Synthesis and Characterization of Nanometal Oxide-Biochar Derived from Date Palm Waste for Adsorption of Manganese and Iron from Contaminated Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Production of Raw Biochar
2.3. Preparation of Nanometal Oxide-Biochar (NMO)
2.4. Characterization
2.5. Static Adsorption Process of Manganese and Iron
2.6. Modeling for Adsorption Process
2.6.1. Isotherm Modeling
2.6.2. Kinetic Modeling
2.6.3. Column Modeling
3. Results and Discussion
3.1. Surface and Chemical Analysis of NMO and Biochar
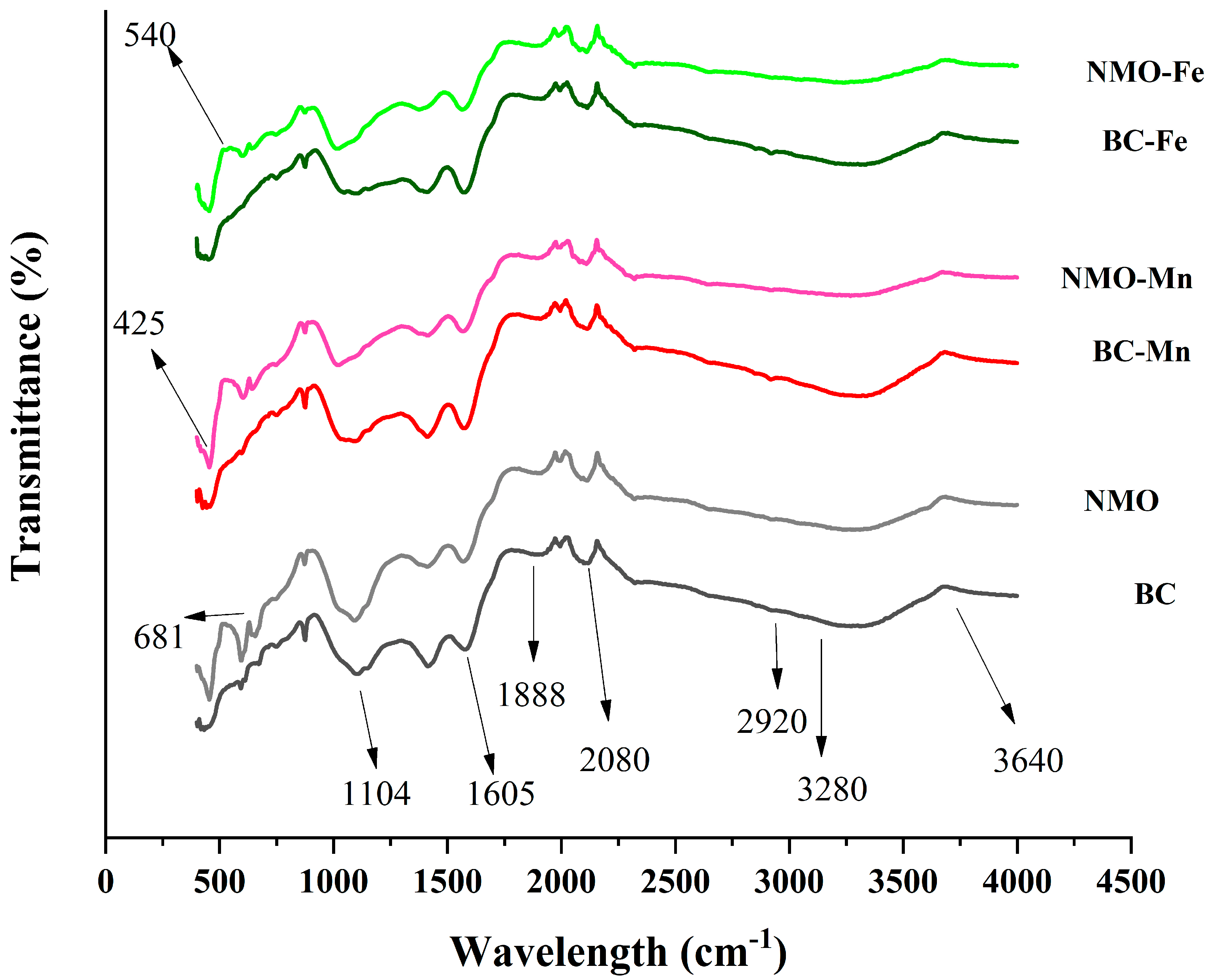
3.1.1. FTIR Spectroscopic Analysis
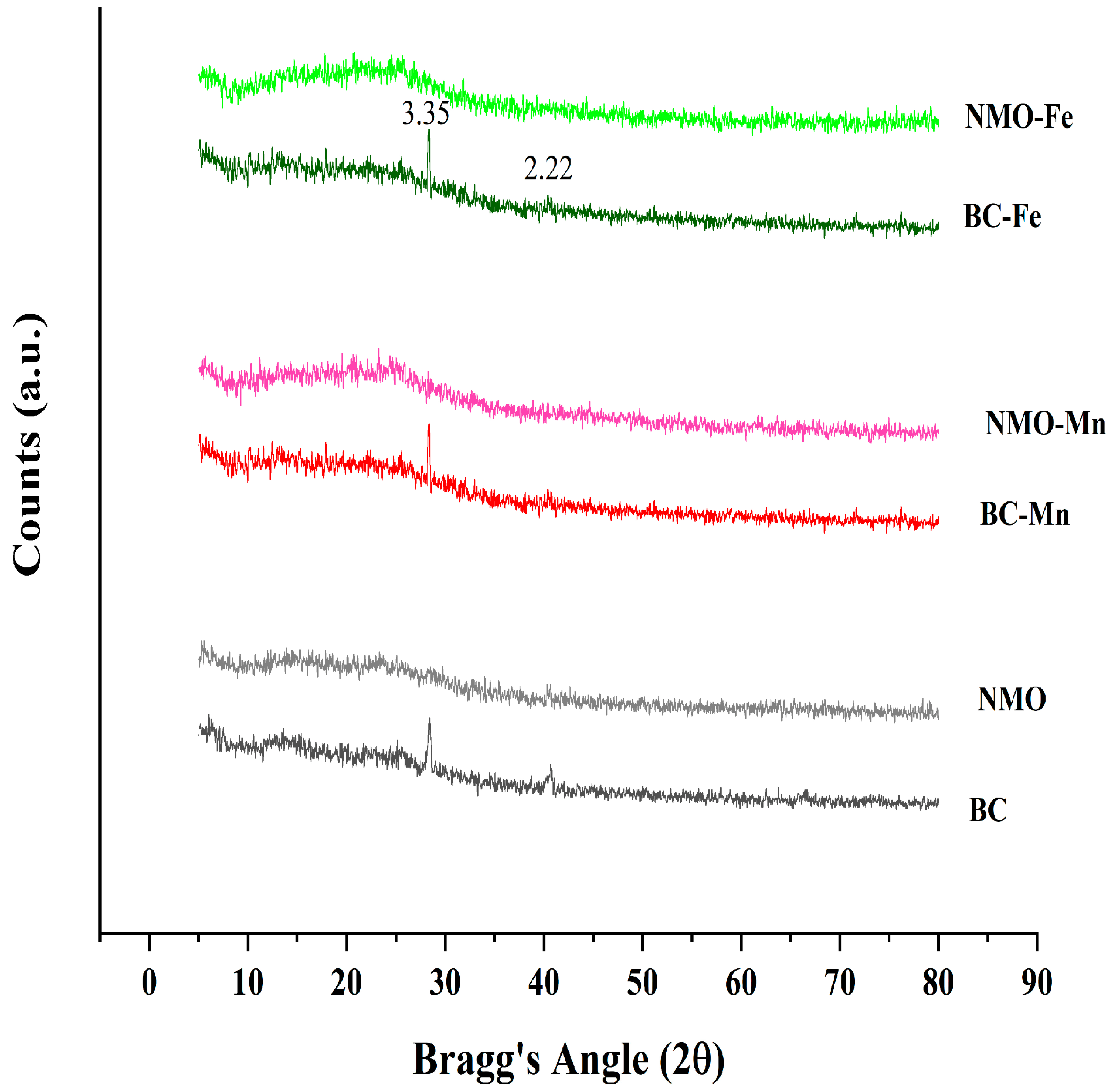
3.1.2. XRD Analysis
3.1.3. SEM and EDX Analysis
3.1.4. BET Surface Area of the NMO and Biochar
3.2. Static Study for Manganese and Iron
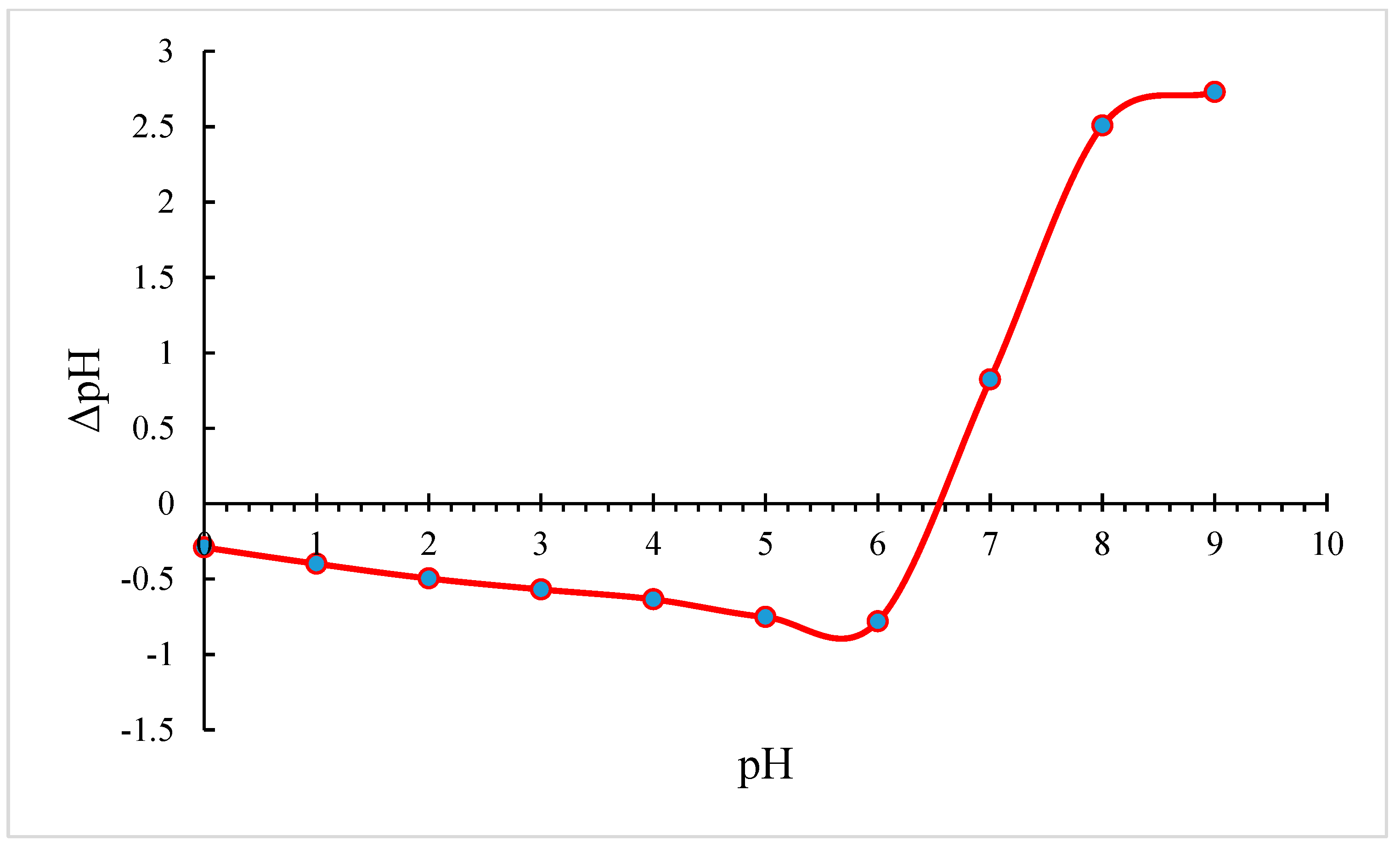
3.2.1. Impact of pH on Manganese and Iron on NMO and BC
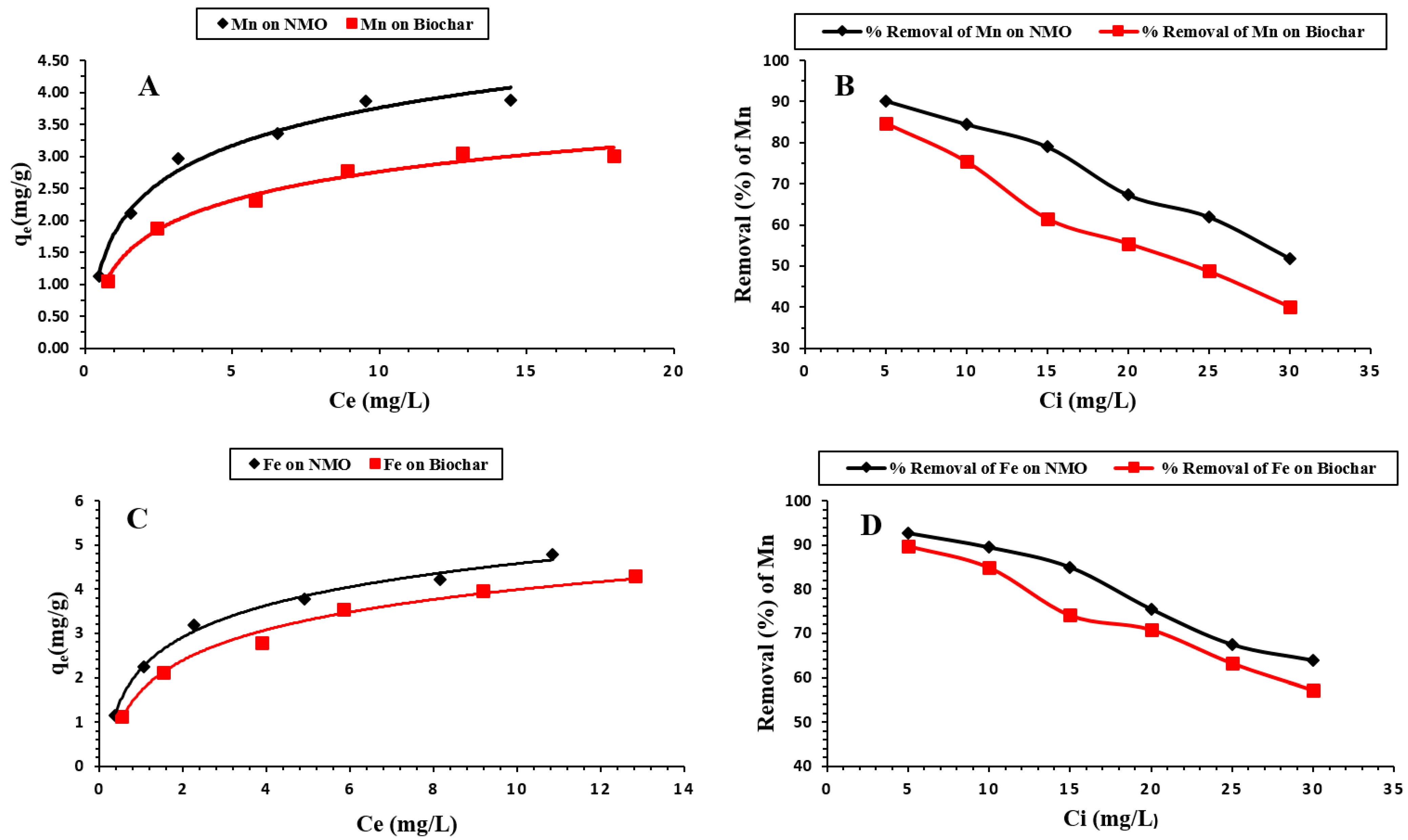
3.2.2. Impact of Initial Manganese and Iron Concentrations on NMO and BC
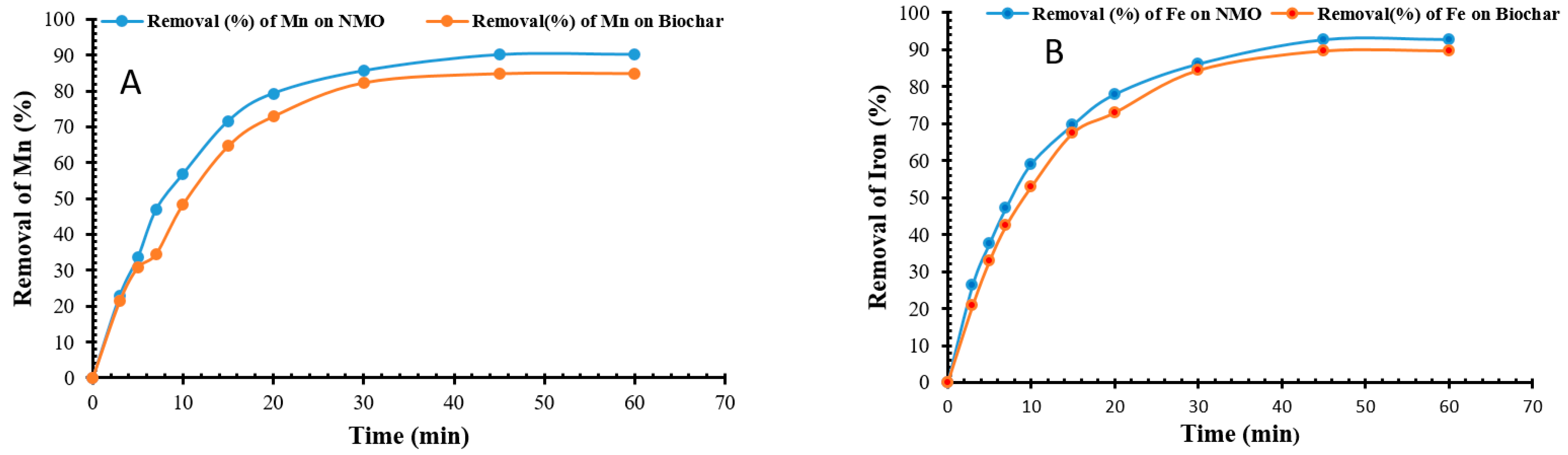
3.2.3. Impact of Contact Time on NMO and BC
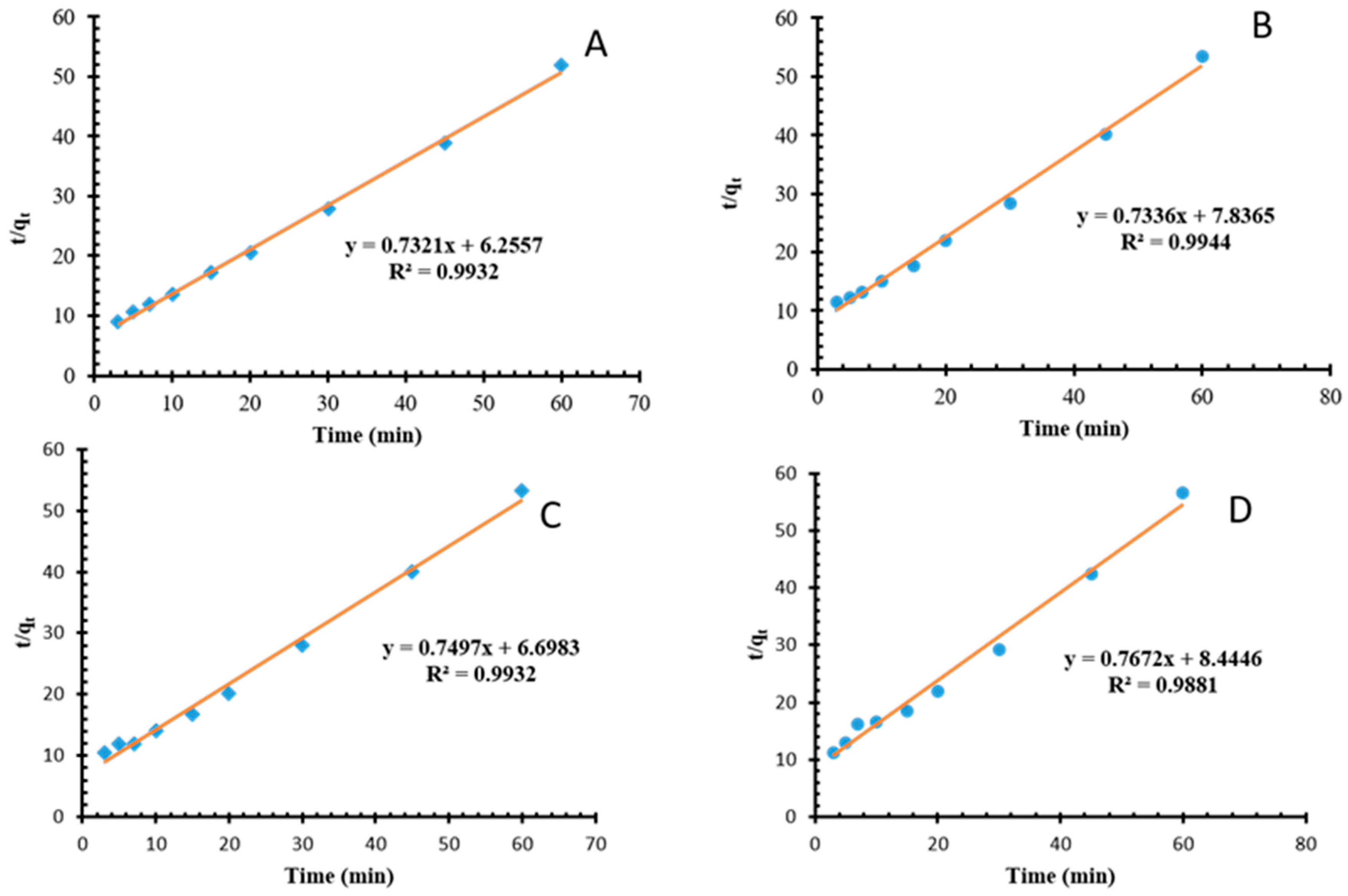
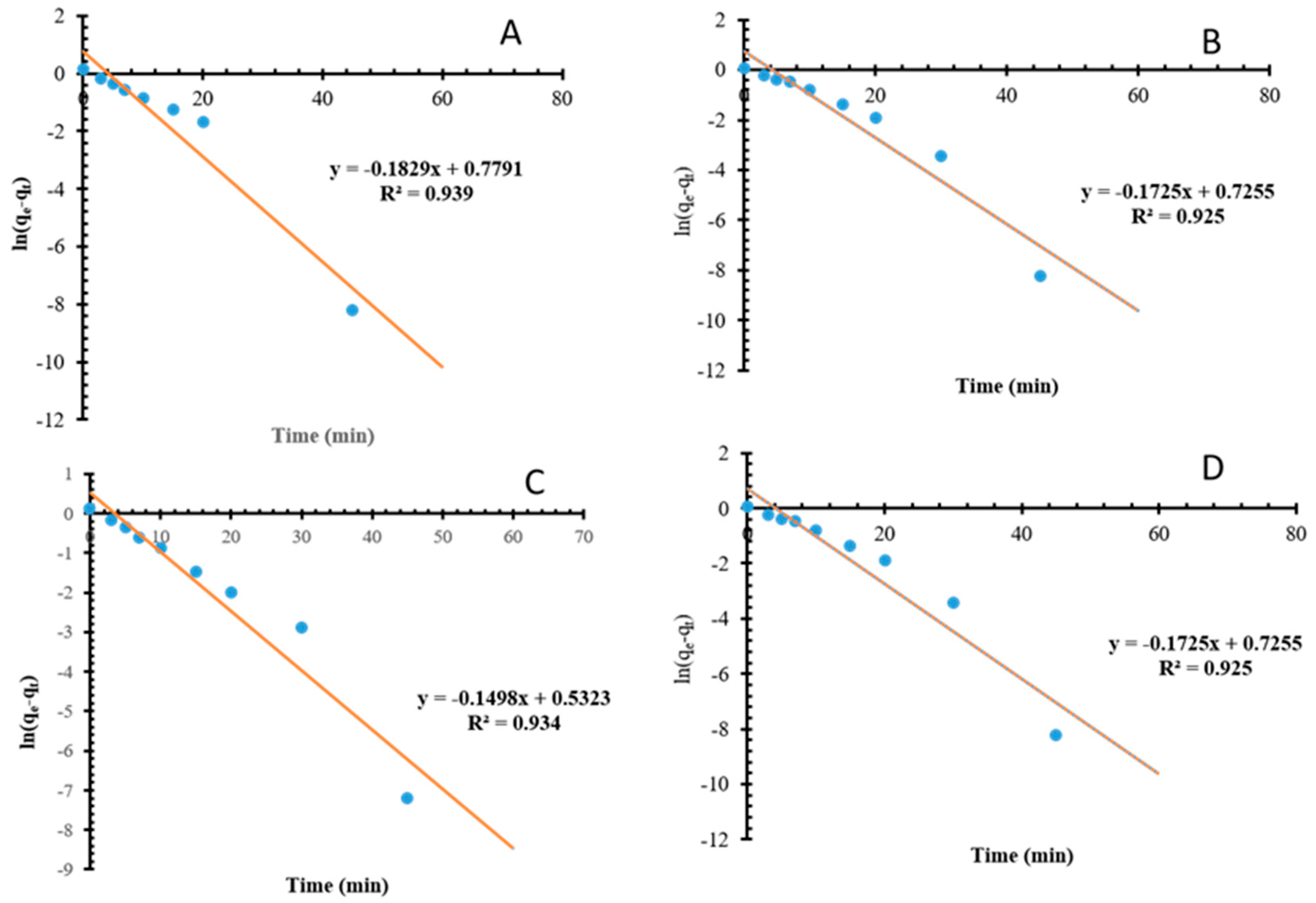
3.2.4. Kinetic Modeling of Mn and Fe on NMO and Biochar
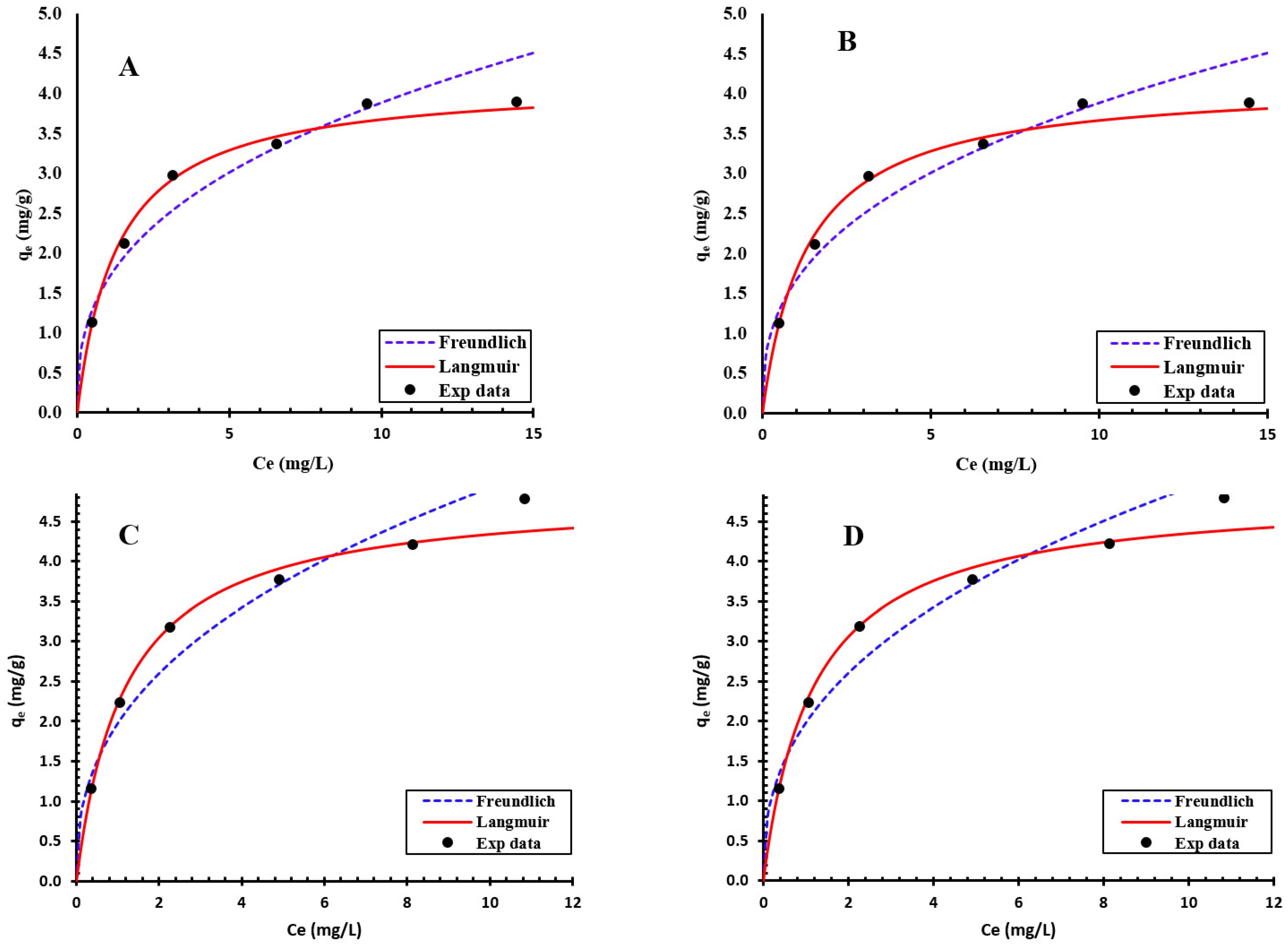
3.3. Isotherm Modeling
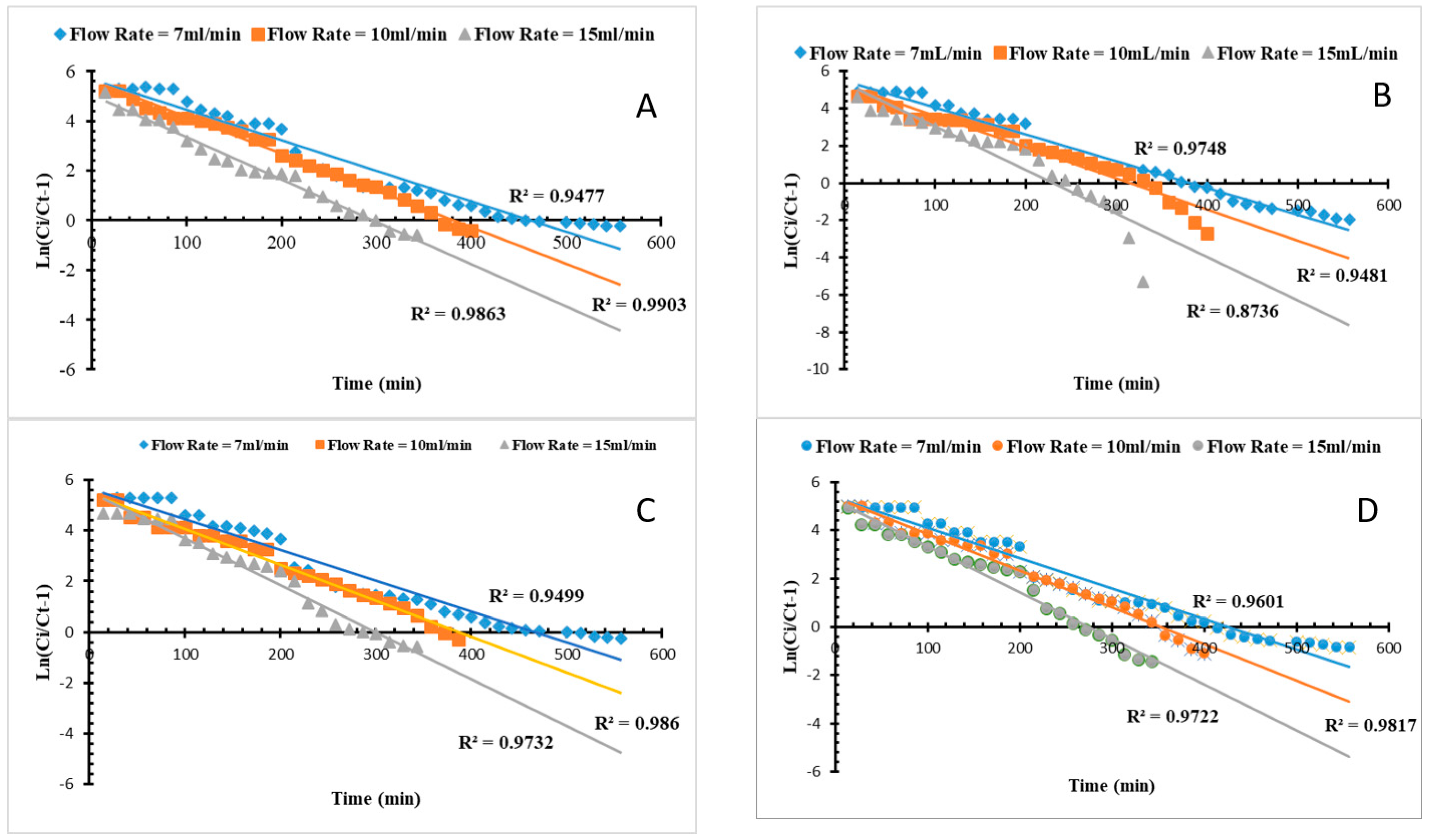
3.4. Column Modeling for Mn and Fe Sorption
Thomas and Adams–Bohart Model Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ce | Equilibrium concentration (mg·L−1) |
| Ci | Initial concentration (mg·L−1) |
| Ct | Concentration at the time (mg·L−1) |
| DPW | Date palm waste |
| K1 | Rate constant (min−1) |
| K2 | Rate constant (g·mg−1·min−1) |
| Kab | Adams–Bohart kinetic constant (mg·L−1) |
| Kf | Freundlich Adsorption (mg/g) |
| Kth | Kinetic coefficient for Thomas Model (L/mg·mn) |
| M | Mass of adsorbent (g) |
| N | Adsorbent intensity |
| No | Saturation concentration (mg·L−1) |
| BC | Biochar |
| NMO | Nanometal Oxide Biochar |
| Qo | Maximum monolayer adsorption (mg/g) |
| qe | Quantity of solute adsorbed (mg/g) |
| qt | Solute adsorbed a t time (mg/g) |
| Qth | Flow rate (mL·min−1) |
| qth | Thomas’s adsorption capacity (mg/g) |
| RL | Langmuir isotherm constant |
| T | Time (min) |
| V | Flow rate (mL/min) |
| vL | Linear velocity (cm/s) |
| Z | Depth of column (cm) |
Appendix A
References
- Akl, M.A.; Yousef, A.M.; AbdElnasser, S. Removal of Iron and Manganese in Water Samples Using Activated Carbon Derived from Local Agro-Residues. J. Chem. Eng. Process Technol. 2013, 4, 1–10. [Google Scholar] [CrossRef]
- Zareh, M.M.; Keshk, A.A. Chemical Studies on the Water Quality in Tabuk City, Saudi Arabia. Int. J. Environ. Agric. Res. 2016, 2, 27–37. [Google Scholar]
- WHO Guidelines for Drinking-Water Quality, 4th Edition, Incorporating the 1st Addendum. WHO. Int. Available online: https://www.who.int/publications/i/item/9789241549950 (accessed on 2 July 2023).
- Kwakye-Awuah, B.; Sefa-Ntiri, B.; Von-Kiti, E.; Nkrumah, I.; Williams, C. Adsorptive Removal of Iron and Manganese from Groundwater Samples in Ghana by Zeolite Y Synthesized from Bauxite and Kaolin. Water 2019, 11, 1912. [Google Scholar] [CrossRef]
- Sizirici, B.; Fseha, Y.H.; Yildiz, I.; Delclos, T.; Khaleel, A. The Effect of Pyrolysis Temperature and Feedstock on Date Palm Waste Derived Biochar to Remove Single and Multi-Metals in Aqueous Solutions. Sustain. Environ. Res. 2021, 31, 1–16. [Google Scholar] [CrossRef]
- Akbari Zadeh, M.; Daghbandan, A.; Abbasi Souraki, B. Removal of Iron and Manganese from Groundwater Sources Using Nano-Biosorbents. Chem. Biol. Technol. Agric. 2022, 9, 3. [Google Scholar] [CrossRef]
- Basak, S.; Das, R.K.; Banerjee, A.; Paul, S.; Pathak, S.; Duttaroy, A.K. Maternal Obesity and Gut Microbiota Are Associated with Fetal Brain Development. Nutrients 2022, 14, 4515. [Google Scholar] [CrossRef] [PubMed]
- Fseha, Y.H.; Sizirici, B.; Yildiz, I. Manganese and Nitrate Removal from Groundwater Using Date Palm Biochar: Application for Drinking Water. Environ. Adv. 2022, 8, 100237. [Google Scholar] [CrossRef]
- Nazal Al-Shemary, A.; Dawi Al-Otabi, K.; Mosa Al-Omran, A. Analytical Groundwater Quality Assessment for Drinking and Agriculture Purposes in Al-Jouf Region, Kingdom of Saudi Arabia. Int. J. Plant Soil Sci. 2022, 34, 1–17. [Google Scholar] [CrossRef]
- Therdkiattikul, N.; Ratpukdi, T.; Kidkhunthod, P.; Chanlek, N.; Siripattanakul-Ratpukdi, S. Manganese-Contaminated Groundwater Treatment by Novel Bacterial Isolates: Kinetic Study and Mechanism Analysis Using Synchrotron-Based Techniques. Sci. Rep. 2020, 10, 13391. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, S.; Du, X.; Cheng, X.; Fu, M.; Hou, N.; Li, D. Immobilization of Iron-and Manganese-Oxidizing Bacteria with a Biofilm-Forming Bacterium for the Effective Removal of Iron and Manganese from Groundwater. Bioresour. Technol. 2016, 220, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Homoncik, S.C.; MacDonald, A.M.; Heal, K.V.; Dochartaigh, B.É.Ó.; Ngwenya, B.T. Manganese Concentrations in Scottish Groundwater. Sci. Total Environ. 2010, 408, 2467–2473. [Google Scholar] [CrossRef]
- Wasserman, G.A.; Liu, X.; Parvez, F.; Ahsan, H.; Levy, D.; Factor-Litvak, P.; Kline, J.; van Geen, A.; Slavkovich, V.; LoIacono, N.J. Water Manganese Exposure and Children’s Intellectual Function in Araihazar, Bangladesh. Environ. Health Perspect. 2006, 114, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Ayati, A.; Tanhaei, B.; Beiki, H.; Krivoshapkin, P.; Krivoshapkina, E.; Tracey, C. Insight into the Adsorptive Removal of Ibuprofen Using Porous Carbonaceous Materials: A Review. Chemosphere 2023, 323, 138241. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.I.; Ayati, A.; Farghali, M.; Krivoshapkin, P.; Tanhaei, B.; Karimi-Maleh, H.; Krivoshapkina, E.; Taheri, P.; Tracey, C.; Al-Fatesh, A.; et al. Advanced Adsorbents for Ibuprofen Removal from Aquatic Environments: A Review; Springer International Publishing: Berlin/Heidelberg, Germany, 2023; ISBN 0123456789. [Google Scholar]
- Pakade, V.E.; Tavengwa, N.T.; Madikizela, L.M. Recent Advances in Hexavalent Chromium Removal from Aqueous Solutions by Adsorptive Methods. RSC Adv. 2019, 9, 26142–26164. [Google Scholar] [CrossRef]
- Lingamdinne, L.P.; Choi, J.-S.; Angaru, G.K.R.; Karri, R.R.; Yang, J.-K.; Chang, Y.-Y.; Koduru, J.R. Magnetic-Watermelon Rinds Biochar for Uranium-Contaminated Water Treatment Using an Electromagnetic Semi-Batch Column with Removal Mechanistic Investigations. Chemosphere 2022, 286, 131776. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.S.; Wong, C.S. A New Method for Heavy Metals and Aluminium Detection Using Biopolymer-Based Optical Biosensor. IEEE Sens. J. 2014, 15, 471–475. [Google Scholar] [CrossRef]
- Li, H.; Xiong, J.; Xiao, T.; Long, J.; Wang, Q.; Li, K.; Liu, X.; Zhang, G.; Zhang, H. Biochar Derived from Watermelon Rinds as Regenerable Adsorbent for Efficient Removal of Thallium (I) from Wastewater. Process Saf. Environ. Prot. 2019, 127, 257–266. [Google Scholar] [CrossRef]
- Daňo, M.; Viglašová, E.; Štamberg, K.; Galamboš, M.; Galanda, D. Pertechnetate/Perrhenate Surface Complexation on Bamboo Engineered Biochar. Materials 2021, 14, 486. [Google Scholar] [CrossRef] [PubMed]
- Teo, S.C.; Wong, L.S. Whole Cell-Based Biosensors for Environmental Heavy Metals Detection. Annu. Res. Rev. Biol. 2014, 4, 2663–2674. [Google Scholar] [CrossRef]
- Idrees, M.; Batool, S.; Ullah, H.; Hussain, Q.; Al-Wabel, M.I.; Ahmad, M.; Hussain, A.; Riaz, M.; Ok, Y.S.; Kong, J. Adsorption and Thermodynamic Mechanisms of Manganese Removal from Aqueous Media by Biowaste-Derived Biochars. J. Mol. Liq. 2018, 266, 373–380. [Google Scholar] [CrossRef]
- Bakly, S.; Al-Juboori, R.A.; Bowtell, L. Macadamia Nutshell Biochar for Nitrate Removal: Effect of Biochar Preparation and Process Parameters. C 2019, 5, 47. [Google Scholar] [CrossRef]
- Yahya, M.D.; Aliyu, A.S.; Obayomi, K.S.; Olugbenga, A.; Abdullahi, U. Column Adsorption Study for the Removal of Chromium and Manganese Ions from Electroplating Wastewater Using Cashew Nutshell Adsorbent. Cogent Eng. 2020, 7, 1748470. [Google Scholar] [CrossRef]
- Marcelo, L.R.; de Gois, J.S.; da Silva, A.A.; Cesar, D.V. Synthesis of Iron-Based Magnetic Nanocomposites and Applications in Adsorption Processes for Water Treatment: A Review. Environ. Chem. Lett. 2021, 19, 1229–1274. [Google Scholar] [CrossRef]
- Jiang, M.; Yang, W.; Zhang, Z.; Yang, Z.; Wang, Y. Adsorption of Three Pharmaceuticals on Two Magnetic Ion-Exchange Resins. J. Environ. Sci. 2015, 31, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Faiad, A.; Alsmari, M.; Ahmed, M.M.Z.; Bouazizi, M.L.; Alzahrani, B.; Alrobei, H. Date Palm Tree Waste Recycling: Treatment and Processing for Potential Engineering Applications. Sustainability 2022, 14, 1134. [Google Scholar] [CrossRef]
- Antal, M.J.; Grønli, M. The Art, Science, and Technology of Charcoal Production. Ind. Eng. Chem. Res. 2003, 42, 1619–1640. [Google Scholar] [CrossRef]
- Dixit, A.; Gupta, S.; Dai Pang, S.; Kua, H.W. Waste Valorisation Using Biochar for Cement Replacement and Internal Curing in Ultra-High Performance Concrete. J. Clean. Prod. 2019, 238, 117876. [Google Scholar] [CrossRef]
- Alrowais, R.; Bashir, M.T.; Sikandar, M.A.; Khan, M.A. Synthesis and Performance Evaluation of Olive Fruit Waste Resin for Removal of Fluoride from Aqueous Solution: Batch and Column Modeling. Desalination Water Treat. 2022, 252, 261–275. [Google Scholar] [CrossRef]
- Bashir, M.T.; Ali, S.; Idris, A.; Harun, R. Kinetic and Thermodynamic Study of Nitrate Adsorption from Aqueous Solution by Lignocellulose-Based Anion Resins. Desalination Water Treat. 2017, 62, 20136. [Google Scholar] [CrossRef]
- Noura, A.L.; Abu-Sharar, T.M. Kinetics of Fluoride Adsorption onto Native and Mg(OH)2-Amended Limestone. Appl. Water Sci. 2021, 11, 37. [Google Scholar] [CrossRef]
- Mondal, A.; Dubey, B.K.; Arora, M.; Mumford, K. Porous Media Transport of Iron Nanoparticles for Site Remediation Application: A Review of Lab Scale Column Study, Transport Modelling and Field-Scale Application. J. Hazard. Mater. 2021, 403, 123443. [Google Scholar] [CrossRef] [PubMed]
- Keränen, A.; Leiviskä, T.; Hormi, O.; Tanskanen, J. Removal of Nitrate by Modified Pine Sawdust: Effects of Temperature and Co-Existing Anions. J. Environ. Manag. 2015, 147, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Chia, C.H.; Gong, B.; Joseph, S.D.; Marjo, C.E.; Munroe, P.; Rich, A.M. Imaging of Mineral-Enriched Biochar by FTIR, Raman and SEM–EDX. Vib. Spectrosc. 2012, 62, 248–257. [Google Scholar] [CrossRef]
- Joe, I.H.; Vasudevan, A.K.; Aruldhas, G.; Damodaran, A.D.; Warrier, K.G.K. FTIR as a Tool to Study High-Temperature Phase Formation in Sol–Gel Aluminium Titanate. J. Solid State Chem. 1997, 131, 181–184. [Google Scholar] [CrossRef]
- Pal, M.; Roy, B.; Pal, M. Structural Characterization of Borate Glasses Containing Zinc and Manganese Oxides. J. Mod. Phys. 2011, 2, 1062–1066. [Google Scholar] [CrossRef]
- Yakabuskie, P.A.; Joseph, J.M.; Keech, P.; Botton, G.A.; Guzonas, D.; Wren, J.C. Iron Oxyhydroxide Colloid Formation by Gamma-Radiolysis. Phys. Chem. Chem. Phys. 2011, 13, 7198–7206. [Google Scholar] [CrossRef]
- Wu, W.; Yang, M.; Feng, Q.; McGrouther, K.; Wang, H.; Lu, H.; Chen, Y. Chemical Characterization of Rice Straw-Derived Biochar for Soil Amendment. Biomass Bioenergy 2012, 47, 268–276. [Google Scholar] [CrossRef]
- Medellin-Castillo, N.A.; Leyva-Ramos, R.; Padilla-Ortega, E.; Perez, R.O.; Flores-Cano, J.V.; Berber-Mendoza, M.S. Adsorption Capacity of Bone Char for Removing Fluoride from Water Solution. Role of Hydroxyapatite Content, Adsorption Mechanism and Competing Anions. J. Ind. Eng. Chem. 2014, 20, 4014–4021. [Google Scholar] [CrossRef]
- Cedrún-Morales, M.; Ceballos, M.; Polo, E.; Del Pino, P.; Pelaz, B. Nanosized Metal–Organic Frameworks as Unique Platforms for Bioapplications. Chem. Commun. 2023, 59, 2869–2887. [Google Scholar] [CrossRef] [PubMed]
- Rehman, I.U.; Zahoor, M.; Bashir, M.T.; Ali, J.; Khan, F.A.; Sher, M. Removal of Cu2+ from Aqueous Solution by Activated Carbon Prepared from Sawdust and Nutshells. Desalination Water Treat. 2018, 126, 171–180. [Google Scholar] [CrossRef]
- Gorgievski, M.; Božić, D.; Stanković, V.; Štrbac, N.; Šerbula, S. Kinetics, Equilibrium and Mechanism of Cu2+, Ni2+ and Zn2+ Ions Biosorption Using Wheat Straw. Ecol. Eng. 2013, 58, 113–122. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, J.; He, T.; Wu, J. Corn Stalks Char from Fast Pyrolysis as Precursor Material for Preparation of Activated Carbon in Fluidized Bed Reactor. Bioresour. Technol. 2014, 167, 551–554. [Google Scholar] [CrossRef]
- Han, R.; Wang, Y.; Zhao, X.; Wang, Y.; Xie, F.; Cheng, J.; Tang, M. Adsorption of Methylene Blue by Phoenix Tree Leaf Powder in a Fixed-Bed Column: Experiments and Prediction of Breakthrough Curves. Desalination 2009, 245, 284–297. [Google Scholar] [CrossRef]
- Jahangiri-Rad, M.; Jamshidi, A.; Rafiee, M.; Nabizadeh, R. Adsorption Performance of Packed Bed Column for Nitrate Removal Using PAN-Oxime-Nano Fe2O3. J. Environ. Health Sci. Eng. 2014, 12, 90. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, M.M. Water Chemistry; Waveland Press: Long Grove, IL, USA, 2014; ISBN 1478627018. [Google Scholar]
- Fu, F.; Wang, Q. Removal of Heavy Metal Ions from Wastewaters: A Review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Vries, D.; Bertelkamp, C.; Kegel, F.S.; Hofs, B.; Dusseldorp, J.; Bruins, J.H.; De Vet, W.; Van den Akker, B. Iron and Manganese Removal: Recent Advances in Modelling Treatment Efficiency by Rapid Sand Filtration. Water Res. 2017, 109, 35–45. [Google Scholar] [CrossRef] [PubMed]

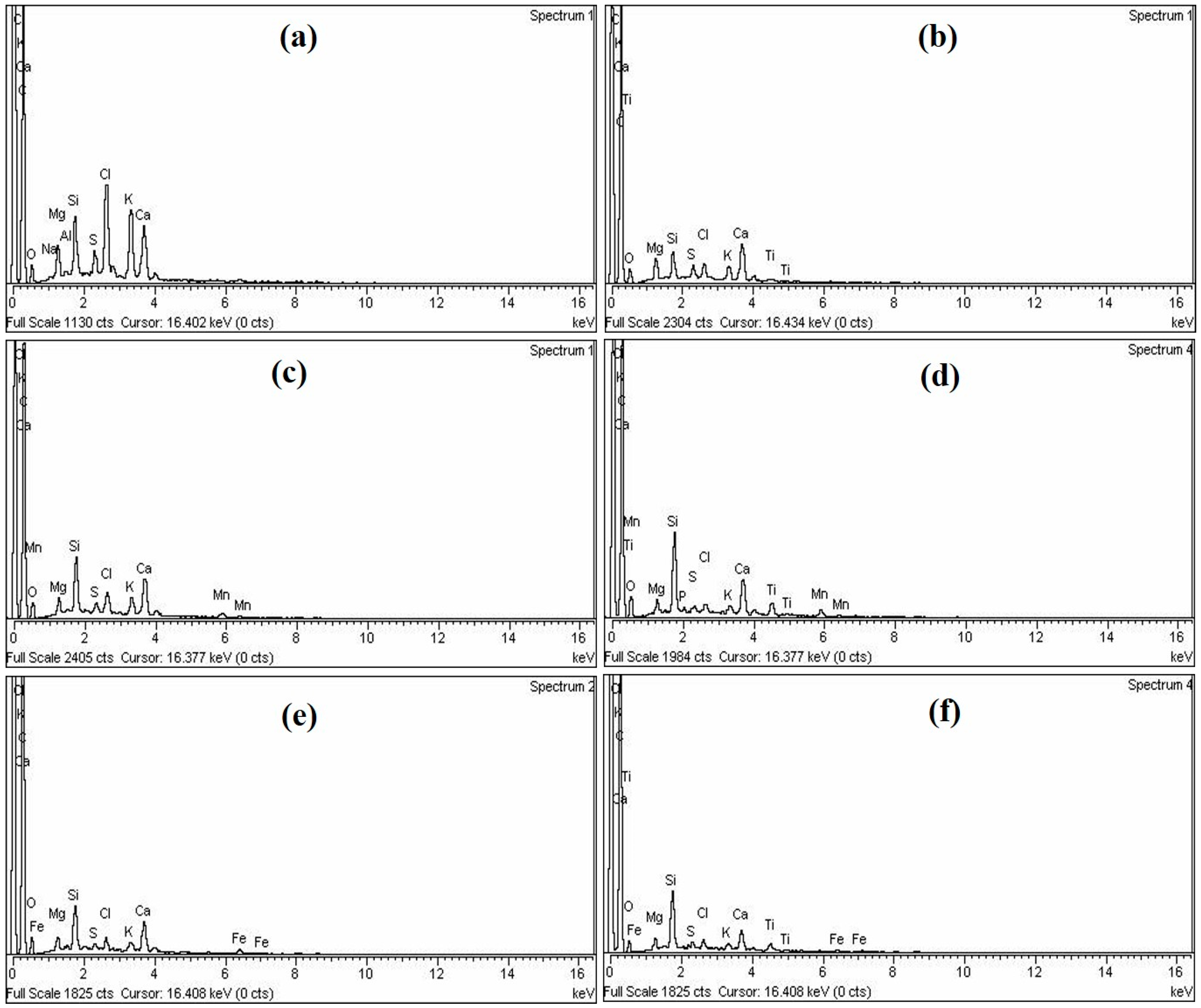


| Description | C | O | Si | Cl | K | Ca | Mn | Fe | Ti |
|---|---|---|---|---|---|---|---|---|---|
| BC | 75.68 | 7.32 | 2.09 | 4.77 | 4.17 | 3.44 | --------- | --------- | -------- |
| NMO | 77.82 | 11.47 | 1.59 | 1.12 | 1.29 | 4.06 | --------- | --------- | 0.33 |
| BC-Mn | 78.60 | 9.32 | 2.74 | 1.60 | 1.48 | 3.35 | 0.88 | --------- | -------- |
| NMO-Mn | 73.49 | 13.60 | 4.10 | 0.60 | 0.66 | 3.15 | 1.23 | --------- | 1.74 |
| BC-Fe | 81.48 | 8.95 | 2.43 | 1.38 | 0.81 | 2.86 | --------- | 0.90 | --------- |
| NMO-Fe | 74.05 | 13.09 | 4.76 | 0.80 | 0.82 | 3.20 | --------- | 1.29 | 1.18 |
| Description | BET Area (m2·g−1) | Pore Size (nm) | Pore VAv (cm3·g−1) |
|---|---|---|---|
| Biochar | 32.43 | 5.72 | 0.0774 |
| NMO | 33.15 | 5.15 | 0.0791 |
| Metal | Adsorbent | qe (Exp.) | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
|---|---|---|---|---|---|---|---|---|
| mg/g | K1 (min−1) | qe (mg/g) | R2 | K2 (g/mg-min) | qe (mg/g) | R2 | ||
| Mn | NMO | 1.127 | 0.1498 | 1.7028 | 0.934 | 0.08391 | 1.334 | 0.993 |
| Mn | Biochar | 1.061 | 0.1725 | 2.0657 | 0.925 | 0.06970 | 1.303 | 0.988 |
| Fe | NMO | 1.158 | 0.1829 | 2.17951 | 0.939 | 0.0857 | 1.366 | 0.993 |
| Fe | Biochar | 1.122 | 0.1599 | 2.05238 | 0.891 | 0.0687 | 1.363 | 0.991 |
| Metal | Adsorbent | Isotherm | Parameters | Adsorption |
|---|---|---|---|---|
| Mn | NMO | Langmuir | Q0 (mg/g) | 4.151 |
| b (L/mg) | 0.75295 | |||
| R2 | 0.996 | |||
| Freundlich | Kf (mg/g) | 1.6629 | ||
| N | 2.718 | |||
| R2 | 0.943 | |||
| Biochar | Langmuir | Q0 (mg/g) | 3.169 | |
| b (L/mg) | 0.65374 | |||
| R2 | 0.992 | |||
| Freundlich | Kf (mg/g) | 1.26124 | ||
| N | 2.965 | |||
| R2 | 0.962 | |||
| Fe | NMO | Langmuir | Q0 (mg/g) | 4.859 |
| b (L/mg) | 0.84597 | |||
| R2 | 0.998 | |||
| Freundlich | Kf (mg/g) | 1.9765 | ||
| N | 2.525 | |||
| R2 | 0.955 | |||
| Biochar | Langmuir | Q0 (mg/g) | 4.3334 | |
| b (L/mg) | 0.67221 | |||
| R2 | 0.991 | |||
| Freundlich | Kf (mg/g) | 1.6039 | ||
| N | 2.424 | |||
| R2 | 0.978 |
| Metal | Adsorbent | Q (mL/min) | Adams–Bohart Model | Thomas Model | ||||
|---|---|---|---|---|---|---|---|---|
| kab (mg·L−1) | N0 (mg·L−1) | R2 | Kth (mL/mg·min) | q0 (mg/g) | R2 | |||
| Fe | NMO | 7 | 0.0002089 | 21518 | 0.919 | 0.24748 | 32.289 | 0.950 |
| 10 | 0.0003587 | 17448 | 0.988 | 0.40634 | 27.008 | 0.986 | ||
| 15 | 0.0005958 | 15177 | 0.970 | 0.73034 | 22.245 | 0.973 | ||
| Biochar | 7 | 0.0002031 | 20575 | 0.917 | 0.25672 | 29.539 | 0.960 | |
| 10 | 0.0003575 | 16736 | 0.988 | 0.42923 | 24.919 | 0.982 | ||
| 15 | 0.0005634 | 14696 | 0.975 | 0.74271 | 20.516 | 0.972 | ||
| Mn | NMO | 7 | 0.0002100 | 21375 | 0.916 | 0.24999 | 31.971 | 0.948 |
| 10 | 0.0003665 | 17382 | 0.992 | 0.41979 | 26.732 | 0.990 | ||
| 15 | 0.0005635 | 15456 | 0.978 | 0.68789 | 22.683 | 0.986 | ||
| Biochar | 7 | 0.0002089 | 19609 | 0.912 | 0.29016 | 26.599 | 0.975 | |
| 10 | 0.0003501 | 16073 | 0.986 | 0.45819 | 22.651 | 0.948 | ||
| 15 | 0.0005459 | 14021 | 0.976 | 0.91707 | 17.024 | 0.874 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alrowais, R.; Bashir, M.T.; Sikandar, M.A.; Hayet Khan, M.M.; Alwushayh, B.; Ghazy, A.; Uddin, M.A.; Iqbal, J. Synthesis and Characterization of Nanometal Oxide-Biochar Derived from Date Palm Waste for Adsorption of Manganese and Iron from Contaminated Water. Water 2023, 15, 3603. https://doi.org/10.3390/w15203603
Alrowais R, Bashir MT, Sikandar MA, Hayet Khan MM, Alwushayh B, Ghazy A, Uddin MA, Iqbal J. Synthesis and Characterization of Nanometal Oxide-Biochar Derived from Date Palm Waste for Adsorption of Manganese and Iron from Contaminated Water. Water. 2023; 15(20):3603. https://doi.org/10.3390/w15203603
Chicago/Turabian StyleAlrowais, Raid, Muhammad Tariq Bashir, Muhammad Ali Sikandar, Md. Munir Hayet Khan, Bandar Alwushayh, Ahmed Ghazy, Md. Alhaz Uddin, and Javed Iqbal. 2023. "Synthesis and Characterization of Nanometal Oxide-Biochar Derived from Date Palm Waste for Adsorption of Manganese and Iron from Contaminated Water" Water 15, no. 20: 3603. https://doi.org/10.3390/w15203603
APA StyleAlrowais, R., Bashir, M. T., Sikandar, M. A., Hayet Khan, M. M., Alwushayh, B., Ghazy, A., Uddin, M. A., & Iqbal, J. (2023). Synthesis and Characterization of Nanometal Oxide-Biochar Derived from Date Palm Waste for Adsorption of Manganese and Iron from Contaminated Water. Water, 15(20), 3603. https://doi.org/10.3390/w15203603







