Microplastics in the Syr Darya River Tributaries, Uzbekistan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling
2.3. Laboratory Processing of Samples
2.4. Quantatitative Analysis and Particle Characterization
2.5. Quality Assurance and Quality Control
3. Results
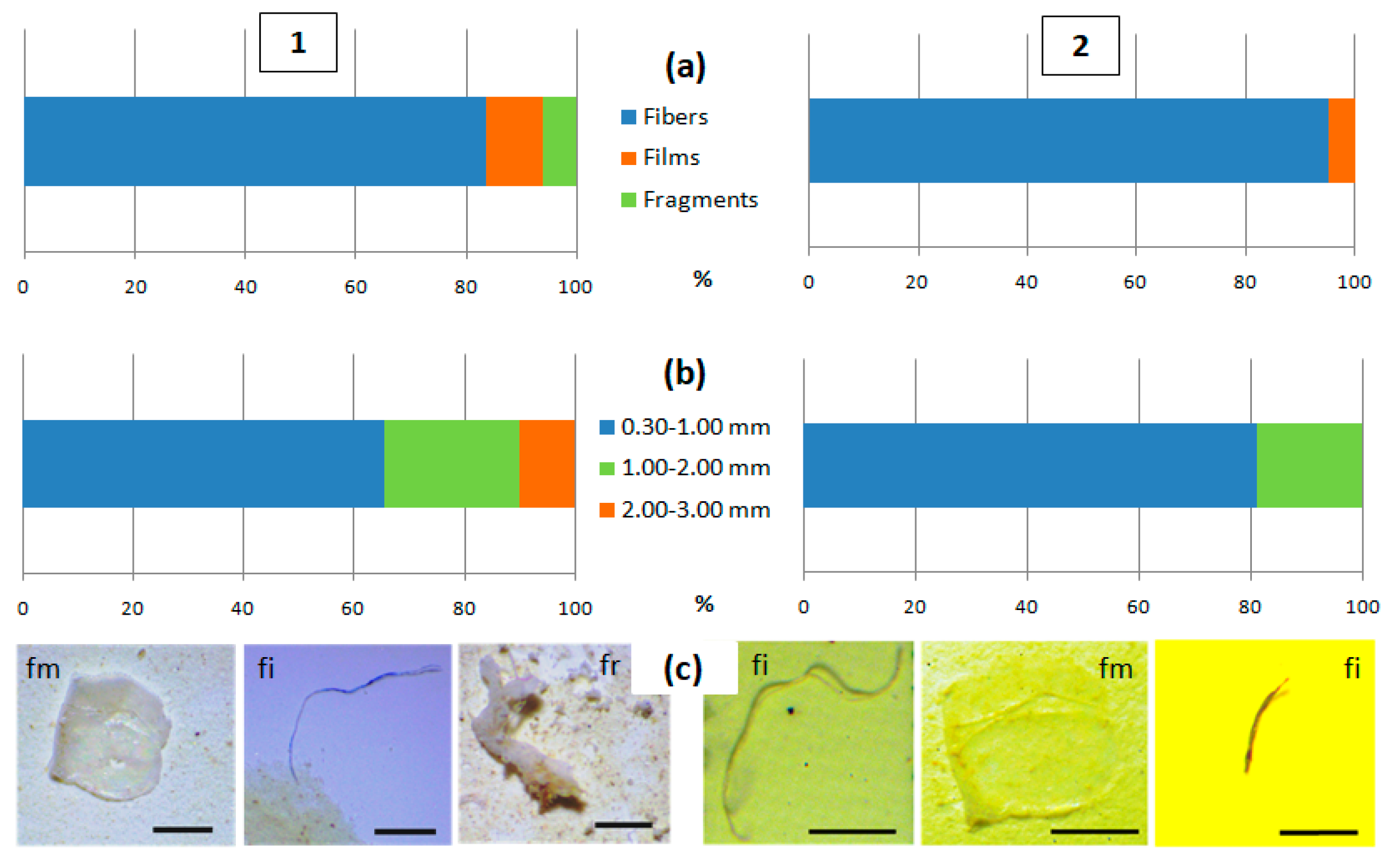
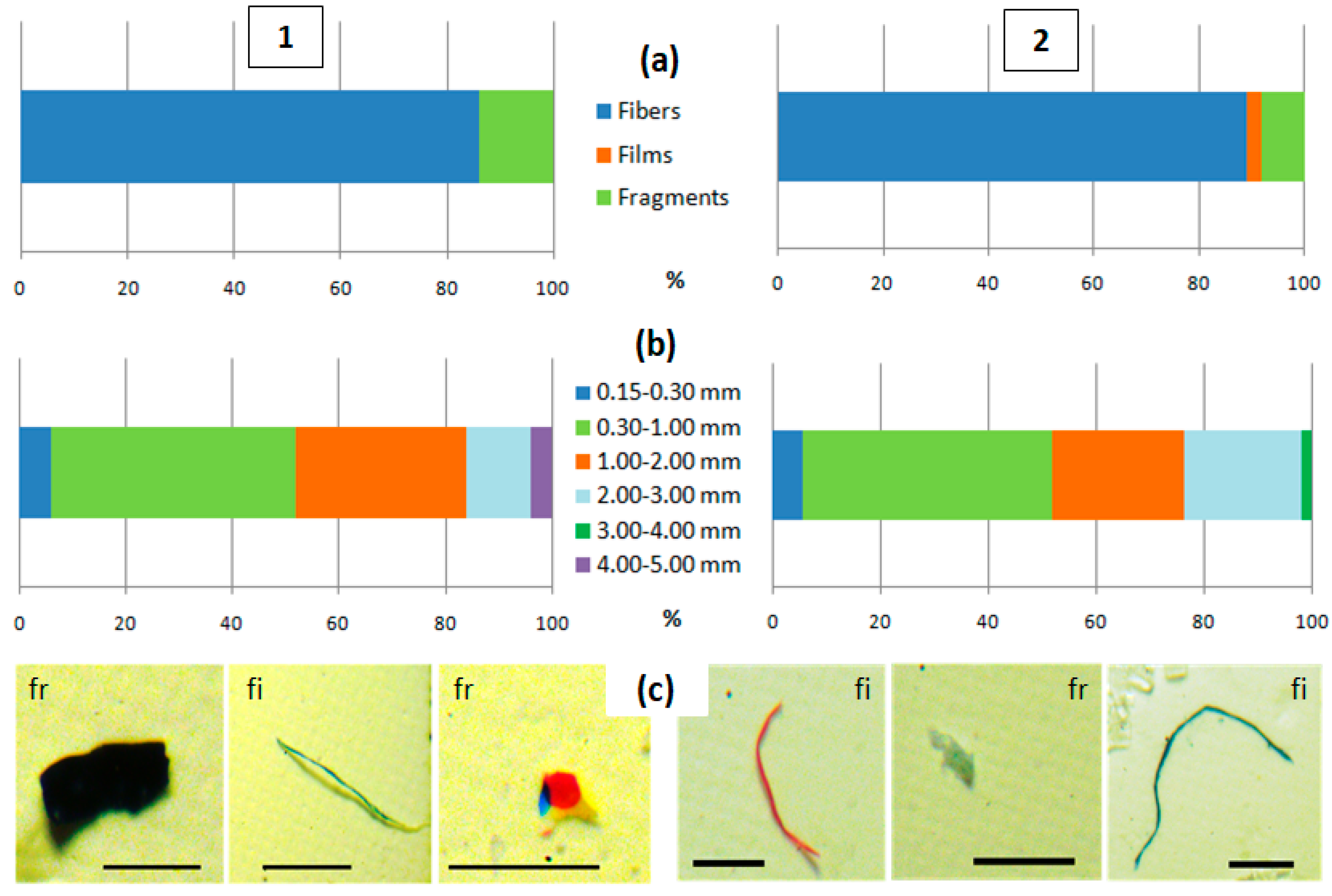
3.1. Quantitative Content and Morphology of MPs in Surface Water of Kara Darya and Chirchiq Rivers
3.2. MP Content in Benthic Sediments of Kara Darya and Chirchiq Rivers and Sediment Characteristics
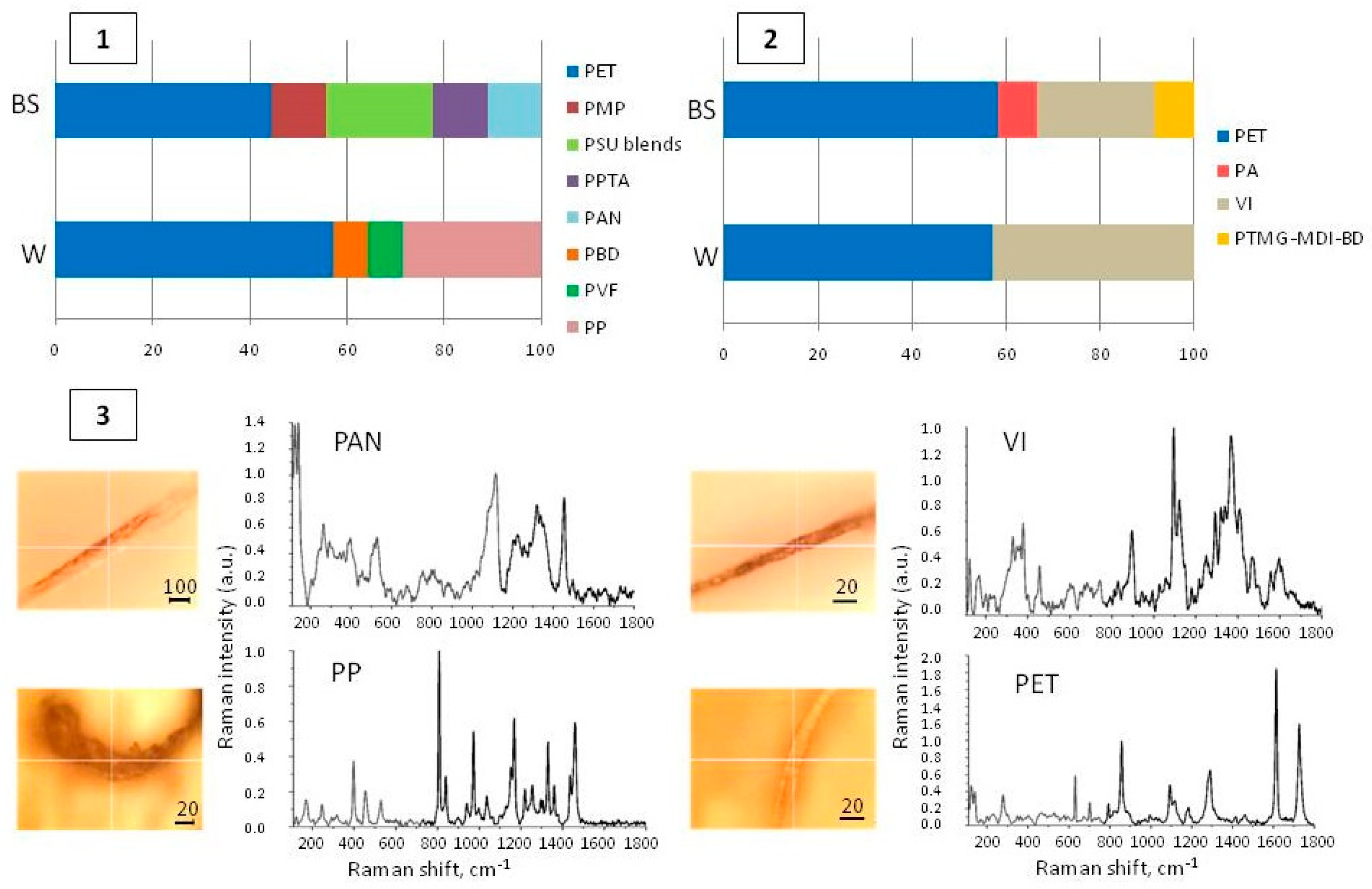
3.3. Polymer Composition of MPs Extracted from Water and Benthic Sediments of the Syr Darya Tributaries
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Horton, A.A. Plastic Pollution: When Do We Know Enough? J. Hazard. Mater. 2022, 422, 126885. [Google Scholar] [CrossRef] [PubMed]
- Versteeg, F.G.; Hegeman, N.C.; Sebakhy, K.O.; Picchioni, F. RAFT Polymerization of a Biorenewable/Sustainable Monomer via a Green Process. Macromol. Rapid Commun. 2022, 43, 2200045. [Google Scholar] [CrossRef] [PubMed]
- Sebakhy, K.O.; Gavrilov, M.; Valade, D.; Jia, Z.; Monteiro, M.J. Nanoparticles of Well-Defined 4-Arm Stars Made Using Nanoreactors in Water. Macromol. Rapid Commun. 2014, 35, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Plastics Europe. Enabling a Sustainable Future. Plastics—The Facts. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2022 (accessed on 20 September 2023).
- Sun, J.; Zhu, Z.R.; Li, W.H.; Yan, X.; Wang, L.K.; Zhang, L.; Jin, J.; Dai, X.; Ni, B.J. Revisiting Microplastics in Landfill Leachate: Unnoticed Tiny Microplastics and Their Fate in Treatment Works. Water Res. 2021, 190, 116784. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Bank, M.S.; Hansson, S.V. The Plastic Cycle: A Novel and Holistic Paradigm for the Anthropocene. Environ. Sci. Technol. 2019, 53, 7177–7179. [Google Scholar] [CrossRef]
- Wright, S.L.; Kelly, F.J. Plastic and Human Health: A Micro Issue? Environ. Sci. Technol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef]
- Lebreton, L.C.M.; Van Der Zwet, J.; Damsteeg, J.W.; Slat, B.; Andrady, A.; Reisser, J. River plastic emissions to the world’s oceans. Nat. Commun. 2017, 8, 15611. [Google Scholar] [CrossRef]
- Kumar, P.; Inamura, Y.; Bao, P.N.; Abeynayaka, A.; Dasgupta, R.; Abeynayaka, H.D.L. Microplastics in Freshwater Environment in Asia: A Systematic Scientific Review. Water 2022, 14, 1737. [Google Scholar] [CrossRef]
- Ziajahromi, S.; Neale, P.A.; Leusch, F.D.L. Wastewater treatment plant effluent as a source of microplastics: Review of the fate, chemical interactions and potential risks to aquatic organisms. Water Sci. Technol. 2016, 74, 2253–2269. [Google Scholar] [CrossRef]
- Periyasamy, A.P.; Tehrani-Bagha, A. A review on microplastic emission from textile materials and its reduction techniques. Polym. Degrad. Stab. 2022, 199, 109901. [Google Scholar] [CrossRef]
- Rochman, C.M.; Kross, S.M.; Armstrong, J.B.; Bogan, M.T.; Darling, E.S.; Green, S.J.; Smyth, A.R.; Veríssimo, D. Scientific Evidence Supports a Ban on Microbeads. Environ. Sci. Technol. 2015, 49, 10759–10761. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Song, B.; Liang, J.; Niu, Q.; Zeng, G.; Shen, M.; Deng, J.; Luo, Y.; Wen, X.; Zhang, Y. Microplastics and associated contaminants in the aquatic environment: A review on their ecotoxicological effects, trophic transfer, and potential impacts to human health. J. Hazard. Mater. 2021, 405, 124187. [Google Scholar] [CrossRef]
- D’Avignon, G.; Gregory-Eaves, I.; Ricciardi, A. Microplastics in lakes and rivers: An issue of emerging significance to limnology. Environ. Rev. 2022, 30, 228–244. [Google Scholar] [CrossRef]
- Chen, H.; Qin, Y.; Huang, H.; Xu, W. A Regional difference analysis of microplastic pollution in global freshwater bodies based on a regression model. Water 2020, 12, 1889. [Google Scholar] [CrossRef]
- AWI-LITTERBASE. Available online: https://litterbase.awi.de (accessed on 20 September 2023).
- UNECE (United Nations Economic Commission for Europe). Second Assessment of Transboundary Rivers, Lakes and Groundwaters; United Nations Publications: Geneva, Switzerland; New York, NY, USA, 2011; p. 428. ISBN 9789210549950. [Google Scholar]
- Shoergashova, S. Microplastic pollution of water bodies. Univers. Tech. Sci. Electron. Sci. J. 2020, 7, 15–17. [Google Scholar]
- Frank, Y.A.; Vorobiev, D.S.; Kayler, O.A.; Vorobiev, E.D.; Kulinicheva, K.S.; Trifonov, A.A.; Soliman Hunter, T. Evidence for microplastics contamination of the remote tributary of the Yenisei River, Siberia—The pilot study results. Water 2021, 13, 3248. [Google Scholar] [CrossRef]
- Masura, J.; Baker, J.; Foster, G.; Arthur, C. Laboratory Methods for the Analysis of Microplastics in the Marine Environment: Recommendations for Quantifying Synthetic Particles in Waters and Sediments; NOAA Technical Memorandum NOS-OR&R-48; NOAA Marine Debris Division: Silver Spring, MD, USA, 2015; p. 39. [CrossRef]
- Sutherland, R.A. Loss-on-ignition estimates of organic matter and relationships to organic carbon in fluvial bed sediments. Hydrobiologia 1998, 389, 153–167. [Google Scholar] [CrossRef]
- ISO 11277:2020; Soil Quality—Determination of Particle in Mineral Soil Material—Method by Sieving and Sedimentation. 3rd ed. Technical Committee ISO/TC 190/SC 3 Chemical and Physical Characterization: Geneva, Switzerland, 2020; p. 38.
- Frias, J.P.G.L.; Nash, R. Microplastics: Finding a consensus on the definition. Mar. Pollut. Bull. 2019, 138, 145–147. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Mohamed Nor, N.H.; Hermsen, E.; Kooi, M.; Mintenig, S.M.; De France, J. Microplastics in freshwaters and drinking water: Critical review and assessment of data quality. Water Res. 2019, 155, 410–422. [Google Scholar] [CrossRef]
- Kachinskyj, N.A. Physics of Soil; Vysshaya Shkola: Moscow, USSR, 1965; p. 323. ISBN 978-5-4458-5455-5. [Google Scholar]
- Yasinskii, S.V.; Venitsianov, E.V.; Kashutina, E.A.; Sidorova, M.V.; Ershova, A.A.; Makeeva, I.N. Contribution of microparticles to the transport of pollution by rivers and groundwaters in a large city. IOP Conf. Ser. Earth Environ. Sci. 2021, 834, 012047. [Google Scholar] [CrossRef]
- Ding, L.; Mao, R.F.; Guo, X.; Yang, X.; Zhang, Q.; Yang, C. Microplastics in surface waters and sediments of the Wei River, in the northwest of China. Sci. Total Environ. 2019, 667, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Simon-Sanchez, L.; Grelaud, M.; Garcia-Orellana, J.; Ziveri, P. River Deltas as hotspots of microplastic accumulation: The case study of the Ebro River (NW Mediterranean). Sci. Total Environ. 2019, 687, 1186–1196. [Google Scholar] [CrossRef] [PubMed]
- Leslie, H.A.; Brandsma, S.H.; Van Velzen, M.J.M.; Vethaak, A.D. Microplastics en route: Field measurements in the Dutch river delta and Amsterdam canals, wastewater treatment plants, North Sea sediments and biota. Environ. Int. 2017, 101, 133–142. [Google Scholar] [CrossRef]
- Zobkov, M.; Belkina, N.; Kovalevski, V.; Zobkova, M.; Efremova, T.; Galakhina, N. Microplastic abundance and accumulation behavior in Lake Onego sediments: A journey from the river mouth to pelagic waters of the large boreal lake. J. Environ. Chem. Eng. 2020, 8, 104367. [Google Scholar] [CrossRef]
- Zobkov, M.B.; Chubarenko, I.P.; Esiukova, E.E.; Belkina, N.A.; Kovalevsky, V.V.; Zobkova, M.V.; Efremova, T.A.; Galakhina, N.E. Lakes as accumulators of microplastics on their way from land to the World Ocean: A review of research. Izv. RGS 2021, 153, 68–86. [Google Scholar] [CrossRef]
- Szymanska, M.; Obolewski, K. Microplastics as contaminants in freshwater environments: A multidisciplinary review. Ecohydrol. Hydrobiol. 2020, 20, 333–345. [Google Scholar] [CrossRef]
- Frank, Y.; Ershova, A.; Batasheva, S.; Vorobiev, E.; Rakhmatullina, S.; Vorobiev, D.; Fakhrullin, R. Microplastics in Freshwater: A Focus on the Russian Inland Waters. Water 2022, 14, 3909. [Google Scholar] [CrossRef]
- Pozdnyakov, S.R.; Ivanova, E.V.; Guzeva, A.V.; Shalunova, E.P.; Martinson, K.D.; Tikhonova, D.A. Studying the Concentration of Microplastic Particles in Water, Bottom Sediments and Subsoils in the Coastal Area of the Neva Bay, the Gulf of Finland. Water Resour 2020, 47, 599–607. [Google Scholar] [CrossRef]
- Cowger, W.; Gray, A.B.; Eriksen, M.; Moore, C.; Thiel, M. Evaluating wastewater effluent as a source of microplastics in environmental samples. In Microplastics in Water and Wastewater; Karapanagioti, H.K., Kalavrouziotis, I.K., Eds.; IWA Publishing: London, UK, 2019; pp. 109–131. [Google Scholar] [CrossRef]
- Kabir, A.H.M.E.; Sekine, M.; Imai, T.; Yamamoto, K.; Kanno, A.; Higuchi, T. Assessing small-scale freshwater microplastics pollution, land-use, source-to-sink conduits, and pollution risks: Perspectives from Japanese rivers polluted with microplastics. Sci. Total Environ. 2021, 768, 144655. [Google Scholar] [CrossRef]
- Liu, Y.; You, J.; Li, Y.; Zhang, J.; He, Y.; Breider, F.; Tao, S.; Liu, W. Insights into the horizontal and vertical profiles of microplastics in a river emptying into the sea affected by intensive anthropogenic activities in Northern China. Sci. Total Environ. 2021, 779, 146589. [Google Scholar] [CrossRef] [PubMed]
- Strady, E.; Kieu-Le, T.-C.; Gasperi, J.; Tassin, B. Temporal dynamic of anthropogenic fibers in a tropical river-estuarine system. Environ. Pollut. 2020, 259, 113897. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.K.H.; Lee, K.K.; Tang, K.H.D.; Yap, P.S. Microplastics in the freshwater and terrestrial environments: Prevalence, fates, impacts and sustainable solutions. Sci. Total Environ. 2020, 719, 137512. [Google Scholar] [CrossRef] [PubMed]
- De Falco, F.; Gullo, M.P.; Gentile, G.; Di Pace, E.; Cocca, M.; Gelabert, L.; Brouta-Agnésa, M.; Rovira, A.; Escudero, R.; Villalba, R.; et al. Evaluation of microplastic release caused by textile washing processes of synthetic fabrics. Environ. Pollut. 2018, 236, 916–925. [Google Scholar] [CrossRef]
- Textile Exchange. Preferred Fiber & Materials Market Report. Available online: http://textileexchange.org/app/uploads/2022/10/Textile-Exchange_PFMR_2022.pdf (accessed on 20 September 2023).
- Elhawary, I.A. Fibre to Yarn. In Textiles and Fashion; Elsevier: Amsterdam, The Netherlands, 2015; pp. 191–212. [Google Scholar]

| River | Mean ± SE | Min–Max | SD | Cv, % |
|---|---|---|---|---|
| Kara Darya | 4.28 ± 0.09 | 4.19–4.46 | 0.16 | 3.60 |
| Chirchiq | 0.95 ± 0.36 | 0.27–1.49 | 0.62 | 65.5 |
| River (Items) | Mean ± SE | Min–Max | SD | Cv, % |
|---|---|---|---|---|
| Kara Darya (items per kg−1/items per m−2) | 333 ± 11.5/ 22,160 ± 768 | 313–353/ 20,830–23,490 | 20.0/1330 | 6.00 |
| Chirchiq (items per kg−1/items per m−2) | 244 ± 28.9/ 4459 ± 527 | 187–273/ 3405–4986 | 50.0/913 | 20.5 |
| River | LOI, % | Sand Fractions, µm | Clay Fractions, µm | ||||
|---|---|---|---|---|---|---|---|
| 1000–250 | 250–50 | 50–10 | 10.0–5.00 | 5.00–1.00 | <1.00 | ||
| Kara Darya | 7.55 | 84.3 | 4.02 | 2.40 | 1.76 | 2.56 | 4.96 |
| Chirchiq | 10.4 | 94.9 | 1.24 | 1.12 | 2.08 | 0.32 | 0.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frank, Y.; Khusanov, A.; Yuldashov, M.; Vorobiev, E.; Rakhmatullina, S.; Rednikin, A.; Tashbaev, S.; Mamatkarimova, S.; Ruchkina, K.; Namozov, S.; et al. Microplastics in the Syr Darya River Tributaries, Uzbekistan. Water 2023, 15, 3698. https://doi.org/10.3390/w15203698
Frank Y, Khusanov A, Yuldashov M, Vorobiev E, Rakhmatullina S, Rednikin A, Tashbaev S, Mamatkarimova S, Ruchkina K, Namozov S, et al. Microplastics in the Syr Darya River Tributaries, Uzbekistan. Water. 2023; 15(20):3698. https://doi.org/10.3390/w15203698
Chicago/Turabian StyleFrank, Yulia, Alijon Khusanov, Mansur Yuldashov, Egor Vorobiev, Svetlana Rakhmatullina, Alexey Rednikin, Sherzodbek Tashbaev, Sarvinoz Mamatkarimova, Kristina Ruchkina, Sirojiddin Namozov, and et al. 2023. "Microplastics in the Syr Darya River Tributaries, Uzbekistan" Water 15, no. 20: 3698. https://doi.org/10.3390/w15203698
APA StyleFrank, Y., Khusanov, A., Yuldashov, M., Vorobiev, E., Rakhmatullina, S., Rednikin, A., Tashbaev, S., Mamatkarimova, S., Ruchkina, K., Namozov, S., Turaev, L., Sobirov, J., Yuldashev, A., & Vorobiev, D. (2023). Microplastics in the Syr Darya River Tributaries, Uzbekistan. Water, 15(20), 3698. https://doi.org/10.3390/w15203698








