A Review of Analytical Methods and Technologies for Monitoring Per- and Polyfluoroalkyl Substances (PFAS) in Water
Abstract
:1. Introduction
| Carbon Number | Type | Carboxylate Ion (or Acid Form) | Sulfonate Ion (or Acid Form) |
|---|---|---|---|
| 4 | Short chain | Perfluorobutanoate (PFBA) | Perfluorobutane sulfonate (PFBS) |
| 5 | Perfluoropentanoate (PFPeA) | Perfluoropentane sulfonate (PFPeS) | |
| 6 | Perfluorohexanoate (PFHxA) | Perfluorohexane sulfonate (PFHxS) | |
| 7 | Perfluoroheptanoate (PFHpA) | Perfluoroheptane sulfonate (PFHpS) | |
| 8 | Long chain | Perfluorooctanoate (PFOA) | Perfluorooctane sulfonate (PFOS) |
| 9 | Perfluorononanoate (PFNA) | Perfluorononane sulfonate (PFNS) | |
| 10 | Perfluorodecanoate (PFDA) | Perfluorodecane sulfonate (PFDS) |
2. Method
2.1. Semi-Systematic Literature Review
2.2. Sources of Information and Screening Process
2.3. Publication Distribution
3. Results and Discussion
3.1. PFAS Monitoring Methodologies and Technologies
3.2. Effectiveness of the Chromatographic Technique
3.2.1. Types of PFASs Detected
3.2.2. Elimination of Background Levels and/or Pre-Treatment
3.2.3. Limit of Detection
3.2.4. Analysis of Various Types of Samples
- Pre-treatment of soil samples should be focused on capturing PFAS with diverse properties, especially hydrophobic compounds, and cationic, anionic, or zwitterionic species [54].
- Background interferences should be cleaned up, as some recoveries can exceed 100%, showing high background interference [20].

3.3. Effectiveness of Alternative Methods and Techniques
3.3.1. Types of PFAS Detected
3.3.2. Elimination of Background Levels and/or Pre-Treatment
3.3.3. Limit of Detection
3.3.4. Analysis of Various Types of Samples
3.4. Effectiveness of Emerging Sensor-Based Technology
3.4.1. Types of PFAS Detected
3.4.2. Elimination of Background Levels and/or Pre-Treatment
3.4.3. Limit of Detection
3.4.4. Analysis of Various Types of Samples
| Item | Type of PFAS | Samples | (LOD) | References |
|---|---|---|---|---|
| Chromatographic Technique | ||||
| Multiple monolithic fibre solid-phase microextraction (MMF-SPME)-HPLC-MS/MS | PFCA | Tap water, river water, wastewater, and milk samples | 0.4–12.1 ng/L | [39,95] |
| Dispersive liquid–liquid microextraction (DLLME)- HPLC-MS/MS | Medium- and long-chain PFASs (CF2 > 5) | Water and urine samples | 0.6–8.7 ng/L | [20,96] |
| Vortex-assisted liquid–liquid microextraction (VALLME)-LC-MS | PFOS | Tap, river, and well water samples | 1.6 ng/L | [54,97] |
| Ice concentration linked with extractive stirrer (ICECLES)-HPLC-MS/MS | PFHxA, PFOA, and PFHpA | Drinking water samples | 0.05–0.3 ng/L | [98] |
| Acrodisc Filter multiple reaction monitoring (MRM)-UPLC-MS/MS | PFOS, PFOA, PFNA, and PFBS | Tap water and surface water samples | 7–40 ng/mL | [99] |
| SPE extraction- UHPLC/(-) ESI-MS/MS | PFCAs, PFSAs, and perfluoro ethers | Surface water samples | 0.48–1.68 ng/L | [100] |
| Sensor-based technology | ||||
| Biosensor, Colorimetric, Electrochemical, Electrochemiluminescence Fluorescence Nanoparticle Optical Fibre Photoelectrochemical Spectrophotometric | PFOS, PFOA, PFBS, GenX, 6:2 FTS, and others | Mostly water samples | Below 10 ng/L but mostly by incorporating chromatographic techniques | [20,65,66,85,94] |
| Alternative methods and techniques | ||||
| Total oxidisable precursor (TOP) assays | Total oxidisable PFASs | Water, surface/subsurface soil and groundwater samples | 0.5–7.9 ng/L | [2,6,20,54,64,67,74,78,101,102,103,104] |
| Fluorine-19 nuclear magnetic resonance (19F NMR) spectroscopy | Total organic fluorine (TOF) and total fluorine (TF, organic and inorganic) | [2,20,54,102] | ||
| Inductively coupled plasma mass spectrometry (ICP-MS) | Fluorine-specific detection of PFASs after LC separation | [102] | ||
| Continuum source molecular absorption spectroscopy (CS-MAS) | Total fluorine | [102] | ||
| X-ray photoelectron spectroscopy (XPS) | Fluorine/organic fluorine detection | [20] | ||
| Particle-induced gamma-ray emission (PIGE) spectroscopy | Total fluorine measurements of HFPO-DA, PFBS, PFPeA, PFHxA, PFHxS, PFHpA, PFOA, PFOS, PFNA, and PFDA | Drinking water samples | <50 ng/L | [2,20,54,105] |
| 3D-printed cone spray ionisation (3D-PCSI) | PFBA, PFHpA, PFOA, 6:2FTS, PFNA, PFOSA, PFOS, PFDA, PFUdA, PFDoA, and PFTrDA | Soil and sediment matrices | 100 ng/L | [76] |
| High-resolution graphite furnace continuum source molecular absorption spectrometry (HR GF-MAS) | Total fluorine measurement of PFCA | Seawater, river water, and effluent samples | Without SPE: 0.1 mg/L With SPE: 300 ng/L | [106] |
| Laser thermal desorption (LDTD) coupled with Orbitrap HRMS | PFBA, PFPeA, PFHxA, PFHpA, PFOA, PFNA, PFDA, PFUnA, PFDoA, PFBS, PFHxS, PFOS, PFDS, FOSA, 6:2 FTS | Surface water samples | 0.03–0.2 ng/L | [61,107] |
| Total oxidisable precursor (TOP) assays | 29 target analytes including PFUnDA, PFOA, and PFOS | Surface water samples | Method detection limit (MDL): 0.5–7.9 ng/L | [6] |
4. Conclusions
4.1. Limitations
4.2. Future Direction
- Field test device: portable and capable of in situ PFAS analysis.
- Rapid analysis: detecting PFAS at its source in time to take immediate action. Laboratory results for remote sites can take a week or more to arrive.
- Continuous monitoring of a polluted site: ensuring compliance with regulatory standards by monitoring soil, water, and wastewater remediation processes.
- Capable of speciating PFAS molecules: specific, sensitive, and selective against competing ions or molecules to enable operation in harsh environments containing high concentrations of interfering compounds.
- Integrating into a network of smart sensing technology, allowing PFAS contamination mapping and monitoring.
- Using high-resolution cameras and custom applications to analyse sample images and compare them to a calibration curve.
- Utilising GPS tracking and an internet connection to upload results and access online help for on-site assistance, providing rapid and remote response to PFAS monitoring works.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Glossary
| PASF | Perfluoroalkane sulfonyl fluoride |
| PFAAs | Perfluoroalkyl acids |
| PFAIs | Perfluoroalkyl iodides |
| PFCAs | Perfluroalkyl carboxylates (or acid forms) |
| PFSAs | Perfluoroalkane sulfonates (or acid forms) |
| PFPAs | Perfluroalkyl phosphonates (or acid forms) |
| PFPiAs | Perfluroalkyl phosphinates (or acid forms) |
| FTIs | Fluorotelometer iodides |
| PFECAs | Per- and polyfluoroether carboxylates (or acid forms) |
| PFESAs | Per- and polyfluoroether sulfonates (or acid forms) |
| FPs | Fluoropolymers |
| PTFE | Polytetrafluoroethylene |
| PVDF | Polyvinylidene fluoride |
| FEP | Fluorinated ethylene propylene |
| PVF | Polyvinyl fluoride |
References
- Zhang, J.; Pang, H.; Gray, S.; Ma, S.; Xie, Z.; Gao, L. PFAS removal from wastewater by in-situ formed ferric nanoparticles: Solid phase loading and removal efficiency. J. Environ. Chem. Eng. 2021, 9, 105452. [Google Scholar] [CrossRef]
- Menger, R.F.; Funk, E.; Henry, C.S.; Borch, T. Sensors for detecting per- and polyfluoroalkyl substances (PFAS): A critical review of development challenges, current sensors, and commercialization obstacles. Chem. Eng. J. 2021, 417. [Google Scholar] [CrossRef] [PubMed]
- Batayi, B.; Okonkwo, O.J.; Daso, A.P. Poly- and perfluorinated substances in environmental water from the Hartbeespoort and Roodeplaat Dams, South Africa. Water SA 2021, 47. [Google Scholar] [CrossRef]
- Krafft, M.P.; Riess, J.G. Per- and polyfluorinated substances (PFASs): Environmental challenges. Curr. Opin. Colloid. Interface Sci. 2015, 20, 192–212. [Google Scholar] [CrossRef]
- Gao, Y.; Fu, J.; Meng, M.; Wang, Y.; Chen, B.; Jiang, G. Spatial distribution and fate of perfluoroalkyl substances in sediments from the Pearl River Estuary, South China. Mar. Pollut. Bull. 2015, 96, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Joerss, H.; Schramm, T.R.; Sun, L.; Guo, C.; Tang, J.; Ebinghaus, R. Per- and polyfluoroalkyl substances in Chinese and German river water—Point source- and country-specific fingerprints including unknown precursors. Environ. Pollut. 2020, 267, 115567. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Song, B.; Zhong, H.; Ma, X.; Wang, Y.; Ma, D.; Lu, Y.; Gao, W.; Wang, Y.; Jiang, G. Legacy and emerging per- and polyfluoroalkyl substances (PFAS) in the Bohai Sea and its inflow rivers. Environ. Int. 2021, 156, 106735. [Google Scholar] [CrossRef]
- Bugsel, B.; Bauer, R.; Herrmann, F.; Maier, M.E.; Zwiener, C. LC-HRMS screening of per- and polyfluorinated alkyl substances (PFAS) in impregnated paper samples and contaminated soils. Anal. Bioanal. Chem. 2022, 414, 1217–1225. [Google Scholar] [CrossRef]
- Tan, K.Y.; Lu, G.H.; Yuan, X.; Zheng, Y.; Shao, P.W.; Cai, J.Y.; Zhao, Y.R.; Zhu, X.H.; Yang, Y.L. Perfluoroalkyl Substances in Water from the Yangtze River and Its Tributaries at the Dividing Point Between the Middle and Lower Reaches. Bull. Environ. Contam. Toxicol. 2018, 101, 598–603. [Google Scholar] [CrossRef]
- Sim, W.; Park, H.; Yoon, J.K.; Kim, J.I.; Oh, J.E. Characteristic distribution patterns of perfluoroalkyl substances in soils according to land-use types. Chemosphere 2021, 276, 130167. [Google Scholar] [CrossRef]
- Lu, Z.; Song, L.; Zhao, Z.; Ma, Y.; Wang, J.; Yang, H.; Ma, H.; Cai, M.; Codling, G.; Ebinghaus, R.; et al. Occurrence and trends in concentrations of perfluoroalkyl substances (PFASs) in surface waters of eastern China. Chemosphere 2015, 119, 820–827. [Google Scholar] [CrossRef] [PubMed]
- McCord, J.; Strynar, M. Identification of Per- and Polyfluoroalkyl Substances in the Cape Fear River by High Resolution Mass Spectrometry and Nontargeted Screening. Environ. Sci. Technol. 2019, 53, 4717–4727. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Zheng, M.; Liang, Y.; Wang, Y.; Gao, W.; Wang, Y.; Jiang, G. Legacy and emerging per- and polyfluoroalkyl substances (PFAS) in sediments from the East China Sea and the Yellow Sea: Occurrence, source apportionment and environmental risk assessment. Chemosphere 2021, 282, 131042. [Google Scholar] [CrossRef] [PubMed]
- Saleeby, B.; Shimizu, M.S.; Sanchez Garcia, R.I.; Avery, G.B.; Kieber, R.J.; Mead, R.N.; Skrabal, S.A. Isomers of emerging per- and polyfluoroalkyl substances in water and sediment from the Cape Fear River, North Carolina, USA. Chemosphere 2021, 262, 128359. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Son, Y. Perfluoroalkyl substances (PFAS) in surface water and sediments from two urban watersheds in Nevada, USA. Sci. Total Environ. 2021, 751, 141622. [Google Scholar] [CrossRef] [PubMed]
- Knutsen, H.; Maehlum, T.; Haarstad, K.; Slinde, G.A.; Arp, H.P.H. Leachate emissions of short- and long-chain per- and polyfluoralkyl substances (PFASs) from various Norwegian landfills. Environ. Sci. Process Impacts 2019, 21, 1970–1979. [Google Scholar] [CrossRef] [PubMed]
- Guardian, M.G.E.; Boongaling, E.G.; Bernardo-Boongaling, V.R.R.; Gamonchuang, J.; Boontongto, T.; Burakham, R.; Arnnok, P.; Aga, D.S. Prevalence of per- and polyfluoroalkyl substances (PFASs) in drinking and source water from two Asian countries. Chemosphere 2020, 256, 127115. [Google Scholar] [CrossRef] [PubMed]
- Interstate Technology and Regulatory Council, PFAS Fact Sheets. 2020. Available online: https://pfas-1.itrcweb.org/ (accessed on 1 June 2022).
- OUGP Group. Synthesis Paper on per- and Polyfluorinated Chemicals (PFCS); OECD Environment Directorate, Environment, Health and Safety Division: Paris, France, 2013. [Google Scholar]
- Al Amin, M.; Sobhani, Z.; Liu, Y.; Dharmaraja, R.; Chadalavada, S.; Naidu, R.; Chalker, J.M.; Fang, C. Recent advances in the analysis of per- and polyfluoroalkyl substances (PFAS)—A review. Environ. Technol. Innov. 2020, 19, 100879. [Google Scholar] [CrossRef]
- PFAS Investigation & Management Program. 2022. Available online: https://www.defence.gov.au/about/locations-property/pfas (accessed on 1 June 2022).
- Li, J.; He, J.; Niu, Z.; Zhang, Y. Legacy per- and polyfluoroalkyl substances (PFASs) and alternatives (short-chain analogues, F-53B, GenX and FC-98) in residential soils of China: Present implications of replacing legacy PFASs. Environ. Int. 2020, 135, 105419. [Google Scholar] [CrossRef]
- Stoiber, T.; Evans, S.; Naidenko, O.V. Disposal of products and materials containing per-and polyfluoroalkyl substances (PFAS): A cyclical problem. Chemosphere 2020, 260, 127659. [Google Scholar] [CrossRef]
- Zhao, Z.; Xie, Z.; Tang, J.; Sturm, R.; Chen, Y.; Zhang, G.; Ebinghaus, R. Seasonal variations and spatial distributions of perfluoroalkyl substances in the rivers Elbe and lower Weser and the North Sea. Chemosphere 2015, 129, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Lesmeister, L.; Lange, F.T.; Breuer, J.; Biegel-Engler, A.; Giese, E.; Scheurer, M. Extending the knowledge about PFAS bioaccumulation factors for agricultural plants—A review. Sci. Total Environ. 2021, 766, 142640. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Reinhard, M.; Nguyen, T.V.; You, L.; He, Y.; Gin, K.Y. Characterization of occurrence, sources and sinks of perfluoroalkyl and polyfluoroalkyl substances (PFASs) in a tropical urban catchment. Environ. Pollut. 2017, 227, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.R. PFAS in soil and groundwater following historical land application of biosolids. Water Res. 2022, 211, 118035. [Google Scholar] [CrossRef] [PubMed]
- Shen, A.; Lee, S.; Ra, K.; Suk, D.; Moon, H.B. Historical trends of perfluoroalkyl substances (PFASs) in dated sediments from semi-enclosed bays of Korea. Mar. Pollut. Bull. 2018, 128, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Mussabek, D.; Persson, K.M.; Berndtsson, R.; Ahrens, L.; Nakagawa, K.; Imura, T. Impact of the Sediment Organic vs. Mineral Content on Distribution of the Per- and Polyfluoroalkyl Substances (PFAS) in Lake Sediment. Int. J. Environ. Res. Public. Health 2020, 17, 5642. [Google Scholar] [CrossRef] [PubMed]
- Aly, N.A.; Luo, Y.S.; Liu, Y.; Casillas, G.; McDonald, T.J.; Kaihatu, J.M.; Jun, M.; Ellis, N.; Gossett, S.; Dodds, J.N.; et al. Temporal and spatial analysis of per and polyfluoroalkyl substances in surface waters of Houston ship channel following a large-scale industrial fire incident. Environ. Pollut. 2020, 265, 115009. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Ding, G.; Zhang, J.; Wei, L.; Xue, H.; Zhang, N.; Li, Y.; Chen, G.; Sun, Y. Occurrence and distribution of perfluoroalkyl substances (PFASs) in surface water and bottom water of the Shuangtaizi Estuary, China. Environ. Pollut. 2016, 216, 675–681. [Google Scholar] [CrossRef]
- Kwok, K.Y.; Wang, X.H.; Ya, M.; Li, Y.; Zhang, X.H.; Yamashita, N.; Lam, J.C.; Lam, P.K. Occurrence and distribution of conventional and new classes of per- and polyfluoroalkyl substances (PFASs) in the South China Sea. J. Hazard. Mater. 2015, 285, 389–397. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Reinhard, M.; Chen, H.; Gin, K.Y. Fate and transport of perfluoro- and polyfluoroalkyl substances including perfluorooctane sulfonamides in a managed urban water body. Environ. Sci. Pollut. Res. Int. 2016, 23, 10382–10392. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, C.; Zhou, Q.; Yang, S. Occurrence of perfluorinated alkyl substances in sediment from estuarine and coastal areas of the East China Sea. Environ. Sci. Pollut. Res. Int. 2015, 22, 1662–1669. [Google Scholar] [CrossRef] [PubMed]
- Blake, B.E.; Fenton, S.E. Early life exposure to per-and polyfluoroalkyl substances (PFAS) and latent health outcomes: A review including the placenta as a target tissue and possible driver of peri-and postnatal effects. Toxicology 2020, 443, 152565. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Kumar, P.; Mishra, V.; Guijt, R.; Singh, P.; Dumée, L.F.; Sharma, R.S. A review on the sources, occurrence and health risks of per-/poly-fluoroalkyl substances (PFAS) arising from the manufacture and disposal of electric and electronic products. J. Water Process Eng. 2020, 38, 101683. [Google Scholar] [CrossRef]
- USEPA. Drinking Water Health Advisory for Perfluorooctanoic Sulfonate (PFOS); USEPA: Washington, DC, USA, 2016.
- Cordner, A.; De La Rosa, V.Y.; Schaider, L.A.; Rudel, R.A.; Richter, L.; Brown, P. Guideline levels for PFOA and PFOS in drinking water: The role of scientific uncertainty, risk assessment decisions, and social factors. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, K.L.; Hwang, J.H.; Esfahani, A.R.; Sadmani, A.; Lee, W.H. Recent Developments of PFAS-Detecting Sensors and Future Direction: A Review. Micromachines 2020, 11, 667. [Google Scholar] [CrossRef] [PubMed]
- ANZECC. ANZECC Water Quality Guidelines; Australian and New Zealand Guidelines for Fresh and Marine Water Quality; ANZECC: Canberra, ACT, Australia, 2020.
- Commonwealth of Australia. PFAS National Environmental Management Plan Version 2.0; National Chemicals Working Group of the Heads of the EPAs Australia and New Zealand: Wellington, New Zealand, 2020.
- Perfluorinated Chemicals in Food; Food Standard Australia New Zealand: Wellington, New Zealand, 2022.
- Kibbey, T.C.G.; Jabrzemski, R.; O’Carroll, D.M. Supervised machine learning for source allocation of per- and polyfluoroalkyl substances (PFAS) in environmental samples. Chemosphere 2020, 252, 126593. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.J.; Okvitasari, A.R.; Huang, F.Y.; Tsai, C.S. Characteristics, pollution patterns and risks of Perfluoroalkyl substances in drinking water sources of Taiwan. Chemosphere 2021, 264, 128579. [Google Scholar] [CrossRef] [PubMed]
- Barreca, S.; Busetto, M.; Colzani, L.; Clerici, L.; Marchesi, V.; Tremolada, L.; Daverio, D.; Dellavedova, P. Hyphenated High Performance Liquid Chromatography–Tandem Mass Spectrometry Techniques for the Determination of Perfluorinated Alkylated Substances in Lombardia Region in Italy, Profile Levels and Assessment: One Year of Monitoring Activities During 2018. Separations 2020, 7, 17. [Google Scholar] [CrossRef]
- White, N.D.; Balthis, L.; Kannan, K.; De Silva, A.O.; Wu, Q.; French, K.M.; Daugomah, J.; Spencer, C.; Fair, P.A. Elevated levels of perfluoroalkyl substances in estuarine sediments of Charleston, SC. Sci. Total Environ. 2015, 521–522, 79–89. [Google Scholar] [CrossRef]
- Baabish, A.; Sobhanei, S.; Fiedler, H. Priority perfluoroalkyl substances in surface waters—A snapshot survey from 22 developing countries. Chemosphere 2021, 273, 129612. [Google Scholar] [CrossRef]
- Martinez, B.; Da Silva, B.F.; Aristizabal-Henao, J.J.; Denslow, N.D.; Osborne, T.Z.; Morrison, E.S.; Bianchi, T.S.; Bowden, J.A. Increased levels of perfluorooctanesulfonic acid (PFOS) during Hurricane Dorian on the east coast of Florida. Environ. Res. 2022, 208, 112635. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Liang, X.; Liu, H.; Cao, H.; Liu, X.; Jin, Y.; Li, X.; Chen, S.; Wu, X. Activation of Co-O bond in (110) facet exposed Co3O4 by Cu doping for the boost of propane catalytic oxidation. J. Hazard. Mater. 2023, 452, 131319. [Google Scholar] [CrossRef] [PubMed]
- Abunada, Z.; Alazaiza, M.Y.; Bashir, M.J. An overview of per-and polyfluoroalkyl substances (Pfas) in the environment: Source, fate, risk and regulations. Water 2020, 12, 3590. [Google Scholar] [CrossRef]
- Li, X.; Fatowe, M.; Cui, D.; Quinete, N. Assessment of per- and polyfluoroalkyl substances in Biscayne Bay surface waters and tap waters from South Florida. Sci. Total Environ. 2022, 806, 150393. [Google Scholar] [CrossRef] [PubMed]
- Ahmadireskety, A.; Da Silva, B.F.; Awkerman, J.A.; Aufmuth, J.; Yost, R.A.; Bowden, J.A. Per- and polyfluoroalkyl substances (PFAS) in sediments collected from the Pensacola Bay System watershed. Environ. Adv. 2021, 5, 100088. [Google Scholar] [CrossRef]
- Hekster, F.M.; Laane, R.W.; Voogt, P.D. Environmental and toxicity effects of perfluoroalkylated substances. Rev. Environ. Contam. Toxicol. 2003, 179, 99–121. [Google Scholar] [PubMed]
- Nakayama, S.F.; Yoshikane, M.; Onoda, Y.; Nishihama, Y.; Iwai-Shimada, M.; Takagi, M.; Kobayashi, Y.; Isobe, T. Worldwide trends in tracing poly- and perfluoroalkyl substances (PFAS) in the environment. TrAC Trends Anal. Chem. 2019, 121, 115410. [Google Scholar] [CrossRef]
- Hensema, T.J.; Berendsen, B.J.A.; van Leeuwen, S.P.J. Non-targeted identification of per- and polyfluoroalkyl substances at trace level in surface water using fragment ion flagging. Chemosphere 2021, 265, 128599. [Google Scholar] [CrossRef]
- Jamari, N.L.A.; Dohmann, J.F.; Raab, A.; Krupp, E.M.; Feldmann, J. Novel non-targeted analysis of perfluorinated compounds using fluorine-specific detection regardless of their ionisability (HPLC-ICPMS/MS-ESI-MS). Anal. Chim. Acta 2019, 1053, 22–31. [Google Scholar] [CrossRef]
- Janda, J.; Nodler, K.; Brauch, H.J.; Zwiener, C.; Lange, F.T. Robust trace analysis of polar (C2-C8) perfluorinated carboxylic acids by liquid chromatography-tandem mass spectrometry: Method development and application to surface water, groundwater and drinking water. Environ. Sci. Pollut. Res. Int. 2019, 26, 7326–7336. [Google Scholar] [CrossRef]
- Gonzalez de Vega, R.; Cameron, A.; Clases, D.; Dodgen, T.M.; Doble, P.A.; Bishop, D.P. Simultaneous targeted and non-targeted analysis of per- and polyfluoroalkyl substances in environmental samples by liquid chromatography-ion mobility-quadrupole time of flight-mass spectrometry and mass defect analysis. J. Chromatogr. A 2021, 1653, 462423. [Google Scholar] [CrossRef] [PubMed]
- Koelmel, J.P.; Stelben, P.; McDonough, C.A.; Dukes, D.A.; Aristizabal-Henao, J.J.; Nason, S.L.; Li, Y.; Sternberg, S.; Lin, E.; Beckmann, M.; et al. FluoroMatch 2.0-making automated and comprehensive non-targeted PFAS annotation a reality. Anal. Bioanal. Chem. 2022, 414, 1201–1215. [Google Scholar] [CrossRef]
- Mejia-Avendano, S.; Munoz, G.; Sauve, S.; Liu, J. Assessment of the Influence of Soil Characteristics and Hydrocarbon Fuel Cocontamination on the Solvent Extraction of Perfluoroalkyl and Polyfluoroalkyl Substances. Anal. Chem. 2017, 89, 2539–2546. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Chen, Y.; Xue, Q.; Fu, J.; Fu, K.; Fu, J.; Zhang, A.; Cai, Z.; Jiang, G. Trends and perspectives in per-and polyfluorinated alkyl substances (PFASs) determination: Faster and broader. TrAC Trends Anal. Chem. 2020, 133. [Google Scholar] [CrossRef]
- Chen, R.; Li, G.; He, Y.; Pan, L.; Yu, Y.; Shi, B. Field study on the transportation characteristics of PFASs from water source to tap water. Water Res. 2021, 198, 117162. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Darling, S.B.; Chen, J. Selectivity of Per- and Polyfluoroalkyl Substance Sensors and Sorbents in Water. ACS Appl. Mater. Interfaces 2021, 13, 60789–60814. [Google Scholar] [CrossRef]
- John, J.; Coulon, F.; Chellam, P.V. Detection and treatment strategies of per- and polyfluoroalkyl substances (PFAS): Fate of PFAS through DPSIR framework analysis. J. Water Process Eng. 2022, 45, 102463. [Google Scholar] [CrossRef]
- Viada, B.N.; Yudi, L.M.; Arrigan, D.W.M. Detection of perfluorooctane sulfonate by ion-transfer stripping voltammetry at an array of microinterfaces between two immiscible electrolyte solutions. Analyst 2020, 145, 5776–5786. [Google Scholar] [CrossRef]
- Law, C.S.; Wang, J.; Gunenthiran, S.; Lim, S.Y.; Abell, A.D.; Ahrens, L.; Kumeria, T.; Santos, A.; Voelcker, N.H. Real-time detection of per-fluoroalkyl substance (PFAS) self-assembled monolayers in nanoporous interferometers. Sens. Actuators B Chem. 2022, 355, 131340. [Google Scholar] [CrossRef]
- Bell, E.M.; De Guise, S.; McCutcheon, J.R.; Lei, Y.; Levin, M.; Li, B.; Rusling, J.F.; Lawrence, D.A.; Cavallari, J.M.; O’Connell, C.; et al. Exposure, health effects, sensing, and remediation of the emerging PFAS contaminants—Scientific challenges and potential research directions. Sci. Total Environ. 2021, 780, 146399. [Google Scholar] [CrossRef]
- Garg, S.; Kumar, P.; Greene, G.W.; Mishra, V.; Avisar, D.; Sharma, R.S.; Dumee, L.F. Nano-enabled sensing of per-/poly-fluoroalkyl substances (PFAS) from aqueous systems—A review. J. Environ. Manag. 2022, 308, 114655. [Google Scholar] [CrossRef] [PubMed]
- EPA: 8327: Per-and Polyfluoroalkyl Substances (PFAS) by Liquid Chromatography/Tandem Mass Spectrometry (LC/MS/MS). Available online: https://www.nemi.gov/methods/method_summary/13016/ (accessed on 1 June 2022).
- Method 537.1: Determination of Selected Per- and Polyfluorinated Alkyl Substances in Drinking Water by Solid Phase Extraction and Liquid Chromatography/Tandem Mass Spectrometry (LC/MS/MS). Available online: https://cfpub.epa.gov/si/si_public_record_Report.cfm?dirEntryId=343042&Lab=NERL (accessed on 1 June 2022).
- Method 533: Determination of Per- and Polyfluoroalkyl Substances in Drinking Water by Isotope Dilution Anion Exchange Solid Phase Extraction and Liquid Chromatography/Tandem Mass Spectrometry. Available online: https://www.epa.gov/dwanalyticalmethods/method-533-determination-and-polyfluoroalkyl-substances-drinking-water-isotope (accessed on 1 June 2022).
- United States Environmental Protection Agency. PFAS Analytical Methods Development and Sampling Research. Available online: https://www.epa.gov/water-research/pfas-analytical-methods-development-and-sampling-research (accessed on 1 June 2022).
- Trojanowicz, M.; Koc, M. Recent developments in methods for analysis of perfluorinated persistent pollutants. Microchim. Acta 2013, 180, 957–971. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Munoz, G.; Vo Duy, S.; Sauve, S.; Liu, J. Per- and Polyfluoroalkyl Substances in Contaminated Soil and Groundwater at Airports: A Canadian Case Study. Environ. Sci. Technol. 2022, 56, 885–895. [Google Scholar] [CrossRef]
- Neuwald, I.J.; Zahn, D.; Knepper, T.P. Are (fluorinated) ionic liquids relevant environmental contaminants? High-resolution mass spectrometric screening for per- and polyfluoroalkyl substances in environmental water samples led to the detection of a fluorinated ionic liquid. Anal. Bioanal. Chem. 2020, 412, 4881–4892. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.M.; Fedick, P.W. Rapid, low-cost, and in-situ analysis of per- and polyfluoroalkyl substances in soils and sediments by ambient 3D-printed cone spray ionization mass spectrometry. Chemosphere 2021, 272. [Google Scholar] [CrossRef] [PubMed]
- Simon, F.; Gehrenkemper, L.; von der Au, M.; Wittwer, P.; Roesch, P.; Pfeifer, J.; Cossmer, A.; Meermann, B. A fast and simple PFAS extraction method utilizing HR-CS-GFMAS for soil samples. Chemosphere 2022, 295, 133922. [Google Scholar] [CrossRef] [PubMed]
- Al Amin, M.; Sobhani, Z.; Liu, Y.; Dharmarajan, R.; Naidu, R.; Fang, C. Application and confirmation of total oxidizable precursors assay (TOPA) to monitor PFAS with a portable reading kit. In Proceedings of the 8th International Contaminated Site Remediation Conference Incorporating the 2nd International PFAS Conference: Program and Proceedings, CleanUp 2019 Conference, Adelaide, SA, Australia, 8–12 September 2019; pp. 563–564. [Google Scholar]
- Liu, L.; Qu, Y.; Huang, J.; Weber, R. Per- and polyfluoroalkyl substances (PFASs) in Chinese drinking water: Risk assessment and geographical distribution. Environ. Sci. Eur. 2021, 33, 6. [Google Scholar] [CrossRef]
- Glasscott, M.W.; Vannoy, K.J.; Kazemi, R.; Verber, M.D.; Dick, J.E. μ-MIP: Molecularly Imprinted Polymer-Modified Microelectrodes for the Ultrasensitive Quantification of GenX (HFPO-DA) in River Water. Environ. Sci. Technol. Lett. 2020, 7, 489–495. [Google Scholar] [CrossRef]
- Al Amin, M.; Sobhani, Z.; Chadalavada, S.; Naidu, R.; Fang, C. Smartphone-based/Fluoro-SPE for selective detection of PFAS at ppb level. Environ. Technol. Innov. 2020, 18, 100778. [Google Scholar] [CrossRef]
- Fang, C. Using Smartphones to Detect PFAS in Aqueous Film-Forming Foam. Remediation Australasia. 2017. Available online: https://www.remediationaustralasia.com.au/articles/using-smartphones-detect-pfas-aqueous-film-forming-foam (accessed on 1 June 2022).
- Cennamo, N.; D’Agostino, G.; Sequeira, F.; Mattiello, F.; Porto, G.; Biasiolo, A.; Nogueira, R.; Bilro, L.; Zeni, L. A Simple and Low-Cost Optical Fiber Intensity-Based Configuration for Perfluorinated Compounds in Water Solution. Sensors 2018, 18, 3009. [Google Scholar] [CrossRef]
- Karimian, N.; Stortini, A.M.; Moretto, L.M.; Costantino, C.; Bogialli, S.; Ugo, P. Electrochemosensor for Trace Analysis of Perfluorooctanesulfonate in Water Based on a Molecularly Imprinted Poly(o-phenylenediamine) Polymer. ACS Sens. 2018, 3, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Wu, J.; Sobhani, Z.; Amin, M.A.; Tang, Y. Aggregated-fluorescent detection of PFAS with a simple chip. Anal. Methods 2019, 11, 163–170. [Google Scholar] [CrossRef]
- Chatterjee, S.; Motkuri, R.; Truex, M.; Barpaga, D.; Shutthanandan, V.; Han, K.; Enderlin, C.; Wells, B.; Basuray, S.; Reed, M.; et al. Advances in PFAS Monitoring and Remediation Using a Functionalized Material Approach. In Proceedings of the WM2020 Conference, Phoenix, AZ, USA, 8–12 March 2020. [Google Scholar]
- Faiz, F.; Baxter, G.; Collins, S.; Sidiroglou, F.; Cran, M. Polyvinylidene fluoride coated optical fibre for detecting perfluorinated chemicals. Sens. Actuators B Chem. 2020, 312, 128006. [Google Scholar] [CrossRef]
- Kazemi, R.; Potts, E.I.; Dick, J.E. Quantifying Interferent Effects on Molecularly Imprinted Polymer Sensors for Per- and Polyfluoroalkyl Substances (PFAS). Anal. Chem. 2020, 92, 10597–10605. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.B.; Dick, J.E. Towards deployable electrochemical sensors for per- and polyfluoroalkyl substances (PFAS). Chem. Commun. 2021, 57, 8121–8130. [Google Scholar] [CrossRef] [PubMed]
- Savage, D.T.; Briot, N.J.; Hilt, J.Z.; Dziubla, T.D. On the swelling behavior of poly(N-Isopropylacrylamide) hydrogels exposed to perfluoroalkyl acids. J. Polym. Sci. 2021, 59, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Savage, D.T.; Hilt, J.Z.; Dziubla, T.D. Assessing the perfluoroalkyl acid-induced swelling of Forster resonance energy transfer-capable poly(N-isopropylacrylamide) microgels. Soft Matter 2021, 17, 9799–9808. [Google Scholar] [CrossRef] [PubMed]
- Young, N.A.; Lambert, R.L.; Buch, A.M.; Dahl, C.L.; Harris, J.D.; Barnhart, M.D.; Sitko, J.C.; Jordan Steel, J. A Synthetic Biology Approach Using Engineered Bacteria to Detect Perfluoroalkyl Substance (PFAS) Contamination in Water. Mil. Med. 2021, 186, 801–807. [Google Scholar] [CrossRef]
- Lu, D.; Zhu, D.Z.; Gan, H.; Yao, Z.; Luo, J.; Yu, S.; Kurup, P. An ultra-sensitive molecularly imprinted polymer (MIP) and gold nanostars (AuNS) modified voltammetric sensor for facile detection of perfluorooctance sulfonate (PFOS) in drinking water. Sens. Actuators B Chem. 2022, 352, 131055. [Google Scholar] [CrossRef]
- Sahu, S.P.; Kole, S.; Arges, C.G.; Gartia, M.R. Rapid and Direct Perfluorooctanoic Acid Sensing with Selective Ionomer Coatings on Screen-Printed Electrodes under Environmentally Relevant Concentrations. ACS Omega 2022, 7, 5001–5007. [Google Scholar] [CrossRef]
- Huang, Y.; Li, H.; Bai, M.; Huang, X. Efficient extraction of perfluorocarboxylic acids in complex samples with a monolithic adsorbent combining fluorophilic and anion-exchange interactions. Anal. Chim. Acta 2018, 1011, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shi, Y.; Cai, Y. A highly selective dispersive liquid–liquid microextraction approach based on the unique fluorous affinity for the extraction and detection of per-and polyfluoroalkyl substances coupled with high performance liquid chromatography tandem–mass spectrometry. J. Chromatogr. A 2018, 1544, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, A.; Román, I.P.; Canals, A.; Tyrovola, K.; Psillakis, E. Fast screening of perfluorooctane sulfonate in water using vortex-assisted liquid–liquid microextraction coupled to liquid chromatography–mass spectrometry. Anal. Chim. Acta 2011, 691, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Skaggs, C.S.; Logue, B.A. Ultratrace analysis of per- and polyfluoroalkyl substances in drinking water using ice concentration linked with extractive stirrer and high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2021, 1659, 462493. [Google Scholar] [CrossRef] [PubMed]
- Mottaleb, M.A.; Ding, Q.X.; Pennell, K.G.; Haynes, E.N.; Morris, A.J. Direct injection analysis of per and polyfluoroalkyl substances in surface and drinking water by sample filtration and liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2021, 1653, 462426. [Google Scholar] [CrossRef] [PubMed]
- Mulabagal, V.; Liu, L.; Qi, J.; Wilson, C.; Hayworth, J.S. A rapid UHPLC-MS/MS method for simultaneous quantitation of 23 perfluoroalkyl substances (PFAS) in estuarine water. Talanta 2018, 190, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Mattias, S.; Kikuchi, J.; Wiberg, K.; Lutz, A. Spatial distribution and load of per- and polyfluoroalkyl substances (PFAS) in background soils in Sweden. Chemosphere 2022, 295, 133944. [Google Scholar] [CrossRef] [PubMed]
- Camdzic, D.; Dickman, R.A.; Aga, D.S. Total and class-specific analysis of per- and polyfluoroalkyl substances in environmental samples using nuclear magnetic resonance spectroscopy. J. Hazard. Mater. Lett. 2021, 2. [Google Scholar] [CrossRef]
- Katz, D.R.; Sullivan, J.C.; Rosa, K.; Gardiner, C.L.; Robuck, A.R.; Lohmann, R.; Kincaid, C.; Cantwell, M.G. Transport and fate of aqueous film forming foam in an urban estuary. Environ. Pollut. 2022, 300, 118963. [Google Scholar] [CrossRef]
- Charbonnet, J.A.; Rodowa, A.E.; Joseph, N.T.; Guelfo, J.L.; Field, J.A.; Jones, G.D.; Higgins, C.P.; Helbling, D.E.; Houtz, E.F. Environmental Source Tracking of Per- and Polyfluoroalkyl Substances within a Forensic Context: Current and Future Techniques. Environ. Sci. Technol. 2021, 55, 7237–7245. [Google Scholar] [CrossRef]
- Tighe, M.; Jin, Y.; Whitehead, H.D.; Hayes, K.; Lieberman, M.; Pannu, M.; Plumlee, M.H.; Peaslee, G.F. Screening for Per- and Polyfluoroalkyl Substances in Water with Particle Induced Gamma-Ray Emission Spectroscopy. ACS EST Water 2021, 1, 2477–2484. [Google Scholar] [CrossRef]
- Akhdhar, A.; Schneider, M.; Orme, A.; Schultes, L.; Raab, A.; Krupp, E.M.; Benskin, J.P.; Welz, B.; Feldmann, J. The use of high resolution graphite furnace molecular absorption spectrometry (HR -MAS) for total fluorine determination in extractable organofluorines (EOF). Talanta 2020, 209, 120466. [Google Scholar] [CrossRef]
- Munoz, G.; Duy, S.V.; Budzinski, H.; Labadie, P.; Liu, J.; Sauvé, S. Quantitative analysis of poly-and perfluoroalkyl compounds in water matrices using high resolution mass spectrometry: Optimization for a laser diode thermal desorption method. Anal. Chim. Acta 2015, 881, 98–106. [Google Scholar] [CrossRef]
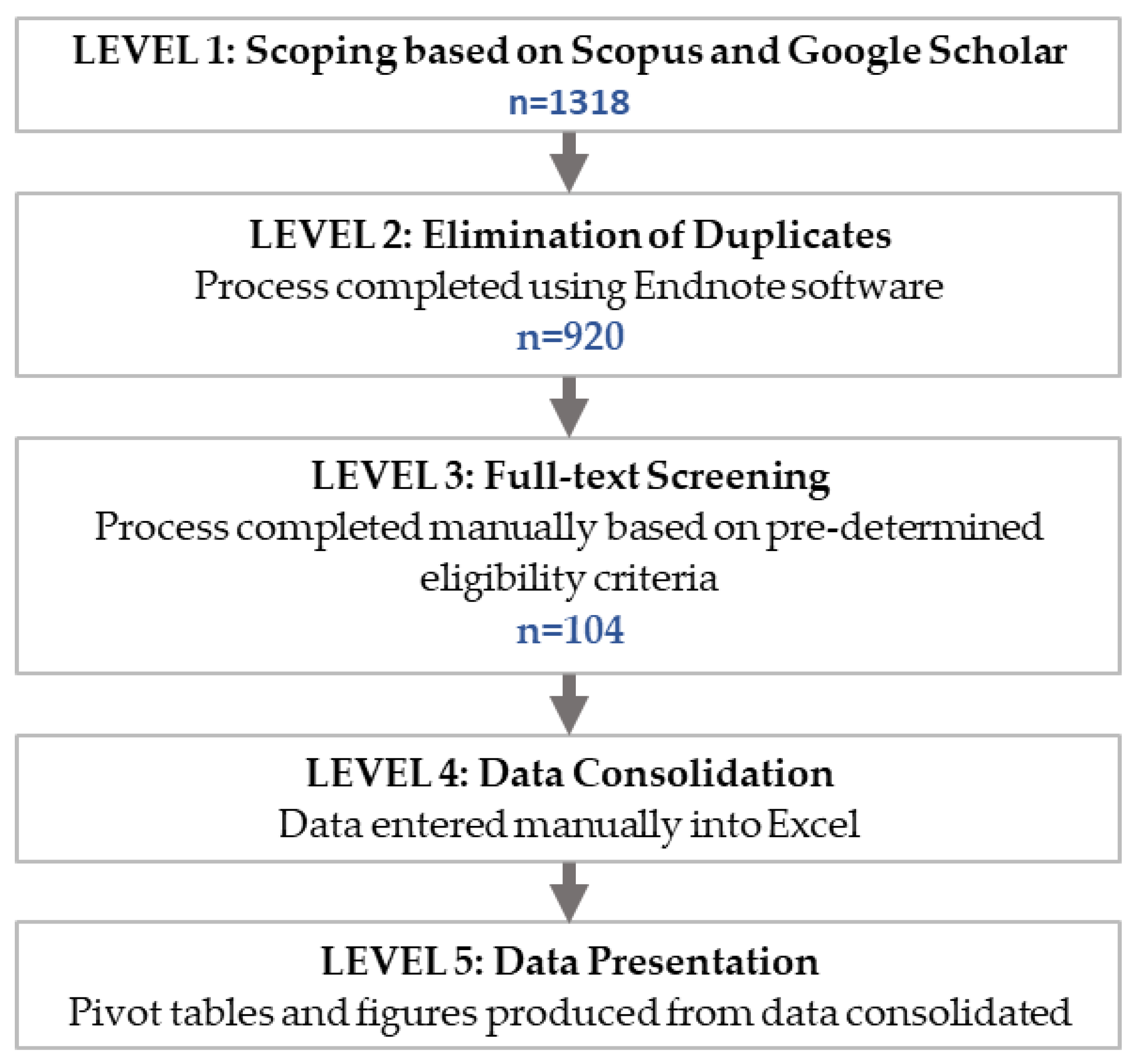

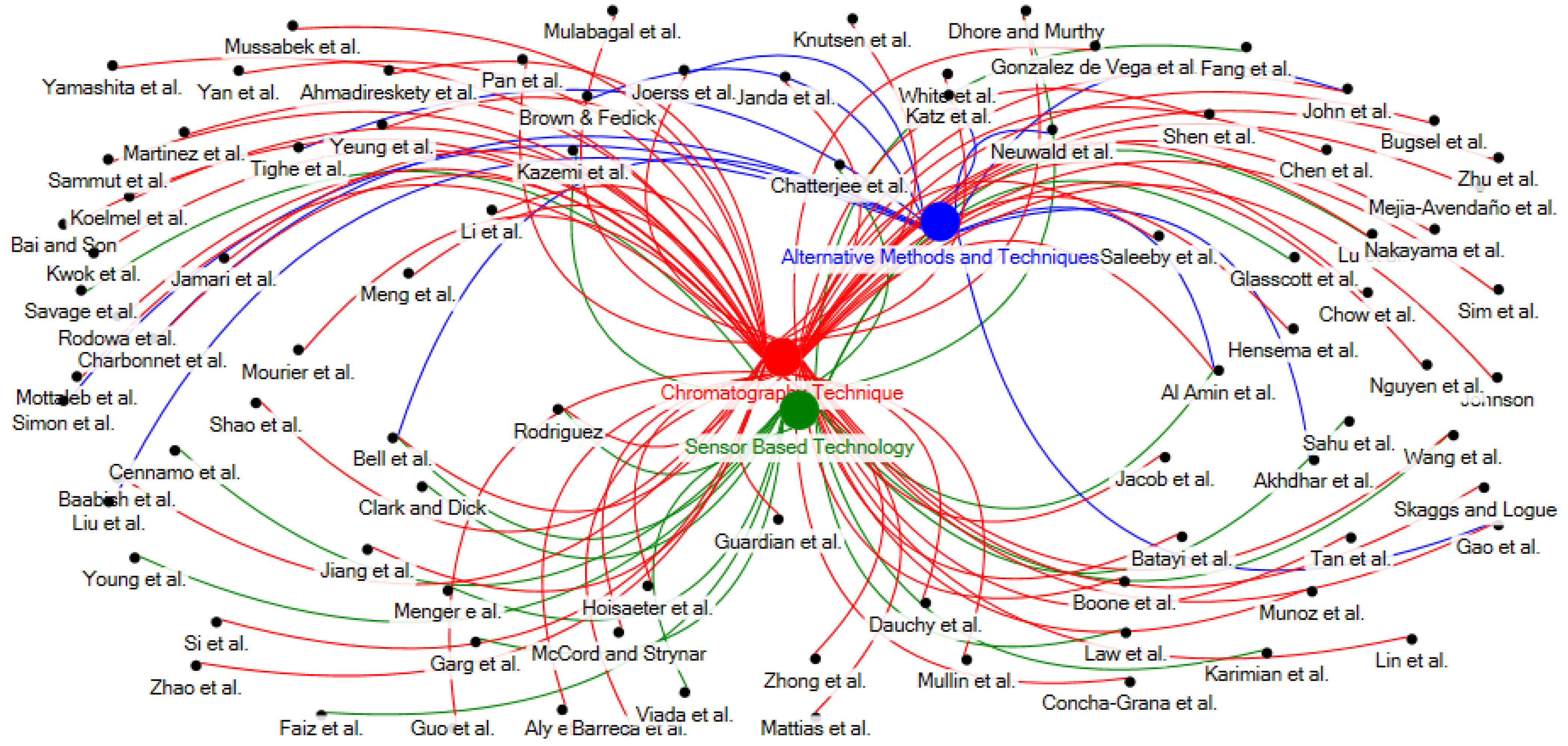
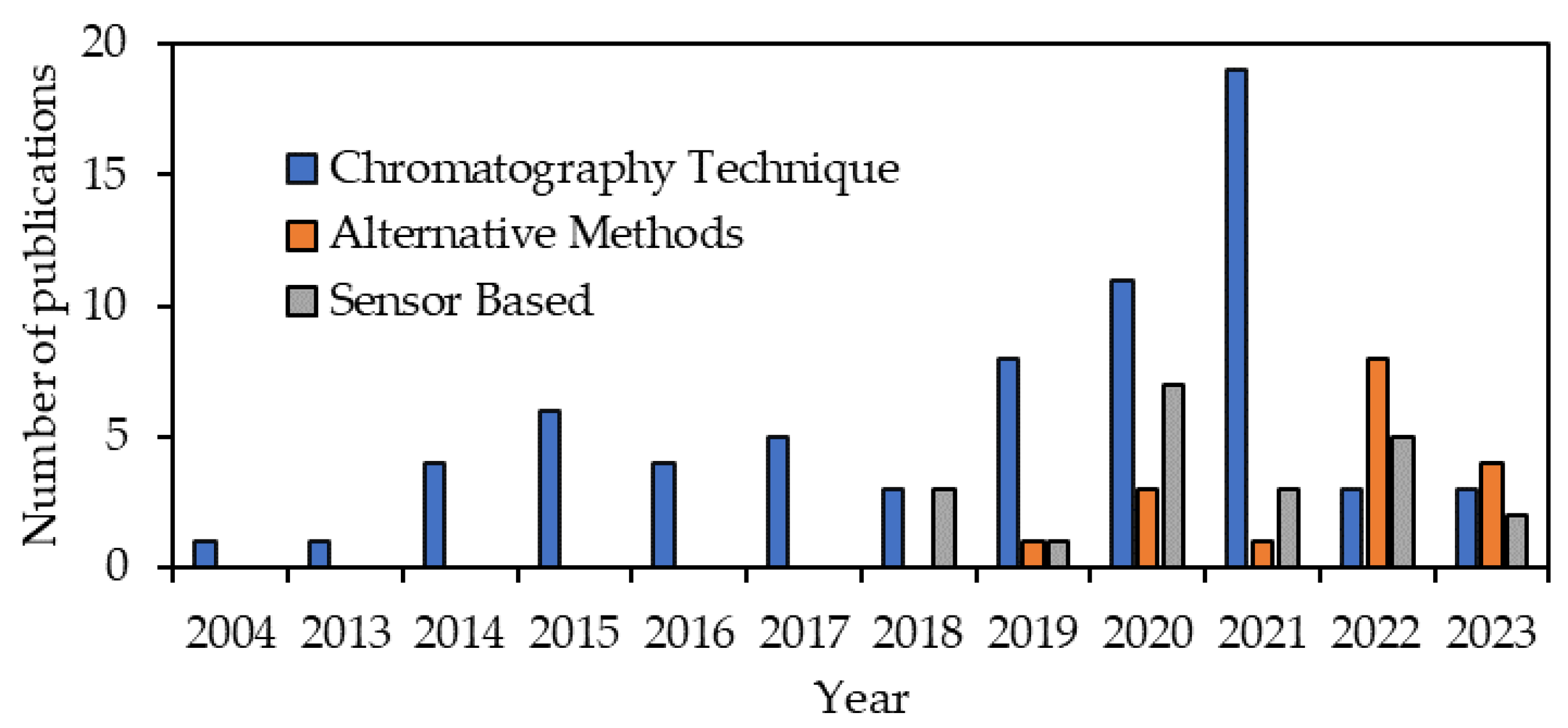
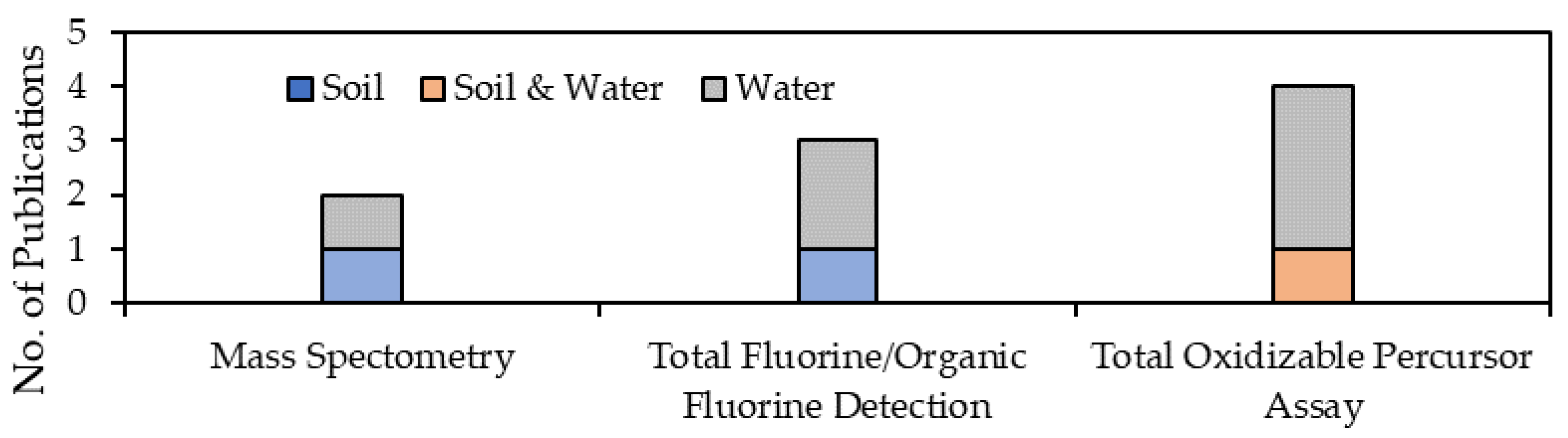
| Research Questions (RQ) | Research Focus |
|---|---|
| RQ 1: What is the purpose of the study? | PFAS monitoring and detection methods |
| RQ 2: When were these data collected? | Studies conducted between 2003 to June 2023. |
| RQ 3: Which sources are considered for PFAS contamination? | Studies conducted on surface waters, tap waters, aqueous waters, soils, or sediments |
| RQ 4: What was the screening process? | Journal and conference publications, full manuscripts and written in English only. |
| Sensor-Based Technology | Optical-based [67,68] | Using optical signals:
|
Nanoparticles-based:
| ||
Dye:
| ||
Optical fibre:
| ||
| Electrochemical-based [63,64] | Using quantifiable electrical signals:
| |
Electrode:
|
| Methods/Techniques | Factor 1 | Factor 2 | Factor 3 | Factor 4 | Disadvantages |
|---|---|---|---|---|---|
| Chromatography | Targeted and non-targeted analytes | Sample extraction and clean-up required | Able to detect analyte concentrations below 10 ng/L | Soil, water, and other various sample types |
|
| Other instrumentation analysis | Targeted and non-targeted analytes | Sample extraction and clean-up required | Some analyses are able to detect analyte concentrations below 10 ng/L (examples: LDTD, TOP), but mostly by incorporating chromatography techniques | Soil, water, and other various sample types |
|
| Sensor-based technology | Targeted analytes only | Sample extraction and clean-up required | Some sensors are able to detect analyte concentrations below 10 ng/L (examples: sensors based on biosensors, and electrochemical, electrochemiluminescence, fluorescence, photoelectrochemical and nanoparticle sensors) | Mainly water, potential for use with soil and other sample types |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nahar, K.; Zulkarnain, N.A.; Niven, R.K. A Review of Analytical Methods and Technologies for Monitoring Per- and Polyfluoroalkyl Substances (PFAS) in Water. Water 2023, 15, 3577. https://doi.org/10.3390/w15203577
Nahar K, Zulkarnain NA, Niven RK. A Review of Analytical Methods and Technologies for Monitoring Per- and Polyfluoroalkyl Substances (PFAS) in Water. Water. 2023; 15(20):3577. https://doi.org/10.3390/w15203577
Chicago/Turabian StyleNahar, Kamrun, Noor Azwa Zulkarnain, and Robert K. Niven. 2023. "A Review of Analytical Methods and Technologies for Monitoring Per- and Polyfluoroalkyl Substances (PFAS) in Water" Water 15, no. 20: 3577. https://doi.org/10.3390/w15203577
APA StyleNahar, K., Zulkarnain, N. A., & Niven, R. K. (2023). A Review of Analytical Methods and Technologies for Monitoring Per- and Polyfluoroalkyl Substances (PFAS) in Water. Water, 15(20), 3577. https://doi.org/10.3390/w15203577







