The Relationship between Total Mercury, Its Fractions and Species Diversity of Diatom Taphocoenoses Deposited in Surface Sediments (Southern Baltic Sea)
Abstract
:1. Introduction
2. Materials and Methods
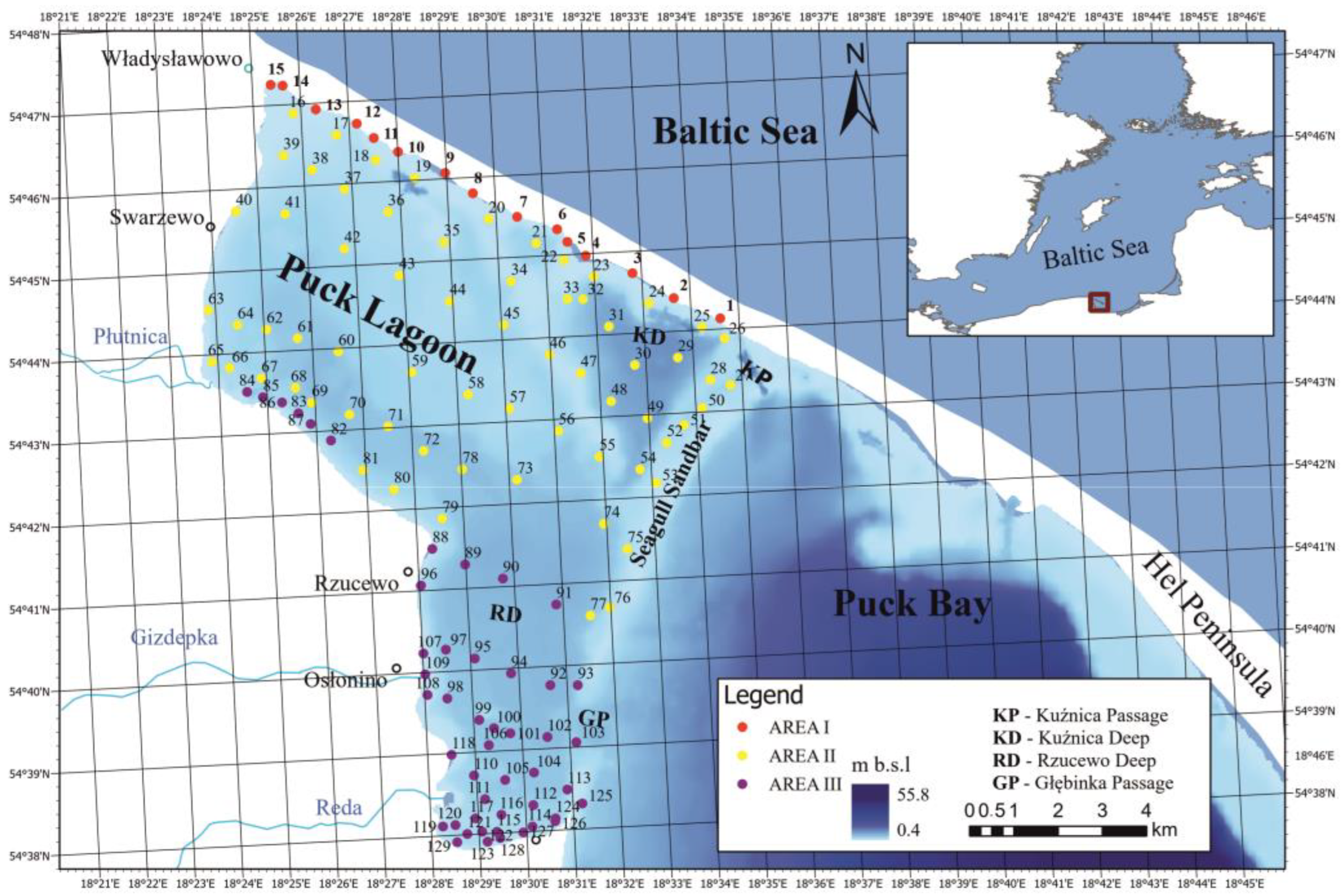
2.1. Study Area
2.2. Sample Collection
2.3. Diatom Analysis
2.4. Hg Analysis
2.5. Loss on Ignition, Wetness and Granulometry Analysis
2.6. Data Processing
3. Results and Discussion
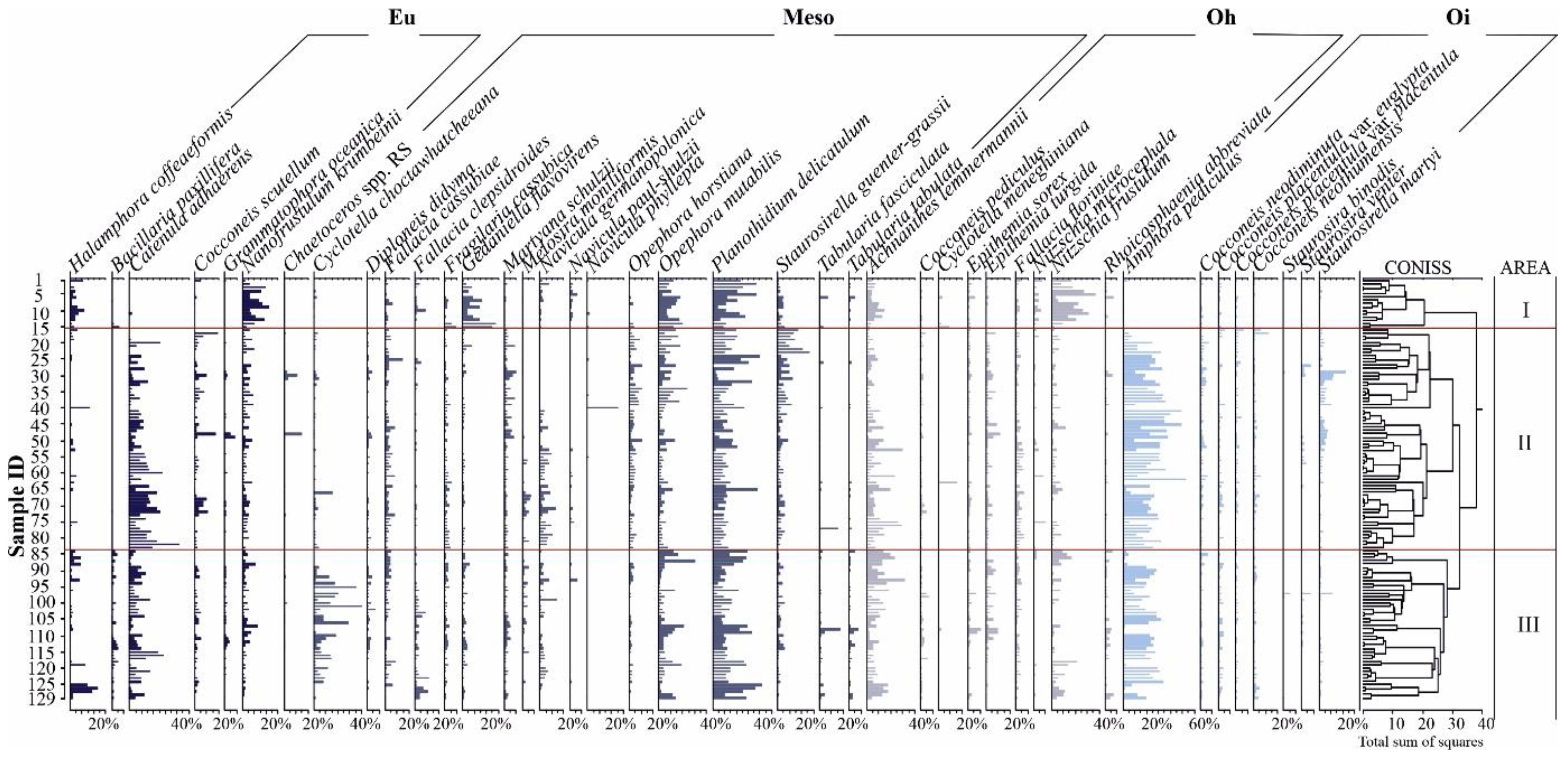
3.1. Diatom Analysis
3.2. Hg in Surface Sediments
Factors Influencing Hg Concentration in the Surface Sediment
3.3. Hg and Diatoms Salinity Regions
3.4. Mercury Versus Diatoms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zahir, F.; Rizwi, S.J.; Haq, S.K.; Khan, R.H. Low dose mercury toxicity and human health. Environ. Toxicol. Pharmacol. 2005, 20, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.B.; Zevenhoven, R.; Brodersen, J.; Hylander, L.D.; Bhattacharya, P. Mercury in waste in the European Union: Sources, disposal methods and risks. Resources. Conserv. Recycl. 2004, 42, 155–182. [Google Scholar] [CrossRef]
- Wyn, B.; Kidd, K.A.; Burgess, N.M.; Curry, R.A. Mercury biomagnification in the food webs of acidic lakes in Kejimkujik National Park and National Historic Site, Nova Scotia. Can. J. Fish. Aquat. Sci. 2009, 66, 1532–1545. [Google Scholar] [CrossRef]
- Falandysz, J. Mercury concentrations in benthic animals and plants inhabiting the Gulf of Gdańsk, Baltic Sea. Sci. Total Environ. 1994, 141, 45–49. [Google Scholar] [CrossRef]
- Lavoie, R.A.; Jardine, T.D.; Chumchal, M.M.; Kidd, K.A.; Campbell, L.M. Biomagnification of mercury in aquatic food webs: A worldwide meta-analysis. Environ. Sci. Technol. 2013, 47, 13385–13394. [Google Scholar] [CrossRef]
- Bełdowska, M.; Zgrundo, A.; Kobos, J. Mercury in the Diatoms of Various Ecological Formations. Water Air Soil Pollut. 2018, 229, 168. [Google Scholar] [CrossRef]
- Kang, W.; Chen, G.; Wang, J.; Huang, L.; Li, R.; Hu, Y.; Tao, J.; Blais, J.M.; Smol, J.P. Assessing the impact of long-term changes in climate and atmospheric deposition on a shallow alpine lake from southeast Tibet. Sci. Total Environ. 2019, 650, 713–724. [Google Scholar] [CrossRef]
- Gosnell, K.J.; Dam, H.G.; Mason, R.P. Mercury and methylmercury uptake and trophic transfer from marine diatoms to copepods and field collected zooplankton. Mar. Environ. Res. 2021, 170, 105446. [Google Scholar] [CrossRef]
- Round, F.E. The Biology of Algae; Cambridge University Press: Cambridge, UK; London, UK; New York, NY, USA; New Rochelle, NY, USA; Melbourne, Australia; Sydney, Australia, 1981; p. 653. [Google Scholar]
- Leblanc, K.; Arístegui, J.; Armand, L.; Assmy, P.; Beker, B.; Bode, A.; Breton, E.; Cornet, V.; Gibson, J.; Gosselin, M.P.; et al. A global diatom database—Abundance, biovolume and biomass in the world ocean. Earth Syst. Sci. Data Discuss. 2012, 5, 147–185. [Google Scholar] [CrossRef]
- Legrand, C.; Fridolfsson, E.; Bertos-Fortis, M.; Lindehoff, E.; Larsson, P.; Pinhassi, J.; Andersson, A. Interannual variability of phyto-bacterioplankton biomass and production in coastal and offshore waters of the Baltic Sea. AMBIO 2015, 44, 427–438. [Google Scholar] [CrossRef]
- Staniszewska, M.; Nehring, I.; Zgrundo, A. The role of phytoplankton composition, biomass and cell volume in accumulation and transfer of endocrine disrupting compounds in the Southern Baltic Sea (The Gulf of Gdansk). Environ. Pollut. 2015, 207, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-S.; Fisher, N.S. Bioaccumulation of methylmercury in a marine diatom and the influence of dissolved organic matter. Mar. Chem. 2017, 197, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, D.; Müller, A.; Csögör, Z.; Frimmel, F.H.; Posten, C. The adsorption kinetics of metal ions onto different microalgae and siliceous earth. Water Res. 2001, 35, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Outridge, P.M.; Stern, G.A.; Hamilton, P.B.; Percival, J.B.; McNeely, R.; Lockhart, W.L. Trace metal profiles in the varved sediment of an Arctic lake. Geochim. Cosmochim. Acta 2005, 69, 4881–4894. [Google Scholar] [CrossRef]
- Zaferani, S.; Pérez-Rodríguez, M.; Biester, H. Diatom ooze—A large marine mercury sink. Science 2018, 361, 797–800. [Google Scholar] [CrossRef]
- Bérard, A.; Dorigo, U.; Mercier, I.; Becker-van Slooten, K.; Grandlean, D.; Leboulonger, C. Comparison of the eco toxicological impact of the traizines Irgarol 1051 and atrazine on micro algal cultures and natural micro algal communities in Lake Geneva. Chemosphere 2003, 53, 935–944. [Google Scholar] [CrossRef]
- Anantharaj, K.; Govindasamy, C.; Natanamurugaraj, G.; Jeyachandran, S. Effect of heavy metals on marine diatom Amphora coffeaeformis (Agardh. Kutz). Glob. J. Environ. Res. 2011, 5, 112–117. [Google Scholar]
- Deng, G.; Zhang, T.; Yang, L.; Wang, Q. Studies of bio uptake and transformation of mercury by a typical unicellular diatom Phaeodactylum tricornutum. Chin. Sci. Bull. 2013, 58, 256–265. [Google Scholar] [CrossRef]
- Naseri, A.; Saadatmand, S.; Noroozi, M.; Asri, Y.; Iranbakhsh, A. Study the mercury biosorption by unicellular diatom Nitzschia capitellata Hustedt. Mod. Phytomorphol. 2020, 14, 70–76. [Google Scholar] [CrossRef]
- Zhong, H.; Wang, W.-X. Controls of Dissolved Organic Matter and Chloride on Mercury Uptake by a Marine Diatom. Environ. Sci. Technol. 2009, 43, 8998–9003. [Google Scholar] [CrossRef]
- Cyberski, J. Hydrologia zlewiska. In Zatoka Pucka; Korzeniewski, K., Ed.; Fundacja Rozwoju UG: Gdańsk, Poland, 1993; pp. 40–71. (In Polish) [Google Scholar]
- Zaborska, A.; Siedlewicz, G.; Szymczycha, B.; Dzierzbicka-Głowacka, L.; Pazdro, K. Legacy and emerging pollutants in the Gulf of Gdańsk (southern Baltic Sea)—Loads and distribution revisited. Mar. Pollut. Bull. 2019, 139, 238–255. [Google Scholar] [CrossRef] [PubMed]
- Chubarenko, B.; Woelfel, J.; Hofmann, J.; Aldag, S.; Beldowski, J.; Burlakovs, J.; Garrels, T.; Gorbunova, J.; Guizani, S.; Kupczyk, A.; et al. Converting beach wrack into a resource as a challenge for the Baltic Sea (an overview). Ocean Coast. Manag. 2021, 200, 105413. [Google Scholar] [CrossRef]
- Pacyna, E.G.; Pacyna, J.M.; Steenhuisen, F.; Wilson, S. Global anthropogenic mercury emission inventory for 2000. Atmos. Environ. 2006, 40, 4048–4063. [Google Scholar] [CrossRef]
- Uścinowicz, S.; Szefer, P.; Sokołowski, K. Trace elements in Baltic Sea sediments. In Geochemistry of Surface Sediments of the Baltic Sea; Uścinowicz, S., Ed.; Polish Geological Survey: Warsaw, Poland, 2011; pp. 214–274. [Google Scholar]
- Kwasigroch, U.; Bełdowska, M.; Jędruch, A.; Saniewska, D. Coastal erosion—A “new” land-based source of labile mercury to the marine environment. Environ. Sci. Pollut. Res. 2018, 25, 28682–28694. [Google Scholar] [CrossRef]
- Jędruch, A.; Falkowska, L.; Saniewska, D.; Durkalec, M.; Nawrocka, A.; Kalisińska, E.; Pacyna, J.M. Status and trends of mercury pollution of the atmosphere and terrestrial ecosystems in Poland. Ambio 2021, 50, 1698–1717. [Google Scholar] [CrossRef]
- Pempkowiak, J. Zarys Geochemii Morskiej; University of Gdansk Press: Gdańsk, Poland, 1997; p. 171. (In Polish) [Google Scholar]
- Pniewski, F.F.; Biskup, P.; Bubak, I.; Richard, P.; Latała, A.; Blanchard, G. Photo-regulation in microphytobenthos from intertidal mudflats and non-tidal coastal shallows. Estuarine. Coast. Shelf Sci. 2015, 152, 153–161. [Google Scholar] [CrossRef]
- Bąk, M.; Witkowski, A.; Żelazna-Wieczorek, J.; Wojtal, A.Z.; Szczepocka, E.; Szulc, K.; Szulc, B. Klucz do Oznaczania Okrzemek w Fitobentosie na Potrzeby Oceny Stanu Ekologicznego wód Powierzchniowych w Polsce; Główny Inspektorat Ochrony Środowiska: Warszawa, Poland, 2012; pp. 20–25. (In Polish) [Google Scholar]
- Urbański, J.; Grusza, G.; Chlebus, N. Fizyczna typologia dna Zatoki Gdańskiej, Atlas Cyfrowy, Sprawozdanie merytoryczne z realizacji projektu badawczego nr 2 PO4E00629. 2007. (In Polish) [Google Scholar]
- Szarafin, T.; Karwik, A.; Uścinowicz, S.; Fac-Beneda, J.; Nowacki, J.; Boniecka, H.; Gajda, A.; Gawlik, W.; Meissner, W.; Bzoma, S. Zbiorcze Sprawozdanie z Analizy Dostępnych Danych i Przeprowadzonych Inwentaryzacji Przyrodniczych (Zebranie i Analiza Wyników Inwentaryzacji, Materiałów Niepublikowanych i Opracowań Publikowanych, Przydatnych do Sporządzenia Projektów Planów); Zatoka Pucka (PLB 220005); Wydawnictwa Wewnętrzne Instytutu Morskiego w Gdańsku Nr 6823. 2014, pp. 190–247. Available online: https://www.researchgate.net/publication/318502945_Fizyczna_typologia_dna_Zatoki_Gdanskiej (accessed on 11 October 2023). (In Polish).
- Szymczak, E. The Role of River Sediment Inflow in Present Sedimentation Proceses in the Puck Lagoon. Ph.D. Thesis, Univ. Gdańsk, Gdańsk, Poland, 2006. (In Polish). [Google Scholar]
- Majewski, A. Charakterystyka Hydrologiczna Estuariowych wód u Polskiego Wybrzeża; Prace PIHM: Warszawa, Poland, 1972; Volume 105, pp. 3–40. (In Polish) [Google Scholar]
- Nowacki, J. Morfometria zatoki. In Zatoka Pucka; Korzeniewski, K., Ed.; Fundacja Rozwoju UG: Gdańsk, Poland, 1993; pp. 71–78. (In Polish) [Google Scholar]
- Cieślikiewicz, W.; Jędrasik, J. Falowanie wiatrowe w Zatoce Puckiej. In Zatoka Pucka; Bolałek, J., Burska, D., Eds.; Wydawnictwo Uniwersytetu Gdańskiego: Gdańsk, Poland, 2022; Tom I; pp. 213–243. (In Polish) [Google Scholar]
- Jędrasik, J.; Cieślikiewicz, W. Cyrkulacja wód w Zatoce Puckiej. In Zatoka Pucka; Bolałek, J., Burska, D., Eds.; Wydawnictwo Uniwersytetu Gdańskiego: Gdańsk, Poland, 2022; Tom I; pp. 183–212. (In Polish) [Google Scholar]
- Nowacki, J. Cyrkulacja i wymiana wód. In Zatoka Pucka; Korzeniewski, K., Ed.; Fundacja Rozwoju UG: Gdańsk, Poland, 1993; pp. 181–205. (In Polish) [Google Scholar]
- Nowacki, J. Termika, zasolenie i gęstość wody. In Zatoka Pucka; Korzeniewski, K., Ed.; Fundacja Rozwoju UG: Gdańsk, Poland, 1993; pp. 79–111. (In Polish) [Google Scholar]
- Szymczak, E.; Rucińska, M.; Szmytkiewicz, A. Osady powierzchniowe Zatoki Puckiej. In Zatoka Pucka; Bolałek, J., Burska, D., Eds.; Wydawnictwo Uniwersytetu Gdańskiego: Gdańsk, Poland, 2022; Tom I; pp. 265–279. (In Polish) [Google Scholar]
- Graca, B.; Witek, Z.; Burska, D.; Białkowska, I.; Pawelec, A.; Bolałek, J. Porewater nutrients (phosphate, ammonia and silicate) in the eastern part of the southern Baltic Sea. Oceanol. Hydrobiol. Stud. 2006, 35, 237–256. [Google Scholar]
- Szmytkiewicz, A. Zróżnicowanie Tempa Sedymentacji Współczesnych Osadów Dennych Zatoki Puckiej. Ph.D. Thesis, Univ. Gdańsk, Gdańsk, Poland, 2016. (In Polish). [Google Scholar]
- Szmytkiewicz, A.; Zalewska, T. Sediment deposition and accumulation rates determined by sediment trap and 210Pb isotope methods in the Outer Puck Bay (Baltic Sea). Oceanologia 2014, 56, 85–106. [Google Scholar] [CrossRef]
- Battarbee, R.W. Diatom analysis. In Handbook of Holocene Palaeoecology and Palaeohydrology; Berglund, B.E., Ed.; John Wiley and Sons Ltd.: London, UK, 1986; pp. 527–570. [Google Scholar]
- Bodén, P. Reproducibility in the Random Settling Method for Quantitative Diatom Analysis. Micropaleontology 1991, 37, 313–319. [Google Scholar] [CrossRef]
- Schrader, H.; Gersonde, R. Diatoms and silico agellates in the eight meters sections of the lower Pleistocene at Capo Rossello. Utrecht Micropaleontol. Bull. 1978, 17, 129–176. [Google Scholar]
- Hustedt, F. Die Kieselalgen Deutschlands, Österreichs und der Schweiz 1–3. In Kryptogamen ora von Deutschland, Österreich und der Schweiz 7; Rabenhorsts, L., Ed.; Akademische Verlerlagsbuchhandlung: Leipzig, Germany, 1927–1966. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 1. Teil: Naviculaceae. In Süßwasserflora von Mitteleuropa 2/1; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; G. Fischer: Stuttgart, Germany; New York, NY, USA, 1986; p. 876. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 2. Teil: Bacillariaceae, Epithemiaceae, Surirellaceae. In Süßwasserflora von Mitteleuropa 2/2; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; G. Fischer: Stuttgart, Germany; New York, NY, USA, 1988; p. 596. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 3. Teil: Centrales, Fragilariaceae, Eunotiaceae. In Süßwasserflora von Mitteleuropa 2/3; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; G. Fischer: Stuttgart, Germany; Jena, Germany, 1991; p. 576. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 4. Teil: Achnanthaceae. Kritische Ergänzungen zu Navicula (Lineolatae) und Gomphonema. Gesamtliteraturverzeichnis. Teil 1–4. In Süßwasserflora von Mitteleuropa 2/4; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Fischer: Stuttgart, Germany; Jena, Germany, 1991; p. 437. [Google Scholar]
- Pankow, H. Ostsee-Algenflora; Fischer: Jena, Germany, 1990. [Google Scholar]
- Lange-Bertalot, H. Navicula sensu stricto. 10 Genera Separated from Navicula sensu lato, Frustulia. In Diatoms of Europe 2; Lange-Bertalot, H., Ed.; A.R.G. Gantner Verlag K.G.: Ruggell, Liechtenstein, 2001; p. 526. [Google Scholar]
- Snoeijs, P. Intercalibration and Distribution of Diatom Species in the Baltic Sea; Opulus Press: Uppsala, Sweden, 1993; Volume 1. [Google Scholar]
- Snoeijs, P.; Balashova, N. Intercalibration and Distribution of Diatom Species in the Baltic Sea; Opulus Press: Uppsala, Sweden, 1998; Volume 5. [Google Scholar]
- Snoeijs, P.; Kasperoviciene, J. Intercalibration and Distribution of Diatom Species in the Baltic Sea; Opulus Press: Uppsala, Sweden, 1996; Volume 4. [Google Scholar]
- Snoeijs, P.; Potapova, M. Intercalibration and Distribution of Diatom Species in the Baltic Sea; Opulus Press: Uppsala, Sweden, 1995; Volume 3. [Google Scholar]
- Snoeijs, P.; Vilbaste, S. Intercalibration and Distribution of Diatom Species in the Baltic Sea; Opulus Press: Uppsala, Sweden, 1994; Volume 2. [Google Scholar]
- Witkowski, A.; Lange-Bertalot, H.; Metzeltin, D. Diatom Flora of Marine Coasts I; A.R.G. Gantner Verlag K.G.: Ruggell, Liechtenstein, 2000. [Google Scholar]
- Kolbe, R.W. Zur Ökologie, Morphologie und Systematik der Brachwasser-Diatomeen; Pflanzenforschung: Berlin, Germany, 1927; 146p. [Google Scholar]
- Vos, P.C.; De Wolf, H. Diatoms as a tool for reconstructing sedimentary environments in coastal wetlands; methodological aspects. Hydrobiologia 1993, 269, 285–296. [Google Scholar] [CrossRef]
- Kolkwitz, R.; Marsson, M. Őkologie der pflanzlichen Saprobien. Ber. Dtsch. Bot. Ges. 1908, 26, 505–519. [Google Scholar]
- Reis, A.T.; Coelho, J.P.; Rodrigues, S.M.; Rocha, R.; Davidson, C.M.; Duarte, A.C.; Pereira, E. Development and validation of a simple thermo-desorption technique for mercury speciation in soils and sediments. Talanta 2012, 99, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Jędruch, A.; Bełdowska, M.; Kwasigroch, U.; Normant-Saremba, M.; Saniewska, D. Mercury fractionation in marine macrofauna using thermodesorption technique: Method and its application. Talanta 2018, 189, 534–542. [Google Scholar] [CrossRef]
- Bełdowska, M.; Saniewska, D.; Gębka, K.; Kwasigroch, U.; Korejwo, E.; Kobos, J. Simple screening technique for determination of adsorbed and absorbed mercury in particulate matter in atmospheric and aquatic environment. Talanta 2018, 182, 340–347. [Google Scholar] [CrossRef]
- Wilman, B.; Saniewska, D.; Pyta, H.; Wysiecki, D.; Bełdowska, M. Mercury fractionation—Problems in method application. Mar. Pollut. Bull. 2023, 187, 114560. [Google Scholar] [CrossRef]
- Kwasigroch, U.; Bełdowska, M.; Jędruch, A.; Łukawska-Matuszewska, K. Distribution and bioavailability of mercury in the surface sediments of the Baltic Sea. Environ. Sci. Pollut. Res. 2021, 28, 35690–35708. [Google Scholar] [CrossRef]
- Łęczyński, L.; Szymczak, E. Fizyczne, Biologiczne i Chemiczne Badania Morskich Osadów Dennych; Jerzy, B., Ed.; Wydawnictwo Uniwersytetu Gdańskiego: Gdańsk, Poland, 2010; pp. 69–118. ISBN 978-83-7326-789-3. (In Polish) [Google Scholar]
- Myślińska, E. Laboratoryjne Badania Gruntów; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2001; ISBN 8301134305. (In Polish) [Google Scholar]
- Gradziński, R.; Kostecka, A.; Radomski, A.; Unrug, R. Zarys Sedymentologii; Wydawnictwa Geologiczne: Warszawa, Poland, 1986. [Google Scholar]
- Grimm, E.C. Tilia, version 2.0.37; Software; Illinois State Museum: Springfield, IL, USA, 2011. [Google Scholar]
- Witak, M.; Pędziński, J.; Oliwa, S.; Hetko, D. Biodiversity of benthic diatom flora in the coastal zone of the Puck Bay (southern Baltic Sea): A case study of Hel Peninsula. Oceanol. Hydrobiol. Stud. 2020, 49, 304–318. [Google Scholar] [CrossRef]
- Zgrundo, A.; Dziengo-Czaja, M.; Bubak, I.; Bogaczewicz-Adamczak, B. Studies of the biodiversity of contemporary diatom assemblages in the Gulf of Gdańsk. Oceanol. Hydrobiol. Stud. 2008, 38 (Suppl. 2), 1–15. [Google Scholar]
- Witak, M. Application of Diatom Biofacies in Reconstructing the Evolution of Sedimentary Basins. Records from the Southern Baltic Sea Differentiated by the Extent of the Holocene Marine Transgressions and Human Impact; Diatom Monographs 12; Witkowski, A., Ed.; A.R.G. Gantner Verlag, K.G.: Ruggell, Liechtenstein, 2010; p. 295. [Google Scholar]
- Bogaczewicz-Adamczak, B.; Kłosińska, D.; Zgrundo, A. Diatoms as indicators of water pollution in the coastal zone of the Gulf of Gdansk (Southern Baltic Sea). Oceanol. Stud. 2001, 30, 59–75. [Google Scholar]
- Witkowski, A. Diatoms of the Puck bay coastal shallows (Poland, Southern Baltic). Nord. J. Bot. 1991, 11, 689–701. [Google Scholar] [CrossRef]
- Dziengo-Czaja, M.; Koss, J.; Matuszak, A. Teratological forms of diatoms (Bacillariophyceae) as indicators of water pollution in the western part of Puck Bay (southern Baltic Sea). Oceanol. Hydrobiol. Stud. 2008, 37, 119–132. [Google Scholar] [CrossRef]
- Hetko, D.; Witak, M.; Oliwa, S. Biodiversity of benthic diatom flora in the coastal zone of the Gulf of Gdańsk: A case study of transect Gdynia-Sopot. Oceanol. Hydrobiol. Stud. 2022. [Google Scholar] [CrossRef]
- Jankowska, H.; Łęczyński, L. Zatoka Pucka; Korzeniewski, K., Ed.; Fundacja Rozwoju UG: Gdańsk, Poland, 1993; pp. 320–327. (In Polish) [Google Scholar]
- Damaszke, M.; Szymczak, E. Transport of the river load from the Błądzikowski stream to the puck lagoon (southern Baltic Sea, Poland). Oceanol. Hydrobiol. Stud. 2013, 42, 216–224. [Google Scholar] [CrossRef]
- Huzarska, K. Spatial distribution of biological and physical sediment parameters in the western Gulf of Gdańsk. Oceanologia 2013, 55, 453–470. [Google Scholar] [CrossRef]
- Saniewska, D.; Gębka, K.; Bełdowska, M.; Siedlewicz, G.; Bełdowski, J. Impact of hydrotechnical works on outflow of mercury from the riparian zone to a river and input to the sea. Mar. Pollut. Bull. 2019, 142, 361–376. [Google Scholar] [CrossRef]
- Latała, A. The chlorophyll ‘’a’’ contents in the surface waters of Gdańsk Bay. Studia I Mater. Oceanol. Stud. KBM PAN 1993, 64, 187–195. [Google Scholar]
- Szarafin, T.; Karwik, A.; Uścinowicz, S.; Fac-Beneda, J.; Nowacki, J.; Boniecka, H.; Gajda, A.; Gawlik, W.; Meissner, W.; Bzoma, S. Zbiorcze Sprawozdanie z Analizy Dostępnych Danych i Przeprowadzonych Inwentaryzacji Przyrodniczych (Zebranie i Analiza Wyników Inwentaryzacji, Materiałów Niepublikowanych i Opracowań Publikowanych, Przydatnych do Sporządzenia Projektów Planów); Zatoka Pucka (PLB 220005); Wydawnictwa Wewnętrzne Instytutu Morskiego w Gdańsku Nr 6757. 2013; pp. 188–244. (In Polish) [Google Scholar]
- Szymczak, E.; Szmytkiewicz, A. Współczesne procesy sedymentacyjne w Zatoce Puckiej. In Zatoka Pucka; Bolałek, J., Burska, D., Eds.; Wydawnictwo Uniwersytetu Gdańskiego: Gdańsk, Poland, 2022; Tom I; pp. 255–264. (In Polish) [Google Scholar]
- Jędruch, A.; Kwasigroch, U.; Bełdowska, M.; Kuliński, K. Mercury in suspended matter of the Gulf of Gdańsk: Origin, distribution and transport at the land–sea interface. Mar. Pollut. Bull. 2017, 118, 354–367. [Google Scholar] [CrossRef]
- Aksentov, K.I.; Sattarova, V.V. Mercury geochemistry of deep-sea sediment cores from the Kuril area, northwest Pacific. Prog. Oceanogr. 2019, 180, 102235. [Google Scholar] [CrossRef]
- Witkowski, A. Mikrofitobentos. In Zatoka Pucka; Korzeniewski, K., Ed.; Fundacja Rozwoju Uniwersytetu Gdańskiego: Gdańsk, Poland, 1993; pp. 395–415. (In Polish) [Google Scholar]
- Prokopowicz, A. Badania nad Metodą Oznaczania rtęci w Wybranych Elementach Środowiska. Ph.D. Thesis, Uniwersytet Śląski, Katowice, Poland, 2007. [Google Scholar]
- Saniewska, D.; Bełdowska, M.; Szymczak, E.; Kuliński, K.; Bełdowski, J.; Voss, M.; Pryputniewicz-Flis, D.; Burska, D. Processes affecting the transformation of mercury in the coastal zone in the vicinity of two river mouths in the southern Baltic Sea. Mar. Chem. 2022, 238, 104065. [Google Scholar] [CrossRef]
- Mason, R.P.; Reinfelder, J.R.; Morel, F.M.M. Bioaccumulation of mercury and methylmercury. Water Air Soil Pollut. 1995, 80, 915–921. [Google Scholar] [CrossRef]
- Mohan, B.S.; Hosetti, B.B. Aquatic plants for toxicity assessment. Environ. Res. 1999, 81, 259–274. [Google Scholar] [CrossRef] [PubMed]
- Smrchek, J.C.; Zeeman, M. Assessing risks to ecological systems from chemicals. In Handbook for Environmental Risk Assessment and Management; Calow, P., Ed.; Blackwell Science Ltd.: London, UK, 1998; Chapter 3; pp. 24–90. [Google Scholar]
- Graca, B.; Łukawska-Matuszewska, K.; Burska, D.; Łęczyński, L.; Bolałek, J. Geochemical changes in aquatic environment caused by deep dredging—A case study: The Puck Bay (Baltic Sea). In Oceanography; Marcelli, M., Ed.; InTechOpen: London, UK, 2012; pp. 259–280. [Google Scholar] [CrossRef]
- Szymczycha, B.; Kłostowska, Ż.; Lengier, M.; Dzierzbicka-Głowacka, L. Significance of nutrient fluxes via submarine groundwater discharge in the Bay of Puck, southern Baltic Sea. Oceanologia 2020, 62, 117–125. [Google Scholar] [CrossRef]
- Hetko, D.; Pędziński, J.; Witak, M. Response of the Diatom Flora of the Hel Peninsula Vicinity, Puck Bay, Baltic Sea, to Anthropopressure. IOP Conf. Ser. Earth Environ. Sci. 2019, 362, 012050. [Google Scholar] [CrossRef]


| Hgtot | HgF1 | HgF2 | HgF3 | HgF4 | HgF5 | Concentration of Diatom Valves | OM | FSF | ||
|---|---|---|---|---|---|---|---|---|---|---|
| All | Min. | 0.1 | 0.0 | 0.0 | <LD * | <LD * | 0.0 | 5.0 | 0.1 | 0.0 |
| Max. | 99.9 | 0.5 | 93.6 | 13.2 | 5.8 | 8.2 | 127.0 | 22.7 | 23.7 | |
| Mean | 4.6 | 0.0 | 2.8 | 1.0 | 0.5 | 0.1 | 31.0 | 1.9 | 2.9 | |
| Median | 1.1 | 0.0 | 0.3 | 0.3 | 0.2 | 0.0 | 24.0 | 1.0 | 0.7 | |
| AREA I | Min. | 0.2 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 8.8 | 0.2 | 0.0 |
| Max. | 2.3 | 0.0 | 1.2 | 1.3 | 0.6 | 0.1 | 71.2 | 1.3 | 1.7 | |
| Mean | 0.8 | 0.0 | 0.4 | 0.2 | 0.1 | 0.0 | 30.4 | 0.6 | 0.5 | |
| Median | 0.6 | 0.0 | 0.3 | 0.1 | 0.1 | 0.0 | 24.5 | 0.6 | 0.2 | |
| AREA II | Min. | 0.1 | 0.0 | 0.0 | <LD * | <LD * | 0.0 | 5.4 | 0.1 | 0.0 |
| Max. | 99.9 | 0.5 | 93.6 | 13.2 | 2.2 | 8.2 | 127.0 | 22.7 | 21.7 | |
| Mean | 6.6 | 0.0 | 4.8 | 1.0 | 0.4 | 0.2 | 31.7 | 2.4 | 2.1 | |
| Median | 1.0 | 0.0 | 0.2 | 0.3 | 0.1 | 0.0 | 24.7 | 1.1 | 0.9 | |
| AREA III | Min. | 0.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 3.8 | 0.2 | 0.0 |
| Max. | 14.7 | 0.1 | 2.4 | 8.3 | 5.8 | 0.4 | 101.7 | 5.2 | 23.7 | |
| Mean | 3.0 | 0.0 | 0.6 | 1.3 | 0.8 | 0.1 | 25.0 | 1.5 | 4.7 | |
| Median | 1.9 | 0.0 | 0.5 | 0.6 | 0.4 | 0.1 | 19.3 | 1.1 | 1.1 |
| Hgtot | HgF1 | HgF2 | HgF3 | HgF4 | HgF5 | OM% | FSF% | Concentration of Diatoms Valves | Wetness | |
|---|---|---|---|---|---|---|---|---|---|---|
| Hgtot | 1.00 | 0.73 | 0.99 | 0.02 | 0.21 | 0.05 | 0.88 | 0.54 | 0.59 | 0.54 |
| HgF1 | 0.73 | 1.00 | 0.64 | 0.31 | 0.28 | 0.19 | 0.72 | 0.38 | 0.33 | 0.54 |
| HgF2 | 0.99 | 0.64 | 1.00 | −0.10 | 0.11 | 0.00 | 0.85 | 0.42 | 0.57 | 0.52 |
| HgF3 | 0.02 | 0.31 | −0.10 | 1.00 | 0.87 | −0.03 | 0.12 | 0.42 | 0.08 | 0.30 |
| HgF4 | 0.21 | 0.28 | 0.11 | 0.87 | 1.00 | −0.02 | 0.23 | 0.48 | 0.14 | 0.28 |
| HgF5 | 0.05 | 0.19 | 0.00 | −0.03 | −0.02 | 1.00 | 0.37 | 0.07 | 0.07 | 0.10 |
| OM% | 0.88 | 0.72 | 0.85 | 0.12 | 0.23 | 0.37 | 1.00 | 0.52 | 0.51 | 0.64 |
| FSF% | 0.54 | 0.38 | 0.42 | 0.42 | 0.48 | 0.07 | 0.52 | 1.00 | 0.30 | 0.59 |
| Concentration of diatoms valves | 0.59 | 0.33 | 0.57 | 0.08 | 0.14 | 0.07 | 0.51 | 0.30 | 1.00 | 0.54 |
| Wetness | 0.54 | 0.54 | 0.52 | 0.30 | 0.28 | 0.10 | 0.64 | 0.59 | 0.54 | 1.00 |
| Hgtot | HgF1 | HgF2 | HgF3 | HgF4 | HgF5 | ||
|---|---|---|---|---|---|---|---|
| AREA I | Hgtot | 1.00 | 0.01 | 0.68 | 0.65 | 0.45 | 0.21 |
| HgF1 | 0.01 | 1.00 | −0.30 | 0.55 | 0.73 | 0.62 | |
| HgF2 | 0.68 | −0.30 | 1.00 | 0.08 | −0.01 | −0.24 | |
| HgF3 | 0.65 | 0.55 | 0.08 | 1.00 | 0.91 | 0.12 | |
| HgF4 | 0.45 | 0.73 | −0.01 | 0.91 | 1.00 | 0.48 | |
| HgF5 | 0.21 | 0.62 | −0.24 | 0.12 | 0.48 | 1.00 | |
| Eu | 0.01 | 0.07 | 0.01 | −0.19 | −0.07 | 0.16 | |
| Meso | 0.52 | 0.10 | 0.46 | 0.52 | 0.54 | 0.26 | |
| Oh | −0.54 | −0.14 | −0.40 | −0.51 | −0.58 | −0.37 | |
| Oi | −0.09 | −0.06 | −0.29 | 0.19 | 0.14 | −0.17 | |
| AREA II | Hgtot | 1.00 | 0.69 | 0.77 | 0.81 | 0.78 | 0.48 |
| HgF1 | 0.69 | 1.00 | 0.70 | 0.56 | 0.52 | 0.05 | |
| HgF2 | 0.77 | 0.70 | 1.00 | −0.03 | 0.16 | −0.01 | |
| HgF3 | 0.81 | 0.56 | −0.03 | 1.00 | 0.69 | −0.04 | |
| HgF4 | 0.78 | 0.52 | 0.16 | 0.69 | 1.00 | −0.07 | |
| HgF5 | 0.48 | 0.05 | −0.01 | −0.04 | −0.07 | 1.00 | |
| Eu | 0.33 | 0.03 | −0.06 | 0.20 | 0.36 | 0.02 | |
| Meso | −0.13 | 0.23 | 0.05 | 0.16 | −0.04 | 0.01 | |
| Oh | 0.00 | −0.09 | −0.10 | −0.06 | −0.04 | −0.02 | |
| Oi | −0.13 | −0.14 | 0.10 | −0.23 | −0.18 | 0.00 | |
| AREA III | Hgtot | 1.00 | 0.52 | 0.44 | 0.93 | 0.91 | 0.50 |
| HgF1 | 0.52 | 1.00 | 0.54 | 0.66 | 0.70 | 0.46 | |
| HgF2 | 0.44 | 0.54 | 1.00 | 0.57 | 0.56 | 0.28 | |
| HgF3 | 0.93 | 0.66 | 0.57 | 1.00 | 0.80 | 0.60 | |
| HgF4 | 0.91 | 0.70 | 0.56 | 0.80 | 1.00 | 0.52 | |
| HgF5 | 0.50 | 0.46 | 0.28 | 0.60 | 0.52 | 1.00 | |
| Eu | −0.01 | −0.22 | −0.35 | −0.11 | −0.19 | −0.01 | |
| Meso | 0.03 | −0.03 | 0.18 | −0.07 | −0.01 | −0.13 | |
| Oh | −0.40 | −0.07 | 0.17 | −0.32 | −0.17 | −0.36 | |
| Oi | 0.31 | 0.19 | −0.12 | 0.39 | 0.22 | 0.44 |
| Hgtot | HgF1 | HgF2 | HgF3 | HgF4 | HgF5 | ||
|---|---|---|---|---|---|---|---|
| AREA II | Hgtot | 1.00 | 0.77 | 0.99 | 0.11 | 0.27 | 0.04 |
| HgF1 | 0.77 | 1.00 | 0.70 | 0.56 | 0.52 | 0.05 | |
| HgF2 | 0.99 | 0.70 | 1.00 | −0.03 | 0.16 | −0.01 | |
| HgF3 | 0.11 | 0.56 | −0.03 | 1.00 | 0.69 | −0.04 | |
| HgF4 | 0.27 | 0.52 | 0.16 | 0.69 | 1.00 | −0.07 | |
| HgF5 | 0.04 | 0.05 | −0.01 | −0.04 | −0.07 | 1.00 | |
| Chaetoceros diadema RS | 0.52 | 0.37 | 0.52 | −0.08 | 0.09 | −0.02 | |
| Chaetoceros seiracanthus RS | 0.03 | −0.01 | 0.01 | −0.01 | 0.03 | 0.44 | |
| Chaetoceros spp. RS | 0.54 | 0.31 | 0.56 | −0.09 | 0.03 | 0.03 | |
| Epithemia adnata | 0.48 | 0.32 | 0.49 | −0.10 | 0.04 | 0.00 | |
| Epithemia frickei | 0.47 | 0.20 | 0.50 | −0.07 | −0.12 | −0.03 | |
| Epithemia goeppertiana | 0.62 | 0.53 | 0.61 | −0.06 | 0.21 | −0.01 | |
| Staurosirella martyi | 0.70 | 0.34 | 0.73 | −0.16 | −0.01 | −0.03 | |
| Fragilaria inflata var. instvantfyi | 0.62 | 0.53 | 0.61 | −0.06 | 0.21 | −0.01 | |
| Navicula cryptocephala | 0.54 | 0.24 | 0.57 | −0.06 | −0.09 | −0.02 | |
| Navicula phyllepta | 0.12 | 0.64 | 0.03 | 0.75 | 0.35 | 0.00 | |
| Pseudostaurosira perminuta | 0.62 | 0.53 | 0.61 | −0.06 | 0.21 | −0.01 | |
| Rhabdonema adriaticum | 0.62 | 0.53 | 0.61 | −0.06 | 0.21 | −0.01 | |
| Rhabdonema minutum | 0.49 | 0.34 | 0.49 | −0.05 | 0.11 | 0.01 | |
| Surirella brebissonii | 0.49 | 0.34 | 0.49 | −0.05 | 0.11 | 0.01 | |
| AREA III | Hgtot | 1.00 | 0.75 | 0.70 | 0.95 | 0.92 | 0.58 |
| HgF1 | 0.75 | 1.00 | 0.54 | 0.66 | 0.70 | 0.46 | |
| HgF2 | 0.70 | 0.54 | 1.00 | 0.57 | 0.56 | 0.28 | |
| HgF3 | 0.95 | 0.66 | 0.57 | 1.00 | 0.80 | 0.60 | |
| HgF4 | 0.92 | 0.70 | 0.56 | 0.80 | 1.00 | 0.52 | |
| HgF5 | 0.58 | 0.46 | 0.28 | 0.60 | 0.52 | 1.00 | |
| Chaetoceros seiracanthus RS | 0.38 | 0.25 | 0.27 | 0.44 | 0.25 | 0.27 | |
| Staurosirella martyi | 0.44 | 0.58 | 0.33 | 0.36 | 0.44 | 0.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hetko, D.; Witak, M.; Bełdowska, M. The Relationship between Total Mercury, Its Fractions and Species Diversity of Diatom Taphocoenoses Deposited in Surface Sediments (Southern Baltic Sea). Water 2023, 15, 3907. https://doi.org/10.3390/w15223907
Hetko D, Witak M, Bełdowska M. The Relationship between Total Mercury, Its Fractions and Species Diversity of Diatom Taphocoenoses Deposited in Surface Sediments (Southern Baltic Sea). Water. 2023; 15(22):3907. https://doi.org/10.3390/w15223907
Chicago/Turabian StyleHetko, Dominika, Małgorzata Witak, and Magdalena Bełdowska. 2023. "The Relationship between Total Mercury, Its Fractions and Species Diversity of Diatom Taphocoenoses Deposited in Surface Sediments (Southern Baltic Sea)" Water 15, no. 22: 3907. https://doi.org/10.3390/w15223907
APA StyleHetko, D., Witak, M., & Bełdowska, M. (2023). The Relationship between Total Mercury, Its Fractions and Species Diversity of Diatom Taphocoenoses Deposited in Surface Sediments (Southern Baltic Sea). Water, 15(22), 3907. https://doi.org/10.3390/w15223907







