Reductive Degradation of N-Nitrosodimethylamine via UV/Sulfite Advanced Reduction Process: Efficiency, Influencing Factors and Mechanism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Solutions
2.2. Batch Experiments
2.3. Analytical Methods
3. Results and Discussion
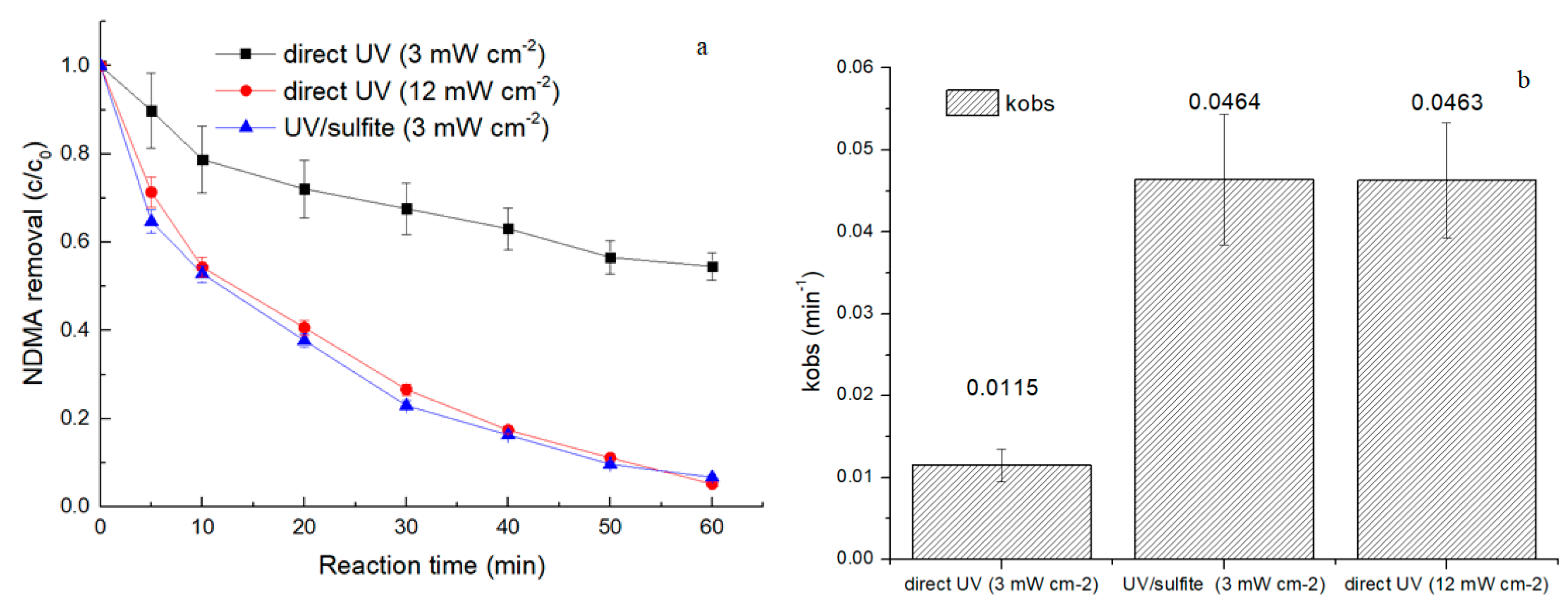
3.1. Comparison between Direct UV Photolysis and UV/Sulfite ARP
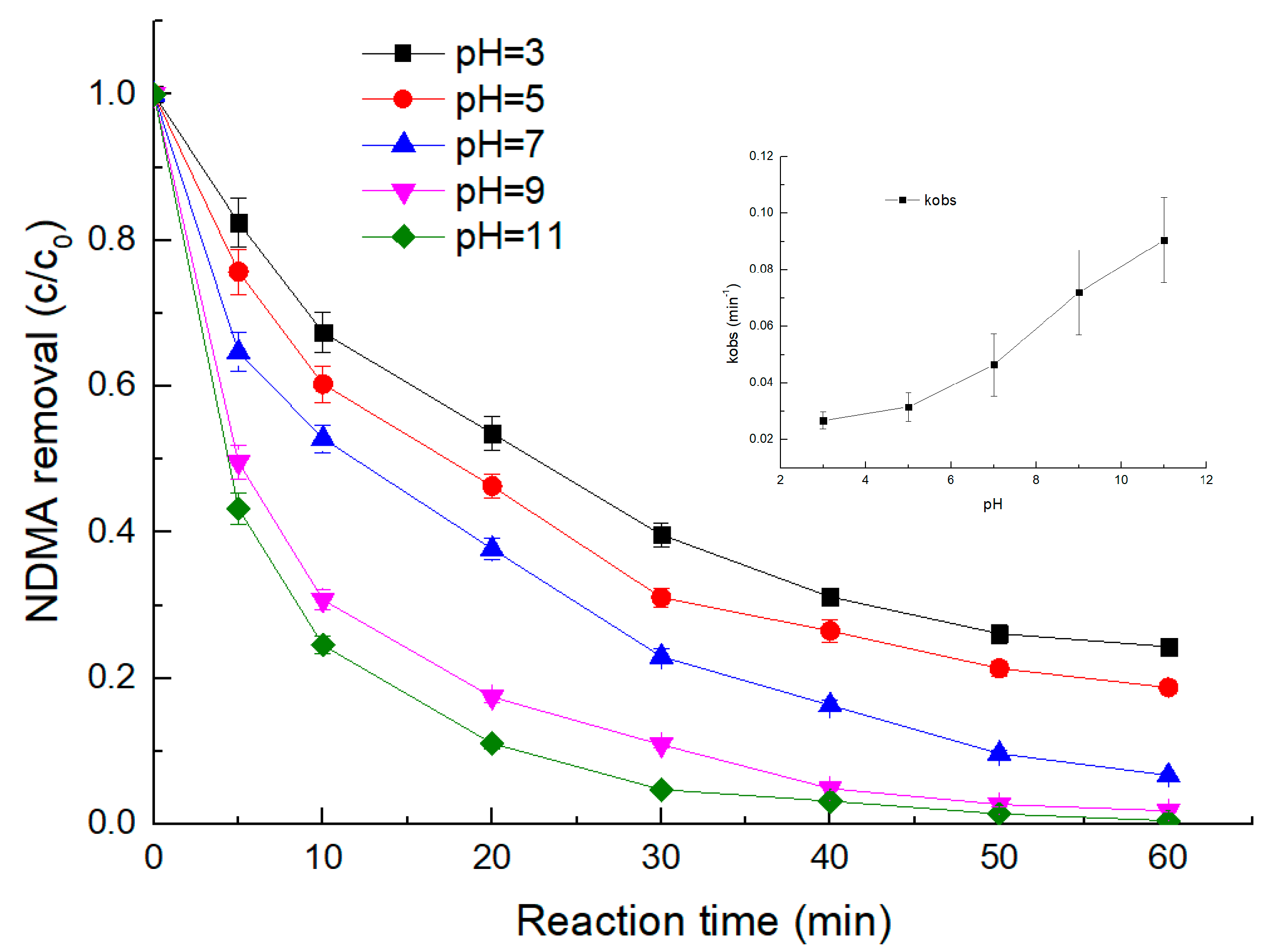
3.2. Effect of pH
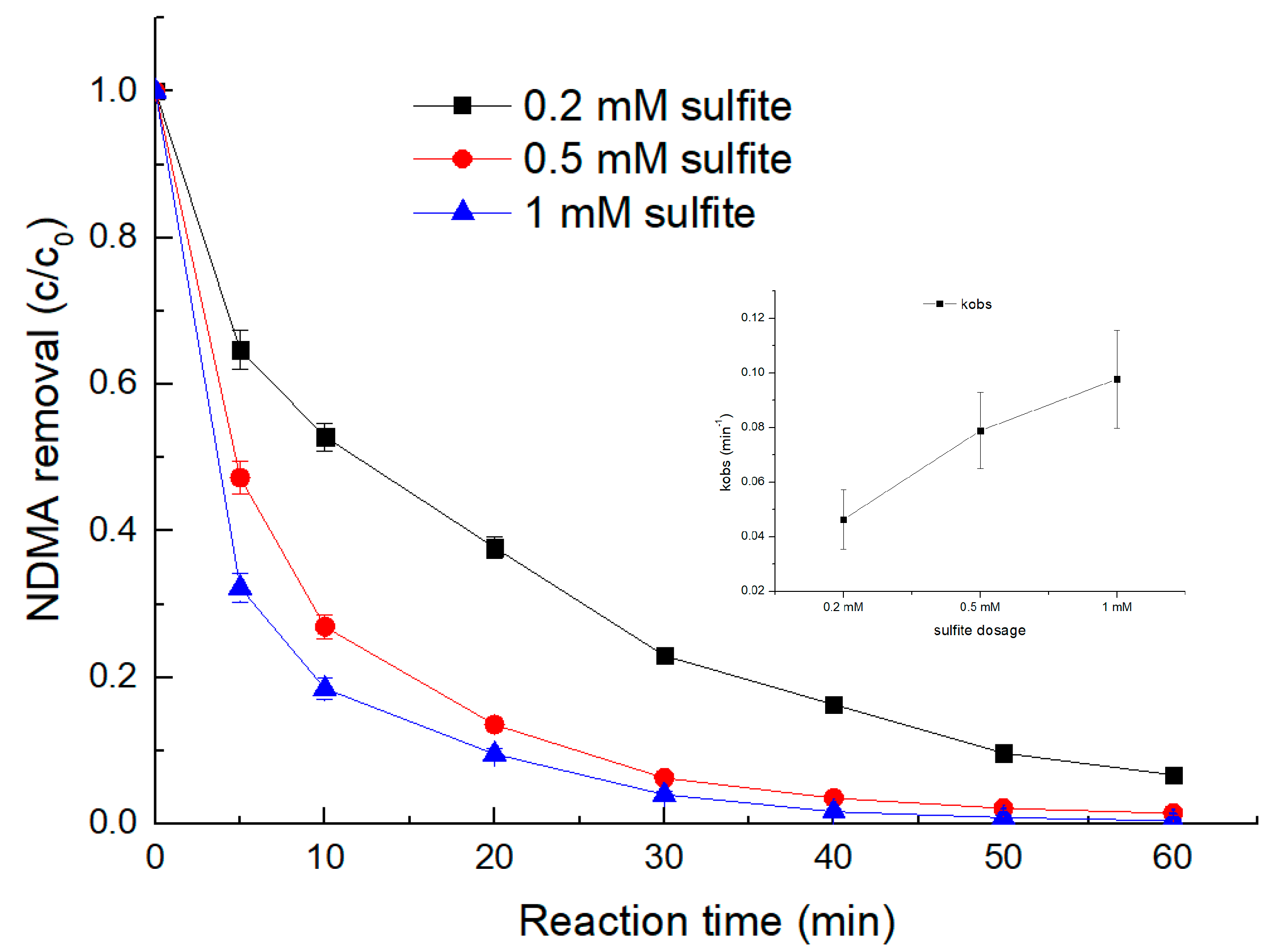
3.3. Effect of Sulfite Dosage
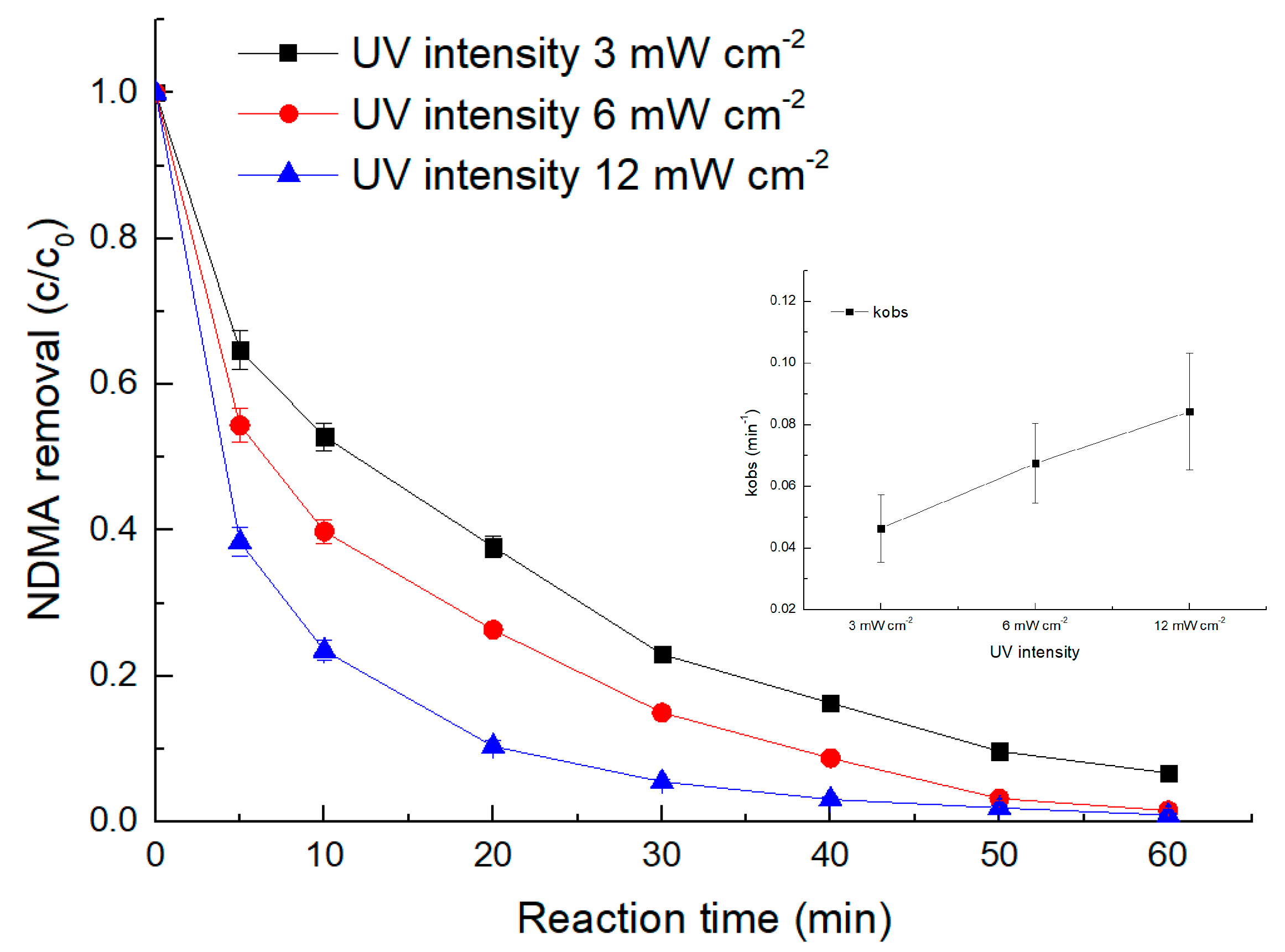
3.4. Effect of UV Intensity
3.5. Effect of Dissolved Oxygen
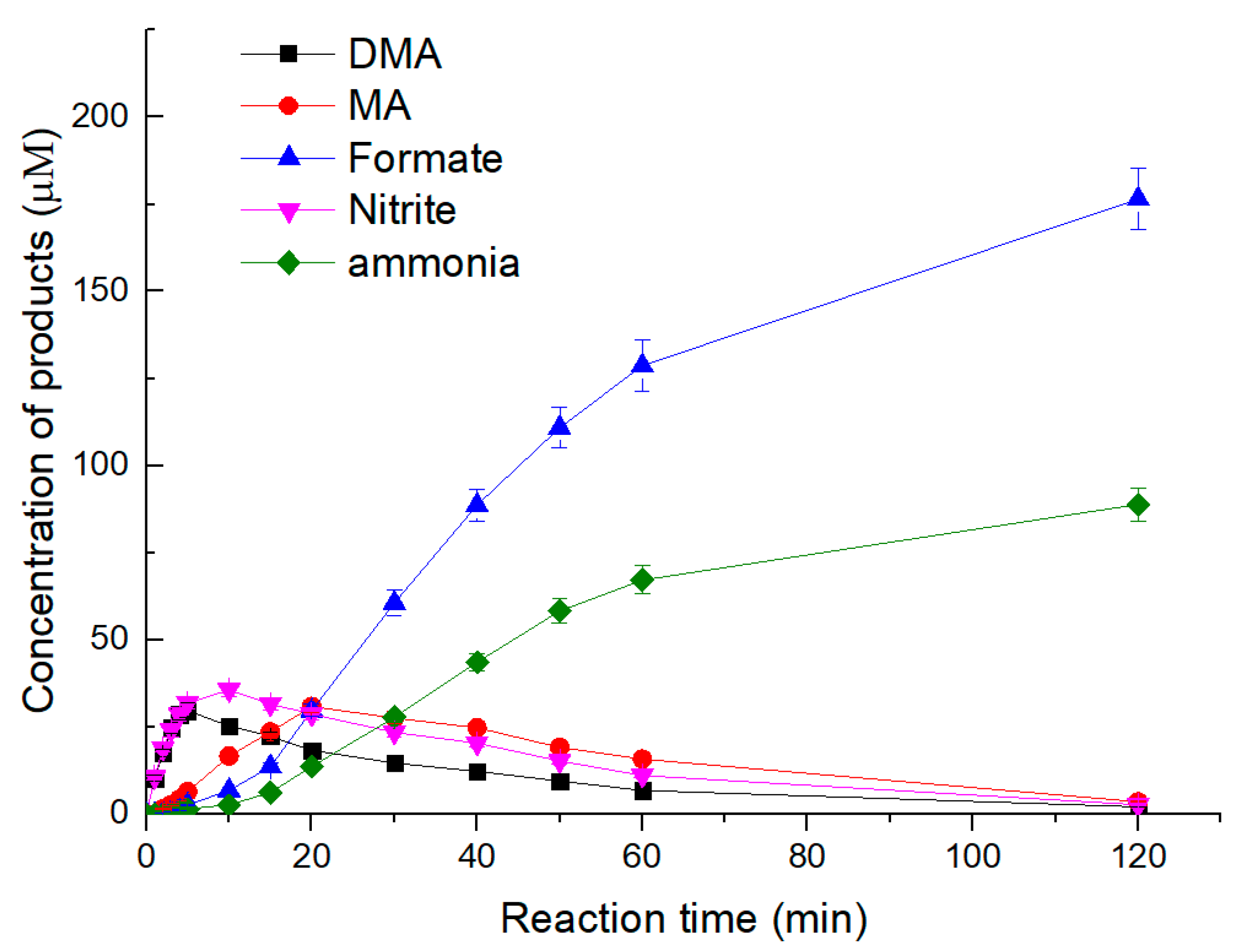
3.6. Degradation Products of NDMA by UV/Sulfite ARP
3.7. Reaction Mechanism
4. Conclusions
- (1)
- The UV/sulfite ARP was an efficient and energy saving method for the reductive degradation of NDMA. An NDMA removal efficiency of 93.29% was achieved via the UV/sulfite ARP, while only 45.48% of NDMA was removed via direct UV photolysis within the same reaction condition.
- (2)
- The degradation of NDMA via the UV/sulfite ARP was favored under alkaline conditions. The removal efficiency of NDMA increased from 21.57% to 66.79% within the initial 5 min of the reaction when the initial pH increased from 3 to 11.
- (3)
- The degradation of NDMA via the UV/sulfite ARP followed pseudo-first-order kinetics. Both increasing the UV light intensity and sulfite dosage led to a proportional increase in the NDMA removal rate.
- (4)
- The presence of dissolved oxygen substantially decreased the removal efficiency of NDMA due to the formation of oxidizing superoxide radicals, which competed with NDMA by capturing the reducing active radicals during the reaction.
- (5)
- The final degradation products of NDMA via the UV/sulfite ARP were formate, ammonia and nitrogen. Some refractory intermediates such as DMA, MA and nitrite were completely decomposed via the UV/sulfite ARP.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Richardson, S.D.; Plewa, M.J.; Wagner, E.D.; Schoeny, R.; DeMarini, D.M. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: A review and roadmap for research. Mutat. Res. 2007, 636, 178–242. [Google Scholar] [CrossRef]
- Plewa, M.J.; Wagner, E.D.; Richardson, S.D. TIC-Tox: A preliminary discussion on identifying the forcing agents of DBP-mediated toxicity of disinfected water. J. Environ. Sci. 2017, 58, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, T.; Masaki, S.; Kodamatani, H.; Ikehata, K. Degradation of N-Nitrosodimethylamine by UV-Based Advanced Oxidation Processes for Potable Reuse: A Short Review. Curr. Pollut. Rep. 2017, 3, 79–87. [Google Scholar] [CrossRef]
- Krasner, S.W.; Mitch, W.A.; McCurry, D.L.; Hanigan, D.; Westerhoff, P. Formation, precursors, control, and occurrence of nitrosamines in drinking water: A review. Water Res. 2013, 47, 4433–4450. [Google Scholar] [CrossRef]
- Luo, Q.; Wang, D.H.; Wang, Z.J. Occurrences of nitrosamines in chlorinated and chloraminated drinking water in three representative cities, China. Sci. Total Environ. 2012, 437, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Prescott, M.; Krasner, S.W.; Guo, Y.B.C. Estimation of NDMA Precursor Loading in Source Water via Artificial Sweetener Monitoring. J. Am. Water Works Assoc. 2017, 109, E243–E251. [Google Scholar] [CrossRef]
- Fujioka, T.; Khan, S.J.; Poussade, Y.; Drewes, J.E.; Nghiem, L.D. N-nitrosamine removal by reverse osmosis for indirect potable water reuse – A critical review based on observations from laboratory-, pilot- and full-scale studies. Sep. Purif. Technol. 2012, 98, 503–515. [Google Scholar] [CrossRef]
- Sgroi, M.; Vagliasindi, F.G.A.; Snyder, S.A.; Roccaro, P. N-Nitrosodimethylamine (NDMA) and its precursors in water and wastewater: A review on formation and removal. Chemosphere 2018, 191, 685–703. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Choi, W.; Kim, Y.G.; Yoon, J. UV Photolytic Mechanism of N-Nitrosodimethylaminein Water: Dual Pathways to Methylamine versus Dimethylamine. Environ. Sci. Technol. 2005, 39, 2101–2106. [Google Scholar] [CrossRef]
- Afzal, A.; Kang, J.; Choi, B.M.; Lim, H.J. Degradation and fate of N-nitrosamines in water by UV photolysis. Int. J. Greenh. Gas Control 2016, 52 (Suppl. C), 44–51. [Google Scholar] [CrossRef]
- Bolton, J.R.; Mayor-Smith, I.; Linden, K.G. Rethinking the Concepts of Fluence (UV Dose) and Fluence Rate: The Importance of Photon-based Units—A Systemic Review. Photochem. Photobiol. 2015, 91, 1252–1262. [Google Scholar] [CrossRef]
- Sakai, H.; Kosaka, K.; Takizawa, S. Degradation of N-Nitrosodimethylamine by Mercury-Free Excimer UV Lamps. Environ. Eng. Sci. 2016, 33, 341–346. [Google Scholar] [CrossRef]
- Li, X.C.; Ma, J.; Liu, G.F.; Fang, J.Y.; Yue, S.Y.; Guan, Y.H.; Chen, L.W.; Liu, X.W. Efficient Reductive Dechlorination of Monochloroacetic Acid by Sulfite/UV Process. Environ. Sci. Technol. 2012, 46, 7342–7349. [Google Scholar] [CrossRef]
- Vellanki, B.P.; Batchelor, B.; Abdel-Wahab, A. Advanced Reduction Processes: A New Class of Treatment Processes. Environ. Eng. Sci. 2013, 30, 264–271. [Google Scholar] [CrossRef]
- Botlaguduru, V.S.V.; Batchelor, B.; Abdel-Wahab, A. Application of UV-sulfite advanced reduction process to bromate removal. J. Water Proc. Eng. 2015, 5, 76–82. [Google Scholar] [CrossRef]
- Jung, B.; Nicola, R.; Batchelor, B.; Abdel-Wahab, A. Effect of low- and medium-pressure Hg UV irradiation on bromate removal in advanced reduction process. Chemosphere 2014, 117, 663–672. [Google Scholar] [CrossRef]
- Bensalah, N.; Nicola, R.; Abdel-Wahab, A. Nitrate removal from water using UV-M/S2O42− advanced reduction process. Int. J. Environ. Sci. Technol. 2014, 11, 1733–1742. [Google Scholar] [CrossRef]
- Cui, J.K.; Gao, P.P.; Deng, Y. Destruction of Per- and Polyfluoroalkyl Substances (PFAS) with Advanced Reduction Processes (ARPs): A Critical Review. Environ. Sci. Technol. 2020, 54, 3752–3766. [Google Scholar] [CrossRef]
- Sun, Z.Y.; Zhang, C.J.; Zhao, X.Y.; Chen, J.; Zhou, Q. Efficient photoreductive decomposition of N-nitrosodimethylamine by UV/iodide process. J. Hazard. Mater. 2017, 329, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.Y.; Xu, L.L.J.; Gan, L.; Qiao, W.C.; Han, J.G.; Mei, X.; Guo, H.; Li, W.; Pei, C.; Gong, H.; et al. Efficient destruction of emerging contaminants in water by UV/S(IV) process with natural reoxygenation: Effect of pH on reactive species. Water Res. 2021, 198, 117143. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Warneck, P. Photodecomposition and photooxidation of hydrogen sulfite in aqueous solution. J. Phys. Chem. 1996, 100, 15111–15117. [Google Scholar] [CrossRef]
- Ye, T.; Wei, Z.; Spinney, R.; Tang, C.J.; Luo, S.; Xiao, R.; Dionysiou, D.D. Chemical structure-based predictive model for the oxidation of trace organic contaminants by sulfate radical. Water Res. 2017, 116, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Velo-Gala, I.; Farre, M.J.; Radjenovic, J.; Gernjak, W. N-Nitrosodimethylamine (NDMA) Degradation by the Ultraviolet/Peroxodisulfate Process. Environ. Sci. Technol. 2019, 6, 106–111. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zha, X.; Wang, S.; Zhang, D. Reductive Degradation of N-Nitrosodimethylamine via UV/Sulfite Advanced Reduction Process: Efficiency, Influencing Factors and Mechanism. Water 2023, 15, 3670. https://doi.org/10.3390/w15203670
Zha X, Wang S, Zhang D. Reductive Degradation of N-Nitrosodimethylamine via UV/Sulfite Advanced Reduction Process: Efficiency, Influencing Factors and Mechanism. Water. 2023; 15(20):3670. https://doi.org/10.3390/w15203670
Chicago/Turabian StyleZha, Xiaosong, Shuren Wang, and Deyu Zhang. 2023. "Reductive Degradation of N-Nitrosodimethylamine via UV/Sulfite Advanced Reduction Process: Efficiency, Influencing Factors and Mechanism" Water 15, no. 20: 3670. https://doi.org/10.3390/w15203670
APA StyleZha, X., Wang, S., & Zhang, D. (2023). Reductive Degradation of N-Nitrosodimethylamine via UV/Sulfite Advanced Reduction Process: Efficiency, Influencing Factors and Mechanism. Water, 15(20), 3670. https://doi.org/10.3390/w15203670







