Microbiological and Physicochemical Quality of Groundwater and Risk Factors for Its Pollution in Ouagadougou, Burkina Faso
Abstract
:1. Introduction
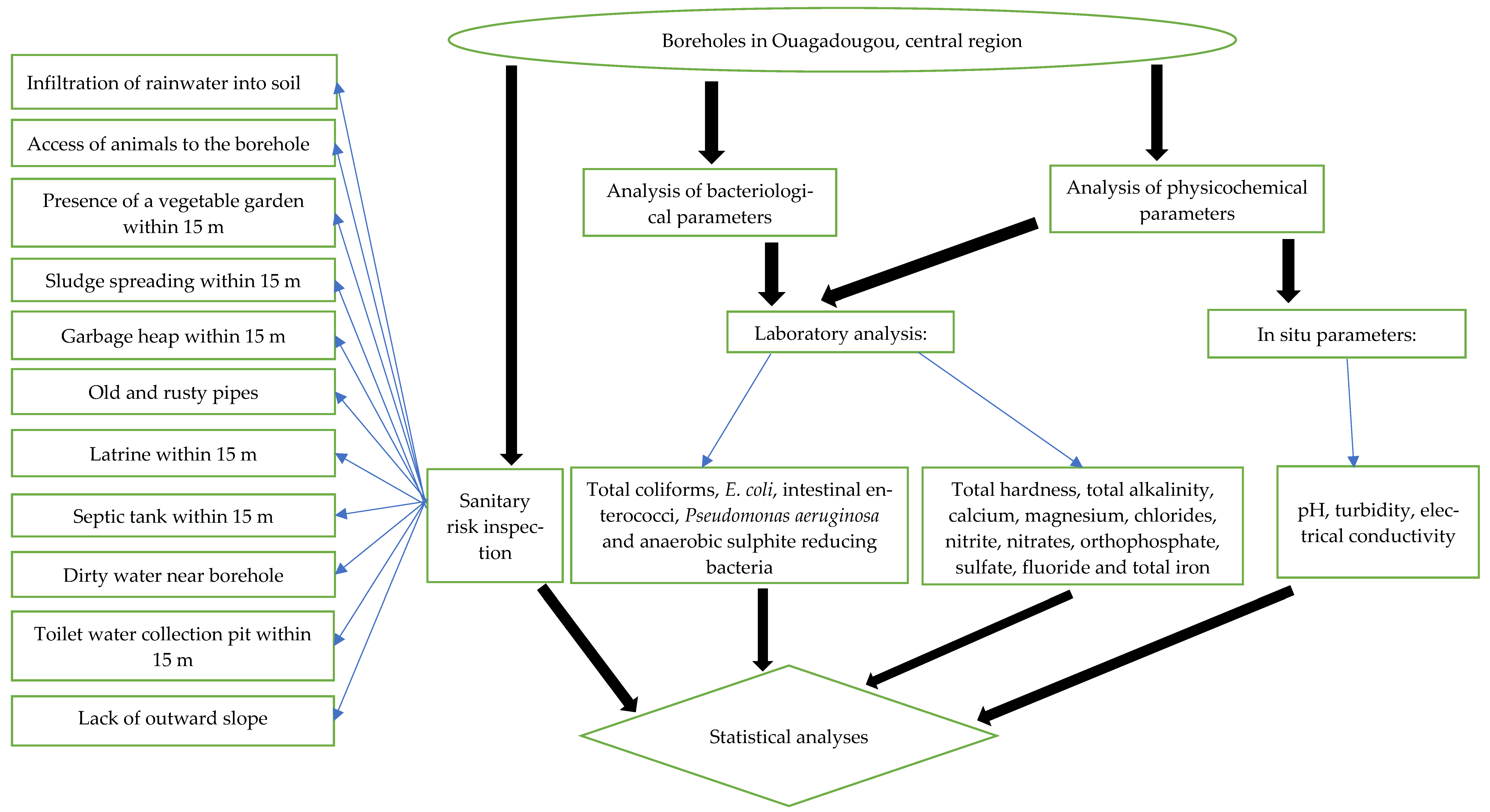
2. Materials and Methods
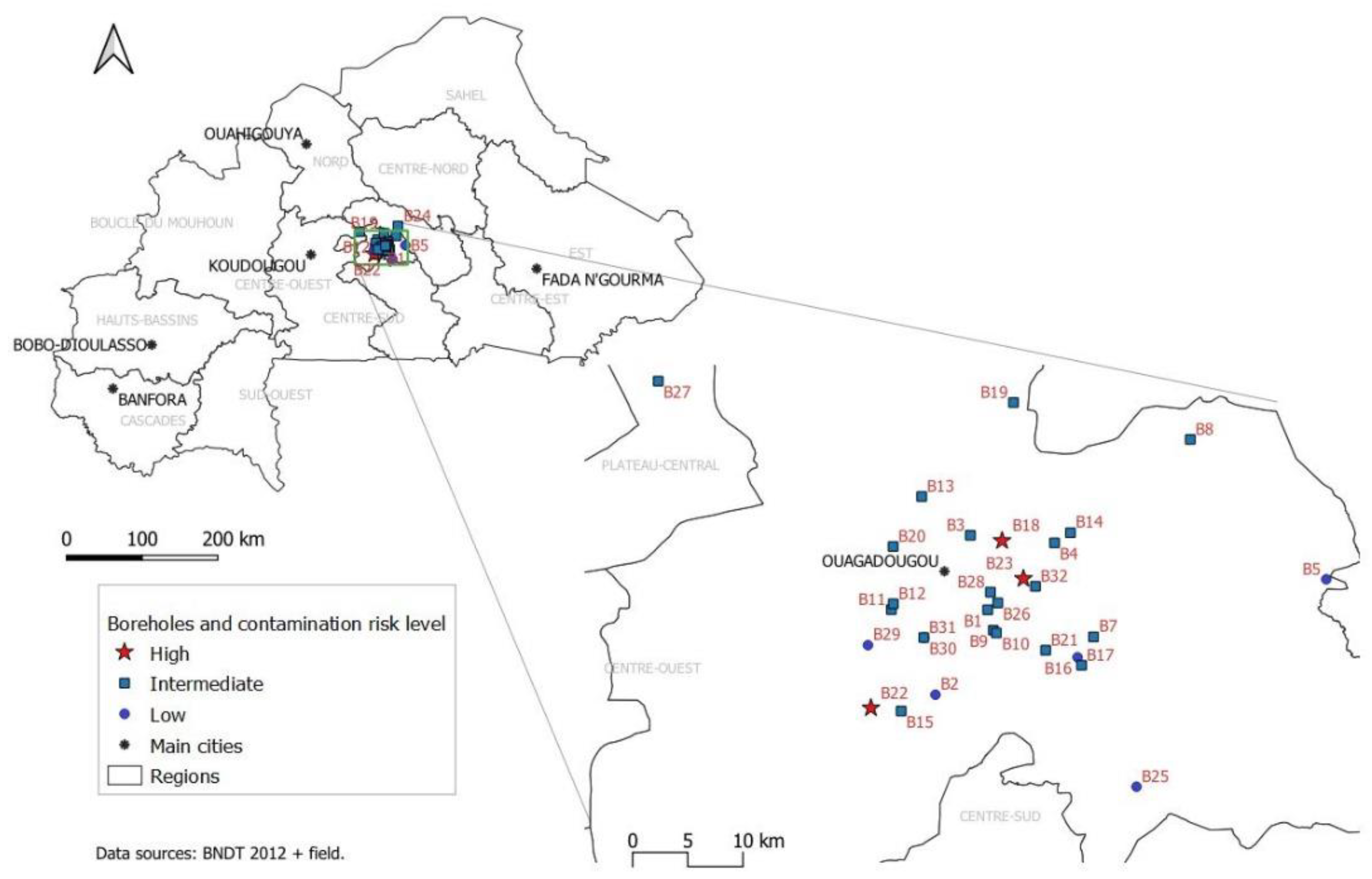
2.1. Study Area and Design
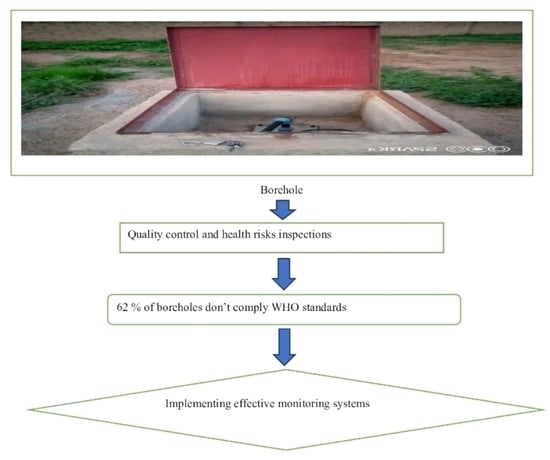
2.2. Sanitary Risk Inspection
2.3. Water Sample Collection
2.4. Microbiological Analyses
2.5. Physicochemical Analyses
2.6. Statistical Analyses
3. Results
3.1. Sanitary Inspection Results
Microbiological Quality of Water Samples
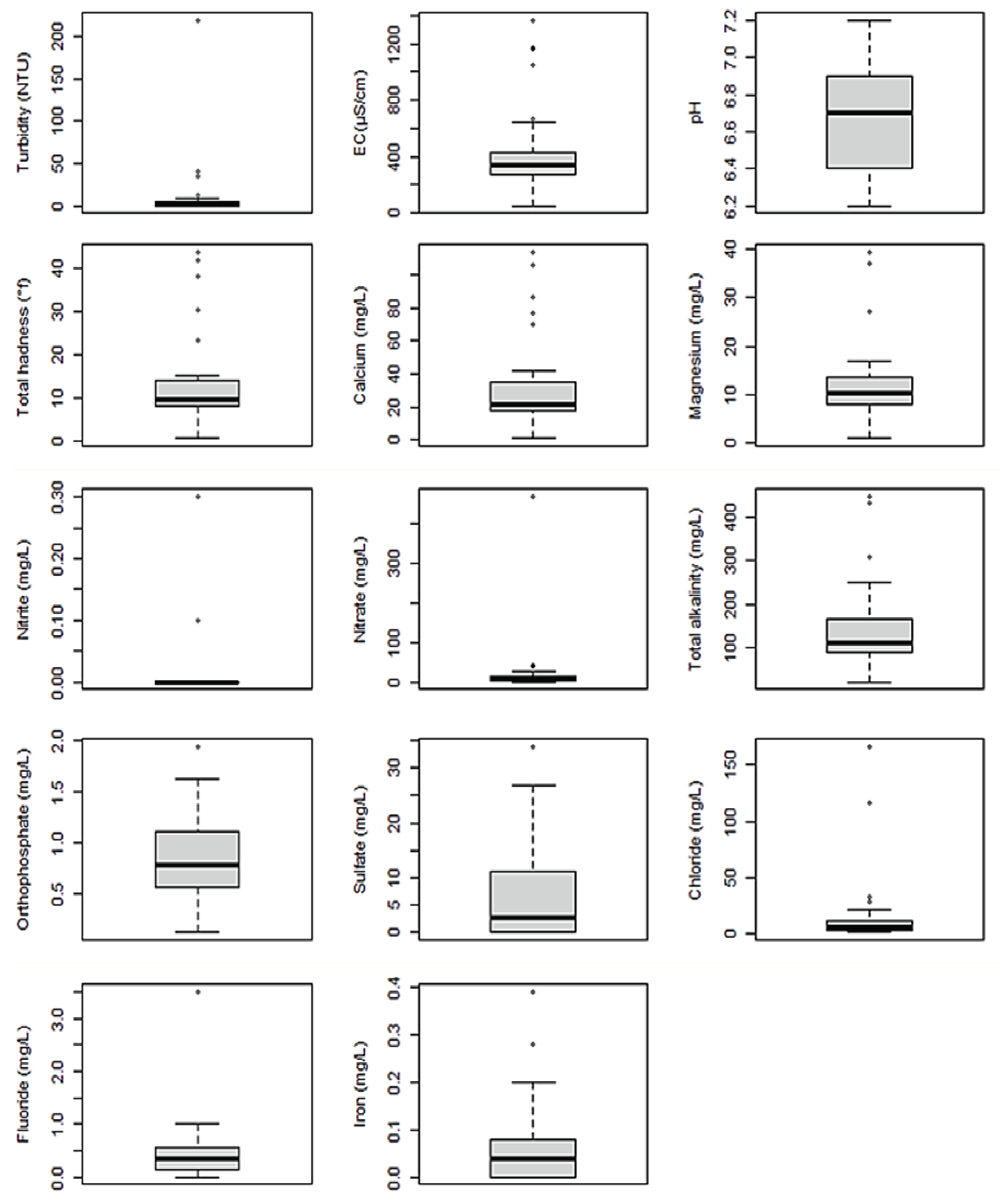
3.2. Physicochemical Quality of Water Samples
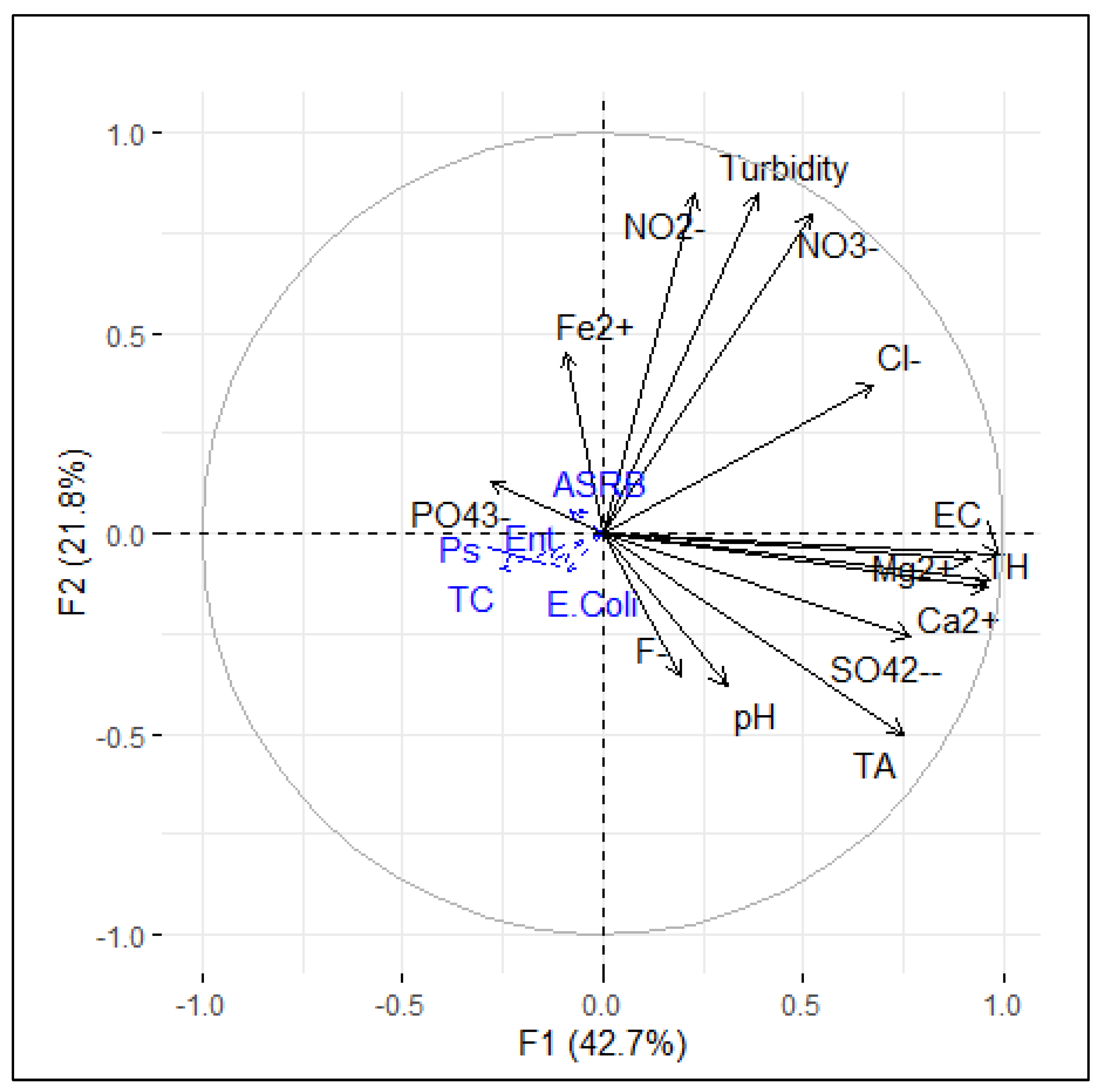
3.3. Overall Water Quality
3.4. Risk Factors for Borehole Contamination
4. Discussion
5. Implications
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- United Nations. The Sustainable Development Goals Report; United Nations: Geneva, Switzerland, 2022; p. 68. [Google Scholar]
- Akoachere, J.T.K.A.; Omam, L.; Massalla, T.N. Assessment of the relationship between bacteriological quality of dug-wells, hygiene behaviour and well characteristics in two cholera endemic localities in Douala, Cameroon. BMC Public Health 2013, 13, 692–703. [Google Scholar] [CrossRef]
- Baye, S.; Eshetie, M.; Denekew, T. Bacteriological and physicochemical quality of drinking water in Wegeda Town, Northwest Ethiopia. J. Environ. Public Health 2021, 2021, 6646269. [Google Scholar] [CrossRef]
- Prüss-Ustün, A.; Bartram, J.; Clasen, T.; Colford, J.M., Jr.; Cumming, O.; Curtis, V.; Bonjour, V.; Dangour, A.D.; De France, J.; Fewtrell, L.; et al. Burden of disease from inadequate water, sanitation and hygiene in low- and middle-income settings: A retrospective analysis of data from 145 countries. Trop. Med. Int. Health 2014, 19, 894–905. [Google Scholar] [CrossRef]
- MacDonald, A.M.; Bonsor, H.C.; O’Dochartaigh, B.E.O.; Taylor, R.G. Quantitative maps of groundwater resources in Africa. Environ. Res. Lett. 2012, 7, 024009. [Google Scholar] [CrossRef]
- Lapworth, D.J.; MacDonald, A.M.; Tijani, M.N.; Darling, W.G.; Gooddy, D.C.; Bonsor, H.C.; Araguás-Araguás, L.J. Residence times of shallow groundwater in West Africa: Implications for hydrogeology and resilience to future changes in climate. Hydrogeol. J. 2013, 21, 673–686. [Google Scholar] [CrossRef]
- Lapworth, D.; Boving, T.; Brauns, B.; Dottridge, J.; Hynds, P.; Kebede, S.; Kreamer, D.; Misstear, B.; Mukherjee, A.; Re, V.; et al. Groundwater quality: Global challenges, emerging threats and novel approaches. Hydrogeol. J. 2023, 31, 15–18. [Google Scholar] [CrossRef]
- Rao, N.S. Seasonal variation of groundwater quality in a part of Guntur District, Andhra Pradesh, India. Environ. Geol. 2006, 2006, 413–429. [Google Scholar] [CrossRef]
- Motlagh, A.M.; Yang, Z.; Saba, H. Groundwater quality. Water Environ. Res. 2020, 92, 1649–1658. [Google Scholar] [CrossRef]
- Conboy, M.; Goss, M. Natural protection of groundwater against bacteria of fecal origin. J. Contam. Hydrol. 2000, 43, 1–24. [Google Scholar] [CrossRef]
- Howard, G.; Pedley, S.; Barrett, M.; Nalubega, M.; Johal, K. Risk factors contributing to microbiological contamination of shallow groundwater in Kampala, Uganda. Water Res. 2003, 37, 3421–3429. [Google Scholar] [CrossRef]
- Foppen, J.W.A.; van Herwerden, M.; Kebtie, M.; Noman, A.; Schijven, J.F.; Stuyfzand, P.J.; Uhlenbrook, S. Transport of Escherichia coli and solutes during waste water infiltration in an urban alluvial aquifer. J. Contam. Hydrol. 2008, 95, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, B.; Abdelhak, M.; Abdeslam, K.; Ahmed, M.; Brahim, Z. Estimation of pollution load of domestic sewage to Oued Bechar (SW Algeria) and its impact on the microbiological quality of groundwater. Procedia Eng. 2012, 33, 261–267. [Google Scholar] [CrossRef]
- Odiyo, J.O.; Makungo, R. Chemical and microbial quality of groundwater in Siloam village, implications to human health and sources of contamination. Int. J. Environ. Res. Public Health 2018, 15, 317. [Google Scholar] [CrossRef] [PubMed]
- DWA. Groundwater Strategy; Department of Water Affairs: Pretoria, South Africa, 2010; p. 64. [Google Scholar]
- Raphael, O.; John, O.O.; Sandra, U.I.; Sunday, A.C. Assessment of borehole water quality consumed in Otukpo and its environs. Int. J. Ecol. Sci. Environ. Eng. 2018, 5, 71–78. [Google Scholar]
- Adamou, H.; Ibrahim, B.; Salack, S.; Adamou, R.; Sanfo, S.; Liersch, S. Physico-chemical and bacteriological quality of groundwater in a rural area of Western Niger: A case study of Bonkoukou. J. Water Health 2020, 18, 77–90. [Google Scholar] [CrossRef]
- Bretzler, A.; Lalanne, F.; Nikiema, J.; Podgorski, J.; Pfenninger, N.; Berg, M.; Schirmer, M. Groundwater arsenic contamination in Burkina Faso, West Africa: Predicting and verifying regions at risk. Sci. Total Environ. 2017, 584–585, 958–970. [Google Scholar] [CrossRef]
- Sako, A.; Ouangaré, C.A.C. Hydrogeochemical characterization and natural background level determination of selected inorganic substances in groundwater from a semi-confined aquifer in Midwestern Burkina Faso, West Africa. Environ. Monit. Assess. 2023, 195, 519–545. [Google Scholar] [CrossRef]
- Faye, M.D.; Kafando, M.B.; Sawadogo, B.; Panga, R.; Ouédraogo, S.; Yacouba, H. Groundwater Characteristics and Quality in the Cascades Region of Burkina Faso. Resources 2022, 11, 61. [Google Scholar] [CrossRef]
- Lewis, W.J.; Foster, S.S.D.; Drasar, B.S. The Risk of Groundwater Pollution by On-Site Sanitation in Developing Countries; International Reference Centre for Waste Disposal: Duebendorf, Switzerland, 1982; p. 79. [Google Scholar]
- Graham, J.P.; Matthew, L.P. Pit latrines and their impacts on groundwater quality: A systematic review. Environ. Health Perspect. 2013, 121, 521–530. [Google Scholar] [CrossRef]
- Schijven, J.F.; Hassanizadeh, S.M.; Husman, A.M.D. Vulnerability of unconfined aquifers to virus contamination. Water Res. 2010, 44, 1170–1181. [Google Scholar] [CrossRef] [PubMed]
- Rodier, J.; Legube, B. L’analyse de L’eau, Contrôle et Interprétation, 10th ed.; Dunod: Paris, France, 2016; p. 1759. [Google Scholar]
- Castaing, C.; Billa, M.; Milesi, J.P.; Thieblemont, D.; Le Metour, J.; Egal, E.; Donzeau, M.; Guerrot, C.; Cocherie, A.; Chevremont, P.; et al. Notice Explicative de la Carte Géologique et Minière à 1/1,000,000 du Burkina Faso; Bureau de Recherches Géologiques et Minières: Orléans, France, 2003; p. 148. [Google Scholar]
- Lutterodt, G.; Van de Vossenberg, J.; Hoiting, Y.; Kamara, A.K.; Oduro-Kwarteng, S.; Foppen, J.W.A. Microbial ground water quality status of hand-dug well and boreholes in the Dodowa area of Ghana. Int. J. Environ. Res. Public Health 2018, 15, 730. [Google Scholar] [CrossRef]
- APHA; AWWA; WEF. Standard Methods for Examination of Water and Wastewater, 2nd ed.; American Public Health Association: Washington, DC, USA, 2012; p. 1360. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 30 June 2022).
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Solymos, P.; Stevens, M.; Szoecs, E.; et al. Vegan: Community Ecology 2022, Package. R Package Version 2.6-4. Available online: https://CRAN.R-project.org/package=vegan (accessed on 30 June 2022).
- Edberg, S.C.; Rice, E.W.; Karlin, R.J.; Allen, M.J. Escherichia coli: The best biological drinking water indicator for public health protection. J. Appl. Microbiol. 2000, 88, 106–116. [Google Scholar] [CrossRef]
- Taonameso, S.; Mudau, L.S.; Traoré, A.N.; Potgetier, N. Borehole water: A potential health risk to rural communities in South Africa. Water Supply 2019, 19, 128–136. [Google Scholar] [CrossRef]
- Pantaleo, P.A.; Komakech, H.C.; Mtei, K.M.; Njau, K.N. Contamination of groundwater sources in emerging African towns: The case of Babati town, Tanzania. Water Pract. Technol. 2018, 13, 980–990. [Google Scholar] [CrossRef]
- Rice, E.W.; Covert, T.C.; Wild, D.K.; Berman, D.; Johnson, S.A.; Johnson, C.H. Comparative resistance of Escherichia coli and enterococci to chlorination. J. Environ. Sci. Health A 1996, 28, 89–97. [Google Scholar]
- Kurwadkar, S.; Kanel, S.R.; Nakarmi, A. Groundwater pollution: Occurrence, detection, and remediation of organic and inorganic pollutants. Water Environ. Res. 2020, 92, 659–1668. [Google Scholar] [CrossRef]
- Ouedraogo, I.; Defourny, P.; Vanclooster, M. Mapping the groundwater vulnerability for pollution at the pan African scale. Sci. Total Environ. 2016, 544, 939–953. [Google Scholar] [CrossRef] [PubMed]
- Lapworth, D.J.; Nkhuwa, D.W.C.; Okotto-Okotto, J.; Pedley, S.; Stuart, M.E.; Tijani, M.N.; Wrigh, J. Urban groundwater quality in sub-Saharan Africa: Current status and implications for water security and public health. Hydrogeol. J. 2017, 25, 1093–1116. [Google Scholar] [CrossRef]
- Sharma, B.; Parul; Verma, A.K.; Jain, U.; Yadav, J.K.; Singh, R.; Mishra, R. Occurrence of multidrug resistant Escherichia coli in groundwater of Brij region (UP) and its public health implications. Vet. World 2017, 10, 293–301. [Google Scholar] [CrossRef]
- Razafitsiferana, T.; Bruno, R.; Mihasina, R.; Mahandrimanana, A. Analysis of the Physico-Chemical Parameters of the Water of the River of Mahavavy Situated in the District of Ambilobe, in the Region of Diana (DIEGO SUEREZ). Resour. Environ. 2017, 7, 168–175. [Google Scholar] [CrossRef]
- Atekwana, E.A.; Geyer, C.J. Spatial and temporal variations in the geochemistry of shallow groundwater contaminated with nitrate at a residential site. Environ. Sci. Pollut. Res. Int. 2018, 25, 27155–27172. [Google Scholar] [CrossRef]
- Huan, H.; Hu, L.; Yang, Y.; Jia, Y.; Lian, X.; Ma, X.; Jiang, Y.; Xi, B. Groundwater nitrate pollution risk assessment of the groundwater source field based on the integrated numerical simulations in the unsaturated zone and saturated aquifer. Environ. Int. 2020, 137, 105532–105541. [Google Scholar] [CrossRef] [PubMed]
- World Water Quality Alliance. Assessing Groundwater Quality: A Global Perspective: Importance, Methods and Potential Data Sources; A Report by the Friends of Groundwater in the World Water Quality Alliance. In Proceedings of the 5th Session of the United Nations Environment Assembly, Nairobi, Kenya, 22–23 February 2021. [Google Scholar]
- Goné, D.L.; Savané, I.; Yao, N.A.; Biémi, J. Analysis and Control of the Physicochemical Quality of Groundwater in the Chari Baguirmi Region in Chad. Man. Sci. Nat. 2005, 11, 85–94. [Google Scholar] [CrossRef]
- CEAEQ. Détermination de la Conductivité: Méthode Électrométrique, MA. 115–Cond. 1.1, rév. 1 (Determination of Conductivity: Electrometric Method, MA. 115–Cond. 1.1, rev. 1); Centre d’Expertise en Analyse Environnementale du Québec Ministère du Développement Durable, de l’Environnement et de la Lutte Contre les Changements Climatiques: Québec City, QC, Canada, 2020. [Google Scholar]
- WHO. Guidelines for Drinking-Water Quality, 4th ed; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Bantin, A.B.; Wang, H.; Jun, X. Analysis and control of the physicochemical quality of groundwater in the Chari Baguirmi Region in Chad. Water 2020, 12, 2826. [Google Scholar] [CrossRef]
- Masindi, V.; Foteinis, S. Groundwater contamination in sub-Saharan Africa: Implications for groundwater protection in developing countries. Clean. Ing. Technol. 2021, 2, 100038. [Google Scholar] [CrossRef]
- Sorensen, J.; Lapworth, D.; Nkhuwa, D.; Stuart, M.; Gooddy, D.; Bell, R.; Chirwa, M.; Kabika, J.; Liemisa, M.; Chibesa, M.; et al. Emerging contaminants in urban groundwater Sources in Africa. Water Res. 2014, 72, 51–63. [Google Scholar] [CrossRef]
- Nyenje, P.; Foppen, J.M.; Kulabako, R.; Muwanga, A.; Uhlenbrook, S. Nutrient pollution in shallow aquifers underlying pit latrines and domestic solid waste dumps in urban slums. J. Environ. Manag. 2013, 122, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.J.; Shimizu, Y.; He, K.; Sulaiman, N.M.N. Comparison among different ASEAN water quality indices for the assessment of the spatial variation of surface water quality in the Selangor river basin, Malaysia. Environ. Monit. Assess. 2020, 192, 644–659. [Google Scholar] [CrossRef]
- Wong, Y.J.; Shimizu, Y.; Kamiya, A.; Maneechot, L.; Bharambe, K.P.; Fong, C.S.; Sulaiman, N.M.N. Application of artificial intelligence methods for monsoonal river classification in Selangor river basin, Malaysia. Environ. Monit. Assess. 2021, 193, 438–459. [Google Scholar] [CrossRef]
| Risk Factors for Borehole Water | Borehole Code | Number of Boreholes (%) |
|---|---|---|
| Infiltration of rain water into soil (IRS) | B04, B11, B15, B19, B21, B22, B23, B24, B26, B27, B28, B31, B32 | 13 (40.6) |
| Access of animals to borehole (AAB) | B03, B12, B13, B18, B22, B23, B29 | 7 (21.9) |
| Presence of vegetables garden within 15 m (PVG) | B04, B09, B10, B14 | 4 (12.5) |
| Sludge spreading within 15 m (SS) | B01, B18, B20 | 3 (9.4) |
| Garbage heap within 15 m (GH) | B03, B06, B07, B08, B12, B17, B18, B21, B22, B23, B24 | 11 (34.4) |
| Old and rusty pipe (POR) | B08, B11, B12, B19, B20, B22, B23 | 7 (21.9) |
| Latrine within 15 m (L) | B01, B02, B03, B05, B06, B07, B08, B12, B13, B16, B17, B18, B20, B22, B24, B26, B31, B32 | 18 (56.3) |
| Septic tank within 15 m (ST) | B04, B09, B10, B11, B14, B15, B19, B21, B23, B25, B27, B28, B29, B32 | 14 (43.8) |
| Dirty water near borehole (DWB) | B01, B02, B03, B06, B07, B08, B12, B13, B14, B17, B18, B20, B21, B22, B23, B24, B26 | 17 (53.1) |
| Toilet water collection pit within 15 m (TWCP) | None | 0 (0) |
| Lack of outward slope (LOS) | B01, B03, B04, B06, B07, B08, B09, B10, B13, B14, B15, B17, B18, B19, B20, B21, B22, B23, B24, B25, B26, B27, B28, B31, B32 | 25 (78.1) |
| Parameters | Microbiological Parameters | Physicochemical Parameters | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total Coliforms | EC | IE | PA | ASRBS | pH | Turbidity | Fluoride | Iron | Nitrate | |
| Units | CFU/100 mL | CFU/100 mL | CFU/100 mL | CFU/100 mL | CFU/100 mL | - | NTU | mg/L | mg/L | mg/L |
| Values | B01 (89), B02 (>100), B04 (14), B05 (>100), B06 (8), B07 (14), B08 (>100), B09 (10), B10 (9), B13 (92), B14 (68), B17 (66), B18 (88), B20 (56), B21 (57), B22 (69), B23 (87), B24 (6), B26 (69) | B01 (16), B08 (3), B13 (33), B14 (5), B17 (3), B18 (18), B20 (16), B21 (16), B22 (16), B23 (16), B26 (20) | B18 (3), B26 (1) | B03 (>100), B06 (75), B14 (66), B17 (56) | B18 (12) | B03 (6.2), B04 (6.4), B07 (6.4), B08 (6.3), B09 (6.4), B14 (6.3), B15 (6.3), B16 (6.4), B20 (6.4), B22 (6.4) | B08 (6.02), B12 (218), B14 (9.1), B18 (13.2), B20 (34.4), B22 (34.4), B24 (41) B32 (12.2) | B06 (3.5) | B08 (0.39) | B12 (469.9) |
| Total (%) | 19 (59.4) | 11(34.4) | 2 (6.3) | 4 (12.5) | 1 (3.1) | 10 (31.3) | 7 (21.9) | 1 (3.1) | 1 (3.1) | 1 (3.1) |
| WHO standards | 0 | 0 | 0 | 0 | 0 | 6.5–8.5 | 5 | ≤1.5 | ≤0.3 | ≤50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Traoré, O.; Kpoda, D.S.; Dembélé, R.; Saba, C.K.S.; Cairns, J.; Barro, N.; Haukka, K. Microbiological and Physicochemical Quality of Groundwater and Risk Factors for Its Pollution in Ouagadougou, Burkina Faso. Water 2023, 15, 3734. https://doi.org/10.3390/w15213734
Traoré O, Kpoda DS, Dembélé R, Saba CKS, Cairns J, Barro N, Haukka K. Microbiological and Physicochemical Quality of Groundwater and Risk Factors for Its Pollution in Ouagadougou, Burkina Faso. Water. 2023; 15(21):3734. https://doi.org/10.3390/w15213734
Chicago/Turabian StyleTraoré, Oumar, Dissinviel Stéphane Kpoda, René Dembélé, Courage Kosi Setsoafia Saba, Johannes Cairns, Nicolas Barro, and Kaisa Haukka. 2023. "Microbiological and Physicochemical Quality of Groundwater and Risk Factors for Its Pollution in Ouagadougou, Burkina Faso" Water 15, no. 21: 3734. https://doi.org/10.3390/w15213734
APA StyleTraoré, O., Kpoda, D. S., Dembélé, R., Saba, C. K. S., Cairns, J., Barro, N., & Haukka, K. (2023). Microbiological and Physicochemical Quality of Groundwater and Risk Factors for Its Pollution in Ouagadougou, Burkina Faso. Water, 15(21), 3734. https://doi.org/10.3390/w15213734