Abstract
As contaminants are often present in aquatic environments as mixtures, they may interact with each other and affect living organisms differently than when tested individually. Emerging pollutants such as pharmaceuticals and microplastics can be influenced by various environmental factors, with UV/Vis radiation being among the most significant. This study aimed to evaluate the photodegradation of the antidepressant duloxetine in the presence of four types of microplastics. Acute toxicity was tested using the Spirotox assay, duloxetine concentration was determined using HPLC-DAD analysis, and the resulting photodegradation products were tested using HPLC-MS/MS. Following 1 and 2 h of exposure in a sunlight simulator, the concentrations of duloxetine decreased by nine and thirteen times, respectively, while its toxicity to protozoa decreased by only two and three times. The presence of microplastics in the samples did not affect either the photodegradation process or the toxicity of duloxetine. HPLC-MS/MS analysis revealed the presence of 34 duloxetine photodegradation products. In silico toxicity analysis using the T.E.S.T. program for the protozoan Tetrahymena pyriformis indicated that one-third of the photoproducts were as toxic, and two products were found to be much more toxic than duloxetine. The high toxicity of one of these compounds was confirmed using the Spirotox test.
1. Introduction
Duloxetine (DLX), a serotonin and norepinephrine reuptake inhibitor, is an antidepressant pharmaceutical approved for medical use in the U.S. and EU in 2004. DLX quickly gained high popularity and in 2020, it was ranked twenty-seventh among the top 300 best-selling drugs and was the sixth among antidepressants in the U.S., with an annual sales of 22.5 million packages [1]. It is used to treat not only major depressive disorders but also neuropathic pain and fibromyalgia. The consumption of antidepressants has increased significantly in recent years, largely due to the economic crisis and the SARS-CoV-2 pandemic [2].
Although widely used worldwide, DLX is rarely detected in wastewater and surface waters [3,4,5]. One of the reasons is its relatively short period of use, and another is its susceptibility to photodegradation [3,6]. Reports on DLX photostability are contradictory, although it is important to note that most research has been conducted on drug formulations rather than aqueous solutions [7].
New groups of emerging pollutants have been detected in the aquatic environment, with microplastics (MPs) receiving significant attention in recent years. Due to their durability and increasing global usage, plastics have become ubiquitous environmental pollutants in both soil and aquatic (both marine and freshwater) environments [8,9,10]. According to the most common definition, MPs are particles ranging in size from 1 to 5000 µm [10,11,12]. Although usually nontoxic, MPs can have a negative impact on ecosystems by interacting with other pollutants. They can adsorb various chemicals, including toxic compounds. The best known are the interactions of MPs with nonpolar compounds like benzo[a]pyren and naphthalene, for which MPs can serve as vectors and transport these substances over long distances or influence their toxicity to aquatic organisms [12,13]. There have been reports on possible interactions of MPs with polar compounds, including pharmaceuticals and personal care products [14,15,16]. The sorption of substances on plastic particles depends on the properties of the medium, the adsorbed substance, and the sorbent [17]. The aging of microplastics, under the influence of sunlight or ultraviolet light, can lead to the release of auxiliary substances, such as plasticizers, the formation of microcracks, and chemical changes in the surface functional groups of MPs [11], and even the formation of persistent free radicals [18]. Aged MPs have a significantly greater sorption capacity for substances, which is possible both by their increasing the specific surface area and forming of hydrophilic groups that establish hydrogen bonds with hydrophilic compounds [19,20].
As mentioned above, DLX is photodegradable. This process can be altered in the presence of MPs. Firstly, the presence of MPs may lead to the generation of free radicals on their surfaces which could accelerate the photodegradation of the drug and change its direction, leading to the formation of other photodegradation products [21]. Secondly, the suspension of MPs could slow down the photodegradation process by acting as a quenching agent and reducing radiation exposure to DLX [7]. To the best of our knowledge, the impact of MPs on the photodegradation of selective serotonin reuptake inhibitors (SSRIs), including DLX, has not been studied yet.
Like other SSRIs, DLX is toxic to aquatic organisms with an EC50 of around 1 mg L−1 [22]. In our previous study, we found that it reduced the number of vacuoles formed by ciliated protozoa Spirostomum ambiguum even at a concentration as low as 0.063 mg L−1 [23]. Products formed during photodegradation may differ in toxicity compared to the parent compounds [24,25,26]. Therefore, to comprehensively assess the biological activity of the photodegradation mixture, it is necessary to conduct biotests in addition to chemical analysis [27]. Postreaction toxicity analysis shows the toxicity caused by the parent compound compared to that caused by the resulting products. Understanding the toxicity of the parent compound helps in evaluating whether the degradation products are also toxic. Unfortunately, due to the absence of standards, in most cases, the toxicity of products can only be estimated using in silico methods with the appropriate algorithms.
The present study aimed to evaluate the influence of four kinds of MPs on the photodegradation of DLX in water: polystyrene (PS), polyethylene terephthalate (PET), polyvinyl chloride (PVC), and phenolic resin (PhR). The concentration of DLX was determined using HPLC-PDA, while the presence of photoproducts was assessed with HPLC coupled with high-resolution MS/MS. The biological activity of the samples was tested with two assays using the protozoan S. ambiguum. A standard Spirotox assay made it possible to evaluate the negative effects of samples on cell morphology and lethality, while an ingestion assay determined the protozoa’s ability to ingest food and form food vacuoles. High concentrations of both the test drug and MPs were used to observe toxic effects and isolate degradation products. The toxicity of DLX and its possible photoproducts toward ciliated protozoan Tetrahymena pyriformis was predicted with the use of S.T.A.R.T. software v. 5.1.2 from the U.S. EPA.
2. Materials and Methods
2.1. Microparticles
MPs were prepared from everyday products such as PS from the black side of a CD box, PET from a red bottle of ketchup, PVC from a tap water pipe, and PhR from laboratory worktops. The materials were prepared according to the procedure described in our previous paper [28]. MP stock suspensions (4 g L−1 of Tyrode’s solution) were homogenized using an ultrasonic water bath (Polsonic, type Sonic 6, Warsaw, Poland) for 20 min at room temperature. The stock suspensions were stored in glass bottles at room temperature in the dark. Immediately before use, the stock suspensions were dispersed in the ultrasonic bath. Baker’s yeast (BY, Saccharomyces cerevisiae) was used as edible particles. Before each analysis, a fresh stock of BY suspension was prepared by adding 10 mg of freeze-dried baker’s yeast to 10 mL of Tyrode’s solution. Working MP suspensions (PET, PS, PVC, and PhR) were prepared just before the tests by carrying out a 10-fold dilution of the stock suspensions with Tyrode’s medium.
2.2. Chemicals
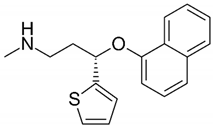
The DLX hydrochloride standard (CAS No. 136434-34-9, Cat. No. D721000) was obtained from Toronto Research Chemicals (Toronto, ON, Canada), while 2-methylnaphthalene-1,4-dione (MNPD) and 2-hydroxynaphthalene-1,4-dione (HNPD) were purchased from Merck (Darmstadt, Germany). These chemicals were of high purity and were stored at −20 °C in original glass bottles The DLX working solution was prepared just before conducting the tests by dissolving 11.0 mg of DLX in 500 mL Tyrode’s medium (22 mg L−1). The solution was dispensed into five glass beakers. Subsequently, 10 mL of MP stock suspensions, prepared according to Section 2.1, were added to four of the beakers. In the control sample, 10 mL of Tyrode’s medium was added, thus achieving a nominal DLX concentration of 20 mg L−1 in each beaker. The chemical structure of DLX and relevant data are shown in Table 1.

Table 1.
DLX chemical structure.
2.3. Photodegradation Experiments
The irradiation process was performed using the SunTest CPS+ apparatus (Klimatest, Warsaw, Poland). The use of an appropriate filter provided full-spectrum light (UV/Vis) similar to the sunlight spectrum (300–800 nm). For the photodegradation experiments, the working suspensions of DLX and MPs (PVC, PET, PS, and PhR) and the control (Tyrode’s medium) were divided into three equal parts and transferred into 40 mL quartz tubes. The first part was not exposed (0 h), while the second and third parts were irradiated in a temperature-controlled chamber using a 1500 W xenon lamp for 1 and 2 h, respectively. The fluence rate was set to 750 W m−2, which corresponds to a dose of 2700 kJ m−2 h−1. The temperature during the experiments was 30–35 °C. All DLX samples were analyzed using HPLC-PDA and HPLC-MS/MS both before exposure and after 1 and 2 h of irradiation.
2.4. Liquid Chromatography with Photodiode Array Detector
The concentration of DLX was measured using a Shimadzu HPLC instrument equipped with two LC-10ATvp pumps, a CTO 10Asvp oven, an SPD-M10A photodiode-array detector (PDA), and an SCL-10A system controller. In the gradient analysis used, phase A consisted of a 0.05% aqueous solution of trifluoroacetic acid, and phase B was with acetonitrile. HPLC-grade acetonitrile and trifluoroacetic acid were purchased from Merck (Darmstadt, Germany), while deionized water was procured by using the Milli-Q® Direct water purification system (Merck, Darmstadt, Germany). The separation process was carried out on a LichroCART 50 × 4 Purospher STAR RP-18 (3 µm) analytical column (Merck, Darmstadt, Germany). The flow rate was 1 mL min−1, and the concentration of phase B, during the analysis, changed according to the following scheme: 0 min: 25%; 1 min: 40%; 5 min: 90%; 6 min: 90%; and 6.10 min: 25%. Quantitative analysis of DLX was carried out at 218 nm. The standard curve of DLX solutions was linear in a concentration range of 0.2–10 mg L−1 (r2 = 0.9970). The LOD value was 0.1 mg L−1. The precision of DLX determination determined at a concentration of 1 mg L−1 was 11%, while the accuracy of determination was 9.5%. Due to the fact that the concentration of unirradiated samples was 20 mg L−1, these samples were diluted twice before determination, and the suspensions were centrifuged at 10,000× g for 3 min.
2.5. Liquid Chromatography with Mass Spectrometer Detector
Solvents, acetonitrile hypergrade for LC-MS (LiChrosolv), and formic acid 98% were provided by Merck (Darmstadt, Germany). Instrumental analysis was performed using a UHPLC Dionex Ultimate 3000 system with a Q-Exactive hybrid quadrupole-orbitrap mass spectrometer system (MS/MS) equipped with heat electrospray ionization (HESI), an online vacuum degasser, a quaternary pump, an autosampler, and a thermostated column compartment. The HESI was operated in positive mode. Full MS scans were acquired over the m/z 70–850 range with a resolution of 70,000 (m/z 200). The target value was set at 3 × 106. Fragmentation was performed in separate runs using a normalized collision energy of 20, 40, and 60 eV. The tandem mass spectrum was acquired with a resolution of 17,500 at m/z 200. The target value was 5 × 104 Standard mass spectrometric conditions for all experiments were as follows: spray voltage: 3.5 kV; sheath gas pressure: 60 arb; aux gas pressure: 20 arb; sweep gas pressure: 0 arb; heated capillary temperature: 320 °C; loop count: 3; isolation window: m/z 1.0; and dynamic exclusion: 6.0 s. For all full-scan measurements, lock-mass ions from ambient air (m/z 445.1200 and 291.2842) were used as internal calibrants. All data collected in profile mode were acquired and processed using Thermo Xcalibur 3.0 software and Compound Discoverer 2.1, respectively. Chromatographic separation was achieved with a Kinetex RP-18 column (100 mm × 4.6 mm, 2.6 µm) supplied by Phenomenex (Torrance, CA, USA) equipped with a security guard. Column temperature was maintained at 40 °C with a flow rate of 0.3 mL min−1. The mobile phases consisted of HPLC-grade water with 0.1% formic acid as eluent A and acetonitrile with 0.1% formic acid as eluent B. Gradient B (%) was as follows: 0 min—5%; 1 min—5%; 13 min—95%; 20 min—95%. The re-equilibration of the column to the initial conditions lasted for 5 min. The volume of injection was 10 µL.
Tentative metabolites were detected using Compound Discoverer Software v.3.3 (Thermo Fisher Scientific, Waltham, MA, USA).
2.6. Protozoan S. ambiguum
The test organism used was the ciliate S. ambiguum, which has been cultured in the Faculty of Pharmacy, Medical University of Warsaw, Poland, for over 35 years. It is one of the largest elongated protozoa [29]. The organisms were cultured in 7 L aquariums with nutrients from the bacterial flora found on oatmeal and alder leaves. Before starting the experiments, the protozoa were washed twice with fresh Tyrode’s medium and then incubated at room temperature for about 1 h to eliminate the culture medium. Tyrode’s medium contains a minimal amount of inorganic components that allow the ciliates to survive up to 8 days and not multiply [29].
2.7. Spirotox Assay
The acute toxicity of the samples was tested using Spirotox assay on the ciliate protozoan S. ambiguum according to the standard operational procedure [29,30]. Tyrode’s medium and a medium with 10% of the appropriate microplastic suspension were used as a diluent. Tests were performed in 24-well polystyrene plates, with two replicates per plate. A series of eleven 1.5-fold dilutions of the test samples and control were prepared directly in the multiwells. Ten organisms were added to each well containing 1 mL of the tested sample. After 24 and 48 h incubations at 25 °C in the dark, test effects including morphological deformations and lethality were observed with a dissection microscope. Based on the observed percentage of test effect, the 24 h-EC20, 24 h-EC50, 48 h-EC20, and 48 h-EC50 values were determined by using the graphical interpolation method.
2.8. Ingestion Studies
To evaluate the influence of DLX and its photodegradation products on the formation of food vacuoles by S. ambiguum, the protozoa were exposed to mixtures containing nonirradiated (0 h) and irradiated (2 h) DLX and MPs. This radiation was carried out in the SunTest CPX+ with the addition of two kinds of food. Baker’s yeast (BY) was used as a natural, edible food and PS microspheres (PS-MP) were served as artificial, inedible particles with the same diameter as that of BY. The selection of microparticles given was made based on previous studies [28]. Four kinds of MPs were applied, namely PET, PVC, PS, and PhR at a concentration of 106 particles mL−1 with the same diameter as BY. The control contained the protozoa in Tyrode’s medium. DLX concentration corresponded to the 0.33 EC20 nontoxic value obtained in the Spirotox experiment. The tests were carried out in 6-well polystyrene plates, with each well containing 10 mL of the sample and 100 protozoa. After a 24 h incubation, the protozoa were immobilized and the number of food vacuoles was determined according to the procedure outlined in Section 2.9. The test was performed in triplicate.
2.9. Counting of the Food Vacuoles in the Protozoa
To immobilize the protozoa, 10–20 ciliates were transferred from the test wells to 1 mL of 0.4 mM nickel nitrate solution in Tyrode’s medium. After 5–10 min, the immobilized protozoa were placed onto a microscopic slide and covered with a coverslip. Photographs were taken within a timeframe of less than 30 min, at 200× magnification. Keyence VHX 7000 digital microscope (KEYENCE International, Mechelen, Belgium) was used to observe the ciliates and their food vacuoles. For the vacuole counting process, dedicated image analysis software was used.
2.10. In Silico Toxicity Assay
The Toxicity Estimation Software Tool (T.E.S.T., Washington, DC, USA) was used to estimate the toxicity of chemicals. T.E.S.T. uses the quantitative structure–activity relationships (QSAR) methodology to predict toxicity measures based on the molecular descriptors of the tested compound. T.E.S.T. v. 5.1.2 was obtained as a free download from the U.S. EPA [31]. In this research, the T. pyriformis 50% growth inhibition concentration (48 h-IGC50) model was used to predict the toxicity of both DLX and its degradation products towards protozoa.
2.11. Data Treatment
Toxicity values EC50 and EC20 represent the concentrations of the tested sample that cause 50% and 20% toxic effects, respectively. When evaluating nonirradiated samples with a single substance (DLX), these values were expressed in mg L−1. However, when analyzing irradiated samples containing a mixture of DLX and photodegradation products, they were expressed as a percentage of the highest concentration sample. The values were then converted into toxicity units (TUs) according to the following formula:
TU = 100/EC50
The toxicity of mixtures is expressed using the concentration addition approach according to the formula:
where:
TUmix = TUDLX + TUprod
TUDLX = CDLX/EC50 DLX
TUprod is the toxicity units of unidentified photodegradation products, CDLX is the concentration of DLX in the sample (mg L−1), and EC50DLX is the EC50 of DLX (mg L−1).
Statistical analysis was conducted using Statistica (version 13, TIBCO Software Inc., Palo Alto, CA, USA). The normality of the distribution was checked using the Kolmogorov–Smirnov (K–S) test with Lillefors correction (K–S–L) and the Shapiro–Wilk (S–W) test. The homogeneity of variances was checked using Levene’s test. To compare the relationship between the DLX solution and the DLX solution with microplastics, a one-way analysis of variance (ANOVA) was used. For all tests, the significance threshold was set at p < 0.05. If either the condition of normality of distribution or that of homogeneity of variance was not met, statistical analysis was performed with nonparametric tests.
3. Results
3.1. Duloxetine Toxicity in the Spirotox Assay
The toxicity of DLX towards S. ambiguum was tested in both Tyrode’s medium and Tyrode’s medium containing 10% MP suspension. The concentration of MP suspensions remained the same across all DLX dilutions and the negative control and was around 106 particles mL−1. No toxic effects were observed in the negative controls indicating that all MPs at the concentration of 106 particles mL−1 were not toxic to the protozoa. DLX 24 h-EC50 values ranged from 0.87 to 1.11 mg L−1, while 24 h-EC20 values were 20% lower (Table 2). Over the subsequent 24 h of incubation, toxicity increased slightly. Therefore, for the remainder of this paper, only 24 h-EC50 values were taken into account. The presence of MPs in the samples did not affect the toxicity of DLX, as the results of DLX in the MP suspensions did not differ by more than 20% when compared to the DLX solution (Table 2).

Table 2.
Toxicity of duloxetine in the presence of different microplastics tested in the Spirotox assay. The values expressed in mg L−1.
3.2. Photodegradation of Duloxetine
The DLX solutions in Tyrode’s medium with and without MP suspensions were irradiated in the SunTest CPX+ for 1 and 2 h. During the irradiation process, DLX concentrations were determined using HPLC-PDA, and toxicity was tested using the Spirotox assay. The 24 h-EC50 values of the irradiated samples were calculated and expressed as percentages, assuming that 100% corresponded to the initial DLX concentration of 20 mg L−1.
During exposure to simulated sunlight, DLX concentrations dropped to 10–14% and 6–8% of the initial level after the first and second hour of irradiation, respectively (Figure 1a). There were no statistical differences in DLX concentration between samples with MPs and those in Tyrode’s solution, indicating that MPs had no effect on the drug’s photodegradation. The toxicity of the samples decreased to only 42–67% and 25–46% after 1 and 2 h of irradiation, respectively, compared to the nonirradiated samples (Figure 1b). Considering the concentration addition approach, it was assumed that the toxicity of DLX and its degradation products add up and this leads to the products’ toxicity having a significant share in the overall toxicity of the mixture (Table 3). It is important to note that especially after 2 h of irradiation, DLX alone would not have caused significant toxic reactions in the protozoa, as was indicated by TUDLX values less than 1. The highest decrease in toxicity was observed in the case of PhR, followed by PS and PVC, while the results for the PET suspension were close to the control sample. However, the TUmix values obtained in the DLX–MP suspensions did not differ significantly when compared to the DLX solution in Tyrode’s medium.
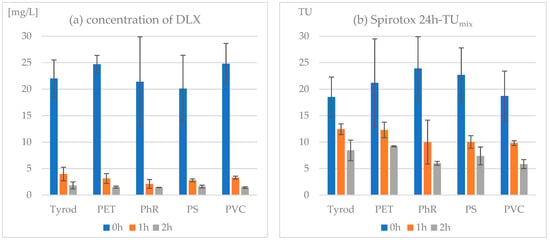
Figure 1.
Photodegradation of DLX in the presence of microplastics: (a) concentration of DLX determined by HPLC-PDA and (b) toxicity of samples in Spirotox assay.

Table 3.
Toxicity of DLX samples during photodegradation in the presence of microplastics. The 24 h-TU values are based on 24 h-EC50 values from the Spirotox assay and the concentration of DLX determined by HPLC-PDA.
3.3. Ingestion Assay
The ingestion assay aimed to assess the influence of the sample on the ability of the protozoan S. ambiguum to ingest food and create food vacuoles. The protozoa were exposed simultaneously to the samples along with either edible (BY) or inedible (PS-MP) particles for a duration of 24 h. Only one initial DLX concentration, equivalent to 0.33 of the 24 h-EC20 value as determined in the Spirotox test, was used. For each suspension, two sets of tests were conducted: one with nonirradiated samples and the other with samples irradiated for 2 h using the SunTest CPS+. The protozoa demonstrated a keen ability to form food vacuoles, both from natural food (Figure 2a) and artificial food (Figure 2b), although the number of vacuoles was much lower in the second case (Figure 3).
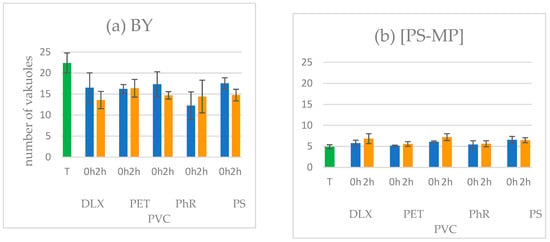
Figure 2.
Number of food vacuoles created by the protozoan S. ambiguum in nonirradiated (0 h) and irradiated (2 h) DLX suspensions in the presence of MPs. Initial concentration of DLX = 1/3 EC20. The suspensions were incubated for 24 h with (a) baker’s yeast (BY) and (b) polystyrene microspheres (PS-MP). T—control sample without DLX.
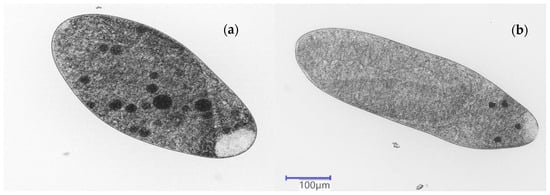
Figure 3.
Food vacuoles created by the protozoan S. ambiguum in the DLX suspensions. Initial concentration of DLX = 1/3 EC20. The suspensions were incubated for 24 h with (a) BY and (b) PS.
The number of food vacuoles containing BY in all DLX samples was 20–40% lower when compared to those in Tyrode’s medium. The lowest number was in the case of nonirradiated samples (0 h) with PS (Figure 2a), whereas in the case of inedible PS-MP particles, the number of food vacuoles remained relatively uniform across all samples. Importantly, the presence of all tested MPs did not influence the formation of food vacuoles in the DLX samples, irrespective of whether edible or inedible particles had been added.
3.4. Analyses of Photoproducts with HPLC-MS/MS
Thirty-four DLX degradation products were detected in the samples that had been irradiated in the SunTest CPS+. Their plausible molecular formulas are shown in Table 4. The presence of MPs in the samples did not lead to the generation of different degradation products compared to those observed in pure Tyrode’s medium. However, the samples exhibited variations in peak intensity, suggesting the possibility of different kinetics in the formation of these products (Figure 4). For example, after 1 h of irradiation, a higher abundance of M122b, M171, and M325b was present in PET than in PhR or PS. Moreover, some products reached their maximum peak area after 1 h (such as M172, M277a, M279, and M293), while some peaked after 2 h (such as M126a, M181b, and M197a).

Table 4.
Tentative identification of degradation products of DLX and their MS parameters.
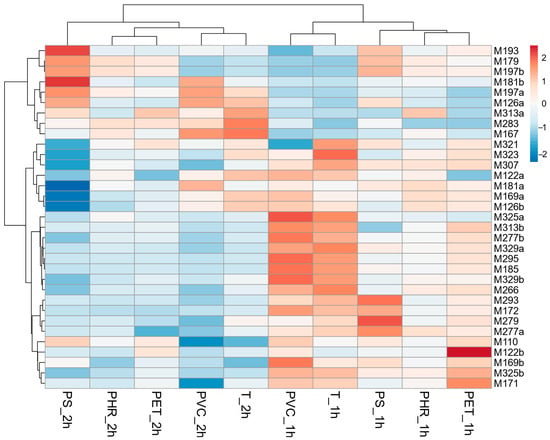
Figure 4.
Clustering result of detected degradation products and experiment variant, i.e., time of irradiation and microplastic type (using Euclidean distance and clustering algorithm using Ward’s method). The level of degradants is presented as a heatmap (red indicates a higher peak area, and blue indicates a lower peak area than the average in the row).
3.5. Toxicity Assessment of DLX Photoproducts
The toxicity of DLX photoproducts toward the protozoan T. pyriformis was predicted based on their chemical structure using T.E.S.T, made available by the U.S. EPA (Table 5). Out of the four algorithms available in T.E.S.T., the “nearest neighbor” and “consensus” methods yielded the most significant toxicity predictions. In the “nearest neighbor method”, predicted toxicity is estimated by taking an average of the results from the three chemicals in the training set that are most similar to the test chemical. On the other hand, the “consensus method” is recommended for its precision, as it provides the most accurate predictions by taking an average of the predicted toxicities from all QSAR methods applied.

Table 5.
Predicted toxicity to T. pyriformis (48 h-IGC50) calculated in Toxicity Estimation Software Tool (T.E.S.T.).
The toxicity of derivatives containing a carbon backbone identical to DLX (comprising 18 or 17 carbon atoms) was either similar or at most two times higher than that of the parent compound. A significant breakdown of the molecule resulted in a substantial reduction in the predicted toxicity of the compound toward protozoa. However, there were two exceptions, namely compounds M172 and M174, whose predicted toxicities were 17 and 12 times higher than that of DLX. Based on MS/MS data, these are probably 2-methylnaphthalene-1,4-dione (MNPD) and 2-hydroxynaphthalene-1,4-dione (HNPD) (Figure 5).
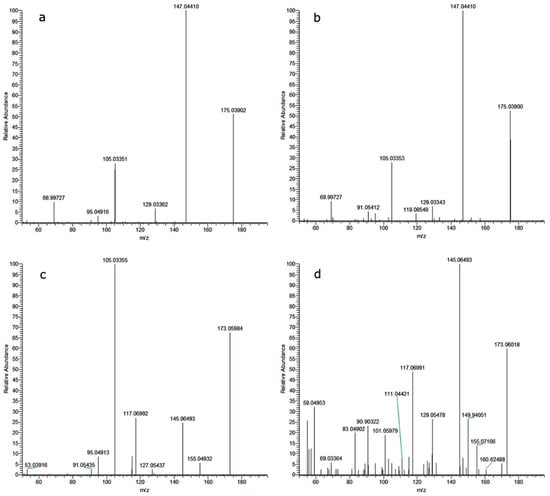
Figure 5.
MS/MS spectrum of 2-hydroxynaphtalene-1,4- dione (a) analytical standard, (b) M174, and 2-methylnaphthalene-1,4-dione (c) analytical standard, (d) M172.
After receiving the results of the in silico analysis, the MNPD and HNPD standards were purchased, and their toxicity was tested in the standard Spirotox assay. The MNPD and HNPD 24 h-EC50 values were 0.164 ± 0.017 and 2.00 ± 0.32 µg L−1, respectively, indicating that MNPD was seven times more toxic, while HNPD was two times less toxic than DLX.
Based on the MS/MS data of the analytical standard, the identification of the compound as 2-methylnaphthalene-1,4-dione (MNPD) is not definitively precluded (Figure 5). Nevertheless, it is worth noting that the MS2 fragments, deemed crucial for compound elucidation, exhibit significant variations in their intensities between the analytical standard and M172. Of particular interest, however, are the results obtained following the photodegradation of the MNPD analytical standard. A rapid decline in the concentration of MNPD was observed, accompanied by the generation of two distinct categories of compounds: (1) compounds characterized by the monoisotopic mass of MNPD and similar MS2 fragmentation patterns, albeit with differing intensity profiles; (2) the emergence of 2-hydroxynaphthalene-1,4-dione (2-HNP). It is noteworthy that the spectral profiles of the analytical standard for 2-HNP and the compound denoted as M174, discovered during the degradation of DLX and MNPD, exhibit remarkable similarities.
4. Discussion
4.1. Irradiation of DLX
DLX is considered to be stable in abiotic conditions when kept in the dark [3,32,33]. However, it undergoes transformations when exposed to UV/Vis light [33]. In our study, DLX was irradiated in the sunlight simulator, and its concentration decreased to below 10% of the initial level after 2 h of irradiation. Datar and Waghmare [34] observed significant degradation of DLX after 33 min irradiation within the range of 200–400 nm. Similar results were obtained by Osawa et al. [3], where DLX was completely degraded after 30 min irradiation with UV light (450 W medium-pressure mercury–vapor lamp, irradiance of 0.37 W cm−2). The significantly lower and slower degradation of DLX in our experiment can be attributed to the application of much lower irradiance (750 W m−2), and the restriction of the light spectrum to a range above 300 nm, similar to sunlight.
The degradation of DLX under the influence of light is a free radical reaction similar to most photodegradation reactions, and it may be affected by the presence of other substances. Polymer microparticles present in the environment were earlier considered to be chemically inert particles. However, in recent years, it has been observed that they can sorb chemicals onto their surface [35] and even generate free radicals [11,36,37]. Environmentally persistent free radicals were detected on microplastics containing conjugated benzene rings, such as PS and phenolic resins [21]. When ingested, these microparticles can cause damage to organisms by inducing oxidative stress. Photoaged polyethylene, containing free radicals, caused toxic effects in the nematode Caenorhabditis elegans [37]. In our study, DLX was irradiated alongside four types of MPs composed of monomers containing phenolic rings. We expected that the presence of the phenolic rings might lead to faster degradation of the study drug and/or greater toxicity to protozoa. However, no statistically significant changes were observed in either the changes in degradation ratio or toxicity under any circumstances. It should be noted that the exposure time of C. elegans was much longer (45–60 d) [37], than the exposure time of S. ambiguum in our study. The conducted experiments indicate that even the presence of high, though not environmentally relevant, concentrations of microplastics did not affect the toxicity of DLX to protozoa, both before and after 2 h irradiation in a sunlight simulator.
4.2. DLX Photodegradation Products
Analysis of the samples using HPLC-MS/MS showed the presence of 34 transformation products of DLX. In previous studies, the detection of DLX photodegradation products ranged from five to seventeen compounds, depending on the analytical techniques employed [3,6,34]. Osawa et al. [3] identified nine compounds, which were mainly products of DLX hydroxylation and epoxidation processes. Our study confirmed the presence of the derivatives identified by Osawa et al. [3] and Santoke et al. [38] with molecular masses of 313 and 329 Da, corresponding to hydroxy- or epoxy- and dihydroxy-DLX. On the other hand, contrary to [38], we did not detect 1-naphthol, but identified its oxidation products, NMPD and HMPD. Notably, 1,4-Naphthoquinone was identified as a photodegradation product originating from both naphthalene and 1-naphthol, with 1-naphthol being more photolabile and undergoing subsequent transformations faster than 1,4-naphthoquinone [39]. Vialation et al. [40] observed that 7-hydroxy-1,4-naphthoquinone was one of the main products of naphthalene photodegradation, formed by the transformation of the photolabile 1,4-naphthoquinone.
4.3. Toxicity of the DLX Derivatives
The toxicity of the irradiated samples to S. ambiguum decreased at a much slower rate than the concentration of DLX, which proves that the products of photodegradation are also toxic to protozoa. By using the toxicity units and the concentration addition approach, it was observed that the toxicity of the irradiated samples was also caused by the photoproducts, especially after 2 h of irradiation when DLX concentration fell below the EC50 value.
The in silico toxicity of the identified DLX transformation products was assessed using T.E.S.T. with the T. pyriformis IGC50 endpoint (Table 5). The algorithm was based on the dataset of 96 h Tetratox chronic toxicity assay. Organisms differ in their sensitivity to chemicals. In our previous study [41], we compared the 24 h acute toxicity data for S. ambiguum (Spirotox) with the IGC50 values from Tetratox. The sensitivity of Spirotox was at least equivalent or even better in the case of aliphatic alcohols and phenols, despite the different durations of the tests and their different endpoints. The IGC50 value calculated in T.E.S.T. for DLX was comparable to the Spirotox 24 h-EC50 value. Thus, it may be predicted that the toxicity of the transformation products would be also similar.
The results of the Spirotox test indicate that MNPD is seven times more toxic than DLX, which confirms the predictions obtained by the T.E.S.T. algorithm for T. pyriformis. Previous research has demonstrated the high toxicity of 1,4-naphthoquinone derivatives to both procaryotes and eucaryotes [42,43,44]. Among 1,4-naphthoquinone derivatives substituted at carbon 2, MNPD was the most toxic to Allivibrio fischeri (previously known as Photobacterium phosphoreum) luminescent bacteria, with an EC50 = 0.226 mg L−1 [42]. The Daphnia magna LC50 = 0.49 mg L−1 value for MNPD was also similar to the Spirotox EC50 value [43]. The primary mechanism of the toxic effects of naphthoquinones is related to reversible redox reactions between the quinone and hydroquinone forms [44], resulting in the generation of free radicals [43].
4.4. Ecological Consequences and Perspectives for Future Research
Aquatic organisms which feed through filtration, including protozoa, are exposed to xenobiotics not only when they are dissolved in water but also when they are sorbed onto particles. The toxic effect of xenobiotics can be direct or indirect through the formation of their transformation products or the generation of free radicals. Our study has demonstrated that the presence of even high concentrations of microplastics does not significantly impact the phototransformation of DLX and its toxicity to protozoa. However, it is important to note that we only used freshly prepared microplastics in our study. Given that aged microplastics can be more toxic and more reactive, similar studies with aged materials should be conducted in the future.
Our research indicates that products resulting from the photodecomposition of a substance may show much greater biological activity than their parent compounds. Considering that many xenobiotics, including pharmaceuticals, contain naphthalene in their chemical structure, future research should focus on the possibility of the formation of highly toxic naphthoquinones.
Author Contributions
Conceptualization, J.C., A.D., J.G. and G.N.-J.; methodology, J.C., A.D., J.G. and G.N.-J.; software, J.C., J.G. and G.N.-J.; validation, J.C., J.G. and G.N.-J.; formal analysis, J.C., A.D., J.G. and G.N.-J.; investigation, J.C., A.D., N.C., J.G. and G.N.-J.; resources, J.C. and G.N.-J.; data curation, A.D., J.G. and G.N.-J.; writing—original draft preparation, J.C., A.D., J.G. and G.N.-J.; writing—review and editing, J.C., A.D. and G.N.-J.; visualization, J.C. and G.N.-J.; supervision, G.N.-J.; project administration, G.N.-J.; funding acquisition, G.N.-J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science Center in Poland (grant number: 2019/35/B/NZ8/01388).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of this study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Available online: www.clincalc.com (accessed on 31 May 2023).
- Fernandez, J.P.; Almeida, C.M.R.; Salgado, M.A.; Carvalho, M.F. Pharmaceutical compounds in aquatic environments—Occurrence, fate and bioremediation prospective. Toxics 2021, 9, 257. [Google Scholar] [CrossRef] [PubMed]
- Osawa, R.A.; Carvalho, A.P.; Monteiro, O.C.; Florêncio, M.H. Degradation of duloxetine: Identification of transformation products by UHPLC-ESI(+)-HRMS/MS, in silico toxicity and wastewater analysis. J. Environ. Sci. 2019, 82, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Schultz, M.M.; Furlong, E.T. Trace analysis of antidepressant pharmaceuticals and their select degradates in aquatic matrixes by LC/ESI/MS/MS. Anal. Chem. 2008, 80, 1756–1762. [Google Scholar] [CrossRef] [PubMed]
- Schultz, M.M.; Furlong, E.T.; Kolpin, D.W.; Werner, S.L.; Schoenfuss, H.L.; Barber, L.B.; Blazer, V.S.; Norris, D.O.; Vaida, A.M. Antidepressant pharmaceuticals in two U.S. effluent-impacted streams: Occurrence and fate in water and sediment, and selective uptake in fish neural tissue. Environ. Sci. Technol. 2010, 44, 1918–1925. [Google Scholar] [CrossRef]
- Chadha, R.; Bali, A.; Bansal, G. Characterization of stress degradation products of duloxetine hydrochloride employing LC-UV/PDA and LC-MS/TOF studies. J. Pharm. Biomed. Anal. 2016, 121, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Trawiński, J.; Skibiński, R. Studies on photodegradation process of psychotropic drugs: A review. Environ. Sci. Pollut. Res. 2017, 24, 1152–1199. [Google Scholar] [CrossRef]
- Akdogan, Z.; Guven, B. Microplastics in the environment: A critical review of current understanding and identification of future research needs. Environ. Pollut. 2019, 254, 113011. [Google Scholar] [CrossRef]
- Abeynayaka, A.; Werellagama, I.; Ngoc-Bao, P.; Hengesbaugh, M.; Gajanayake, P.; Nallaperuma, B.; Karkour, S.; Bui, X.T.; Itsubo, N. Microplastics in wastewater treatment plants. Curr. Dev. Biotechnol. Bioengin. 2022, 311–337. [Google Scholar] [CrossRef]
- Santos, L.H.M.L.M.; Rodríguez-Mozaz, S.; Barceló, D. Microplastics as vectors of pharmaceuticals in aquatic organisms—An overview of their environmental implications. CSCEE 2021, 3, 100079. [Google Scholar] [CrossRef]
- Li, C.; Jiang, B.; Guo, J.; Sun, C.; Shi, C.; Huang, S.; Liu, W.; Wu, C.; Zhang, Y. Aging Process of Microplastics in the Aquatic Environments: Aging Pathway, Characteristic Change, Compound Effect, and Environmentally Persistent Free Radicals Formation. Water 2022, 14, 3515. [Google Scholar] [CrossRef]
- Hartmann, N.B.; Hüffer, T.; Thompson, R.C.; Hassellöv, M.; Verschoor, A.; Daugaard, A.E.; Rist, S.; Karlsoon, T.; Brennholt, N.; Cole, M.; et al. Are we speaking the same language? Recommendations for a definition and categorization framework for plastic debris. Environ. Sci. Technol. 2019, 53, 1039–1047. [Google Scholar] [CrossRef]
- Martin, J.; Santos, J.L.; Aparicio, I.; Alonso, E. Microplastics and associated emerging contaminants in the environment: Analysis, sorption mechanisms and effects of co-exposure. Trends Environ. Anal. Chem. 2022, 35, e00170. [Google Scholar] [CrossRef]
- Atugoda, T.; Vithanage, M.; Wijesekara, H.; Balan, N.; Sarmah, A.K.; Bank, M.S.; You, S.; Ok, Y.S. Interactions between microplastics, pharmaceuticals and personal care products: Implications for vector transport. Environ. Int. 2021, 49, 106367. [Google Scholar] [CrossRef]
- Ma, J.; Zhao, J.; Zhu, Z.; Li, L.; Yu, F. Effect of microplastic size on the adsorption behavior and mechanism of triclosan on polyvinyl chloride. Environ. Pollut. 2019, 254, 113104. [Google Scholar] [CrossRef] [PubMed]
- Puckowski, A.; Ćwięk, W.; Mioduszewska, K.; Stepnowski, P.; Białk-Bielińska, A. Sorption of pharmaceuticals on the surface of microplastics. Chemosphere 2021, 263, 127976. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, X.; Zhou, X.; Kong, X.; Tao, S.; Xing, B. Sorption of four hydrophobic organic compounds by three chemically distinct polymers: Role of chemical and physical composition. Environ. Sci. Technol. 2012, 46, 7252–7259. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, H.; Zhao, J.; Luo, X.; Wang, Z.; Xing, B. Photodegradation elevated the toxicity of polystyrene microplastics to grouper (Epinephelus moara) through disrupting hepatic lipid homeostasis. Environ. Sci. Technol. 2020, 54, 6202–6212. [Google Scholar] [CrossRef] [PubMed]
- Menéndez-Pedriza, A.; Jaumot, J. Interaction of environmental pollutants with microplastics: A critical review of sorption factors, bioaccumulation and ecotoxicological effects. Toxics 2020, 8, 40. [Google Scholar] [CrossRef]
- Kong, F.; Xu, X.; Xue, Y.; Gao, Y.; Zhang, L.; Wang, L.; Jiang, S.; Zhang, Q. Investigation of the adsorption of sulfamethoxazole by degradable microplastics artificially aged by chemical oxidation. Arch. Environ. Contam. Toxicol. 2021, 81, 155–165. [Google Scholar] [CrossRef]
- Zhu, K.; Jia, H.; Zhao, S.; Xia, T.; Guo, X.; Wang, T.; Zhu, L. Formation of environmentally persistent free radicals on microplastics under light irradiation. Environ. Sci. Technol. 2019, 53, 8177–8186. [Google Scholar] [CrossRef]
- Minguez, L.; Pedelucq, J.; Farcy, E.; Ballandonne, C.; Budzinski, H.; Halm-Lemeille, M.P. Toxicities of 48 pharmaceuticals and their freshwater and marine environmental assessment in Northwestern France. Environ. Sci. Pollut. Res. 2016, 23, 4992–5001. [Google Scholar] [CrossRef] [PubMed]
- Chojnacka, J.; Drobniewska, A.; Lenga, W.; Misztal, J.; Wawryniuk, M.; Nałęcz-Jawecki, G. The mutual effect of microparticles and antidepressants on the protozoan Spirostomum ambiguum (Müller, 1786) Ehrenberg, 1835. Water 2023, 15, 552. [Google Scholar] [CrossRef]
- Li, F.H.; Yao, K.; Lv, W.Y.; Liu, G.G.; Chen, P.; Huang, H.P.; Kang, Y.P. Photodegradation of ibuprofen under UV-Vis irradiation: Mechanism and toxicity of photolysis products. Bull. Environ. Contam. Toxicol. 2015, 94, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ma, L.-Y.; Li, L.-S.; Xu, L. Photodegradation kinetics, transformation, and toxicity prediction of ketoprofen, carprofen, and diclofenac acid in aqueous solutions. Environ. Toxicol. Chem. 2017, 36, 3232–3239. [Google Scholar] [CrossRef] [PubMed]
- Uzelac, M.M.; Armaković, S.J.; Armaković, S.; Cetojević-Simin, D.D.; Agbaba, J.; Binić, N.D. The role of environmental waters ionic composition and UV-LED radiation on photodegradation, mineralization and toxicity of commonly used beta-blockers. J. Mol. Struct. 2022, 1249, 131579. [Google Scholar] [CrossRef]
- Spina, M.; Venancio, W.; Rodrigues-Silva, C.; Pivetta, R.C.; Diniz, V.; Rath, S.; Guimaraes, J.R. Degradation of antidepressant pharmaceuticals by photoperoxidation in diverse water matrices: A highlight in the evaluation of acute and chronic toxicity. Environ. Sci. Pollut. Res. 2021, 28, 24034–24045. [Google Scholar] [CrossRef]
- Nałęcz-Jawecki, G.; Chojnacka, J.; Wawryniuk, M.; Drobniewska, A. Influence of Nano and Small Microplastics on Ciliated Protozoan Spirostomum ambiguum (Müller, 1786) Ehrenberg, 1835. Water 2021, 13, 2857. [Google Scholar] [CrossRef]
- Nałęcz-Jawecki, G.; Sawicki, J. Toxicity of inorganic compounds in the Spirotox test: A miniaturized version of the Spirostomum ambiguum test. Arch. Environ. Contam. Toxicol. 1998, 34, 1–5. [Google Scholar] [CrossRef]
- Nałęcz-Jawecki, G. Spirotox Test—Spirostomum ambiguum Acute Toxicity Test. In Small-Scale Freshwater Toxicity Investigations: Toxicity Test Methods; Blaise, C., Férard, J.-F., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 299–322. [Google Scholar]
- Available online: https://www.epa.gov/chemical-research/toxicity-estimation-software-tool-test (accessed on 31 May 2023).
- Valimana-Traverso, J.; Amariei, G.; Boltes, K.; Garcia, M.A.; Marina, M.L. Stability and toxicity studies for duloxetine and econazole on Spirodela polyrhyza using chiral capillary electrophoresis. J. Hazard. Mater. 2019, 374, 203–210. [Google Scholar] [CrossRef]
- Valimana-Traverso, J.; Amariei, G.; Boltes, K.; Garcia, M.A.; Marina, M.L. Enantiomer stability and combined toxicity of duloxetine and econazole on Daphnia magma using real concentrations determined by capillary electrophoresis. Sci. Total Environ. 2019, 670, 770–778. [Google Scholar] [CrossRef]
- Datar, P.A.; Waghmare, R.U. Development and validation of an analytical method for the stability of duloxetine hydrochloride. J. Taibah Univ. Sci. 2014, 8, 357–363. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Su, F.; Wang, Y.; Peng, L.; Liu, D. Adsorption behaviour of microplastics on the heavy metal Cr(VI) before and after ageing. Chemosphere 2022, 302, 134865. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Zhu, K.; Ma, H.; Liu, J.; Zhang, C.; Dai, Y.; Jia, H. Sulfur-containing persistent free radicals and reactive species on photoaged microplastics: Identification and the formation mechanism. Environ. Sci. Technol. 2023, 57, 8680–8690. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Jiang, Y.; Gu, Y.; Ding, P.; Wang, C.; Pan, R.; Shi, C.; Zeng, L.; Chen, X.; Li, H. The generation of environmentally persistent free radicals on photoaged microbeads from cosmetics enhances the toxicity via oxidative stress. Environ. Int. 2023, 174, 107875. [Google Scholar] [CrossRef]
- Santoke, H.; Song, W.; Cooper, W.J.; Peake, B.M. Advanced oxidation treatment and photochemical fate of selected antidepressant pharmaceuticals in solutions of Suwannee River humic acid. J. Hazard. Mater. 2012, 217–218, 382–390. [Google Scholar] [CrossRef]
- Brussol, C.; Duane, M.; Carlier, P.; Kotzias, D. Photo-induced OH reactions of naphthalene and its oxidation products on SiO2. Environ. Sci. Pollut. Res. 1999, 6, 138–140. [Google Scholar] [CrossRef]
- Vialation, D.; Richard, C.; Baglio, D.; Paya-Perez, A.-B. Mechanism of the photochemical transformation of naphthalen in water. J. Photochem. Photobiol. A Chem. 1999, 123, 15–19. [Google Scholar] [CrossRef]
- Nałęcz-Jawecki, G.; Sawicki, J. Spirotox—A new tool for testing the toxicity of volatile compounds. Chemosphere 1999, 38, 3211–3218. [Google Scholar] [CrossRef]
- Ding, F.; Guo, J.; Li, Z.; Li, L.Y.; Zhang, J.Y.; Zhang, J.H.; Lian, J.; Song, W.H.; Zhu, L. Evaluation and structure-activity relationship study of acute toxicity of naphthoquinones to Photobacterium phosphoreum, Photobacterium T3B. Bull. Environ. Contam. Toxicol. 2010, 85, 116–120. [Google Scholar] [CrossRef]
- Barata, C.; Varo, I.; Navarro, J.C.; Arun, S.; Porte, C. Antioxidant enzyme activities and lipid peroxidation in the freshwater cladoceran Daphnia magna exposed to redox cycling compounds. Comp. Biochem. Physiol. C 2005, 140, 175–186. [Google Scholar] [CrossRef]
- Klotz, L.-O.; Hou, X.; Jacob, C. 1,4-Naphthoquinones: From oxidative damage to cellular and inter-cellular signalling. Molecules 2014, 19, 14902–14918. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).