Macroinvertebrate Spatial Diversity Patterns of Shore Habitats in Italian High-Altitude Natural and Permanent Lakes and Ponds
Abstract
1. Introduction
2. Materials and Methods
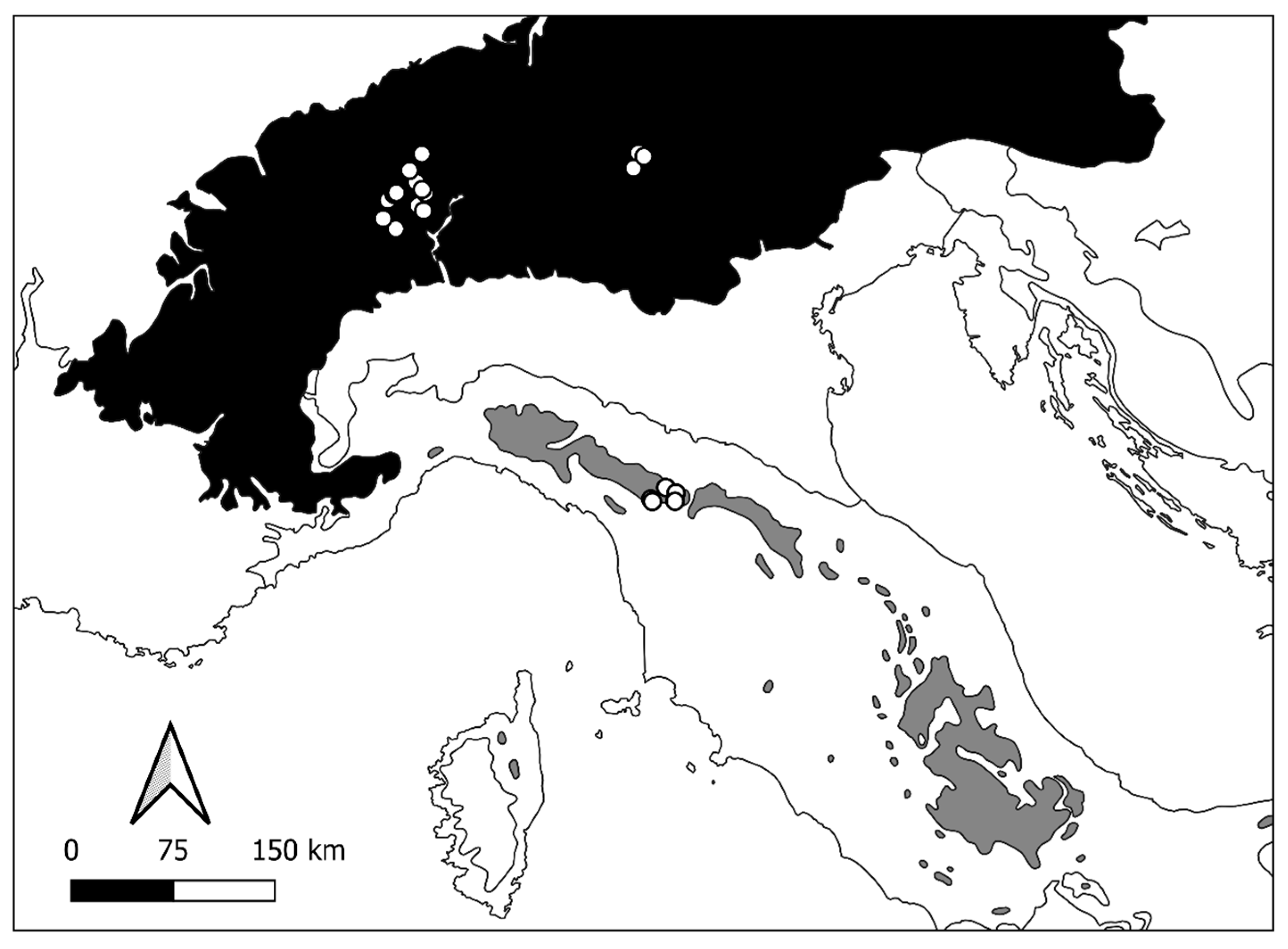
2.1. Study Area
2.2. Bibliographic Search
2.3. Statistical Approaches
3. Results
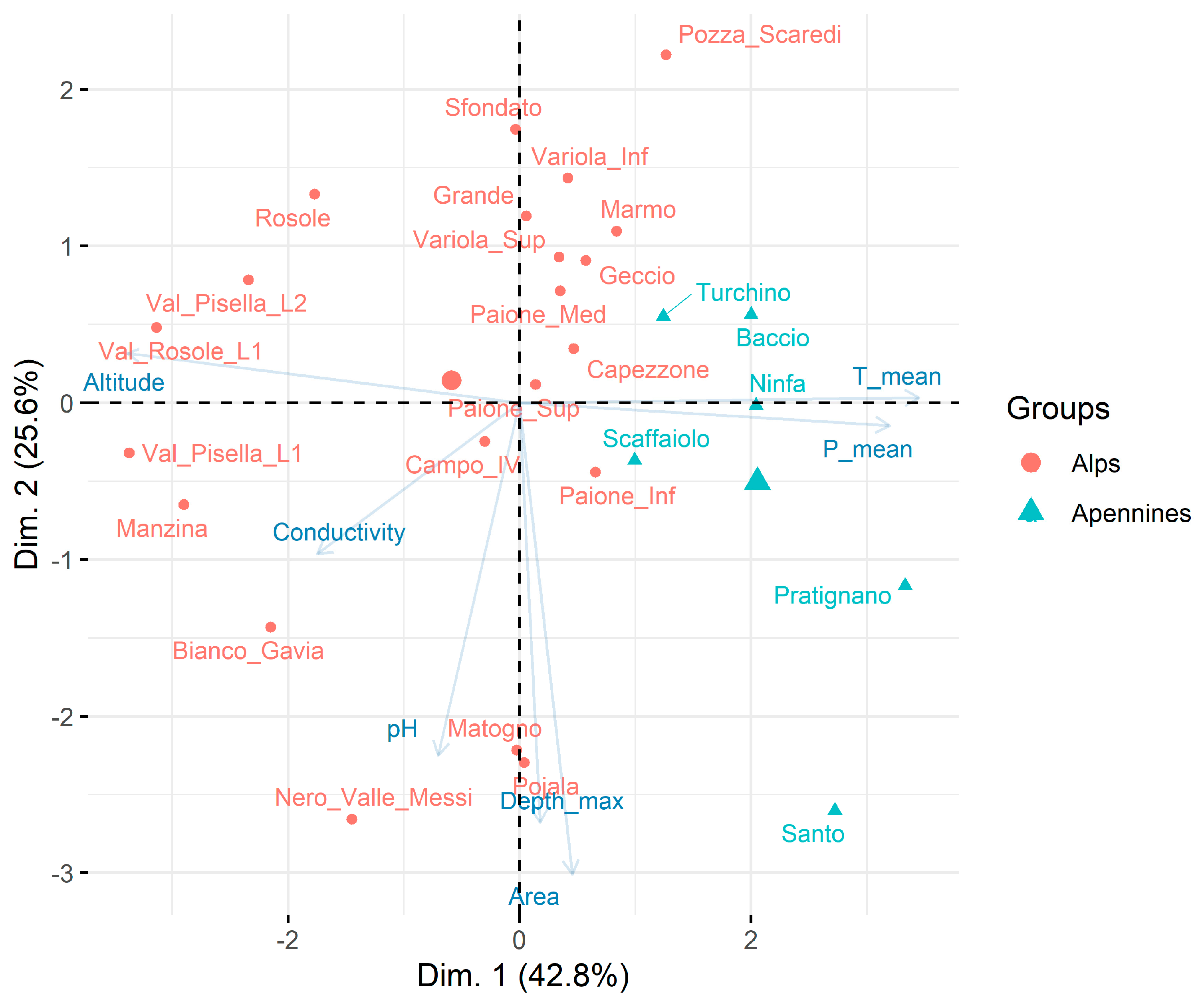
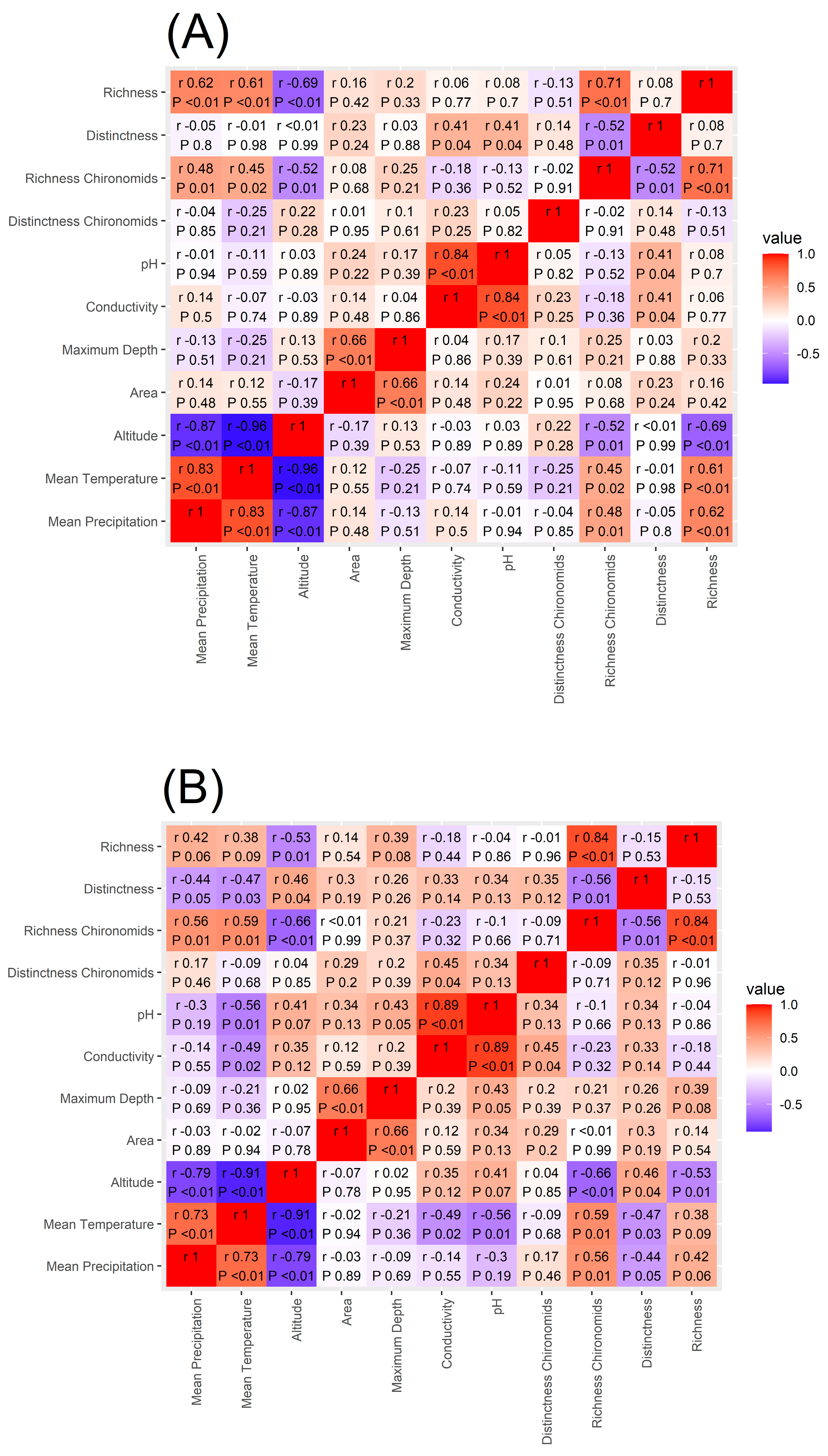
3.1. Environmental Characterisation
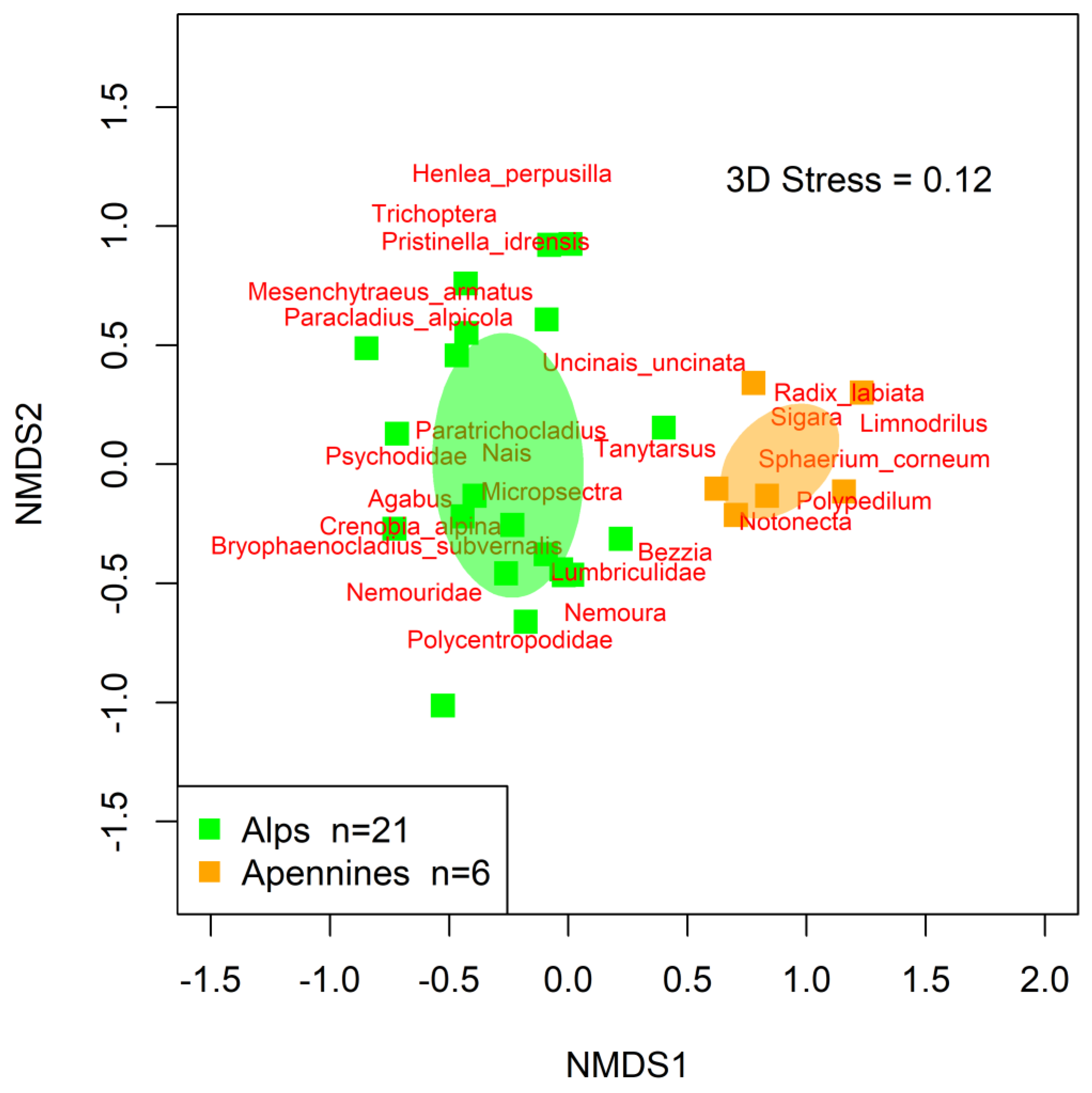
3.2. Macroinvertebrate Assemblage Structure
4. Discussion
4.1. Environmental Characterisation
4.2. Macroinvertebrate Assemblage Structure
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kernan, M.; Catalan, J.F.; Ventura, M.; Curtis, C.J. A biological survey of high mountain and high latitude lakes across Europe: Aims, sampling strategy, methods and main achievements. Adv. Limnol. 2009, 62, 3–16. [Google Scholar] [CrossRef]
- Battarbee, R.V. Mountain lakes, pristine o polluted? Limnetica 2005, 24, 1–8. [Google Scholar] [CrossRef]
- Battarbee, R.V.; Patrick, S.; Kernan, M.; Psenner, R.; Thies, H.; Grimalt, J.; Rosseland, B.O.; Wathne, B.; Catalan, J.; Mosello, R.; et al. High Mountain Lakes and Atmospherically Transported Pollutants. In Global Change and Mountain Regions: An Overview of Current Knowledge; Huber, U.M., Bugmann, H.K.M., Reasoner, M.A., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 113–121. [Google Scholar]
- Machate, O.; Schmeller, D.S.; Schulze, T.; Brack, W. Review: Mountain lakes as freshwater resources at risk from chemical pollution. Environ. Sci. Eur. 2023, 35, 3. [Google Scholar] [CrossRef]
- Rogora, M.; Frate, L.; Carranza, M.L.; Freppaz, M.; Stanisci, A.; Bertani, I.; Bottarin, R.; Brambilla, A.; Canullo, R.; Carbognani, M.; et al. Assessment of climate change effects on mountain ecosystems through a cross-site analysis in the Alps and Apennines. Sci. Total Environ. 2018, 624, 429–1442. [Google Scholar] [CrossRef]
- Sutela, T.; Aroviita, J.; Keto, A. Assessing ecological status of regulated lakes with littoral macrophyte, macroinvertebrate and fish assemblages. Ecol. Indic. 2013, 24, 185–192. [Google Scholar] [CrossRef]
- Caruso, B.S.; King, R.; Newton, S.; Zammit, C. Simulation of Climate Change Effects on Hydropower Operations in Mountain Headwater Lakes, New Zealand. River Res. Appl. 2017, 33, 147–161. [Google Scholar] [CrossRef]
- Thompson, R.; Kamenik, C.; Schmidt, R. Ultra-sensitive Alpine lakes and climate change. J. Limnol. 2005, 64, 139–152. [Google Scholar] [CrossRef]
- Salerno, F.; Gambelli, S.; Viviano, G.; Thakuri, S.; Guyennon, N.; D’Agata, C.; Diolaiuti, G.; Smiraglia, C.; Stefani, F.; Bocchiola, D.; et al. High alpine ponds shift upwards as average temperatures increase: A case study of the Ortles-Cevedale mountain group (Southern Alps, Italy) over the last 50 years. Glob. Planet Chang. 2014, 120, 81–91. [Google Scholar] [CrossRef]
- Sadro, S.; Melack, J.M.; Sickman, J.O.; Skeen, K. Climate warming response of mountain lakes affected by variations in snow. Limnol. Oceanogr. Lett. 2019, 4, 9–17. [Google Scholar] [CrossRef]
- Fjellheim, A.; Boggero, A.; Nocentini, A.M.; Rieradevall, M.; Raddum, G.G.; Schnell, Ø.A. Distribution of benthic invertebrates in relation to environmental factors—A study of European remote alpine lake ecosystems. Verh. Int. Ver. Limnol. 2000, 27, 484–488. [Google Scholar] [CrossRef]
- Fjellheim, A.; Raddum, G.G.; Vandvik, V.; Cogalniceanu, D.; Bitusik, P.; Boggero, A.; Brancelj, A.; Dumnicka, E.; Galas, J.; Gâldean, N.; et al. Diversity and distribution patterns of benthic invertebrates along alpine gradients. A study of remote European freshwater lakes. Adv. Limnol. 2009, 62, 167–190. [Google Scholar] [CrossRef]
- Carlson, B.Z.; Corona, M.C.; Dentant, C.; Bonet, R.; Thuiller, W.; Choler, P. Observed long-term greening of alpine vegetation—A case study in the French Alps. Environ. Res. Lett. 2017, 12, 114017. [Google Scholar] [CrossRef]
- Gobiet, A.; Kotlarski, S.; Beniston, M.; Heinrich, G.; Rajczak, J.; Stoffel, M. 21st century climate change in the European Alps—A review. Sci. Total Environ. 2014, 493, 1138–1151. [Google Scholar] [CrossRef]
- Boggero, A. Macroinvertebrates of Italian mountain lakes: A review. Redia J. Zool. 2018, 101, 35–45. [Google Scholar] [CrossRef]
- Allan, J.D.; Castillo, M.M. Stream Ecology: Structure and Function of Running Waters; Springer: Dordrecht, The Netherlands, 2007; pp. 135–161. [Google Scholar]
- Reice, S.R.; Wohlenberg, M. Monitoring freshwater benthic macroinvertebrates and benthic processes: Measures for assessment of ecosystem health. In Freshwater Biomonitoring and Benthic Macroinvertebrates; Rosenberg, D.M., Resh, V.H., Eds.; Chapman and Hall: New York, NY, USA, 1992; pp. 287–305. [Google Scholar]
- Boggero, A.; Zaupa, S.; Musazzi, S.; Rogora, M.; Dumnicka, E.; Lami, A. Environmental factors as drivers for macroinvertebrate and diatom diversity in Alpine lakes: New insights from the Stelvio National Park (Italy). J. Limnol. 2019, 78, 147–162. [Google Scholar] [CrossRef]
- Armitage, P.D.; Pardo, I. Impact assessment of regulation at the reach level using macroinvertebrate information from mesohabitats. Regul. Rivers Res. Manag. 1995, 10, 147–158. [Google Scholar] [CrossRef]
- Catalán, J.; Camarero, L.; Felip, M.; Pla, S.; Ventura, M.; Buchaca, T.; Bartumeus, F.; de Mendoza, G.; Miró, A.; Casamayor, E.O.; et al. High mountain lakes: Extreme habitats and witnesses of environmental changes. Limnetica 2006, 25, 551–584. [Google Scholar] [CrossRef]
- Catalán, J.; Curtis, C.; Kernan, M. Remote European mountain lake ecosystems: Regionalisation and ecological status. Freshw. Biol. 2009, 54, 2419–2432. [Google Scholar] [CrossRef]
- Füreder, L.; Ettinger, R.; Boggero, A.; Thaler, B.; Thies, H. Macroinvertebrate Diversity in Alpine Lakes: Effects of Altitude and Catchment Properties. Hydrobiologia 2006, 562, 123–144. [Google Scholar] [CrossRef]
- Marcheggiani, S.; Cesarini, G.; Puccinelli, C.; Chiudioni, F.; Mancini, L.; Angelici, C.; Martinoli, M.; Tancioni, L. An Italian local study on assessment of the ecological and human impact of water abstraction. Microchem. J. 2019, 149, 104016. [Google Scholar] [CrossRef]
- Morales-Molino, C.; Steffen, M.; Samartin, S.; van Leeuwen, J.F.N.; Hürlimann, D.; Vescovi, E.; Tinner, W. Long-Term Responses of Mediterranean Mountain Forests to Climate Change, Fire and Human Activities in the Northern Apennines (Italy). Ecosystems 2021, 24, 1361–1377. [Google Scholar] [CrossRef]
- Di Giorgio, M.; Zuppa, A.M. Macrobenthos dei laghetti di quota del Gran Sasso e del tratto sorgentizio del torrente Nora Appennino abruzzese, nota preliminare. In Monitoraggio Biologico Del Gran Sasso; Cicolani, B., Ed.; Consorzio di ricerca Gran Sasso: Teramo, Italy, 1996; pp. 284–289. [Google Scholar]
- Ruggiero, A.; Solimini, A.G.; Mutschlechner, A.; Anello, M.; Carchini, G. Aspetti limnologici del Lago Racollo (1568 m s.l.m.), Campo Imperatore (AQ). Studi Trentini Di Sci. Nat. Acta Biol. 2001, 78, 173–180. [Google Scholar]
- Ruggiero, A.; Solimini, A.G.; Carchini, G. Limnological aspects of an Apennine shallow lake. Ann. Limnol. Int. J. Lim. 2004, 40, 89–99. [Google Scholar] [CrossRef]
- Osella, G.; Pannunzio, G. Macrobenthos dei laghetti del Gran Sasso d’Italia. Quad. Mus. Civ. Di Stor. Nat. Ferrara 2013, 1, 57–67. [Google Scholar]
- Khamis, K.; Hannah, D.M.; Clarvis, M.H.; Brown, L.E.; Castella, E.; Milner, A.M. Alpine aquatic ecosystem conservation policy in a changing climate. Environ. Sci. Policy 2014, 43, 39–55. [Google Scholar] [CrossRef]
- Velle, G.; Bodin, C.L.; Arle, J.; Austnes, K.; Boggero, A.; Bojkova, J.; Fornaroli, R.; Fölster, J.; Goedkoop, W.; Jones, I.; et al. Responses of Benthic Invertebrates to Chemical Recovery from Acidification; NIVA report SNO 7881-2023. ICP Waters report 153/2023; NIVA: Bergen, Norway, 2023. [Google Scholar]
- Ansaloni, I.; Prevedelli, D.; Ruocco, M.; Simonini, R. Checklist of benthic macroinvertebrates of the Lago Pratignano (northern Apennines, Italy): An extremely rich ecosystem. Check List 2016, 12, 1821. [Google Scholar] [CrossRef]
- Nelson, S.J.; Hovel, R.A.; Daly, J.; Gavin, A.; Dykema, S.; McDowell, W.H. Northeastern mountain ponds as sentinels of change: Current and emerging research and monitoring in the context of shifting chemistry and climate interactions. Atmos. Environ. 2021, 264, 118694. [Google Scholar] [CrossRef]
- European Environment Agency. Europe’s Environment: The Third Assessment; Environmental assessment report 10; EEA: Luxembourg, 2003. [Google Scholar]
- Boggero, A.; Füreder, L.; Lencioni, V.; Simcic, T.; Thaler, B.; Ferrarese, U.; Lotter, A.F.; Ettinger, R. Littoral Chironomid communities of Alpine lakes in relation to environmental factors. Hydrobiologia 2006, 562, 145–165. [Google Scholar] [CrossRef]
- Ansaloni, I.; Benassi, A.; Manzieri, A.M.; Ruocco, M.; Sala, L.; Tintorri, A. Il Lago della Ninfa (Appennino modenese): Comunità macrozoobentonica, fauna vertebrata e considerazioni ecologiche. Atti Soc. Nat. Mat. Modena 2015, 146, 249–262. [Google Scholar]
- British Standards Institution. Guidance Standard on Assessing the Hydromorphological Features of Lakes; BS EN 16039:2011 Water quality; BSI: London, UK, 2011. [Google Scholar]
- Körner, C. A re-assessment of high elevation treeline positions and their explanation. Oecologia 1998, 115, 445–459. [Google Scholar] [CrossRef]
- de Jong, Y. Fauna Europaea. Fauna Europaea Consortium. 2016. Available online: https://www.gbif.org/dataset/90d9e8a6-0ce1-472d-b682-3451095dbc5a (accessed on 14 September 2023).
- NIVA. International Cooperative Programme for Assessment and Monitoring of Acidification of Rivers and Lakes: Programme Manual; Programme Centre, NIVA: Oslo, Norway, 1987. [Google Scholar]
- ICP Waters Programme Centre. Programme Manual; NIVA report SNO 3547-96; Programme Centre, NIVA: Oslo, Norway, 1996. [Google Scholar]
- ICP Waters Programme Centre. ICP Waters Programme Manual 2010; NIVA SNO 6074-2010. ICP Waters report 105/2010; Programme Centre, NIVA: Oslo, Norway, 2010. [Google Scholar]
- AA.VV. Guide Per il Riconoscimento Delle Specie Animali delle Acque Interne Italiane; CNR Progetto Finalizzato Promozione della qualità dell’ambiente: Verona, Italy; pp. 1977–1985.
- Andersen, T.; Cranston, P.S.; Epler, J.H. (Eds.) Chironomidae of the Holarctic Region: Keys and Diagnoses; Part 1—Larvae; Series Insects Systematics and Evolution Supplement 66; Scandinavian Entomology LTD: Lund, Sweden, 2013. [Google Scholar]
- Wiederholm, T. (Ed.) Chironomidae of the Holartic region Keys and Diagnoses Part II: Pupae; Entomologica Scandinavica; Btj Datfailm: Lund, Sweden, 1986. [Google Scholar]
- Timm, T. A Guide to the Freshwater Oligochaeta and Polychaeta of Northern and Central Europe. Lauterbornia 2009, 66, 1–235. [Google Scholar]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Clarke, K.R.; Warwick, M.R. A taxonomic distinctness index and its statistical properties. J. Appl. Ecol. 1998, 35, 523–531. [Google Scholar] [CrossRef]
- Oksanen, J.; Friendly, F.G.B.M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; et al. Vegan: Community Ecology Package, R Package Version 2.5-6. 2019. Available online: https://CRAN.R-Project.Org/Package=vegan (accessed on 7 July 2023).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- De Cáceres, M.; Legendre, P. Associations between Species and Groups of Sites: Indices and Statistical Inference. Ecology 2009, 90, 3566–3574. [Google Scholar] [CrossRef]
- Dufrěne, M.; Legendre, P. Species Assemblages and Indicator Species: The Need for a Flexible Asymmetrical Approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- Tukey, J.W. Exploratory Data Analysis. In The Concise Encyclopedia of Statistics; Springer: New York, NY, USA, 1977. [Google Scholar] [CrossRef]
- Rogora, M.; Colombo, L.; Lepori, F.; Marchetto, A.; Steingruber, S.; Tornimbeni, O. Thirty years of chemical changes in alpine acid-sensitive lakes in the Alps. Wat. Air Soil Poll. 2013, 224, 1746. [Google Scholar] [CrossRef]
- Poma, G.; Salerno, F.; Roscioli, C.; Novati, S.; Guzzella, L. Persistent organic pollutants in sediments of high-altitude Alpine ponds within Stelvio National Park, Italian Alps. Inland Waters 2017, 7, 34–44. [Google Scholar] [CrossRef]
- Fott, J. (Ed.) Limnology of mountain lakes. In Developments in Hydrobiology; Springer: Dordrecht, The Netherlands, 1994; Volume 93. [Google Scholar]
- Mosquera, P.V.; Hampel, H.; Vazquez, R.F.; Alonso, M.; Catalan, J. Abundance and morphometry changes across the high-mountain lake-size gradient in the tropical Andes of Southern Ecuador. Water Resour. Res. 2017, 53, 7269–7280. [Google Scholar] [CrossRef]
- Seekell, D.A.; Pace, M.L.; Tranvik, L.J.; Verpoorter, C. A fractal-based approach to lake size-distributions. Geophys. Res. Lett. 2013, 40, 517–521. [Google Scholar] [CrossRef]
- Kamenik, C.; Schmidt, R.; Kum, G.; Psenner, R. The Influence of Catchment Characteristics on the Water Chemistry of Mountain Lakes. Arct Antarct Alp. Res. 2001, 33, 404–409. [Google Scholar] [CrossRef]
- Boix, D.; Sala, J.; Moreno-Amich, R. The faunal composition of Espolla Pond (NE Iberian Peninsula): The neglected biodiversity of temporary waters. Wetlands 2001, 21, 577–592. [Google Scholar] [CrossRef]
- Sahuquillo, M.; Poquet, J.M.; Rueda, J.; Miracle, M.R. Macroinvertebrate communities in sediment and plants in coastal Mediterranean water bodies (central Iberian Peninsula). Ann. Limnol. Int. J. Lim. 2007, 43, 11–30. [Google Scholar] [CrossRef]
- Oertli, B.; Indermuehle, N.; Angélibert, S.; Hinden, H.; Stoll, A. Macroinvertebrate assemblages in 25 high Alpine ponds of the Swiss National Park (Cirque of Macun) and relation to environmental variables. Hydrobiologia 2008, 597, 29–41. [Google Scholar] [CrossRef]
- Céréghino, R.; Oertli, B.; Bazzanti, M.; Coccia, C.; Compin, A.; Biggs, J.; Bressi, N.; Grillas, P.; Hull, A.; Kalettka, T.; et al. Biological traits of European pond macroinvertebrates. Hydrobiologia 2012, 689, 51–61. [Google Scholar] [CrossRef]
- Guareschi, S.; Gutiérrez-Cánovas, C.; Picazo, F.; Sánchez-Fernández, D.; Abellán, P.; Velasco, J.; Millán, A. Aquatic Macroinvertebrate Biodiversity: Patterns and Surrogates in Mountainous Spanish National Parks. Aquat. Conserv. 2012, 22, 598–615. [Google Scholar] [CrossRef]
- Novikmec, M.; Veselská, M.; Bitušík, P.; Hamerlík, L.; Matúšová, Z.; Klementová, B.R.; Svitok, M. Checklist of benthic macroinvertebrates of high altitude ponds of the Tatra Mountains (Central Europe) with new records of two species for Slovakia. Check List 2015, 11, 1522. [Google Scholar] [CrossRef]
- Hamerlík, L.; Svitok, M.; Novikmec, M.; Veselská, M.; Bitušík, P. Weak altitudinal pattern of overall chironomid richness is a result of contrasting trends of subfamilies in high-altitude ponds. Hydrobiologia 2017, 793, 67–81. [Google Scholar] [CrossRef]
- Hamerlík, L.; Bitušík, P. The Distribution of Littoral Chironomids along an Altitudinal Gradient in High Tatra Mountain Lakes: Could They Be Used as Indicators of Climate Change? Ann. Limnol. Int. J. Lim. 2009, 45, 145–156. [Google Scholar] [CrossRef]
- Sağlam, N.; Dörücü, M. Observations on the Ecology of the Freshwater Leech Helobdella stagnalis (Hirudinoidea: Glossiphoniidae), New for Turkey. Zool. Middle East 2002, 25, 115–120. [Google Scholar] [CrossRef]
- Boggero, A.; Lencioni, V. Macroinvertebrates assemblages of high altitude lakes, inlets and outlets in the southern. Alps. Arch. Hydrobiol. 2006, 165, 37–61. [Google Scholar] [CrossRef]
- Kelly-Quinn, M.; Bracken, J.J. Ephemeropteran assemblages in Ireland. Ver. Int. Ver. Theoret. Angew. Limnol. 2000, 27, 963–969. [Google Scholar] [CrossRef]
- Oertli, B.; Joye, D.A.; Castella, E.; Juge, R.; Cambin, D.; Lachavanne, J.B. Does pond size matter? The relationship between pond area and biodiversity. Biol. Conserv. 2002, 104, 59–70. [Google Scholar] [CrossRef]
- Schenková, J.; Kroča, J. Seasonal changes of an oligochaetous Clitellata (Annelida) community in a mountain stream. Acta Univ. Carol. Environ. 2007, 21, 143–150. [Google Scholar]
- Di Chiara Paoletti, A.; Sambugar, B. Aquatic Oligochaeta in Italy, with special reference to Naididae. Hydrobiologia 1996, 334, 37–49. [Google Scholar] [CrossRef]
- Schniebs, K.; Glöer, P.; Vinarski, M.V.; Hundsdoerfer, A.K. Intraspecific morphological and genetic variability in the European freshwater snail Radix labiata (Rossmaessler, 1835) (Gastropoda: Basommatophora: Lymnaeidae). Contrib. Zool. 2013, 82, 55–68. [Google Scholar] [CrossRef]
- Mackie, G.L. Biology of Freshwater Corbiculid and Sphaeriid Clams of North America; Bulletin Ohio Biological Survey: Columbus, OH, USA, 2007; Volume 15. [Google Scholar]
- Ilg, C.; Oertli, B. How Can We Conserve Cold Stenotherm Communities In Warming Alpine Ponds? Hydrobiologia 2014, 723, 53–62. [Google Scholar] [CrossRef]
- Reiss, F. Verbreitung lakustriner Chironomiden (Diptera) des Alpengebiets. Ann. Zool. Fenn. 1968, 5, 119–125. [Google Scholar]
- Tockner, K.; Malard, F.; Burgherr, P.; Robinson, C.T.; Uehlinger, U.; Zah, R.; Ward, J.V. Physico-chemical characterization of channel types in a glacial floodplain ecosystem (Val Roseg, Switzerland). Arch. Hydrobiol. 1997, 140, 433–463. [Google Scholar] [CrossRef]
- Füreder, L.; Schütz, C.; Burger, R.; Wallinger, M. Seasonal Abundance and Community Structure of Chironomidae in Two Contrasting High Alpine Streams. Verh. Int. Ver. Limnol. 2000, 27, 1597–1601. [Google Scholar] [CrossRef]
- Brittain, J.E.; Milner, A.M. (Eds.) Glacier-fed rivers—Unique lotic ecosystems. Freshw. Biol. 2001, 46, 1571–1874. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boggero, A.; Zaupa, S.; Cesarini, G.; Ruocco, M.; Ansaloni, I.; Prevedelli, D.; Fornaroli, R. Macroinvertebrate Spatial Diversity Patterns of Shore Habitats in Italian High-Altitude Natural and Permanent Lakes and Ponds. Water 2023, 15, 3814. https://doi.org/10.3390/w15213814
Boggero A, Zaupa S, Cesarini G, Ruocco M, Ansaloni I, Prevedelli D, Fornaroli R. Macroinvertebrate Spatial Diversity Patterns of Shore Habitats in Italian High-Altitude Natural and Permanent Lakes and Ponds. Water. 2023; 15(21):3814. https://doi.org/10.3390/w15213814
Chicago/Turabian StyleBoggero, Angela, Silvia Zaupa, Giulia Cesarini, Matteo Ruocco, Ivano Ansaloni, Daniela Prevedelli, and Riccardo Fornaroli. 2023. "Macroinvertebrate Spatial Diversity Patterns of Shore Habitats in Italian High-Altitude Natural and Permanent Lakes and Ponds" Water 15, no. 21: 3814. https://doi.org/10.3390/w15213814
APA StyleBoggero, A., Zaupa, S., Cesarini, G., Ruocco, M., Ansaloni, I., Prevedelli, D., & Fornaroli, R. (2023). Macroinvertebrate Spatial Diversity Patterns of Shore Habitats in Italian High-Altitude Natural and Permanent Lakes and Ponds. Water, 15(21), 3814. https://doi.org/10.3390/w15213814








