Parabens and Methylisotiazolinone (MIT): Preservatives with Different Behaviors When Subjected to Ozone and Ultraviolet Light Treatments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Target Solution
2.3. Experimental Set-Up
2.4. Analytical Measurements
3. Results
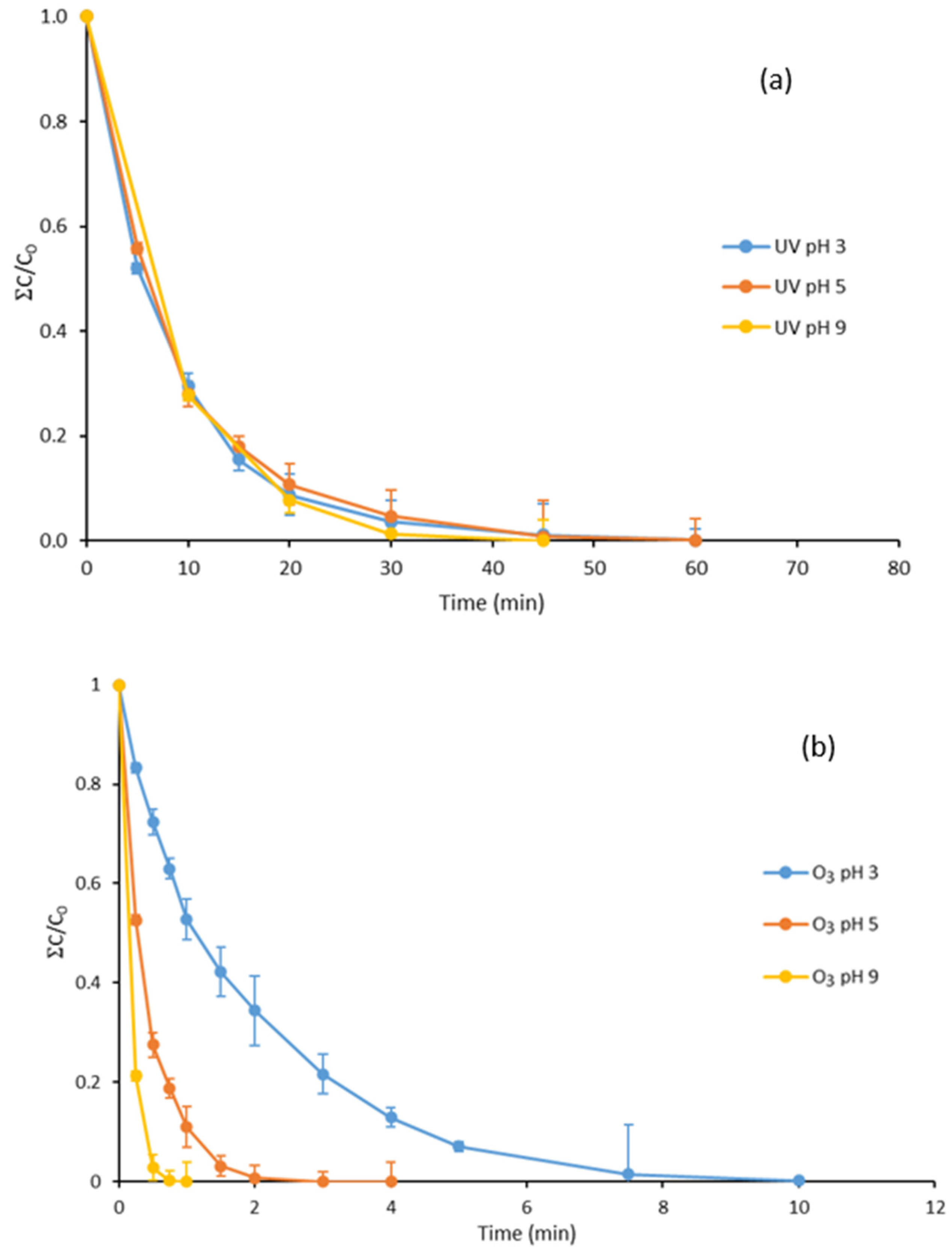
3.1. UV Treatment of Parabens at Different pH Values
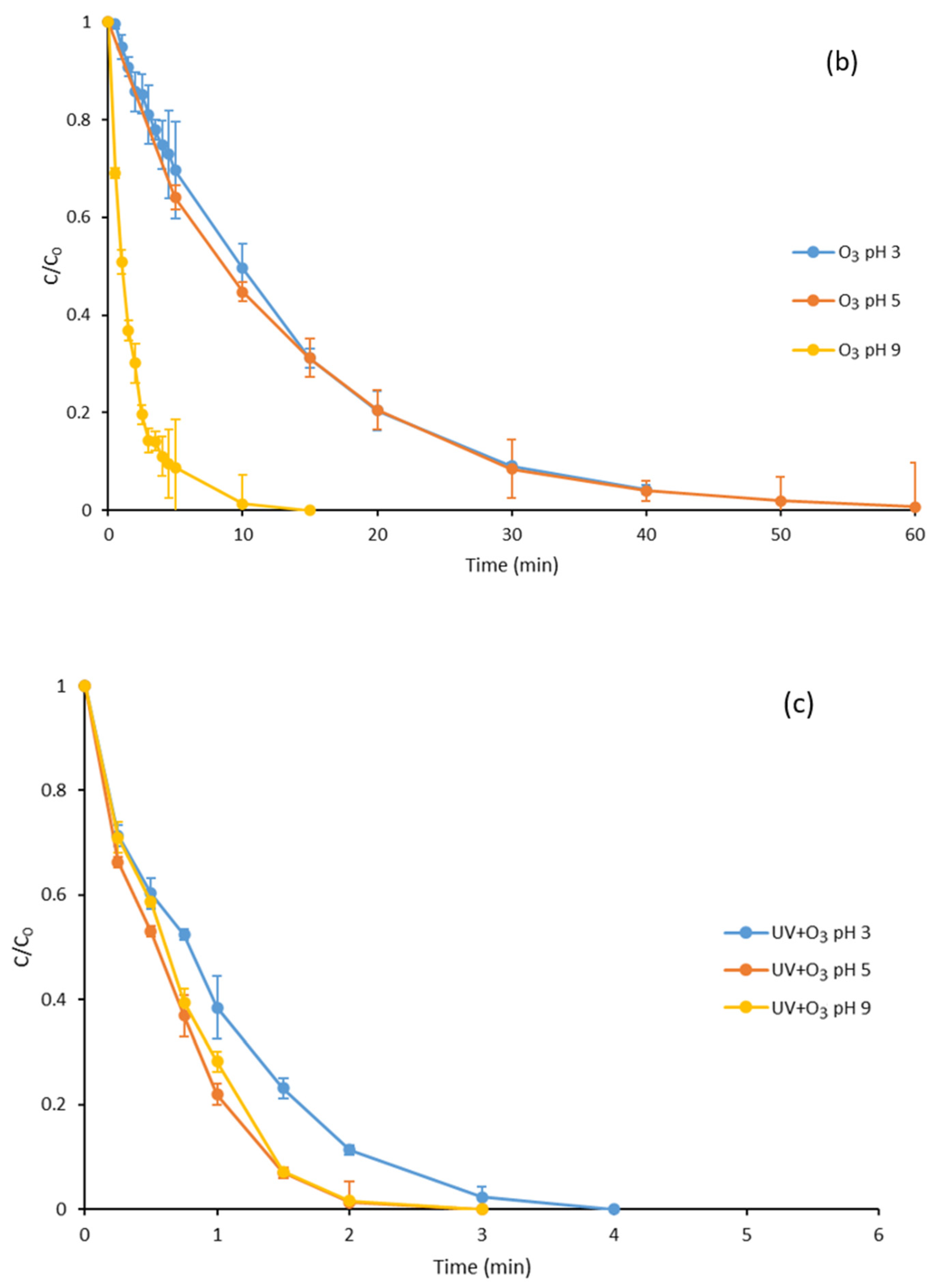
3.2. Ozone Degradation of Parabens at Different pH Values
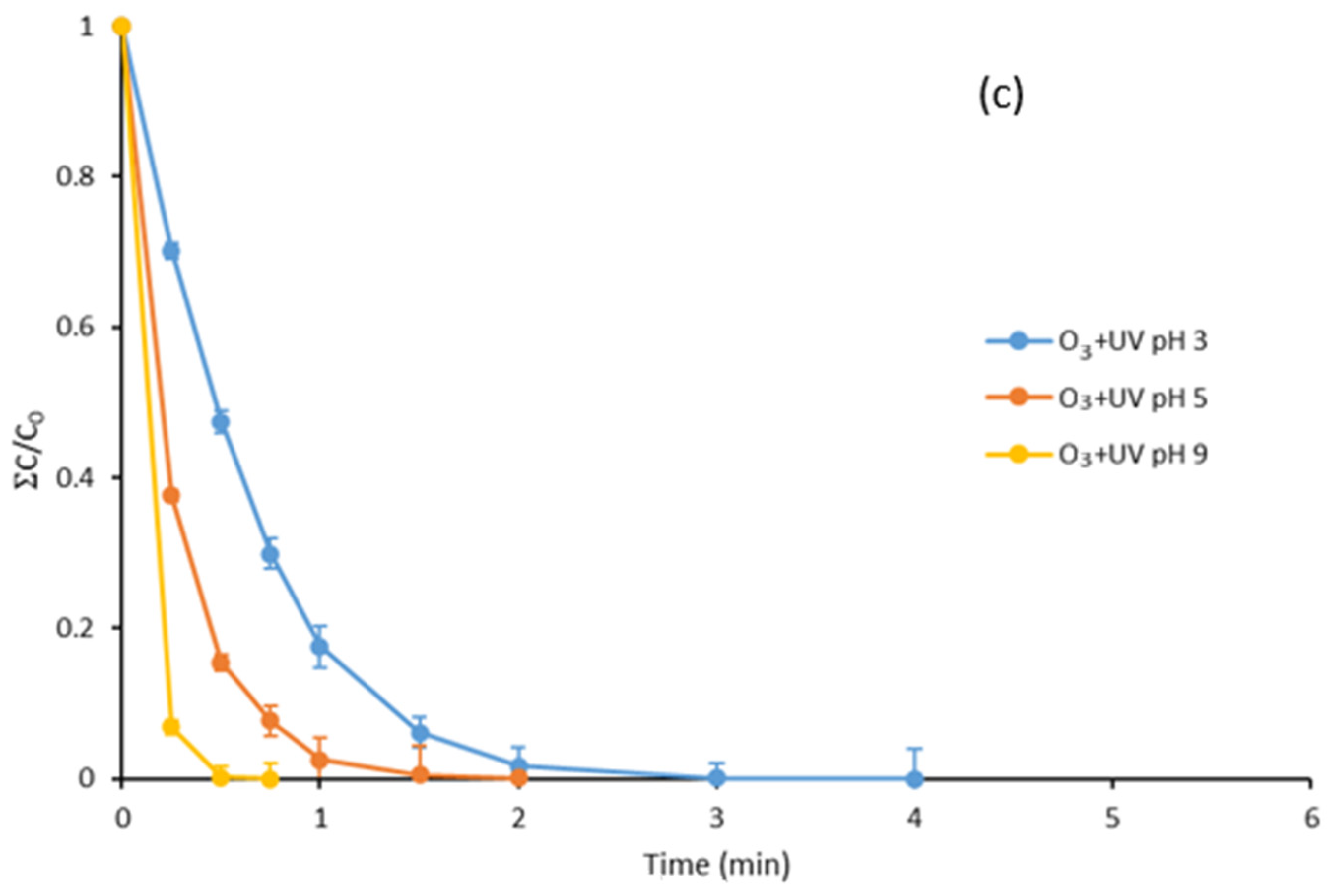
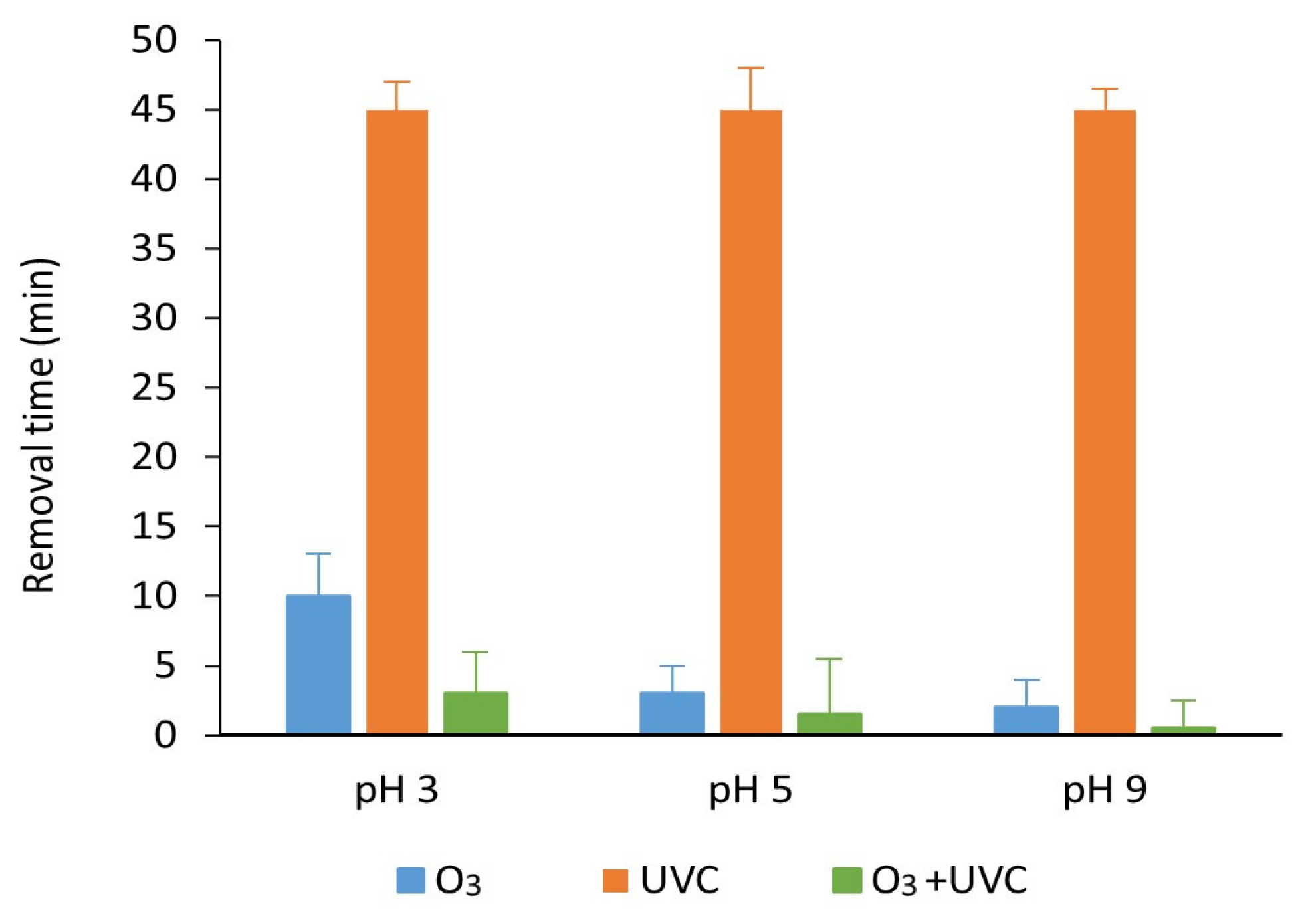
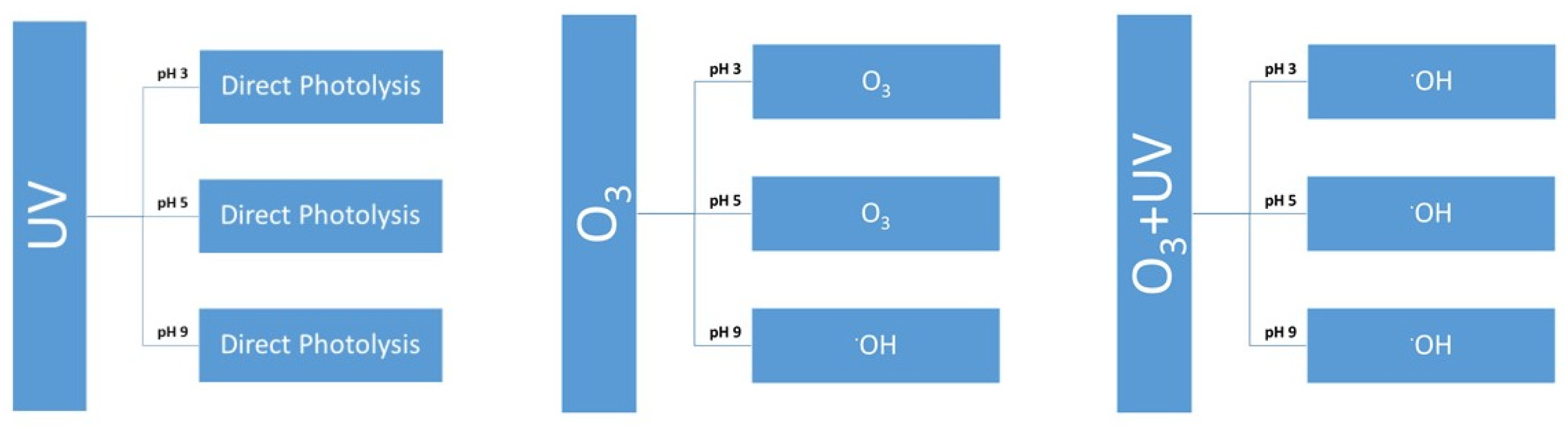
3.3. Degradation with Ozone and UV Light of Paraben Mixture at Different pH Values: Synergistic Effect
3.4. UV Treatment of Methylisothiazolinone at Different pH Values
3.5. Ozone Degradation of Methylisothiazolinone at Different pH Values
3.6. Degradation with Ozone and UV Light of Methylisothiazolinone at Different pH Values
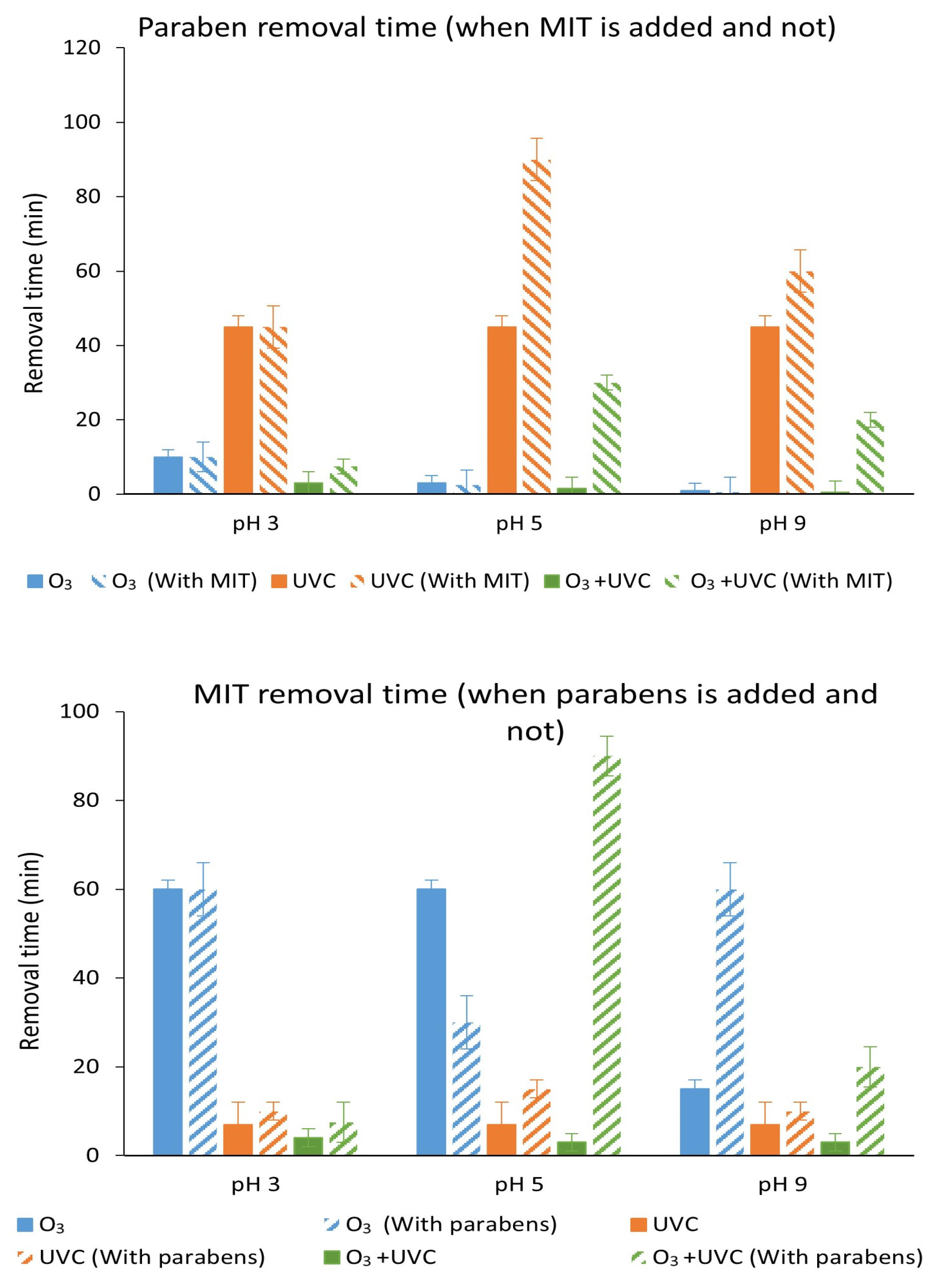
3.7. Degradation with UV Light, Ozone and Ozone Treatment Combined with UV Light of the Mixture of Parabens and Methylisothiazolinone at Different pH Values
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Agüera, A.; Martínez Bueno, M.J.; Fernández-Alba, A.R. New trends in the analytical determination of emerging contaminants and their transformation products in environmental waters. Environ. Sci. Pollut. Res. 2013, 20, 3496–3515. [Google Scholar] [CrossRef]
- Álvarez-Ruiz, R.; Picó, Y. Analysis of emerging and related pollutants in aquatic biota. Trends Environ. Anal. Chem. 2020, 25, e00082. [Google Scholar] [CrossRef]
- Lapworth, D.; Baran, N.; Stuart, M.; Ward, R. Emerging organic contaminants in groundwater: A review of sources, fate and occurrence. Environ. Pollut. 2012, 163, 287–303. [Google Scholar] [CrossRef]
- Bunting, S.; Lapworth, D.; Crane, E.; Grima-Olmedo, J.; Koroša, A.; Kuczyńska, A.; Mali, N.; Rosenqvist, L.; Van Vliet, M.; Togola, A. Emerging organic compounds in European groundwater. Environ. Pollut. 2021, 269, 115945. [Google Scholar] [CrossRef]
- Gaston, L.; Lapworth, D.J.; Stuart, M.; Arnscheidt, J. Prioritization approaches for substances of emerging concern in groundwater: A critical review. Environ. Sci. Technol. 2019, 53, 6107–6122. [Google Scholar] [CrossRef]
- Galindo-Miranda, J.M.; Guízar-González, C.; Becerril-Bravo, E.J.; Moeller-Chávez, G.; León-Becerril, E.; Vallejo-Rodríguez, R. Occurrence of emerging contaminants in environmental surface waters and their analytical methodology—A review. Water Supply 2019, 19, 1871–1884. [Google Scholar] [CrossRef]
- Jiang, J.-Q.; Zhou, Z.; Sharma, V. Occurrence, transportation, monitoring and treatment of emerging micro-pollutants in waste water—A review from global views. Microchem. J. 2013, 110, 292–300. [Google Scholar] [CrossRef]
- Gkika, D.A.; Mitropoulos, A.C.; Lambropoulou, D.A.; Kalavrouziotis, I.K.; Kyzas, G.Z. Cosmetic wastewater treatment technologies: A review. Environ. Sci. Pollut. Res. 2022, 29, 75223–75247. [Google Scholar] [CrossRef]
- de García, S.O.; Pinto, G.P.; Encina, P.G.; Mata, R.I. Consumption and occurrence of pharmaceutical and personal care products in the aquatic environment in Spain. Sci. Total Environ. 2013, 444, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Nowak, K.; Jabłońska, E.; Ratajczak-Wrona, W. Controversy around parabens: Alternative strategies for preservative use in cosmetics and personal care products. Environ. Res. 2021, 198, 110488. [Google Scholar] [CrossRef]
- Chen, M.-H.; Yu, B.; Zhang, Z.-F.; Ma, W.-L. Occurrence of parabens in outdoor environments: Implications for human exposure assessment. Environ. Pollut. 2021, 282, 117058. [Google Scholar] [CrossRef] [PubMed]
- Vale, F.; Sousa, C.A.; Sousa, H.; Santos, L.; Simões, M. Parabens as emerging contaminants: Environmental persistence, current practices and treatment processes. J. Clean. Prod. 2022, 347, 131244. [Google Scholar] [CrossRef]
- Amat, A.M.; Arques, A.; López-Pérez, M.F.; Nacher, M.; Palacios, S. Effect of methylisothiazolinone on biological treatment: Efficiency of SBRs and bioindicative studies. Environ. Eng. Sci. 2015, 32, 479–485. [Google Scholar] [CrossRef]
- Silva, V.; Silva, C.; Soares, P.; Garrido, E.M.; Borges, F.; Garrido, J. Isothiazolinone biocides: Chemistry, biological, and toxicity profiles. Molecules 2020, 25, 991. [Google Scholar] [CrossRef]
- Abad-Gil, L.; Lucas-Sánchez, S.; Gismera, M.J.; Sevilla, M.T.; Procopio, J.R. Determination of paraben-, isothiazolinone-and alcohol-type preservatives in personal care products by HPLC with dual (diode-array and fluorescence) detection. Microchem. J. 2021, 160, 105613. [Google Scholar] [CrossRef]
- Duarte-Alvarado, V.; Santos-Juanes, L.; Arques, A.; Amat, A.M. Mild Fenton Processes for the Removal of Preservatives: Interfering Effect of Methylisothiazolinone (MIT) on Paraben Degradation. Catalysts 2022, 12, 1390. [Google Scholar] [CrossRef]
- Nowak, M.; Zawadzka, K.; Lisowska, K. Occurrence of methylisothiazolinone in water and soil samples in Poland and its biodegradation by Phanerochaete chrysosporium. Chemosphere 2020, 254, 126723. [Google Scholar] [CrossRef]
- Paijens, C.; Bressy, A.; Frère, B.; Tedoldi, D.; Mailler, R.; Rocher, V.; Neveu, P.; Moilleron, R. Urban pathways of biocides towards surface waters during dry and wet weathers: Assessment at the Paris conurbation scale. J. Hazard. Mater. 2021, 402, 123765. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment–A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef]
- Marin, M.; Lhiaubet-Vallet, V.; Santos-Juanes, L.; Soler, J.; Gomis, J.; Arques, A.; Amat, A.; Miranda, M. A Photophysical approach to investigate the photooxidation mechanism of pesticides: Hydroxyl radical versus electron transfer. Appl. Catal. B Environ. 2011, 103, 48–53. [Google Scholar] [CrossRef]
- Cako, E.; Wang, Z.; Castro-Munoz, R.; Rayaroth, M.P.; Boczkaj, G. Cavitation based cleaner technologies for biodiesel production and processing of hydrocarbon streams: A perspective on key fundamentals, missing process data and economic feasibility—A review. Ultrason. Sonochem. 2022, 88, 106081. [Google Scholar] [CrossRef]
- Castro-Munoz, R.; Boczkaj, G.; Jafari, S.M. The role of hydrodynamic cavitation in tuning physicochemical properties of food items: A comprehensive review. Trends Food Sci. Technol. 2023, 134, 192–206. [Google Scholar] [CrossRef]
- Ajiboye, T.O.; Oyewo, O.A.; Onwudiwe, D.C. Photocatalytic removal of parabens and halogenated products in wastewater: A review. Environ. Chem. Lett. 2021, 19, 3789–3819. [Google Scholar] [CrossRef]
- Zúñiga-Benítez, H.; Peñuela, G.A. Methylparaben removal using heterogeneous photocatalysis: Effect of operational parameters and mineralization/biodegradability studies. Environ. Sci. Pollut. Res. 2017, 24, 6022–6030. [Google Scholar] [CrossRef] [PubMed]
- Lucas, M.S.; Peres, J.A. Removal of emerging contaminants by Fenton and UV-driven advanced oxidation processes. Water Air Soil. Pollut. 2015, 226, 273. [Google Scholar] [CrossRef]
- Zúñiga-Benítez, H.; Muñoz-Calderón, A.; Peñuela, G.A. Removal of a mix of benzophenones and parabens using solar photo-Fenton and a cylinder parabolic collector in aqueous solutions. J. Environ. Chem. Eng. 2018, 6, 7347–7357. [Google Scholar] [CrossRef]
- Tay, K.S.; Rahman, N.A.; Abas, M.R.B. Ozonation of parabens in aqueous solution: Kinetics and mechanism of degradation. Chemosphere 2010, 81, 1446–1453. [Google Scholar] [CrossRef]
- Huang, N.; Shao, W.-T.; Wang, W.-L.; Wang, Q.; Chen, Z.-Q.; Wu, Q.-Y.; Hu, H.-Y. Removal of methylisothiazolinone biocide from wastewater by VUV/UV advanced oxidation process: Kinetics, mechanisms and toxicity. J. Environ. Manag. 2022, 315, 115107. [Google Scholar] [CrossRef]
- Russo, D.; Cochran, K.H.; Westerman, D.; Puma, G.L.; Marotta, R.; Andreozzi, R.; Richardson, S.D. Ultrafast photodegradation of isoxazole and isothiazolinones by UV254 and UV254/H2O2 photolysis in a microcapillary reactor. Water Res. 2020, 169, 115203. [Google Scholar] [CrossRef]
- Cui, J.; Cai, S.; Zhang, S.; Wang, G.; Gao, C. Degradation of a non-oxidizing biocide in circulating cooling water using UV/persulfate: Kinetics, pathways, and cytotoxicity. Chemosphere 2022, 289, 133064. [Google Scholar] [CrossRef]
- Gómez-Rodríguez, P.; Calza, P.; Fabbri, D.; Medana, C.; van-Grieken, R.; López-Muñoz, M.-J. Photocatalytic degradation of methylisothiazolinone in water by TiO2 and TiO2/persulfate systems with simulated solar radiation. Catal. Today 2023, 413, 113942. [Google Scholar] [CrossRef]
- Yang, Z.-W.; Wang, W.-L.; Lee, M.-Y.; Wu, Q.-Y.; Guan, Y.-T. Synergistic effects of ozone/peroxymonosulfate for isothiazolinone biocides degradation: Kinetics, synergistic performance and influencing factors. Environ. Pollut. 2022, 294, 118626. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, J.C.; Bessegato, G.G.; Zanoni, M.V.B. Efficiency comparison of ozonation, photolysis, photocatalysis and photoelectrocatalysis methods in real textile wastewater decolorization. Water Res. 2016, 98, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Amat, A.; Arques, A.; Miranda, M.; López, F. Use of ozone and/or UV in the treatment of effluents from board paper industry. Chemosphere 2005, 60, 1111–1117. [Google Scholar] [CrossRef] [PubMed]
- Bennett, G.F. Ozone Reaction Kinetics for Water and Wastewater Systems; Fernando, J.B., Ed.; Lewis Publishers (A CRC Press Company): Boca Raton, FL, USA, 2006; 384p, ISBN 1-56670-629-7. [Google Scholar]
- Von Sonntag, C.; Von Gunten, U. Chemistry of Ozone in Water and Wastewater Treatment; IWA Publishing: London, UK, 2012. [Google Scholar]
- Deborde, M.; Rabouan, S.; Duguet, J.-P.; Legube, B. Kinetics of aqueous ozone-induced oxidation of some endocrine disruptors. Environ. Sci. Technol. 2005, 39, 6086–6092. [Google Scholar] [CrossRef] [PubMed]
- Amat, A.M.; Arques, A.; Beneyto, H.; García, A.; Miranda, M.A.; Seguí, S. Ozonisation coupled with biological degradation for treatment of phenolic pollutants: A mechanistically based study. Chemosphere 2003, 53, 79–86. [Google Scholar] [CrossRef] [PubMed]
- García-Ballesteros, S.; Mora, M.; Vicente, R.; Vercher, R.; Sabater, C.; Castillo, M.; Amat, A.; Arques, A. A new methodology to assess the performance of AOPs in complex samples: Application to the degradation of phenolic compounds by O3 and O3/UV-A–Vis. Chemosphere 2019, 222, 114–123. [Google Scholar] [CrossRef]
- Wang, W.-L.; Chen, Z.; Du, Y.; Zhang, Y.-L.; Zhou, T.-H.; Wu, Q.-Y.; Hu, H.-Y. Elimination of isothiazolinone biocides in reverse osmosis concentrate by ozonation: A two-phase kinetics and a non-linear surrogate model. J. Hazard. Mater. 2020, 389, 121898. [Google Scholar] [CrossRef]
- Li, A.; Wu, Q.-Y.; Tian, G.-P.; Hu, H.-Y. Effective degradation of methylisothiazolone biocide using ozone: Kinetics, mechanisms, and decreases in toxicity. J. Environ. Manag. 2016, 183, 1064–1071. [Google Scholar] [CrossRef]


| Treatment | pH 3 | pH 5 | pH 9 |
|---|---|---|---|
| O3 | 0.589 | 2.642 | 8.254 |
| UVC | 0.107 | 0.110 | 0.153 |
| O3 + UVC | 2.260 | 3.709 | 12.937 |
| Synergy | 3.247 | 1.348 | 1.539 |
| Treatment | pH 3 | pH 5 | pH 9 |
|---|---|---|---|
| O3 | 0.084 | 0.082 | 0.419 |
| UVC | 0.732 | 0.863 | 0.657 |
| O3 + UVC | 1.229 | 2.120 | 2.055 |
| Synergy | 1.506 | 2.243 | 1.909 |
| Compound | ε (M−1cm−1) |
|---|---|
| Methylparaben | 15,123 |
| Ethylparaben | 14,562 |
| Propylparaben | 20,572 |
| Butylparaben | 14,218 |
| Propylparaben | 17,119 |
| MIT | 4213 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Timoner, R.; Duarte-Alvarado, V.; Castillo, M.Á.; Santos-Juanes, L.; Arques, A.; Amat, A.M. Parabens and Methylisotiazolinone (MIT): Preservatives with Different Behaviors When Subjected to Ozone and Ultraviolet Light Treatments. Water 2023, 15, 3837. https://doi.org/10.3390/w15213837
López-Timoner R, Duarte-Alvarado V, Castillo MÁ, Santos-Juanes L, Arques A, Amat AM. Parabens and Methylisotiazolinone (MIT): Preservatives with Different Behaviors When Subjected to Ozone and Ultraviolet Light Treatments. Water. 2023; 15(21):3837. https://doi.org/10.3390/w15213837
Chicago/Turabian StyleLópez-Timoner, Rubén, Victoria Duarte-Alvarado, María Ángeles Castillo, Lucas Santos-Juanes, Antonio Arques, and Ana María Amat. 2023. "Parabens and Methylisotiazolinone (MIT): Preservatives with Different Behaviors When Subjected to Ozone and Ultraviolet Light Treatments" Water 15, no. 21: 3837. https://doi.org/10.3390/w15213837






