Abstract
Reclaimed water irrigation can effectively alleviate the shortage of water resources in arid and semi-arid areas; however, reclaimed water contains organic pollutants that may enter the agricultural production environment through irrigation, such as endogenous estrogens, so people have always paid attention to the safety of reclaimed water irrigation. In this paper, we studied the effects of reclaimed water irrigation, groundwater irrigation, and alternating irrigation of the two water sources on grain quality, as well as endogenous estrogen concentrations of winter wheat and topsoil in the North China Plain during 2015–2016. The results show that the concentrations of crude protein, total soluble sugar, crude ash, crude starch, and reduction-type vitamin C (reduction-type VC) in the winter wheat grains were 12.5–16.4%, 0.85–2.15%, 1.85–3.28%, 61.5–75.0%, and 4.9–16.0 mg/kg, respectively. There were no significant differences in these quality indexes among the irrigation treatments (p > 0.05). The concentrations of endogenous estrogen in the surface soil and winter wheat grain under all irrigation treatments were 0.34–4.01 μg/kg and below the limits of detection (ND)–3.71 μg/kg, respectively. There were no significant differences in the concentrations of endogenous estrogen in the soil and wheat grain among the different irrigation treatments (p > 0.05). The bioconcentration factor (BCF) of the endogenous estrogen in the soil–winter wheat system was 0.08–1.90, and there was no significant difference in the BCF among the irrigation treatments (p > 0.05). Compared with groundwater irrigation, reclaimed water irrigation did not significantly affect endogenous estrogen concentrations in the soil and winter wheat, as well as the bioconcentration factors.
1. Introduction
Water resources are indispensable strategic resources for the development of human life and production. Because of the shortage of water resources, people are gradually beginning to use reclaimed water to irrigate crops. Reclaimed water irrigation mainly refers to the harmless treatment of domestic sewage and industrial wastewater for irrigation in agriculture, gardens, and other areas [1]. Reclaimed water is mainly suitable for non-drinking water use [2]. Scholars widely believe that reclaimed water contains nitrogen, phosphorus, potassium, and other nutrients, which can enhance soil fertility and crop productivity [3], so reclaimed water is often used in agricultural irrigation in many countries [4].
However, the sources of reclaimed water are mostly industrial wastewater and urban sewage, and there are some pollutants in the water, such as endogenous estrogens, which cannot be entirely removed in sewage treatment plants (STPs) [5]. For example, Zhou et al. [6] found that the estrogen concentration in the untreated water and treated water of the Gao Beidian STP were 263.4 ng/L and 15.7 ng/L, respectively. Endogenous estrogens mainly include natural estrone (E1), 17β-estradiol (17β-E2), estriol (E3), and synthetic estrogen (ethinyl estradiol, EE2). E1, E2, E3, and EE2 were detected at the outlets of STPs in different countries (i.e., the UK, Germany, the Netherlands, and Brazil), the corresponding concentrations were 0.2–196.7 ng/L, 0.1–64 ng/L, 0.4–39.1 ng/L, and 0.59–5.6 ng/L, respectively [7,8,9,10]. Scholars have also detected E1, E2, E3, and EE2 at the outlets of six STPs in China, and the corresponding concentrations were 10.1–29.4 ng/L, 1.5–10.8 ng/L, ND–7.6 ng/L, and ND–9.7 ng/L, respectively [11].
Endogenous estrogens interfere with the endocrine system even at low concentrations (0.26–2.4 ng/L) [12]; thus, endogenous estrogens have attracted increasing attention [13]. Studies have shown that endogenous estrogens can be taken up by crops from polluted soil and interact with organic matter in the soil, threatening the ecological environment and creating further risks to human health [14]. Wei et al. found that higher concentrations (50–100 μg/L) of endogenous estrogens resulted in lower germination rate (1.43–7.58%) of brassica seeds and decreased the root length (15.23–24.91%), inhibiting the seedling growth [15]. High concentrations (>1000 μg/L) of endogenous estrogens also caused cell death in wheat and affected its growth [16]. If the level of estrogen in the environment is higher than its safety threshold, it will increase the risk of cancer in humans and induce cardiovascular disease [17]. In addition, EE2 reduces fish biomass and destroys the aquatic food chain [18]. Therefore, people are concerned about the safety of endogenous estrogens.
Endogenous estrogens in reclaimed water may migrate to different media with water [19]. In recent years, endogenous estrogens have been found in rivers, lakes, soils, and crops. For example, the concentrations of E1, E2, E3, and EE2 in China’s rivers were 2.88–33 ng/L, 0.13–51 ng/L, 53 ng/L, and 58 ng/L, respectively [20,21]. These endogenous estrogens could enter the soil through irrigation [22], and the estrogen concentration found in farmland soil irrigated with reclaimed water was 38.5 ± 0.9 ng/L [23]. The concentrations of E1 and E2 in lettuce and wheat (planted in pots) irrigated with wastewater were 0.7–75 μg/kg and 50–100 μg/kg [24,25]. The concentrations of E1 and E2 in maize planted in an aqueous solution (the concentrations of E1 and E2 in the aqueous solution were both 0.22 μM/mL) were ND–0.15 μmol/g and ND–0.2 μmol/g [26]. The concentration of E2 in wheat was 0–80 μg/kg, which was planted in an aqueous solution with an E2 concentration of 100 μg/L [16]. The BCFs (bioconcentration factors) of E1 and E2 for Echinodorus horemanii and Eichhornia crassipes under hydroponic conditions were 4.1–13.4 and 2.2–10.1, respectively [27].
Currently, most studies on reclaimed water irrigation are based on hydroponic experiments and pot experiments [25]; however, field conditions are more complicated than hydroponic and pot conditions, and the results based on hydroponic and pot experiments cannot completely reflect the actual estrogens levels of migration in soil and crops. Furthermore, the hydroponic solution used was mostly a nutrient solution with estrogen, the irrigation water used in the pot experiments was mostly a mixture of pure water and estrogen, and the mixed water and nutrient solution in the hydroponic and pot experiments were different from the irrigation water in actual production systems. Moreover, there are few studies on the migration of endogenous estrogens in soil–plant systems under actually reclaimed water irrigation based on field experiments. Therefore, this paper analyzed the effects of reclaimed water irrigation on the estrogen concentrations in soil and grain based on a field experiment using actual reclaimed water conditions.
2. Materials and Methods
2.1. Site Information and Experimental Design
The experiment was carried out at the Yong Ledian Experimental Station of the Beijing Institute of Water Science and Technology from October 2014 to June 2016. The geographical coordinates of the experimental station are 114°20′ E and 39°20′ N and approximately 12 m above sea level. The region has a warm temperate, semi-humid, continental, and monsoonal climate. Over the past 30 years, the average annual temperature was 11–12 °C; the average annual sunshine hours was 2459 h; the average annual precipitation was 565 mm; and the precipitation was mainly from June to August. In the experiment, the length and width of each plot were set to 3 m and 2 m, respectively, and a 1 meter deep geomembrane was buried around the plot to eliminate the lateral flow of soil moisture. The soil texture was silty loam. The groundwater depth was approximately 8 m during the experiment.
In this study, winter wheat was taken as the research object. During the two-year experiment, the winter wheat varieties included Jimai 22 (the local farmers adjusted their planting variety in 2015–2016, so we also replaced Jimai 22 with Lunxuan 518 in 2015–2016), Zhongmai 175, Shimai 15, Nongda 211, and Shifu 20. The row spacing of winter wheat was set to 15 cm. Except for irrigation water sources, the other field management measures were the same for all treatments, as shown in Table 1. Considering the actual situation (i.e., some of the ditches in many areas were recharged with reclaimed water, other ditches’ flows were cut off at times, and reclaimed water flowed into the river), each variety received contained three irrigation treatments—reclaimed water irrigation, groundwater irrigation, and alternating irrigation of the two water sources. Each treatment had three replicates, and a total of forty-five experimental plots were randomly arranged. The reclaimed water used for irrigation was the secondary effluent of the Gao Beidian STP, which uses air-aeration-activated sludge treatment [28]. The groundwater was collected from the well of the test station, and the alternating water was a mixture of reclaimed water and groundwater (V/V=1:1). The water quality of the three sources is shown in Table 2. The estrogen concentration in the reclaimed water (2.87–26.88 ng/L) in this study was similar to the values (15.7 ng/L) detected at the effluent of the Gao Beidian wastewater treatment plant by Zhou et al. [6]. There was a significant difference (p < 0.05) in the estrogen concentrations between the reclaimed water and groundwater (Table 2). The conventional water quality indicators (such as suspended matter, pH, EC, and BOD5) of irrigation water used in the test satisfied the provisions of “The reuse of urban recycling water quality of farmland irrigation water” [29].

Table 1.
Field management of winter wheat for testing.

Table 2.
Quality of reclaimed water and groundwater.
During the experiment, no other fertilizers (phosphatic and potassic fertilizers) and no other agricultural chemicals (herbicides, insecticides, and fungicides) were applied to the experimental site, and weeds in the wheat fields were manually removed.
2.2. Experimental Observations and Methods
- (1)
- Grain yield of winter wheat
The wheat grains in the whole plot were threshed after the winter wheat harvesting, and the grains were weighed after natural air drying (the grain moisture content was approximately 6%).
- (2)
- Grain and soil sampling
After wheat harvest, soil samples (0–20 cm) were collected from the fields planted with Jimai 22 (replaced with Lunxuan 518 in 2016), and 5 subsamples were taken from each plot and then mixed into 1 sample, for a total of 9 soil samples. For the wheat grains in 2015, 5 subsamples were mixed into 1 sample in each plot, for a total of 45 crop samples; a total of 18 grain samples were collected from Lunxuan 518 and Shifu 20 using the same method in 2016. The soil and winter wheat grains were wrapped with aluminum foil to avoid secondary pollution.
- (3)
- Determination of the organic pollutants in grains and soil
Sample pretreatment: Winter wheat grain and soil samples were freeze-dried and sieved using 2 mm screen meshes. Taking 5 g of each sample (dry mass) and adding 0.1 mg/kg of the internal standard, the sample and substitute were stirred evenly and the estrogen in the winter wheat and soil samples were extracted using microwave extraction. The microwave extractant was a mixture of acetone and methanol with a volume ratio of 3: 1. The extraction temperature was set to 100 °C. The temperature gradient first climbed for 10 min, then maintained for 30 min, and finally cooled for 20 min. After the extraction, the supernatant was removed and put into a glass flask. The above process was repeated twice, and the supernatant was combined twice. The dehydrated extract was concentrated to 5 mL using a rotary evaporator (50 °C) and a nitrogen blowing instrument (50 °C), and then purified water was added to 100 mL. After mixing, the extraction fluid was activated with an Oasis HLB solid-phase extraction column with a volume ratio of 1: 1 methanol and pure water. The solid-phase extraction column was eluted with 10 mL 10% methanol aqueous solution and then with 10 mL pure methanol. The eluent was placed in a constant temperature water bath at 37 °C and concentrated to 0.5 mL with nitrogen. The ultrapure water was added to a constant volume of 1 mL. After adding the internal standard, it was filtered through a 0.22 μm filter membrane and transferred to a 1.5 mL sample bottle. Finally, it was stored in a refrigerator until detection.
Determination of the samples: High-performance liquid chromatography–triple quadrupole tandem mass spectrometer (UPLC–MS–MS–8040, Shimadzu, Kyouto, Japan) was used to determine the concentrations of estrogen in the soil and grain, including E1, EE2, E2, and E3. Table 3 shows the basic properties of estrogen. The chromatographic conditions in the experiment were set as follows: The mobile phase was 0.1% formic acid aqueous solution (A) and methanol/acetonitrile (B, 1/1, V/V, containing 0.1% formic acid). The chromatographic column size was 2.11 mm × 30 mm × 3 μm. The mass spectrometry conditions were set as follows: Electrospray ionization (ESI) in the positive ion mode was used, and the source temperature and desolvation temperature were set at 120 °C and 500 °C, respectively. The monitoring mode was the single-ion monitoring (SIM) mode. The qualitative analysis of the sample was determined by the sample’s characteristic peak and retention time. Finally, the quantitative analysis of the sample was carried out according to its base peak area.

Table 3.
Properties of the endogenous estrogens.
- (4)
- Quality control and assurance
Three blank samples were used for each batch of samples. The test results for the blank reagent were lower than the test’s detection limit. A blank reagent should be used every 12 h to detect whether the experimental instrument is contaminated. In this experiment, the detection limits of the endogenous estrogens for the soil and wheat grain samples were 0.021–0.25 μg/kg.
- (5)
- Determination of the quality of the grains
For the determination of the crude protein concentration and crude ash concentration in the wheat grains, the Kjeldahl method of determination and near-infrared were used [31]. For the determination of the total soluble sugar and starch in the wheat grains, enthrone colorimetric and coupled spectrophotometer assay methods were used [32]. For the quality determination of the reduction-type VC in the wheat grains, high performance liquid chromatography was used [33].
2.3. Data Calculation and Analysis
- (1)
- Calculation of the bioconcentration factors (BCFs)
The bioconcentration factors (BCFs) of the endogenous estrogens in the soil–winter wheat system were calculated as follows:
where Cp is the endogenous estrogen concentration in winter wheat grain, and Cs is the endogenous estrogen concentration in the topsoil (expressed as dry weight, μg/kg).
- (2)
- Data analysis
The data and graph were processed using Microsoft Excel 2010 software. SPSS 20.0 software was used for the statistical analysis of the endogenous estrogen concentrations in the soil and wheat grains in this experiment, including the LSD (Least-Significant Difference) method for the significance difference analysis (p = 0.05). (some data can be found in the Supplementary Materials).
3. Results
3.1. Estrogen Concentrations in the Surface Soil with Different Water Quality Irrigation
Figure 1 shows the total estrogen concentrations in the 0–20 cm soil in the winter wheat harvest in 2015 and 2016. The estrogen concentrations in the surface soil for the reclaimed water irrigation treatment, groundwater irrigation treatment, and alternating irrigation treatment were 0.62–4.01, 0.35–3.11, and 0.34–2.71 μg/kg, respectively. The significance analysis in this paper showed no significant difference in surface-soil estrogen concentrations among different water quality irrigation treatments (p = 0.178–0.294) in the same wheat harvest time, indicating there was no significant effect of reclaimed irrigation water on the concentrations of estrogens in the soil. The significance analysis showed that significant differences were found in the surface-soil estrogen concentrations between the two wheat harvest times for the same water quality irrigation treatments (p = 0.001–0.026).
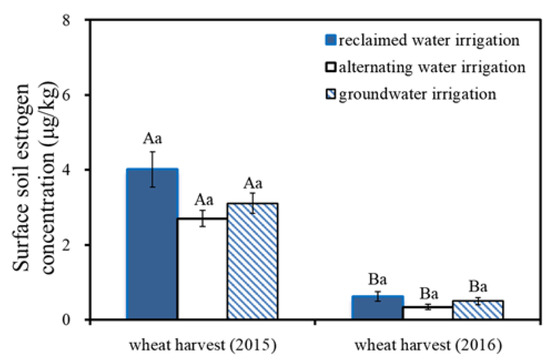
Figure 1.
The total concentrations of estrogen in 0–20 cm soil at wheat harvest under different irrigation water quality treatments. Note: The different capital letters on the column indicate a significant difference among the same treatments (p < 0.05) at different harvest times. The same lowercase letter on column indicates that there was no significant difference among different treatments (p > 0.05) at the same harvest time.
3.2. Grain Quality and Yield of Winter Wheat with Different Water Irrigation Treatments
Figure 2 shows the grain quality indexes of the winter wheat under different water quality irrigation treatments. The concentrations of crude protein, total soluble sugar, crude ash, crude starch, and reduction-type VC in the winter wheat grains for all treatments were 12.5–16.37%, 0.85–2.15%, 1.85–3.28%, 61.5–75.0%, and 4.9–16.0 mg/kg, respectively. For the same wheat variety, the significance analysis showed that no significant difference in the quality indexes was found among the different water quality irrigation treatments (p > 0.05), indicating there was no significant effect of the reclaimed irrigation water on the wheat grain quality.
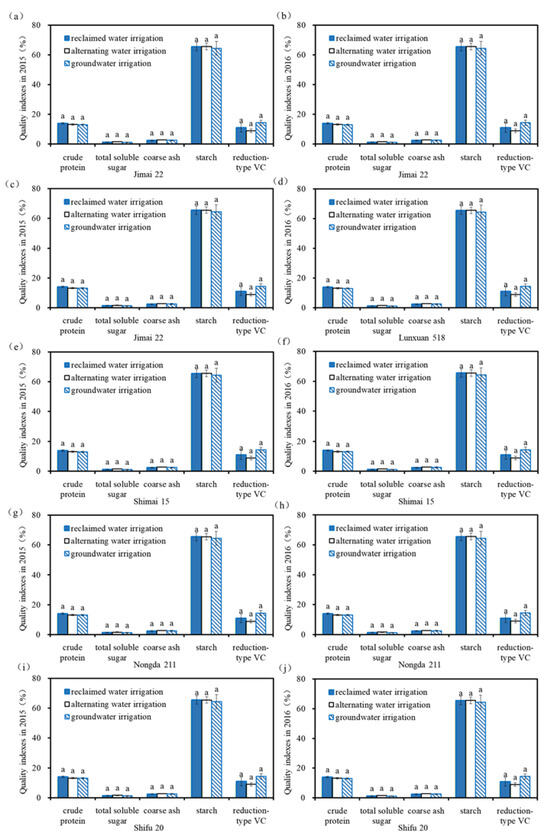
Figure 2.
Quality indexes of wheat grain under different irrigation water quality treatments. Note: (a,c,e,g,i) quality indexes of different wheat varieties in 2015 (Jimai 22, Zhongmai 175, Shimai 15, Nongda 211, Shifu 20); (b,d,f,h,j) quality indexes of different wheat varieties in 2016 (Lunxuan 518, Zhongmai 175, Shimai 15, Nongda 211, Shifu 20). The same lowercase letters above the columns indicate no significant difference among the three treatments for wheat quality (p > 0.05).
Figure 3 shows the grain yields of winter wheat under different water quality irrigation treatments in 2015–2016. The winter wheat yields under different water quality treatments were 4.35–7.08 t/ha. There was no significant difference in the wheat grain yield under different water quality irrigation treatments (p > 0.05) for each wheat variety.
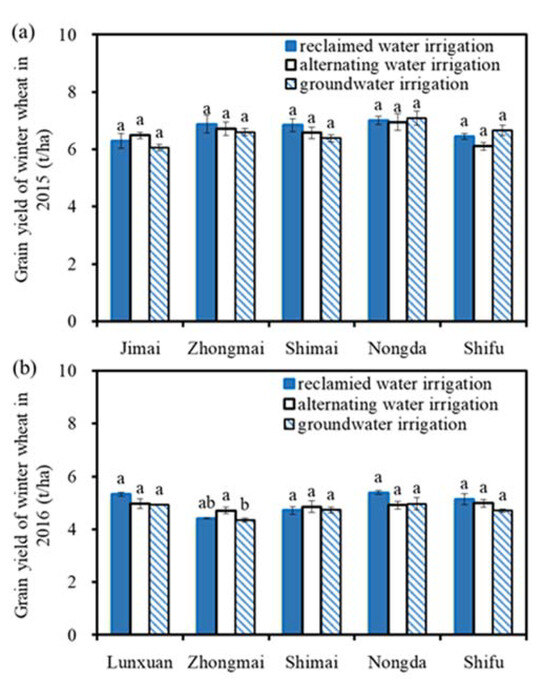
Figure 3.
Grain yields of winter wheat in 2015 and 2016: (a) represents the grain yield of winter wheat in 2015; (b) represents the grain yield of winter wheat in 2016. Note: Different lowercase letters above the columns indicate significant differences among the three treatments for each variety (p < 0.05).
3.3. Estrogen Concentrations in Winter Wheat Grains with Different Water Quality Irrigation
Figure 4 shows the estrogen concentrations of winter wheat grain irrigated with different water quality treatments. The estrogen concentrations of the winter wheat grain under reclaimed water, groundwater, and alternating irrigation treatments were ND–3.71 μg/kg, ND–1.95 μg/kg, and 0.20–2.62 μg/kg, respectively. For the same wheat variety, the significance analysis showed that there was no significant difference in the estrogen concentrations of wheat grains irrigated with different water quality treatments (p > 0.05). This indicates that the reclaimed water irrigation had no significant effect on the estrogen concentrations in the wheat grains. For the same irrigation water quality treatments, the significance analysis showed that there was no significant difference in the estrogen concentrations in the wheat grains among the different wheat varieties (p > 0.05), which indicates that the reclaimed irrigation water had no significant effect on the estrogen concentration in the wheat grain of different wheat varieties. There was no significant difference in the grain estrogen concentration among the different irrigation water quality treatments for the different wheat varieties (p > 0.05).
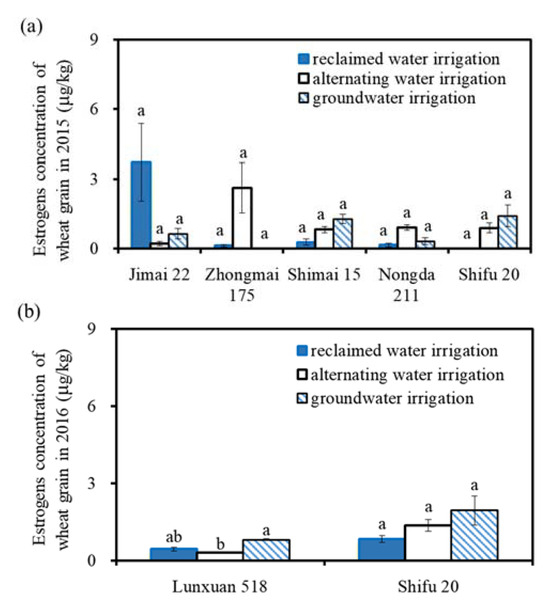
Figure 4.
Estrogens concentration in the wheat grain under different irrigation water quality treatments. Note: The same capital letters above the columns indicate that there was no significant difference in the grain estrogen concentrations among the different wheat varieties for the same irrigation water quality (p > 0.05). The same lowercase letters above the columns indicate that there was no significant difference in the grain estrogen concentration among the different irrigation water quality treatments for the same wheat variety (p > 0.05).
3.4. Estrogen Components and Bioconcentration Factors in the Soil—Winter Wheat System with Different Water Quality Irrigation Treatments
Figure 5 shows the concentrations of estrogen components in the surface soil and wheat grains. The concentrations of E1 and E3 in the soil were 0.20–1.28 μg/kg and ND–2.73 μg/kg, respectively. The concentrations of E1 and E3 in the wheat grain were ND–3.71 μg/kg and ND–1.71 μg/kg, respectively. In this study, E2 and EE2 were below the limits of detection in both the soil and crop samples, and E2 and EE2 were not detected in the irrigation water either. In 2015, the detection rates of E1 and E3 in the soil were 55.6% and 100%, respectively. In 2016, the detection rates of E1 and E3 in the soil were 100% and 11.1%, respectively. In 2015, only E1 could be detected in the wheat grains (the detection rate was 53.3%). In 2016, the detection rates of E1 and E3 in the wheat grains were 94.4% and 38.9%, respectively.
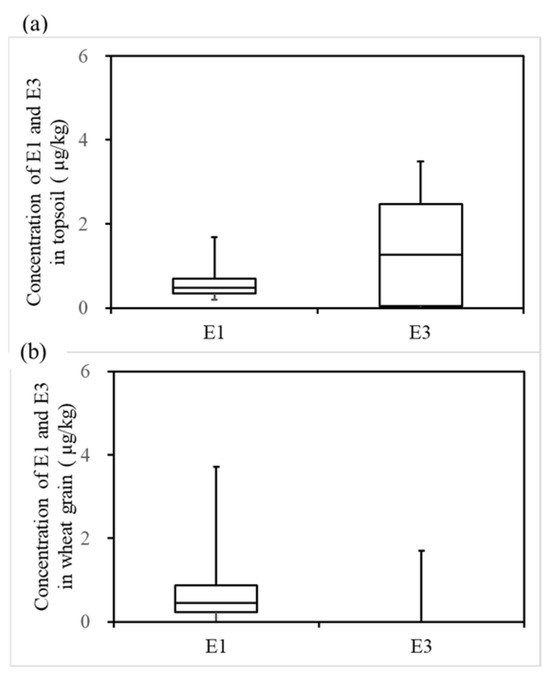
Figure 5.
E1 and E3 concentrations in surface soil and wheat grain in 2015–2016: (a) concentrations of E1 and E3 in the soil, (b) concentrations of E1 and E3 in the wheat grain.
Figure 6 shows the bioconcentration factors of estrogen in the soil–wheat grain system. The bioconcentration factors reflect the process of endogenous estrogens transferring from the soil to crops and are calculated by the ratio of the concentration of endogenous estrogens in a certain organ of the crop to the concentration of endogenous estrogen in the soil [24]. The bioconcentration factors in 2015 and 2016 were 0.08–1.56 and 0.98–1.9, respectively. The significance analysis showed that different irrigation treatments had no significant effect on the bioconcentration factors (p = 0.281–0.289).
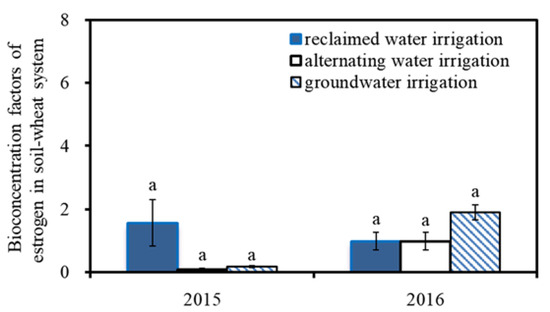
Figure 6.
Bioconcentration factors of estrogen in soil–wheat system. The same lowercase letters above the columns indicates that there was no significant difference in the BCFs among the different wheat varieties for the same irrigation water quality (p > 0.05).
4. Discussion
In this paper, estrogens were detected in the soil of all three treatments, which indicates that estrogens can enter the soil through irrigation water. The estrogen concentrations in the surface soil in 2016 were significantly lower than those in the soil in 2015. The reason was that the estrogen concentration in the water in 2016 (11.75 ng/L) was lower than the estrogen concentration in the water in 2015 (14.99 ng/L). The other reason may be due to the increased amount of irrigation water in 2016 (Table 1), causing the leaching of estrogen into the soil layer below 20 cm. Finlay-Moore et al. [34] found that the estrogen concentration in the soil decreased from 0.67 μg/kg to 0.07 μg/kg with the increased amount of irrigation water. The results in this paper are similar to that of Finlay-Moore et al. [34]. Other scholars have detected the concentrations of estrogens in soil with reclaimed irrigation. For example, Chen et al. [35] and Scherr et al. [36] found that the concentrations of estrogens in 0–20 cm farmland soil (irrigated with reclaimed water) were 0–5 μg/kg and 0–3.9 μg/kg, respectively. The concentrations of estrogen in this study are comparable with those in the soil irrigated with reclaimed water in the above research [35,36].
The crude protein concentration of the wheat grain (12.5–16.37%) in this paper was higher than the crude protein concentration of wheat grains (8.3–9.6%) with nitrogen application less than 120 kg/ha, and it was close to the crude protein concentration (11.8–13.2%) of wheat grain with a nitrogen application rate of 180–240 kg/ha [37]. The main reason could be that the crude protein concentration of wheat grain is mainly related to the amount of nitrogen application [38]. The crude protein concentration (8.5–13.8%) of the wheat grain increased with the increase in the nitrogen application rate (0–240 kg/ha) [39]. Because of the sufficient nitrogen application in this study, the crude protein concentration of the wheat grain was similar to the high value reported by Litke et al. [39]. Crude starch is an important component of wheat grain. The crude starch concentration of the wheat grain (61.5–75.0%) in this paper was similar to the values reported by Wioletta et al. [40] detected the quality of four different wheat varieties (common wheat, spelt wheat, Emmer wheat, and Einkorn wheat), and found that the crude protein concentrations of wheat were 11.0%, 12.8%, 15.4%, and 18.1%, and the crude ash concentrations of wheat were 1.52%, 1.86%, 2.16%, and 2.65%, respectively. Dai et al. [41] found that the crude starch concentrations of wheat under an ordinary irrigation mode were 66.96–69.25%; the concentrations of crude protein in the wheat were 11.09–12.21%. The crude protein content and crude starch content in wheat grains in this paper are consistent with the values obtained by Wioletta et al. [40] and Dai et al. [41], who found that the crude starch concentrations of wheat under a full irrigation mode were 66.96–69.25%.
The yields of winter wheat in this paper (4.35–7.08 t/ha) were similar to that of Li et al. [42], who reported that wheat grain yields with sufficient water and N supply were 5.03–7.04 t/ha. In this paper, the grain yield of the winter wheat in 2016 was lower than that in 2015; however, it may be due to the increased accumulated temperature in 2016 (2474 °C and 2527 °C in 2015 and 2016, respectively). When the effective accumulated temperature of wheat exceeded 2500 °C, this would have produced a negative impact on the yield of the winter wheat [43,44]; on the other hand, there was extremely low-temperature weather in the Beijing district from 22 January to 24 January 2016 (the daily minimum temperature changed from −15.2 °C to −10.9 °C, and the daily maximum temperature changed from −10.9 °C to −3.9 °C). Liu et al. [45] showed that wheat yields were reduced by 24.3–34.5% when the minimum temperature was lower than −4 °C. The winter wheat in 2016 may have suffered from freezing damage, causing a decreased yield.
Other scholars have also conducted experiments on estrogen concentrations in different crops. Wei et al. [46] and Lu et al. [47] detected the estrogen concentrations in different crops (greengrocery, radish, lettuce, tomato, potato, and citrus) in the local market, and found that the E1 concentrations in greengrocery, radish, and citrus were 0.21–1.85 μg/kg. The E2 concentrations in lettuce, tomato, potato, and citrus were 1.89 ± 1.72 μg/kg, <0.06 μg/kg, 1.36 ± 0.49 μg/kg, and 2.31 ± 1.12 μg/kg, respectively. This indicates that the E3 was not detected for all crops. The estrogen concentrations in wheat grains (ND–3.71 μg/kg) in this paper are similar to the values in these above studies [46,47]. In addition, At the same time, Li et al. [48] irrigated eggplant, beans, carrots, and cabbage with reclaimed water, underground water, and alternated water, and they found that the estrogen concentrations were 26.34–61.88, 3.34–7.67, 12.15–72.04, and 0.94–2.40 μg/kg, respectively, and the three irrigation methods had no significant effect on the estrogen concentrations in the vegetables. Li et al. [49] have also investigated the estrogen concentrations in wheat with different reclaimed water irrigation histories (30a and 40a) in a southeastern suburb of Beijing and found that the estrogen concentration in wheat grains was 1.384–2.075 μg/kg, and there was no significant effect of the reclaimed water irrigation history on the estrogen concentration in the wheat grains. The results of this paper and Li et al.’s [48,49] indicate that reclaimed water irrigation had no significant effect on the estrogen concentrations in different crops in Beijing (the concentrations of endogenous estrogen in reclaimed water were in the range of 2.87–26.88 ng/L. In this paper, we only used the reclaimed water collected from Gao Beidian STP; however, because of the different sources of sewage in different regions and the different sewage treatment processes used in different STPs [50], the quality of reclaimed water collected from different STPs are different from each other, so it is necessary to study the effects of reclaimed water collected from other STPs on the migration of endogenous estrogens in soil plants.
E2 and EE2 were not detected in the irrigation water in this paper, and this could be explained by the following reasons. First, EE2 is a synthetic estrogen and mainly produced from industrial wastewater, while most of the wastewater in Gao Beidian STP is domestic sewage [28], so the concentration of EE2 is low. Second, E2 and EE2 could be metabolized into E1 in the process of sewage treatment in the STP [51]. Only E1 and E3 could be detected in the soil and wheat grain, which is because the irrigation water (reclaimed water, alternating water, and groundwater) in this study only detected E1 and E3, as E2 and EE2 were below the limits of detection. When the octanol/water partition coefficients (log Kow) are greater than 3.5, estrogens have higher hydrophobicity and are more likely to accumulate in the soil and crop roots [14]; on the other hand, E1 would be transformed into E3 under the action of microorganisms, so the concentration of E3 in the soil was higher than that of E1 in the soil. The main migration route of estrogen in a soil–crop system is primarily divided into two processes. First, organic pollutants in the soil solution were taken up by the root system. Then, the organic pollutants migrate to the crop tissues under the action of transpiration tension [52]. Studies have shown that the liposolubility of estrogens is an important factor affecting the accumulation of estrogens in crops [53]. It is difficult to transport organic pollutants from roots to shoots and kernel through the vascular tissue of crops due to the high hydrophobicity, so E3 cannot easily enter wheat grains through wheat tissues [24], and so the concentration of E3 in wheat is lower than E1 in wheat.
It was found that the concentration of E1 in lettuce irrigated with wastewater was 0.7–6.7 μg/kg [54]. Li et al. [48] found that the bioconcentration factors of endogenous estrogens in carrots and cabbages were 0.08–0.3 and 1.57–5.52, respectively. In this paper, the bioconcentration factors of wheat estrogen were close to that of carrot and cabbage.
5. Conclusions
This paper studied the effects of different irrigation water quality conditions on the concentrations of endogenous estrogens in topsoil and winter wheat grain, as well as on wheat grain quality and yields. From the results, there was no significant difference in wheat grain quality, yield, bioconcentration factor, and the concentration of endogenous estrogens in the surface soil and wheat grain among the different irrigation water quality treatments (i.e., reclaimed water irrigation, alternating irrigation, and groundwater irrigation) in this paper. This study provides a reasonable basis for the utilization of reclaimed water and the conservation of water resources. Because the wheat grain yields were different according to the water quality of STPs, which also affects the various growth characteristics of different crops, future studies could focus on field experiments based on reclaimed water collected from different STPs and using different crops.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w15203671/s1, Table S1: Estrogen concentration in topsoil with different irrigation quality in 2015; Table S2: Estrogen concentration in topsoil with different irrigation quality in 2016; Table S3: Winter wheat quality index with different irrigation quality in 2015; Table S4: Winter wheat quality index with different irrigation quality in 2016; Table S5: Estrogen concentration in winter wheat grain with different irrigation quality in 2015; Table S6: Estrogen concentration in winter wheat grain with different irrigation quality in 2016.
Author Contributions
H.L. conceived and designed the experiments; Y.L. performed the experiments; Y.C., T.L., Z.Z. and Y.W. analyzed the data and wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of Jiangsu Province, grant number: BK20200941, the Natural Science Foundation of Jiangsu Province, grant number: BK20210824, the Green Yang Jinfeng project in Yangzhou, grant number: YZLYJF–2020PHD070, and the Science and Technology Projects of Public Welfare of Ningbo, the grant number: 2022S097.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors of this paper expresse our most sincere gratitude to all the staff of Tongzhou Experimental Base for the technical support during our experiment.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, L.; Lou, C.H.; Li, Y.; Ma, N. Effect of reclaimed water irrigation on Antioxidant Enzyme Gene Expression in wheat. J. Shanxi Agric. Sci. 2020, 48, 649–676. (In Chinese) [Google Scholar]
- Gil–Meseguer, E.; Bernabé-Crespo, M.B.; Gómez-Espín, J.M. Recycled sewage-a water resource for dry regions of southeastern Spain. Water Resour. Manag. 2019, 33, 725–737. [Google Scholar] [CrossRef]
- Wang, G.; Du, Z.; Ning, H.; Liu, H.; Abubakar, S.A.; Gao, Y. Changes in GHG Emissions Based on Irrigation Water Quality in Short-Term Incubated Agricultural Soil of the North China Plain. Agriculture 2021, 11, 1268. [Google Scholar] [CrossRef]
- Ma, M.; Rao, K.; Wang, Z. Occurrence of estrogenic effects in sewage and industrial wastewaters in Beijing, China. Environ. Pollut. 2008, 147, 331–336. [Google Scholar] [CrossRef]
- Guo, X.X.; Luo, H.; Li, N. Status and prospect of reclaimed water reuse in China. Pearl River 2023, 4, 45–52. (In Chinese) [Google Scholar]
- Zhou, Y.; Zha, J.; Xu, Y.; Lei, B.; Wang, Z. Occurrences of six steroid estrogens from different effluents in Beijing, China. Environ. Monit. Assess. 2012, 184, 1719–1729. [Google Scholar] [CrossRef]
- Koh, Y.K.K.; Chiu, T.Y.; Boobis, A.; Cartmell, E.; Scrimshaw, M.D.; Lester, J.N. Treatment and removal strategies for estrogens from wastewater. Environ. Technol 2008, 29, 245–267. [Google Scholar] [CrossRef] [PubMed]
- Mohagheghian, A.; Nabizadeh, R.; Mesdghinia, A.; Rastkari, N.; Mahvi, A.H.; Alimohammadi, M.; Yunesian, M.; Ahmadkhaniha, R.; Nazmara, S. Distribution of estrogenic steroids in municipal wastewater treatment plants in Tehran, Iran. Environ. Health Sci. Eng. 2014, 12, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Gin, K.Y.H.; Lin, A.Y.C.; Reinhard, M. Impacts of emerging organic contaminants on freshwater resources: Review of recent occurrences, sources, fate and effects. Sci. Total Environ. 2010, 408, 6062–6069. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, G.P.; de Souza, N.C.; Vidal, C.B.; Alves, J.A.; Firmino, P.I.M.; Nascimento, R.F.; dos Santos, A.B. Occurrence and removal of estrogens in Brazilian wastewater treatment plants. Sci. Total Environ. 2014, 490, 288–295. [Google Scholar] [CrossRef]
- Huang, B.; Li, X.; Sun, W.; Ren, D.; Li, X.; Li, X.N.; Liu, Y.; Li, Q.; Pan, X.J. Occurrence, removal, and fate of progestogens, androgens, estrogens, and phenols in six sewage treatment plants around Dianchi Lake in China. Environ. Sci. Pollut. Res. 2014, 21, 12898–12908. [Google Scholar] [CrossRef]
- Wu, Q.; Lam, C.W.J.; Kwok, K.Y.; Tsui, M.M.P.; Lam, P.K.S. Occurrence and fate of endogenous steroid hormones, alkylphenol ethoxylates, bisphenol A and phthalates in municipal sewage treatment systems. J. Environ. Sci. 2017, 61, 49–58. [Google Scholar] [CrossRef]
- Sweeney, M.F.; Hasan, N.; Soto, A.M.; Sonnenschein, C. Environmental endocrine disruptors: Effects on the human male reproductive system. Rev. Endocr. Metab. Disord. 2015, 16, 341–357. [Google Scholar] [CrossRef] [PubMed]
- Adeel, M.; Song, X.M.; Wang, Y.Y.; Francis, D.; Yang, Y.S. Environmental impact of estrogens on human, animal and plant life: A critical review. Environ. Int. 2017, 99, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.C.; Gong, L.; Zhang, L.L.; Chen, M.; Shao, M.C.; Wang, R. Effects of 17β-estradiol stress on seed germination and seeding physiological characteristics of brassica chinensis. Southwest China J. Agric. Sci. 2016, 29, 531–535. (In Chinese) [Google Scholar]
- Chen, X.C.; Li, Y.; Jiang, L.; Hu, B.; Wang, L.; An, S.; Zhang, X. Uptake, accumulation, and translocation mechanisms of steroid estrogens in plants. Sci. Total Environ. 2021, 753, 141979. [Google Scholar] [CrossRef]
- Woclawek-Potocka, I.; Mannelli, C.; Boruszewska, D.; Kowalczyk-Zieba, I.; Wasniewski, T.; Skarzynski, D.J. Diverse effects of phytoestrogens on the reproductive performance: Cow as a model. Int. J. Endocrinol. 2013, 15, 650984. [Google Scholar] [CrossRef]
- Hallgren, P.; Nicolle, A.; Hansson, L.A.; Bronmark, C.; Nikoleris, L.; Hyder, M.; Persson, A. Synthetic estrogen directly affects fish biomass and may indirectly disrupt aquatic food webs. Environ. Toxicol. Chem. 2014, 33, 930–936. [Google Scholar] [CrossRef]
- Ren, J.; Ma, W.F. Effect of trace new organic pollutants on groundwater and research progress on pollution control technology during farm irrigation with reclaimed water. Chin. Soc. Environ. Sci. 2021, 139–144. (In Chinese) [Google Scholar] [CrossRef]
- Lei, K.; Lin, C.Y.; Zhu, Y.; Chen, W.; Pan, H.Y.; Sun, Z.; Sweetman, A.; Zhang, Q.H.; He, M.C. Estrogens in municipal wastewater and receiving waters in the Beijing-Tianjing-Heibei region, China: Occurrence and risk assessment of mixtures. Hazard. Mater. 2020, 389, 121891. [Google Scholar] [CrossRef]
- Ren, C.X.; Tan, X.; Huang, C.M.; Zhao, H.; Lan, W.L. Sources, pollution characteristics, and ecological risk assessment of steroids in Beihai Bay, Guangxi. Water 2022, 14, 1399. [Google Scholar] [CrossRef]
- Bai, X.L. Fate and Transport of Estrogens and Estrogen Conjugates in Manure-Amended Soils. Anim. Manure 2020, 67, 183–199. [Google Scholar]
- Mahjoub, O.; Escande, A.; Rosain, D.; Casellas, C.; Gomez, E.; Fenet, H. Estrogen-like and dioxin-like organic contaminants in reclaimed wasterwater:transfer to irrigated soil and groundwater. Watr Sci. Technol. 2011, 63, 1657–1662. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shargil, D.; Gerstl, Z.; Fine, P.; Nitsan, I.; Kurtzman, D. Impact of biosolids and wastewater effluent application to agricultural land on steroidal hormone content in lettuce plants. Sci. Total Environ. 2015, 505, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.C.; Li, Y.X.; Jiang, L.S.; Jiang, X.M.; Hu, B.Y.; Wang, L.; Zhang, S.W.; Zhang, X.L. Uptake and transport of steroid estrogens in soil-plant systems and their dissipation in rhizosphere: Influence factors and mechanisms. Hazard. Mater. 2022, 42, 128171. [Google Scholar] [CrossRef]
- Card, M.L.; Schnoor, J.L.; Chin, Y. Transformation of natural and synthetic estrogens by maize seedlings. Environ. Sci. Technol. 2013, 47, 5101–5108. [Google Scholar] [CrossRef] [PubMed]
- Pi, N.; Ng, J.Z.; Kelly, B.C. Bioaccumulation of pharmaceutically active compounds and endocrine disrupting chemicals in aquatic macrophytes: Results of hydroponic experiments with Echinodorus horemanii and Eichhornia crassipes. Sci. Total Environ. 2017, 601, 812–820. [Google Scholar] [CrossRef]
- Wang, J.W.; Jiang, Y.; Gao, Q.; Liu, W.Y.; Li, W.; Wang, Y.B. Patterns and practice on comprehensive utilization of wastewater resources in Gaobeidian WWTP. China Water Wastewater 2015, 31, 5–7. (In Chinese) [Google Scholar]
- GB20922–2007; The Reuse of Urban Recycling Water Quality of Farmland Irrigation Water. Standards Press of China: Beijing, China, 2007.
- GB5084–2005; Standards for Irrigation Water Quality. Standards Press of China: Beijing, China, 2005.
- Dziki, D.; Cacak-Pietrzak, G.; Miś, A.; Jończyk, K.; Gawlik-Dziki, U. Influence of wheat kernel physical properties on the pulverizing process. J Food Sci Technol. 2014, 51, 2648–2655. [Google Scholar] [CrossRef]
- Gao, H.Y.; Niu, J.S.; Yang, X.W.; He, D.X.; Wang, C.Y. Impacts of powdery mildew on wheat grain sugar metabolism and starch accumulation in developing grains. Starch 2014, 66, 947–958. [Google Scholar] [CrossRef]
- GB/T 5009.86-2016; Determination of Reductive-Form Ascorbic Acid in Foods. National Health and Family Planning Commission of the People’s Republic of China: Beijing, China, 2016.
- Finlay–Moore, O.; Hartel, P.G.; Cabrera, M.L. 17 beta-estradiol and testosterone in soil and runoff from grasslands amended with broiler litter. Environ. Qual. 2000, 29, 1604–1611. [Google Scholar] [CrossRef]
- Chen, W.P.; Xu, J.; Lu, S.D.; Jiao, W.T.; Wu, L.S.; Andrew, C.C. Fates and transport of PPCPs in soil receiving reclaimed water irrigation. Chemosphere 2013, 93, 2621–2630. [Google Scholar] [CrossRef]
- Scherr, F.F.; Sarmah, A.K.; Di, H.J.; Cameron, K.C. Degradation and metabolite formation of 17β-estradiol-3-sulphate in New Zealand pasture soils. Environ.Int. 2009, 35, 291–297. [Google Scholar] [CrossRef]
- Litke, L.; Gaile, Z.; Ruža, A. Effect of nitrogen rate and forecrop on nitrogen use efficiency in winter wheat (Triticum aestivum). Aron. Res. 2019, 17, 582–592. [Google Scholar]
- Raymbek, A.; Saljnikov, E.; Kenenbayev, S.; Perovic, V.; Cakmak, D. Protein content changes in wheat grain as influenced by nitrogen fertilization. Agrochimeca 2017, 61, 180–189. [Google Scholar] [CrossRef]
- Litke, L.; Gaile, Z.; Ruža, A. Effect of nitrogen fertilization on winter wheat yield and yield quality. Aron. Res. 2018, 16, 500–509. [Google Scholar]
- Wioletta, B.; Anna, J.; Sławomir, S.; Magdalena, S. Comparison of yield, chemical composition and farinograph properties of common and ancient wheat grains. Eur. Food Res. Technol. 2021, 247, 1525–1538. [Google Scholar] [CrossRef]
- Dai, Z.M.; Li, Y.; Zhang, H.; Yan, S.H.; Li, W.Y. Effects of irrigation schemes on the characteristics of starch and protein in wheat (Triticum aestivum L.). Starch Starke 2016, 68, 454–461. [Google Scholar] [CrossRef]
- Li, Y.; Liu, H.J.; Huang, G.H. The effect of N rates on yields and nitrogen use efficiencies during four years of wheat-maize rotation cropping seasons. Agron. J. 2016, 108, 2076–2088. [Google Scholar] [CrossRef]
- Zhong, R.; Ren, Y.K.; Wang, P.R.; Lin, W.; Ren, A.X.; Sun, M.; Gao, Z.Q.; Xu, J.M. Characteristics of climate change during the growth peried of winter wheat and its influence on yield in south Shanxi province. Chin. J. Ecology. 2022, 41, 981–989. (In Chinese) [Google Scholar]
- You, L.Z.; Rosegrant, M.W.; Wood, S.; Sun, D.S. Impact of growing season temperature on wheat productivity in China. Agric. For. Meteorol. 2009, 149, 1009–1014. [Google Scholar] [CrossRef]
- Liu, L.L.; Xia, Y.M.; Liu, B.; Chang, C.Y.; Xiao, L.J.; Shen, J.; Tang, L.; Cao, W.X.; Zhu, Y. Individual and combined effects of jointing and booting low-temperature stress on wheat yield. EUR J. Agron. 2020, 113, 125989. [Google Scholar] [CrossRef]
- Wei, R.C.; Ge, F.; Zheng, Q.; Wang, R. Determination of estrone, estradiol and estriol residues in greengrocery by high performance liquid chromatography–tandem mass spectrometry. Jiangsu J. Agric. Sci. 2013, 29, 880–884. (In Chinese) [Google Scholar]
- Lu, J.; Wu, J.; Stoffella, P.J.; Wilson, P.C. Isotope dilution-gas chromatography/mass spectrometry method for the analysis of alkylphenols, bisphenol A, and estrogens in food crops. J. Chromatogr. A 2012, 1258, 128–135. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Huang, J.X.; Yang, S.L.; Lou, C.H.; Liu, H.L. Effects of irrigation water quality on the contents of polychlorinated biphenyls and endogenous estrogens in soil and vegetables. Res. Pract. Water Probl. Beijing 2018, 41–47. (In Chinese) [Google Scholar]
- Li, Y. Effects of Reclaimed Water Irrigation on Soil Quality and Crops. Post Doctor’ Thesis, China Agricultural University, Beijing, China, 2019. (In Chinese). [Google Scholar]
- Ting, Y.F.; Praveena, S.M. Sources, mechanisms, and fate of steroid estrogens in wastewater treatment plants: A mini review. Environ. Monit. Assess. 2017, 189, 178. [Google Scholar] [CrossRef]
- Zhong, R.Y.; Zou, H.Y.; Gao, J.; Wang, T.; Bu, Q.W.; Wang, Z.L.; Hu, M.; Wang, Z.Y. A critical review on the distribution and ecological risk assessment of steroid hormones in the environment in China. Sci. Total Environ. 2021, 786, 147452. [Google Scholar] [CrossRef]
- Chen, X.C.; Zhang, F.S.; Li, Y.X.; Jiang, X.M.; Zhang, X.L.; Hu, B.Y.; Tong, X.; Xu, K. Transport and transformation of steroid estrogens in soil-plant systems and their toxicological effects on plant. Acta Ecol. Sinica. 2021, 41, 2525–2535. (In Chinese) [Google Scholar]
- Collins, C.; Fryer, M.; Grosso, A. Plant uptake of non-ionic organic chemicals. Environ. Sci. Technol. 2006, 40, 45–52. [Google Scholar] [CrossRef]
- Lin, Q.Q.; Cai, X.D.; Wang, S.Z.; Yang, X.H.; Qiu, R.L.; Huang, X.F.; Zhou, W. Mechanism and influencing factors of plant absorption, migration and metabolism of organic pollutants. J. Agric. Environ. Sci. 2013, 32, 661–667. (In Chinese) [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).