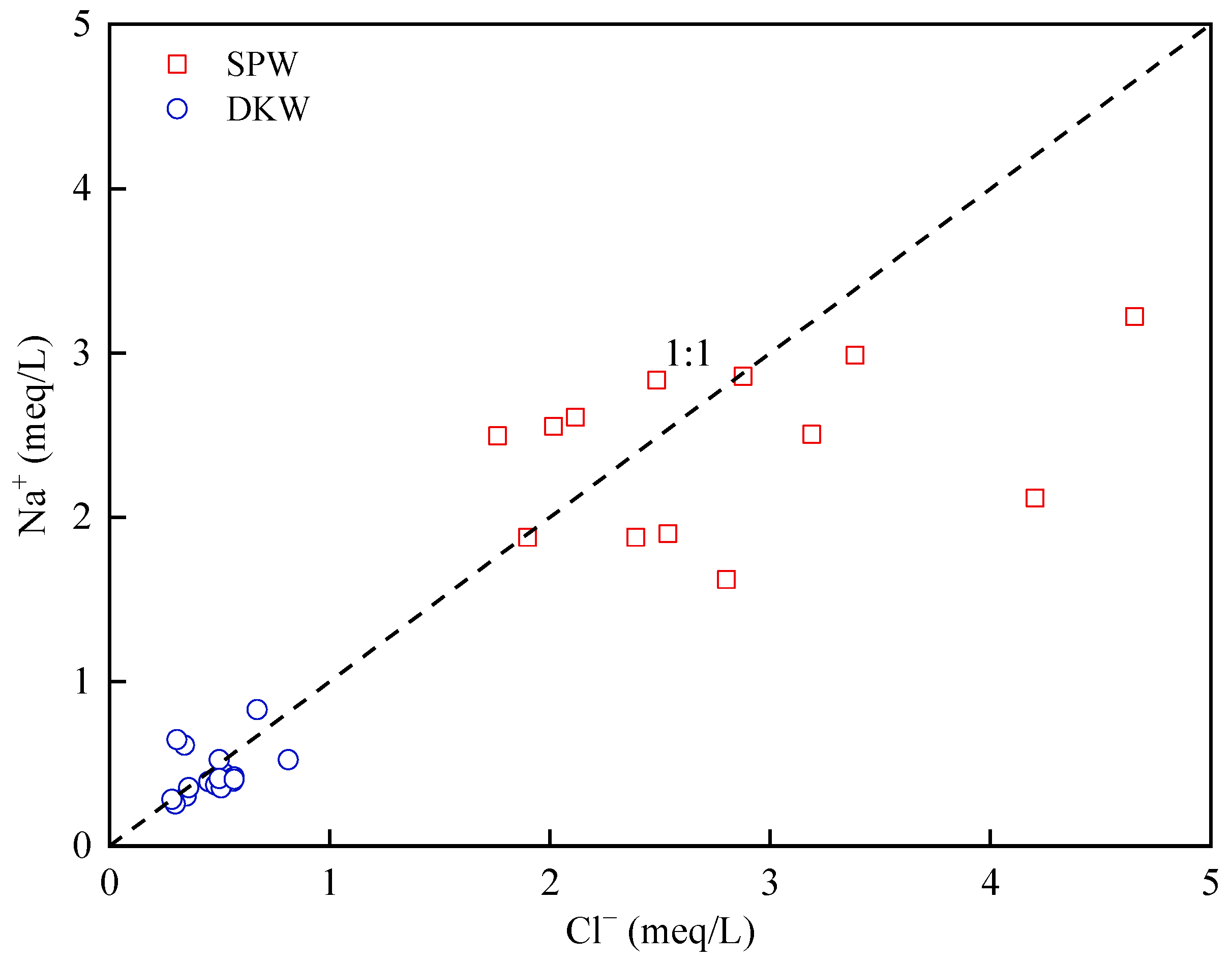
4.1.1. Rock Weathering Processes
4.1.2. Cation Exchange
4.1.3. Anthropogenic Activities
4.2. Groundwater Quality Assessment
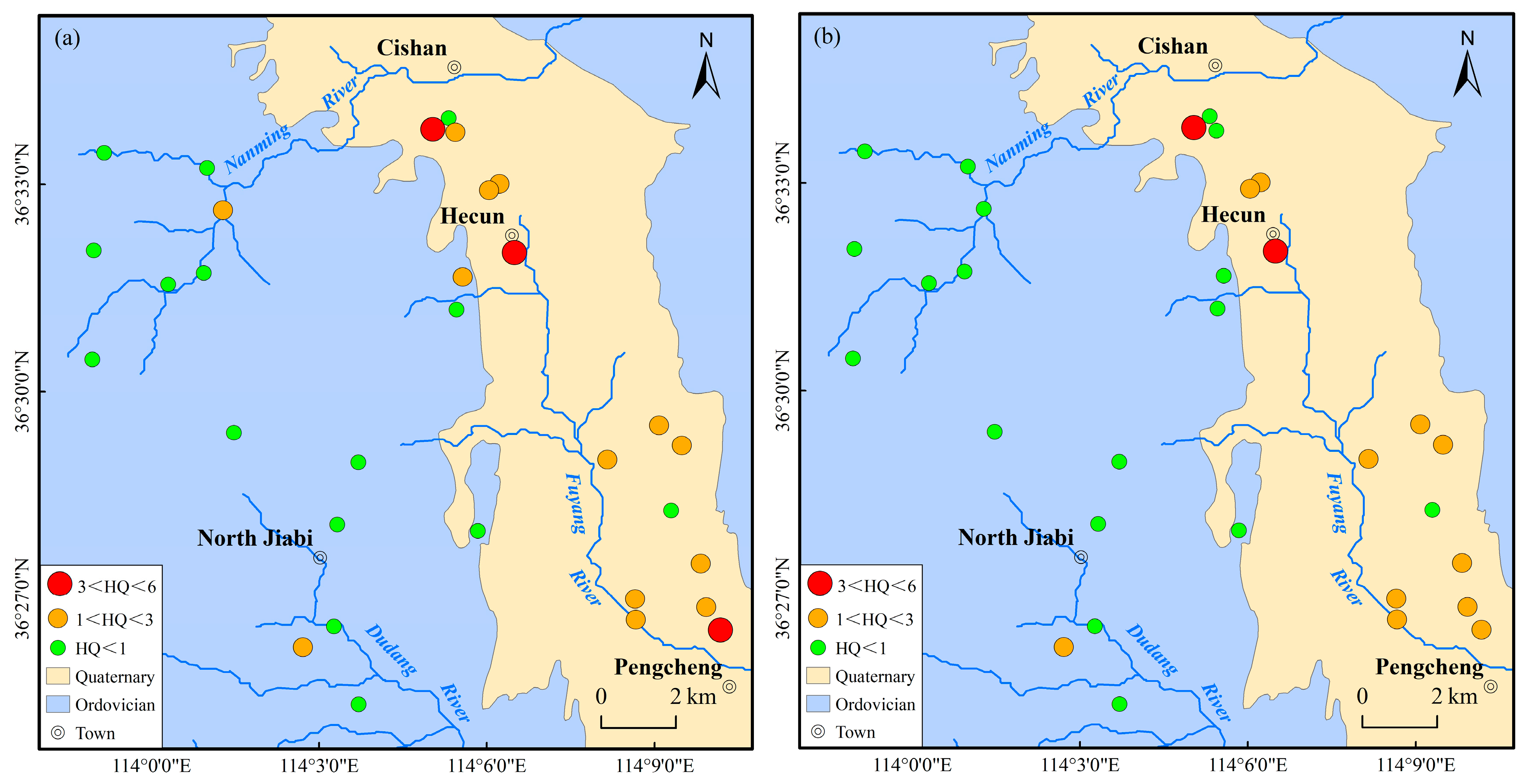
4.3. Health Risk Assessment
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Baciocchi, R.; Berardi, S.; Verginelli, I. Human health risk assessment: Models for predicting the effective exposure duration of on-site receptors exposed to contaminated groundwater. J. Hazard. Mater. 2010, 181, 226–233. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hu, K.; Huang, Y.; Li, H.; Li, B.; Chen, D.; White, R.E. Spatial variability of shallow groundwater level, electrical conductivity and nitrate concentration, and risk assessment of nitrate contamination in North China Plain. Environ. Int. 2005, 31, 896–903. [Google Scholar] [CrossRef]
- Xu, Y.; Xue, X.; Dong, L.; Nai, C.; Liu, Y.; Huang, Q. Long-term dynamics of leachate production, leakage from hazardous waste landfill sites and the impact on groundwater quality and human health. Waste Manag. 2018, 82, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Panhwar, A.H.; Kazi, T.G.; Afridi, H.I.; Shaikh, H.R.; Arain, S.A.; Arain, S.S.; Brahman, K.D. Evaluation of Calcium and Magnesium in Scalp Hair Samples of Population Consuming Different Drinking Water: Risk of Kidney Stone. Biol. Trace Elem. Res. 2013, 156, 67–73. [Google Scholar] [CrossRef]
- Rani, A.; Parashar, K.; Meena, R.; Sharma, S.K.; Tiwari, K.K.; Ajaykumar, V.; Mondal, N.C. Hydrochemical characteristics and potential health risks of nitrate, fluoride, and uranium in Kota district, Rajasthan, India. Environ. Sci. Pollut. Res. 2023, 30, 82485–82505. [Google Scholar] [CrossRef]
- Chakraborti, D.; Rahman, M.M.; Ahamed, S.; Dutta, R.N.; Pati, S.; Mukherjee, S.C. Arsenic contamination of groundwater and its induced health effects in Shahpur block, Bhojpur district, Bihar state, India: Risk evaluation. Environ. Sci. Pollut. Res. 2016, 23, 9492–9504. [Google Scholar] [CrossRef]
- Janardhana Raju, N. Arsenic exposure through groundwater in the middle Ganga plain in the Varanasi environs, India: A future threat. J. Geol. Soc. India 2012, 79, 302–314. [Google Scholar] [CrossRef]
- Chakraborti, D.; Rahman, M.M.; Das, B.; Chatterjee, A.; Das, D.; Nayak, B.; Pal, A.; Chowdhury, U.K.; Ahmed, S.; Biswas, B.K.; et al. Groundwater arsenic contamination and its health effects in India. HydJ 2017, 25, 1165–1181. [Google Scholar] [CrossRef]
- Li, P.; He, X.; Li, Y.; Xiang, G. Occurrence and Health Implication of Fluoride in Groundwater of Loess Aquifer in the Chinese Loess Plateau: A Case Study of Tongchuan, Northwest China. Expo. Health 2019, 11, 95–107. [Google Scholar] [CrossRef]
- Dong, S.; Liu, B.; Shi, X.; Zhang, W.; Li, Z. The spatial distribution and hydrogeological controls of fluoride in the confined and unconfined groundwater of Tuoketuo County, Hohhot, Inner Mongolia, China. Environ. Earth Sci. 2015, 74, 325–335. [Google Scholar] [CrossRef]
- Li, X.; Han, G.; Liu, M.; Song, C.; Zhang, Q.; Yang, K.; Liu, J. Hydrochemistry and Dissolved Inorganic Carbon (DIC) Cycling in a Tropical Agricultural River, Mun River Basin, Northeast Thailand. Int. J. Environ. Res. Public Health 2019, 16, 3410. [Google Scholar] [CrossRef] [PubMed]
- Nadler, A.; Magaritz, M.; Mazor, E. Chemical reactions of sea water with rocks and freshwater: Experimental and field observations on brackish waters in Israel. Geochim. Cosmochim. Acta 1980, 44, 879–886. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, J.-Y.; Kim, K. Geochemical characteristics of fluoride in groundwater of Gimcheon, Korea: Lithogenic and agricultural origins. Environ. Earth Sci. 2011, 63, 1139–1148. [Google Scholar] [CrossRef]
- Mao, H.; Wang, C.; Qu, S.; Liao, F.; Wang, G.; Shi, Z. Source and evolution of sulfate in the multi-layer groundwater system in an abandoned mine—Insight from stable isotopes and Bayesian isotope mixing model. Sci. Total Environ. 2023, 859, 160368. [Google Scholar] [CrossRef]
- Jiang, C.; Zhao, Q.; Zheng, L.; Chen, X.; Li, C.; Ren, M. Distribution, source and health risk assessment based on the Monte Carlo method of heavy metals in shallow groundwater in an area affected by mining activities, China. Ecotoxicol. Environ. Saf. 2021, 224, 112679. [Google Scholar] [CrossRef] [PubMed]
- Sheng, D.; Meng, X.; Wen, X.; Wu, J.; Yu, H.; Wu, M. Contamination characteristics, source identification, and source-specific health risks of heavy metal(loid)s in groundwater of an arid oasis region in Northwest China. Sci. Total Environ. 2022, 841, 156733. [Google Scholar] [CrossRef]
- Morgenstern, U.; Daughney, C.J. Groundwater age for identification of baseline groundwater quality and impacts of land-use intensification—The National Groundwater Monitoring Programme of New Zealand. JHyd 2012, 456–457, 79–93. [Google Scholar] [CrossRef]
- Ben Moussa, A.; Mzali, H.; Zouari, K.; Hezzi, H. Hydrochemical and isotopic assessment of groundwater quality in the Quaternary shallow aquifer, Tazoghrane region, north-eastern Tunisia. Quat. Int. 2014, 338, 51–58. [Google Scholar] [CrossRef]
- Liu, F.; Wang, S.; Wang, L.; Shi, L.; Song, X.; Yeh, T.-C.J.; Zhen, P. Coupling hydrochemistry and stable isotopes to identify the major factors affecting groundwater geochemical evolution in the Heilongdong Spring Basin, North China. J. Geochem. Explor. 2019, 205, 106352. [Google Scholar] [CrossRef]
- Hao, C.-M.; Huang, Y.; Ma, D.-J.; Fan, X. Hydro-geochemistry evolution in Ordovician limestone water induced by mountainous coal mining: A case study from North China. J. Mt. Sci. 2020, 17, 614–623. [Google Scholar] [CrossRef]
- Wang, C.; Liao, F.; Wang, G.; Qu, S.; Mao, H.; Bai, Y. Hydrogeochemical evolution induced by long-term mining activities in a multi-aquifer system in the mining area. Sci. Total Environ. 2023, 854, 158806. [Google Scholar] [CrossRef]
- Gao, M.; Li, X.; Qian, J.; Wang, Z.; Hou, X.; Fu, C.; Ma, J.; Zhang, C.; Li, J. Hydrogeochemical Characteristics and Evolution of Karst Groundwater in Heilongdong Spring Basin, Northern China. Water 2023, 15, 726. [Google Scholar] [CrossRef]
- Al-Omran, A.M.; Aly, A.A.; Al-Wabel, M.I.; Sallam, A.S.; Al-Shayaa, M.S. Hydrochemical characterization of groundwater under agricultural land in arid environment: A case study of Al-Kharj, Saudi Arabia. Arab. J. Geosci. 2015, 9, 68. [Google Scholar] [CrossRef]
- Li, P.; Qian, H.; Wu, J.; Chen, J.; Zhang, Y.; Zhang, H. Occurrence and hydrogeochemistry of fluoride in alluvial aquifer of Weihe River, China. Environ. Earth Sci. 2014, 71, 3133–3145. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C.; Wang, Z. The Hazard Analysis of Water Inrush of Mining of Thick Coal Seam Under Reservoir Based on Entropy Weight Evaluation Method. Geotech. Geol. Eng. 2018, 36, 3019–3028. [Google Scholar] [CrossRef]
- Liu, J.; Gao, Z.; Feng, J.; Wang, M. Identification of the hydrochemical features, genesis, water quality and potential health hazards of groundwater in Dawen River Basin, North China. Ecol. Indic. 2023, 149, 110175. [Google Scholar] [CrossRef]
- Feng, Y.; Fanghui, Y.; Li, C. Improved Entropy Weighting Model in Water Quality Evaluation. Water Resour. Manag. 2019, 33, 2049–2056. [Google Scholar] [CrossRef]
- Abdo, H.G.; Almohamad, H.; Al Dughairi, A.A.; Ali, S.A.; Parvin, F.; Elbeltagi, A.; Costache, R.; Mohammed, S.; Al-Mutiry, M.; Alsafadi, K. Spatial implementation of frequency ratio, statistical index and index of entropy models for landslide susceptibility mapping in Al-Balouta river basin, Tartous Governorate, Syria. Geosci. Lett. 2022, 9, 45. [Google Scholar] [CrossRef]
- Sun, Y.; Liang, X.; Xiao, C. Assessing the influence of land use on groundwater pollution based on coefficient of variation weight method: A case study of Shuangliao City. Environ. Sci. Pollut. Res. 2019, 26, 34964–34976. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, P.; Qian, H. Groundwater Quality Assessment Using Improved Water Quality Index (WQI) and Human Health Risk (HHR) Evaluation in a Semi-arid Region of Northwest China. Expo. Health 2020, 12, 487–500. [Google Scholar] [CrossRef]
- Li, P.; Li, X.; Meng, X.; Li, M.; Zhang, Y. Appraising Groundwater Quality and Health Risks from Contamination in a Semiarid Region of Northwest China. Expo. Health 2016, 8, 361–379. [Google Scholar] [CrossRef]
- Adimalla, N. Groundwater Quality for Drinking and Irrigation Purposes and Potential Health Risks Assessment: A Case Study from Semi-Arid Region of South India. Expo. Health 2019, 11, 109–123. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, C.; Xiao, Q.; Bu, H. Hydrogeochemical characteristics of a closed karst groundwater basin in North China. J. Radioanal. Nucl. Chem. 2020, 325, 365–379. [Google Scholar] [CrossRef]
- Lu, S.; Chen, J.; Zheng, X.; Liang, Y.; Jia, Z.; Li, X. Hydrogeochemical characteristics of karst groundwater in Jinci spring area, north China. Carbonates Evaporites 2020, 35, 68. [Google Scholar] [CrossRef]
- Ma, L.; Huang, T.; Qiu, H.; Yang, Z.; He, X.; Qian, J. Hydrogeochemical characteristic evaluation and irrigation suitability assessment of shallow groundwater in Dangshan County, China. Geosci. J. 2021, 25, 731–748. [Google Scholar] [CrossRef]
- Liu, J.; Gao, Z.; Wang, M.; Li, Y.; Shi, M.; Zhang, H.; Ma, Y. Hydrochemical characteristics and possible controls in the groundwater of the Yarlung Zangbo River Valley, China. Environ. Earth Sci. 2019, 78, 76. [Google Scholar] [CrossRef]
- Ryu, J.-S.; Lee, K.-S.; Chang, H.-W. Hydrochemistry and isotope geochemistry of Song Stream, a headwater tributary of the South Han River, South Korea. Geosci. J. 2007, 11, 157–164. [Google Scholar] [CrossRef]
- Li, S.; Gaillardet, J.; Han, G.; Calmels, D.; Liu, C. Sulfuric acid as a weathering agent of carbonate weathering constrained by δ13C: Examples from Southwest China. Chin. J. Geochem. 2006, 25, 270–271. [Google Scholar] [CrossRef]
- Guo, Y.; Wei, J.; Gui, H.; Zhang, Z.; Hu, M. Evaluation of changes in groundwater quality caused by a water inrush event in Taoyuan coal mine, China. Environ. Earth Sci. 2020, 79, 528. [Google Scholar] [CrossRef]
- Qian, J.; Tong, Y.; Ma, L.; Zhao, W.; Zhang, R.; He, X. Hydrochemical Characteristics and Groundwater Source Identification of a Multiple Aquifer System in a Coal Mine. Mine Water Environ. 2018, 37, 528–540. [Google Scholar] [CrossRef]
- Huang, D.; Liu, Z.; Wang, W. Evaluating the Impaction of Coal Mining on Ordovician Karst Water through Statistical Methods. Water 2018, 10, 1409. [Google Scholar] [CrossRef]
- Martín del Campo, M.A.; Esteller, M.V.; Expósito, J.L.; Hirata, R. Impacts of urbanization on groundwater hydrodynamics and hydrochemistry of the Toluca Valley aquifer (Mexico). Environ. Monit. Assess. 2014, 186, 2979–2999. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Peng, Y.; Li, C.; Gao, Z.; Chen, S. An investigation into the hydrochemistry, quality and risk to human health of groundwater in the central region of Shandong Province, North China. J. Clean. Prod. 2021, 282, 125416. [Google Scholar] [CrossRef]
- Huang, S.; Guo, J.; Xie, Y.; Bian, R.; Wang, N.; Qi, W.; Liu, H. Distribution, sources, and potential health risks of fluoride, total iodine, and nitrate in rural drinking water sources of North and East China. Sci. Total Environ. 2023, 898, 165561. [Google Scholar] [CrossRef] [PubMed]




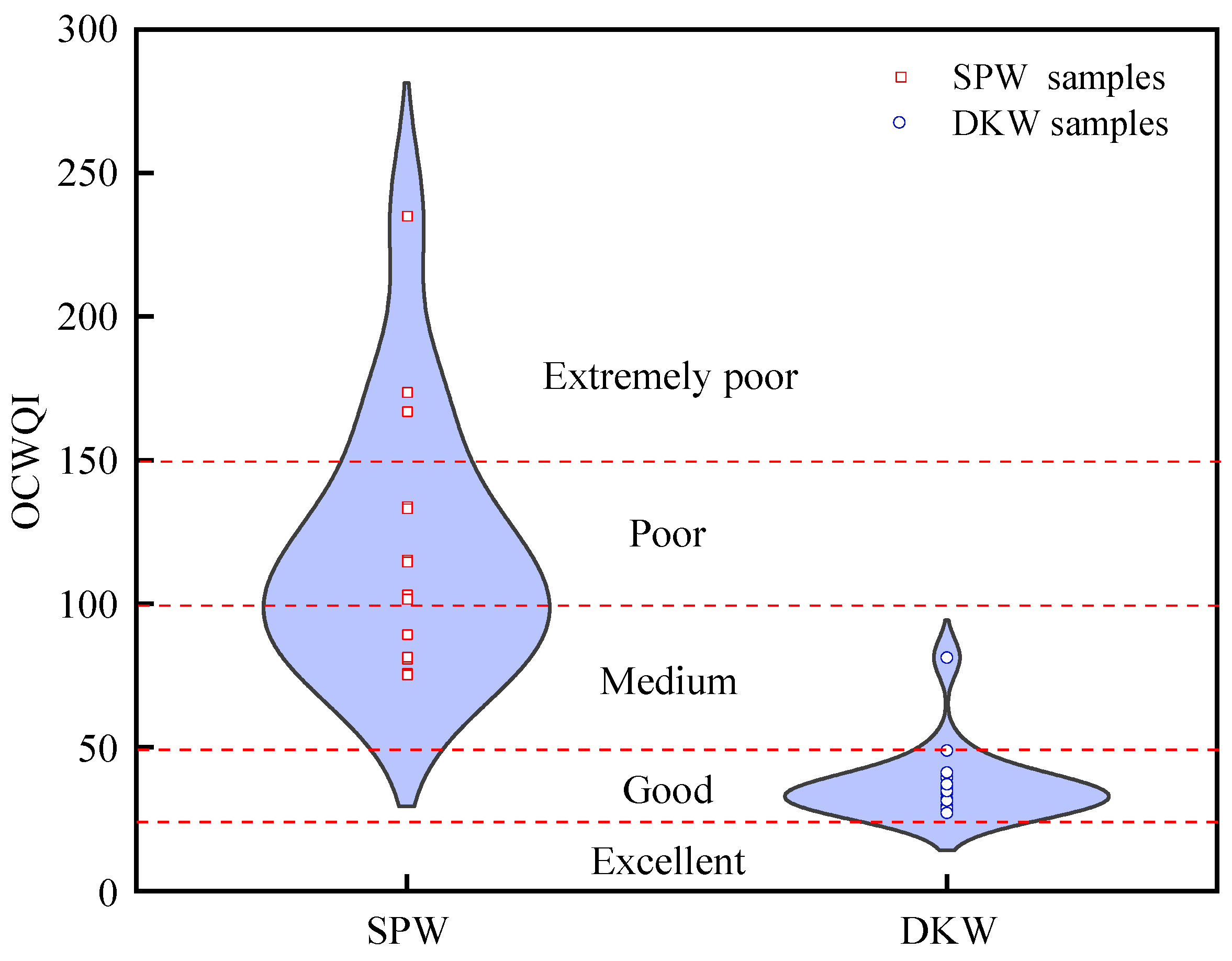
| OCWQI | <25 | 25–50 | 50–100 | 100–150 | >150 |
|---|---|---|---|---|---|
| Classifications | Excellent | Good | Medium | Poor | Extremely poor |
| Parameters | Adults | Children |
|---|---|---|
| IR (in L/day) | 1.5 | 0.78 |
| ED (in year) | 30 | 12 |
| EF (in days/year) | 365 | 365 |
| AWB (in kg) | 65 | 15 |
| AET (in days) | 10,950 | 4380 |
| Components | Min | Max | Mean | Medium | SD | CV | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SPW | DKW | SPW | DKW | SPW | DKW | SPW | DKW | SPW | DKW | SPW | DKW | |
| pH | 6.90 | 7.40 | 7.40 | 8.20 | 7.24 | 7.81 | 7.30 | 7.80 | 0.14 | 0.23 | 0.02 | 0.03 |
| TH | 622.00 | 283.00 | 1610.00 | 808.00 | 936.13 | 384.24 | 885.00 | 351.00 | 270.08 | 123.89 | 0.29 | 0.32 |
| TDS | 897.00 | 326.00 | 3295.00 | 1045.00 | 1447.87 | 452.24 | 1277.00 | 403.00 | 628.16 | 171.45 | 0.43 | 0.38 |
| K+ | 0.89 | 0.57 | 9.56 | 2.29 | 2.80 | 1.25 | 1.78 | 1.07 | 2.38 | 0.53 | 0.85 | 0.42 |
| Na+ | 37.30 | 5.89 | 604.00 | 19.10 | 99.30 | 10.18 | 58.70 | 9.35 | 142.68 | 3.37 | 1.44 | 0.33 |
| Ca2+ | 191.00 | 62.20 | 496.00 | 243.00 | 298.13 | 110.17 | 291.00 | 103.00 | 85.10 | 41.13 | 0.29 | 0.37 |
| Mg2+ | 26.60 | 19.10 | 90.20 | 48.80 | 46.71 | 26.61 | 40.80 | 23.80 | 16.69 | 7.32 | 0.36 | 0.28 |
| Cl− | 62.50 | 10.10 | 172.00 | 28.80 | 108.02 | 16.86 | 99.30 | 17.70 | 37.35 | 5.04 | 0.35 | 0.30 |
| SO42− | 267.00 | 58.00 | 1810.00 | 572.00 | 611.40 | 122.74 | 547.00 | 87.70 | 391.95 | 122.08 | 0.64 | 0.99 |
| HCO3− | 251.00 | 189.00 | 526.00 | 316.00 | 347.67 | 262.24 | 352.00 | 266.00 | 73.66 | 31.35 | 0.21 | 0.12 |
| NO3− | 16.30 | 1.73 | 244.01 | 57.57 | 107.60 | 33.33 | 95.66 | 34.68 | 61.20 | 14.02 | 0.57 | 0.42 |
| F− | 0.23 | 0.15 | 2.40 | 0.52 | 0.50 | 0.27 | 0.34 | 0.25 | 0.54 | 0.10 | 1.08 | 0.38 |
| Components | Wej | Wcj | Woj |
|---|---|---|---|
| pH | 0.2465 | 0.0063 | 0.0472 |
| F− | 0.0535 | 0.1439 | 0.1046 |
| NO3− | 0.1158 | 0.1162 | 0.1384 |
| TH | 0.1302 | 0.0750 | 0.1179 |
| TDS | 0.0893 | 0.1018 | 0.1137 |
| Na+ | 0.0542 | 0.2852 | 0.1482 |
| SO42− | 0.0774 | 0.1478 | 0.1276 |
| Cl− | 0.2330 | 0.1236 | 0.2024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Li, H.; Zhang, F.; Qian, J.; Han, S.; Dai, F. Groundwater Hydrogeochemical Processes and Potential Threats to Human Health in Fengfeng Coal Mining Area, China. Water 2023, 15, 4024. https://doi.org/10.3390/w15224024
Zhang Z, Li H, Zhang F, Qian J, Han S, Dai F. Groundwater Hydrogeochemical Processes and Potential Threats to Human Health in Fengfeng Coal Mining Area, China. Water. 2023; 15(22):4024. https://doi.org/10.3390/w15224024
Chicago/Turabian StyleZhang, Zhiqiang, Haixue Li, Fawang Zhang, Jiazhong Qian, Shuangbao Han, and Fenggang Dai. 2023. "Groundwater Hydrogeochemical Processes and Potential Threats to Human Health in Fengfeng Coal Mining Area, China" Water 15, no. 22: 4024. https://doi.org/10.3390/w15224024
APA StyleZhang, Z., Li, H., Zhang, F., Qian, J., Han, S., & Dai, F. (2023). Groundwater Hydrogeochemical Processes and Potential Threats to Human Health in Fengfeng Coal Mining Area, China. Water, 15(22), 4024. https://doi.org/10.3390/w15224024






