Abstract
The origin of the fauna of Beringia is a notable biogeographical puzzle. Large mussels of the genus Beringiana inhabit both Northeast Asia and the northwestern part of North America and thus provide an important model to investigate the paleobiogeography of Beringia and the past and current intercontinental species exchanges. Data on Beringiana distribution, morphology, genetics, and taxonomy are fragmentary or questionable. In this study, we summarized the data on its distribution in Northeast Asia, performed genetic analysis (cox1), and studied the variation in the shell morphology in samples from four isolated populations, including the putative sympatric species. Over ten large enclaves of Beringiana are currently known in Northeast Asia (east to the Verkhoyansk Range), mostly in the lower reaches of large rivers in northeastern Yakutia, Kamchatka, Magadan Oblast, and Khabarovsk Krai. These enclaves are far apart (several hundreds of kilometers) because the mussel is associated with muddy sand or pebbly sand bottom substrates and spreads through its glochidia, which are parasitic on the fish. Shell morphology can be highly variable even in a single population, overlapping the diagnoses of several Beringiana species (which are currently not recognized as valid). Our analysis of the cox1 sequence in four populations identified all individuals as B. beringiana. We evaluated the possible current and probable Late Pleistocene–Early Holocene freshwater and marine pathways of B. beringiana dispersal through the area of former Beringia, including the current intercontinental migration of glochidia on fish.
1. Introduction
Animal and plant taxa that are found in both Northeast Asia and the northwestern part of North America attract particular attention because these regions belong to ancient Beringia, where a land bridge existed to connect the two continents quite recently. The distribution and ecology of this group of organisms, known as Beringians, helps one to understand many issues in paleogeography and the genesis of flora and fauna in the region of past contacts between the two continents. The history of aquatic fauna of Beringia was recently reviewed in several publications [1,2,3,4,5,6].
The genus Beringiana Starobogatov in Zatrawkin, 1983, comprises large mussels belonging to the family Unionidae. Its taxonomy and ecology are still under dispute. Beringiana is recognized as a Beringian species and is known to inhabit freshwater habitats on both continents. Its range in Asia includes Japan, the south of the Russian Far East, Sakhalin, Kamchatka, the Kuril Islands, and the basins of certain Arctic seas such as the Chukchi, East Siberian, and probably the Laptev seas. These mollusks were also detected in the basins of a few rivers that flow into the Bering Sea and the Sea of Okhotsk. In North America, Beringiana is found in the basins of rivers that flow into the North Pacific.
The taxonomy of the genus Beringiana changed significantly over time, and there is still no consensus. As many as nine species were described based primarily on conchological traits, most of them from the Russian Far East, where multiple species were sometimes observed in the same water bodies. For instance, three sympatric species: Beringiana beringiana (Middendorff, 1851), B. youkonensis (Lea, 1867), and B. kamchatica Bogatov et Starobogatov, 2001, were found in the basins of the Kava and Ola rivers in Magadan Oblast [7,8]. In addition, B. beringiana, B. youkonensis, B. kamchatica, B. derzhavini Bogatov et Starobogatov, 2001, and B. chereshnevi Bogatov et Starobogatov, 2001, were detected in various combinations in water bodies of the Kamchatka River basin [9].
After several revisions [10,11,12], over a dozen species from several synonymized genera (Kunashiria and Arsenievinaia) were included in the genus Beringiana. The number of species in the genus was reduced to four in recent genetic studies. Only one of the species, B. beringiana, is found in Russia, while the greatest diversity (all four species) is observed in Japan [13,14,15]. Bogatov [16] has compared the transversal contours of the shell in B. beringiana and isolated three “comparatory” subspecies and an intraspecific form: B. b. beringiana, B. b. kamchatica, B. b. youkonensis, and B. b. f. compressa (see [17,18] for comparatory method description).
For the southern part of the Russian Far East, there is abundant information on B. beringiana distribution, morphology, and DNA sequences, while much less is known on the northern part of the distribution [7,8,9,13,14,15,19,20,21,22,23].
Maps in the papers of Bolotov et al. [13] and Lopes-Lima et al. [14] depict the continuous distribution of B. beringiana in Yakutia river basins east of the Lena river, in Chukotka, Magadan oblast, and the north of the Khabarovsk krai. However, few B. beringiana samples are known from this region according to the literature and museum collections, and the respective river basins are far apart, separated by many hundreds of kilometers in many cases [8,9,24,25]. Moreover, DNA sequences of only two populations in Northeast Asia are available: one from a lake near the Belaya Gora village in the Indigirka River basin, and the other from the Bol’shaya River in Kamchatka [13,14]. Beringiana has a patchy distribution, so it makes sense to genotype more populations. New species might be found in island populations [26].
Based on this, we set the following objectives:
- To summarize the available data on the Beringiana distribution in Northeast Asia east of the Verkhoyansk Range and the Okhota River basin;
- To study conchological variation in Beringiana, including the cases of several species found in sympatry [7,8] and to study cox1 variation in different morphs;
- To analyze the possible Beringiana dispersion pathways given the high distances between its enclaves and, in particular, the distribution of the species on the two continents.
2. Materials and Methods
The study area included four localities in Northeast Asia. For two of them, this is the first report of Beringiana: the Medvezh’e Lake (Chukotka Autonomous Okrug, left bank area of the Anadyr River) and a lake near the Rezidentsiya village (Khabarovsk Krai, left bank area of the Kukhtuy River). For two other localities, Beringiana populations were reported earlier [7]: the Kava River basin with its Chukcha River tributary (system of the Chukcha, Malaya Chukcha, and Bezymyanka lakes) and the Ola River basin (Chistoe Lake).
B. beringiana is in the regional Red Data Book of Magadan Oblast and the Chukotka Autonomous District [8,22,24], so we examined only dead mussels that were washed up ashore after storms (Figure 1).

Figure 1.
Beringiana beringiana. Magadan Oblast, Chukcha lake.
Genetic methods. Tissue samples for genetic testing were collected from well-preserved dead mussels. Tissue DNA isolation was successful in only eleven mussels (five from Chukcha Lake and two samples from each of the other three populations). DNA was isolated from soft tissue samples fixed with ethanol using BioSilica columns (Novosibirsk, Russia) according to a published protocol [27]. A fragment of the cox1 mitochondrial gene was amplified with the universal primers LCOm and HCO [28] within project no. FWNR-2022-0022. DNA sequencing with BigDye 3.1 (Applied Biosystems, Waltham, Massachusetts, USA) was performed at the SB RAS Genomics Core Facility. The same primers were used for amplicon sequencing in both directions.
Sequencing data were analyzed and edited using Chromas 2.6.6 (http://technelysium.com.au; accessed on 10 February 2023). A haplotype network was constructed using the program Network 10.2.0.0 [29]. Molecular genetic indices (nucleotide and haplotype diversity, mismatch distribution) were calculated in Arlequin v.3.0 [30]. Maximum likelihood trees were built by RAxML v. 8.2.12 [31] with the GRTCAT model with 1000 bootstrap repetitions.
Shell morphology variation was studied in both live mussels, which were then returned to their water bodies, and dead ones collected on lake banks. In total, we examined 12 mussels near the village of Rezidentsiya, 31 in the Kava River basin, 18 in the Ola River basin, and 10 in the Anadyr River basin. Morphometric analysis was carried out as described in [16,19] using the identification keys of [9,10]. We measured the length (L), height (H), and convexity (B) of the shells; the distances between the apex (M) and the columella (N), and between the apex and the anterior margin of the shell (Lm).
Beringiana distribution map in Northeast Asia was constructed using literature data, the collections of the Zoological Institute (Saint Petersburg), survey data, and original materials.
Statistical analysis was performed by standard methods using Statistica 10 for Windows. Values are reported as mean ± SE. Comparison of morphological values was performed by the Mann–Whitney U-test. The level of significance was set at p ≤ 0.05.
3. Results
3.1. Distribution
Data on the species distribution in Northeast Asia are summarized in Figure 2. We mapped all sites of Beringiana reports currently known in northeastern Yakutia, Chukotka, amchatka, Magadan Oblast, and northernmost Khabarovsk Krai, that is, an area east of the river Indigirka and Suntar-Khayata ranges.
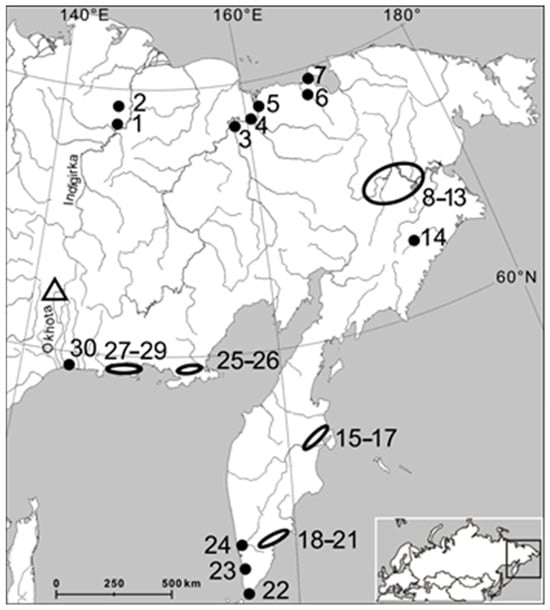
Figure 2.
Detection sites (black dots and circles) currently known for mussels of the genus Beringiana in Northeast Asia. A bifurcation of the Del’kyu River is shown with a triangle. See Table S1 (References [32,33,34,35,36] are cited in the supplementary materials) for numerical designations.
3.2. Genetic Analysis
Cox1 sequences were obtained for 11 soft tissue samples of Beringiana mussels from four localities (Figure 3) and compared to GenBank sequences. All individuals were assigned to B. beringiana. All GenBank data available for the species were combined with our data, and a haplotype network was constructed for a pooled sample (Figure 4). In total, 11 haplotypes and 15 variable sites were observed in 39 sequences of the pooled sample. The majority (24 out of 39) sequences belonged to the same haplotype, while the other haplotypes were represented by 1 to 4 mussels each.
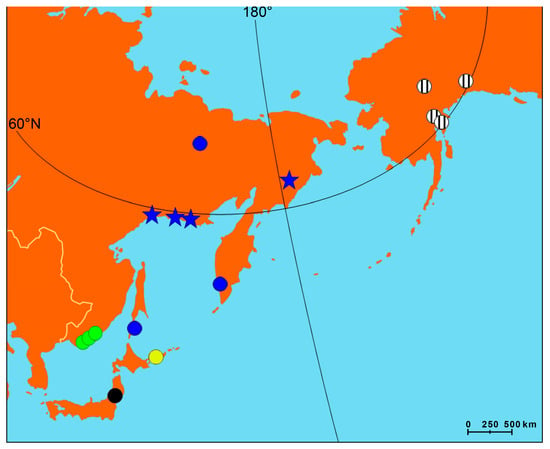
Figure 3.
Beringiana beringiana populations with available cox1 data. Circles, GenBank; stars, this study. See Figure 4 for color legend.
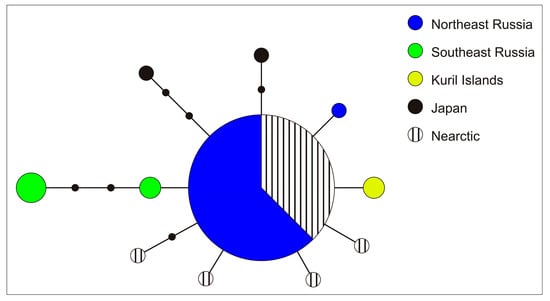
Figure 4.
Cox1 haplotype network of B. beringiana. Missing haplotypes are shown as dots.
Haplotype diversity for the whole dataset amounted to 0.6167 (SD, 0.0885), and nucleotide diversity was 0.002582 (SD, 0.00177). Both Tajima’s D (−1.85995) and Fu’s Fs (−5.06095) values were negative, with p-values of 0.012 and 0.004, respectively. For mismatch distribution, the observed frequency distribution and that expected under the population expansion model differed with borderline p = 0.05. Since southern haplotypes differed from northern ones, we calculated these indices for a dataset of 28 specimens that excluded the populations from Japan and Primorye. Tajima’s D was −2.09646 (p-value = 0.00200) and Fu’s Fs, −4.61217 (p-value < 10−5). The observed mismatch distribution almost perfectly matched the expected one, with no statistical differences between the two distributions.
We constructed a phylogenetic tree for the available dataset of the four Beringiana species, B. beringiana, B. japonica, B. fukuharai, and B. gosannensis (Figure 5). The datasets for the latter three species were taken from GenBank; all specimens were from various parts of the Japanese Archipelago. The monophyly of all four species was supported by bootstrap. Species identification of only one specimen was probably incorrect: accession LC519019 was submitted as B. japonica, while it was grouped together with B. beringiana.
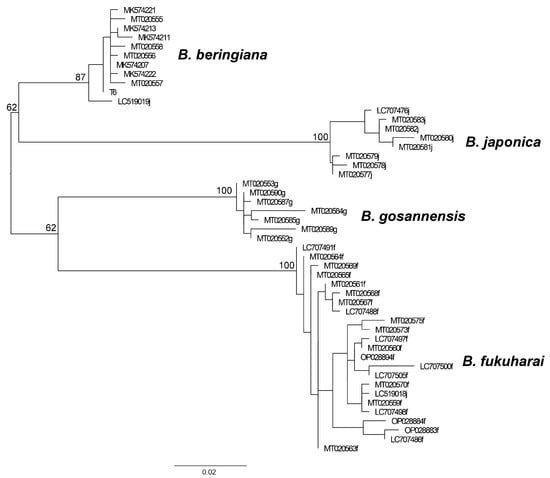
Figure 5.
Phylogenetic tree constructed using the maximum likelihood method for the Beringiana species. The tree is rooted at midpoint. Specimen names refer to GenBank accession or to our internal numbers. Numbers near branches indicate bootstrap support.
3.3. Conchological Analysis
Shell morphological traits used in the taxonomy of the genus Beringiana include not only descriptive characters (shape, umbo sculpture, nacre color, etc.) but also indices based on measurements and calculations. The following indices are most often used in identification keys [9,10]:
- -
- The ratio of shell width (B) to shell height (H) characterizes the degree of prominence (a major trait) of the shell;
- -
- The ratio of the distance between the apex (M) and nymph base (N) to the shell width (B);
- -
- The ratio of the distance between the apex and the anterior margin (Lm) to the shell length (L);
- -
- The ratio of the shell height (H) to the shell length (L) characterizes the general shape of the shell.
We calculated the main ratios used for species identification in the studied specimens. The results are summarized in Table 1. Figure 6 shows the variation in the B/H key diagnostic trait, which characterizes the degree of prominence of the shell.

Table 1.
Mean values (mean ± SEM) and coefficients of variation (Cv, %) of the main diagnostic conchological traits in the four Beringiana populations of Northeast Asia.
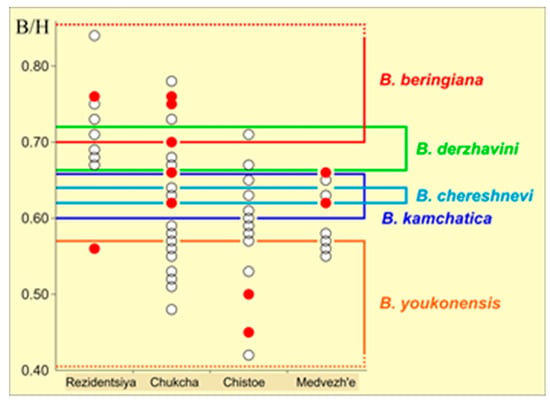
Figure 6.
Diagram of the B/H trait in the studied populations. Vertical axis, B/H value; horizontal axis, populations; colored frames indicate B/H values characteristic for the corresponding species. Individuals included in the genetic analysis are shown with red circles.
Based on the first trait (M − N)/B, the populations formed two groups, Rezidentsiya–Chukcha and Chistoe–Medvezh’e, with trait values being statistically similar within each of the groups and significantly different between the groups (U-test, p = 0.001).
A difference in Lm/L was observed only between the two western populations, Rezidentsiya and Chukcha (U-test, p = 0.0003).
The B/H ratio, which reflects the degree of prominence of the shell, was significantly higher in the Rezidentsiya population compared with the other populations (U-test, p < 0.01 in all cases) (Table 1).
The H/L ratio varied from 0.51 to 0.64 in 97% of the shells examined, i.e., the shells were oval in shape according to Bogatov’s [16] classification. Only two shells (3%) of the Chukcha population had higher values (0.65 and 0.66) and were consequently classified as ovate. Although the shell shape was the same in all populations, the easternmost (Medvezh’e) and westernmost (Rezidentsiya) populations differed in H/L from the populations of Magadan Oblast (U-test, p < 0.01 in both cases) (Table 1).
In each of the samples examined, the variation ranges of the traits were great and exceeded the respective ranges of several species described earlier. The mussels whose species identification was performed genetically in various populations were similarly found in the trait ranges of all the species described earlier (Figure 6).
4. Discussion
4.1. Beringiana beringiana Distribution in Northeast Asia
The published distribution maps of B. beringiana depict a continuous range in the Russian Far East [13]. However, the list of its findings (Figure 2) suggests that it occurs as scattered enclaves, mostly associated with the lower reaches of large rivers (Indigirka, Kolyma, Anadyr, Kamchatka, and their tributaries). Reports of B. beringiana deep in the continent are rare. This species needs sandy or silty river beds, while rock beds prevail in Northeast Asia. Suitable habitats usually form in lower reaches, backwaters, or floodplain lakes.
B. beringiana is found in the middle and upper reaches only in rare cases (e.g., in the rivers Anadyr, Khatyrka, and Kava). These habitats are characterized by a low vertical drop, which results in vast floodplains with many distributaries and through-flowing lakes. This situation is characteristic of the middle reaches of the Anadyr River, in its part parallel to its tributary Mayn River (locations 8 and 9 in Figure 2), as well as in the basin of the mountainous Khatyrka River, where the floodplain Elergytkyn Lake is located in a vast intermountain depression (location 14 in Figure 2).
High population densities of B. beringiana are characteristic of many lakes belonging to the upper and middle basins of the Kava River (locations 27–29 in Figure 2). This territory lies in a vast glacier plain (40–60 km long and 120 km wide) covered by thick sand deposits. Low vertical drops and slow flow favor water heating [37]. B. beringiana was reported from five lakes in this region: Sbornoe, Chukcha, Malaya Chukcha, Bezymyanka, and Zaton, but we believe this list is not exhaustive. Bottom features are constant in through-flowing lakes even during floods, in contrast to backwaters. Due to these factors, the upstream basin of the Kava River probably has a population density of B. beringiana similar to other rivers in Northeast Asia.
Only two known B. beringiana populations in the region are found in large lakes, which are sources of the Ola River. One is Chistoe Lake, located in tundra (approximately 9 × 6.5 km, altitude 91 m) with a gray silt bottom and a substantial area of shallow depths with a sand or fine pebble bottom [37]. The other is the mountainous Kisi Lake (approximately 4 × 1.5 km, altitude 333 m), with the bottom formed by silt, rock grit, and rock fragments [38].
Based on our data collected in this study and the association of B. beringiana with certain bottom types, we believe it is far more widespread than is currently considered. Possible habitats in Chukotka may include the Velikaya and Tumanskaya rivers [24], Krasnoe Lake, and some other water bodies. B. beringiana may be found in many sites of the Kolyma River delta downstream of the Cherskii village because its shells are abundant in floodplain lakes and branches ([25]; S.P. Davydov, personal communication). In the lower reaches of the Indigirka River, shells of B. beringiana are not uncommon in floodplain lakes from the village of Belaya Gora to the Ozhogina River and Ozhogina Lake (S. Yu. Solomov, personal communication); B. beringiana is probably spread down to the river mouth.
Thus, although the range of B. beringiana spans approximately 10° of latitude (59°30′–69°30′) from rivers of the Sea of Okhotsk to downstream regions of the Indigirka, Kolyma, and Anadyr rivers, it is highly fragmented and its populations are often several hundreds of kilometers apart.
4.2. Temperature of B. beringiana Habitats in Northeast Asia
The northeastern part of the B. beringiana range north to the 60th parallel is one of the coldest regions of Eurasia. Winter air temperatures are extremely low, and the climatic summer (a period when average daily air temperatures are higher than 15 °C) is absent in many of its parts.
The differences between the duration of the ice-free period in the northernmost (lower reaches of the Rauchua River) and the southernmost (lower reaches of the Kukhtuy River) known B. beringiana localities exceed 50–60 days [39]. The average July water temperatures are similar throughout the region (ca. 10–12 °C), up to 14 °C only in the middle part of the Kolyma River basin [40]. However, Beringiana habitats stand out: they have average ice-free periods, but much higher summer water temperatures. No special studies were performed on the subjects, but we are aware that many of these locations are traditionally used for bathing.
We should note that B. beringiana is able to live in habitats heated by thermal springs. Derzhavin [41] reported a population from a warm river in Kamchatka near the Paratunka village, which even in late winter was hotter than 11 °C. In the major part of Northeastern Asia, these mollusks spend winter burrowing in bottom sediments at low positive temperatures.
Thus, although B. beringiana inhabits cold regions of Northeast Asia, it is confined to the warm and shallow water bodies in this region.
4.3. Genetic Studies
Our genetic analysis of four populations from Northeast Asia confirmed that they belong to the same species, B. beringiana. Genetic variation in B. beringiana is low, as is evident from the haplotype network (Figure 4), suggesting recent divergence of populations and their dispersal through the current range. The network corresponds to a classical star-like cluster [42], in which a central haplotype is usually considered ancestral and the others are thought to be its derivatives formed by accumulating substitutions. It is also possible to assume in our case that the large size of the central (“basic”) haplotype in the network is explained by the fact that more mussels were examined in samples from northern populations compared with southern ones. While the same haplotype is found in Northeast Asia, Sakhalin, and Alaska, various haplotypes are characteristic of Primorye, the Kuril Islands, and Japan. A center of genetic diversity of B. beringiana is likely to be in the south, but the available data on southern regions are insufficient for deciding whether the center should be sought on the continent or islands. This question will be resolved by extensive sampling.
Tajima’s D and Fu’s Fs measures were negative and deviated significantly from neutrality, supporting recent population expansion. However, mismatch distribution analysis for the total B. beringiana sample showed that the differences between the observed distributions and those expected for the population expansion model had p-value = 0.05, i.e., at the border of statistical significance. This was due to the specimens from the southern locations. For the sample of the northern populations (without Primorye and the Japanese archipelago), the observed mismatch profile almost perfectly fitted the distribution expected under the recent expansion model. Given the available data, we can state that the Northern Palearctic and Nearctic part of the distribution was colonized recently. However, as said above, there is still not enough data for the Southern Palearctic to infer the age of the populations and the genetic distances between them.
4.4. Morphological Variation
Highly variable shell morphology was observed in the four studied B. beringiana populations of Northeast Asia. Within-population variation was found to be very high, and the calculated index values fell out of the ranges accepted for the species in the literature earlier [9]. Significant differences in certain key morphological traits were detected even between neighboring populations that live in similar climatic and landscape conditions but are separated by a mountain ridge, as is the case with the populations of the Kukhtuy River basin on the shore of the Sea of Okhotsk (near the village of Rezidentsiya) and the Kava River basin. The ecological factors that determine the shell shape of B. beringiana are difficult to identify because only limited data are available on the environmental conditions of water bodies inhabited by the species in Northeastern Asia. Variation in external traits most likely explains why the sympatry of several species was reported earlier, for example, in the Kava and Ola basins of Magadan Oblast [24]. The variation range observed in the pooled sample from the four populations included all species ranges described in the literature (Figure 6).
Mussels randomly selected for genetic testing were assigned to different species (B. beringiana, B. youkonensis, B. derzhavini, B. chereshnevi, and B. kamchatica) by the set of morphological traits. However, all of them were identified as B. beringiana by cox1 sequence analysis. Our study thus confirmed that high conchological variation, which was reported for the species more than 100 years ago [32,36] lacks taxonomic significance. A similar conclusion was made for freshwater pearl mussels of the genus Margaritifera [43,44].
High morphological diversity and ecological plasticity are characteristic of inhabitants of northern freshwaters but are often not accompanied by genetic differentiation (for a review, see [45]). Evidence for the phenotypic plasticity of shell morphology has been found in other species of Unionidae [46,47,48,49]. This factor possibly underlies the phenotypic diversity in B. beringiana, but experiments are problematic to perform on this protected species.
4.5. Freshwater Dispersal Pathways of B. beringiana
The mosaic distribution of B. beringiana populations across a vast territory (including two continents separated by a strait for 10–12 thousand years) and the identity of haplotypes throughout its range suggest a relatively recent dispersal history of this species. The current range of B. beringiana suggests that this species dispersed by means of ancient river basins that existed during ocean regression. To explain how the species range of Beringian fishes might have formed, Chereshnev [50] and, more recently, Ager [51] proposed paleogeographic schemes of the hydrographic network of Northeastern Asia and a northwestern region of North America (Figure 7). They were based on the works of Soviet and American researchers, primarily, Baranova and Biske [52] and Hopkins [53]. The schemes suggest that the exposed shelf of the Chukchi Sea was drained by rivers of the Kolyma–Chukotka and Chukotka–Alaska complexes. Hopkins [53] believed that Merklin Lake existed in place of the current Bering Strait. The lake was fed by the rivers of Chukotka and Alaska and was drained into the Chukchi Sea. The northern part of the basins of Pacific rivers was drained by the Anadyr complex, paleo-Penzhina, rivers of Taui Bay, and the Okhotsk group of rivers, while the American shore was drained by paleo-Yukon and paleo-Kuskokwim.
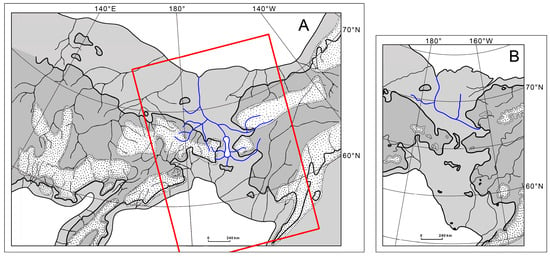
Figure 7.
Hydrography of Beringia in the Late Pleistocene ocean regression: (A) after Chereshnev [50] with changes; (B) after Ager [51]. Dark gray, modern land; light gray, land borders 20–18 thousand years BC; white dotted contours, glaciers; white background, open water; rivers draining both continents are shown in blue. The red frame is the position of Figure 7B.
Thus, during the global 100–120 m ocean regression in the Late Pleistocene, hydrobionts could have moved between Chukotka and Alaska through common streams on the exposed shelf.
Based on the schemes, the exchange of freshwater organisms (other than anadromous fish) was unlikely to occur between the mouth populations of Indigirka and Kolyma and between these populations and those of the Pacific coast in Northeast Asia in the Late Pleistocene. The mouth of paleo-Kolyma was far away from the mouth of a great stream that ran on the dry shelf of the Chukchi Sea and drained Chukotka and Alaska.
In Northeastern Asia, the gradual dispersal of B. beringiana along rivers is impeded by the lack of suitable bottom substrates. We believe that long-distance dispersal by means of glochidia is possible not only for anadromous salmons. Grayling, the most abundant fish in the upper reaches of local rivers, might also be among the hosts of B. beringiana glochidia, and it could facilitate their dispersal between flatland watersheds observed in certain species. The Indigirka–Okhota–Kukhtuy river system might have been such a putative zoogeographical channel (Figure 2).
A bifurcation, which is extremely rare in global hydrology, is known for Del’kyu, one of the largest tributaries of Okhota [54]. Approximately 30 km away from its origin (62°08′ N, 141°34′ E; altitude 1400 m), Del’kyu separates into Del’kyu-Okhotskaya (the basin of the Sea of Okhotsk) and Del’kyu-Kuidusunskaya, which is a tributary of the Kuidusun River, which runs into Indigirka (the basin of the East Siberian Sea). The bifurcation region (see Figure 2) is freely passable for boats and certainly fish in years of medium water content [55].
A stream separates from Okhota on its left bank, 32 km away from its mouth, and runs into Khaibas, which is a right tributary of Kukhtuy. The stream ensures a continuous connection of downstream Indigirka with Okhota and Kukhtuy, thus eventually connecting the Pacific and the Arctic Ocean.
Pugachev [54] observed that ichthyofaunal exchange between Okhota and Indigirka is still possible now. Chereshnev [56] (p. 62) did not mention the Del’kyu bifurcation but noted that a pathway from Indigirka through a drainage divide into Okhota and Kukhtuy of the basin of the Sea of Okhotsk was utilized in the spreading of the Siberian species Phoxinus phoxinus, Ph. perenurus, Perca fluviatilis, Carassius carassius jacuticus, Nemacheilus toni, Exos lucius, Salvelinus neiva, and Thymallus arcticus pallasi.
Beringiana beringiana populations are found in the downstream valley lakes of both Indigirka (near the village of Belaya Gora) and Kukhtuy (the village of Rezidentsiya), and their common origin is possible to assume in view of the above. The distance in a straight line between the populations is over 1000 km, and the mussel is so far unknown to inhabit other localities along the line, although its presence there in the past cannot be excluded.
The region of the Del’kyu bifurcation is in an area of recent glaciation and is in the immediate vicinity of the currently greatest mountain glaciers of Northeast Asia [57]. The Okhota–Indigirka continuous water system could apparently not act as a biogeographical channel until the glaciers had regressed [54] to their current level in the most highland part of the Suntar-Khayata Range.
The conclusion does not contradict the molecular genetic data on the genetic similarity of mussels from the downstream regions of Indigirka and Kukhtuy, or from other localities of the species range.
4.6. On the Possibility of B. beringiana Larva Dispersal with Fish across Seas
Since molecular data suggest that Beringiana populations from both continents diverged relatively recently, it is reasonable to investigate whether its larvae (glochidia) might spread through seas as parasites of migrating fish.
Eight host fish species are currently known for Beringiana glochidia [20]: the three-spined stickleback Gasterosteus aculeatus Linnaeus, 1758, the ninespine stickleback Pungitius pungitius (Linnaeus, 1758), the pond smelt Hypomesus olidus (Pallas, 1814), the coho salmon Oncorhynchus kisutch (Walbaum, 1792), the sockeye salmon O. nerka (Walbaum, 1792), the dolly varden Salvelinus malma (Walbaum, 1792), the white char S. albus Glubokovsky, 1977, and the whitespotted char S. leucomaenis (Pallas, 1814). All but one species (white char) are euryhaline, living both in rivers and marine waters, and could therefore be capable of transferring glochidia between distant river mouths through the sea.
It is unclear now how long it takes for B. beringiana glochidia to develop in host fish; glochidia are only known to infest fish in Kamchatka in July and August [58]. There are no data as to whether B. beringiana glochidia survive on gills in marine water. Glochidia of the freshwater pear mussel Margaritifera margaritifera remain viable on Atlantic salmon gills in the sea [59]. A spreading of M. margaritifera through the sea has been confirmed in a phylogenetic study [60]. Taken together, the above data indicate that dissemination of B. beringiana glochidia from one river basin to another through the sea on fish gills might be possible and needs investigation.
Significant distances separate B. beringiana populations inhabiting the mouths of large rivers. For example, the distances between the Indigirka and Kolyma mouths along the shoreline are over 500 km; between the Kolyma and Anadyr mouths, there is over 2000 km; and between the Anadyr and Khatyrka mouths, there is over 1000 km.
Distances of several hundred kilometers can be crossed by anadromous fish and probably within a reasonable time. Transcontinental migration (approximately 1700 km) from the Wulik River in Alaska to Anadyr in Chukotka is known for two dolly wardens (Salvelinus malma) [61]. According to Chereshnev [50], quite intense fish exchange probably exists between S. malma populations of eastern Chukotka and Alaska.
No new haplotype was detected in addition to the known ones in our mtDNA analysis of four B. beringiana populations of Northeast Asia. Similar genetic characteristics were observed for the B. beringiana populations of the region (and other localities of the species range), supporting the idea of recent (Late Pleistocene–Early Holocene) colonization of Northeast Asia by the species. A similar conclusion has been reached in studies of modern fish distributions and paleogeographic reconstructions [62]. Molecular genetic studies have confirmed a recent spreading of certain fish phylogenetic lineages in Northeast Asia [63,64,65,66,67], although genetic data support the long-term existence of endemic salmonids in the El’gygytgyn Lake of Chukotka [68,69].
5. Conclusions
At least 30 B. beringiana populations are currently known in Northeast Asia, and most of them inhabit river mouths that are often several hundred kilometers apart. The patchy distribution of B. beringiana is caused by its paleogeographic history, stringent requirements for muddy sand bottom substrates, and dispersal mode (through parasitic glochidia).
All B. beringiana populations throughout the distribution appear to be genetically similar, which supports the idea of recent Late Pleistocene–Early Holocene colonization of Northeast Asia. Moreover, we found that individuals assigned to different species showed no genetic differences, which supports a high conchological variation in Beringiana that does not have taxonomic consequences.
The geographical isolation of enclaves indicates that glochidia might be dispersed by fish for long distances, both in fresh and marine waters. The available literature data suggest the possibility of long-distance and rapid dispersal, including the transfer between continents.
We suggest that the «Indigirka–Okhota–Kukhtuy» river system might be an important biogeographic channel that connects the biotas of river basins of the two oceans, which warrants further studies of various hydrobionts inhabiting this region.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w15203538/s1, Table S1: Numerical designations used in Figure 2.
Author Contributions
N.A.B.: Methodology, Validation, Formal analysis, Investigation, Resources, Writing—Original Draft, Writing—Review and Editing, Visualization. A.A.M.: Validation, Writing—Review and Editing, Funding acquisition, Project administration. A.N.L.: Resources. S.V.S.: Validation, Formal analysis, Investigation, Resources, Writing—Original Draft, Visualization. T.V.P.: Investigation. D.I.B.: Conceptualization, Methodology, Investigation, Resources, Writing—Original Draft, Writing—Review and Editing, Visualization, Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
The manuscript was prepared with the support of the Russian Science Foundation, project no. 19-14-00066/P.
Data Availability Statement
The initial data for the manuscript are included in the Supplementary Materials.
Acknowledgments
We are grateful to A.V. Alfimov for advice on water temperatures in rivers and lakes; I.V. Vikhrev, S.P. Davydov, A.V. Krechmar, and V.V. Pospekhov for information about new localities of Beringiana in Northeast Asia; R.E. Akimikin, S.I. Grunin, and A.V. Shestakov for help in data collection; and curators of the malacological collection at the Zoological Institute for Catalog materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Campbell, M.A.; Lopéz, J.A. Mitochondrial phylogeography of a Beringian relict: The endemic freshwater genus of blackfish Dallia (Esociformes). J. Fish Biol. 2014, 84, 523–538. [Google Scholar] [CrossRef] [PubMed]
- Neretina, A.N.; Gololobova, M.A.; Neplyukhina, A.A.; Zharov, A.A.; Rogers, C.D.; Horne, D.J.; Protopopov, A.V.; Kotov, A.A. Crustacean remains from the Yuka mammoth raise questions about non-analogue freshwater communities in the Beringian region during the Pleistocene. Sci. Rep. 2020, 10, 859. [Google Scholar] [CrossRef]
- Vinarski, M.V.; Aksenova, O.V.; Bespalaya, Y.V.; Kondakov, A.V.; Tomilova, A.A.; Khrebtova, I.S.; Gofarov, M.Y.; Bolotov, I.N. One Beringian genus less: A reassessment of Pacifimyxas Kruglov & Starobogatov, 1985 (Mollusca: Gastropoda: Lymnaeidae) questions the current estimates of Beringian biodiversity. J. Zool. Syst. Evol. Res. 2021, 59, 44–59. [Google Scholar]
- Novichkova, A.A.; Chertoprud, E.S. The species structure, biogeographical status, and the relation to the Beringian fauna of microcrustaceans (Cladocera, Copepoda) of the Magadan Area (Far East, Russia). Arthropoda Sel. 2022, 31, 283–292. [Google Scholar] [CrossRef]
- Zuykova, E.I.; Sleptzova, L.P.; Bochkarev, N.A.; Kuchko, Y.A.; Sheveleva, N.G.; Zakharov, E.S.; Pestryakova, L.A.; Kotov, A.A. Mitochondrial Lineage Diversity and Phylogeography of Daphnia (Daphnia) (Crustacea: Cladocera) in North-East Russia. Water 2022, 14, 1946. [Google Scholar] [CrossRef]
- Markevich, N.G.; Solovyev, M.M.; Vlasenko, P.G.; Izotova, G.V.; Kashinskaya, E.N.; Bochkarev, N.A.; Politov, D.V.; Melnik, N.O.; Esin, E.V. Phylogeny, Distribution, and Biology of Pygmy Whitefish (Prosopium coulterii) in the Beringia Region (Chukotka). Diversity 2023, 15, 547. [Google Scholar] [CrossRef]
- Prozorova, L.A. Freshwater and terrestrial molluscs of the coast of the Tauyskaya Bay. In Biodiversity of the Tauy Bay of the Sea of Okhotsk; Chereshnev, I.A., Ed.; Dalnauka: Vladivostok, Russia, 2005; pp. 252–261. (In Russian) [Google Scholar]
- Prozorova, L.A. Beringiana beringiana. Beringiana youkonensis. Beringiana kamchatica. In Red Data Book of the Magadan Region; Andreev, A.V., Ed.; Okhotnik: Magadan, Russia, 2019; pp. 24–26. (In Russian) [Google Scholar]
- Bogatov, V.V.; Starobogatov, J.I. Anodontinae (Bivalvia) of the genus Beringiana. Zool. Zhurnal 2001, 80, 26–31. (In Russian) [Google Scholar]
- Starobogatov, Y.I.; Prozorova, L.A.; Bogatov, V.V.; Sayenko, E.M. Mollusks. In Key to Freshwater Invertebrates of Russia and Adjacent Lands; Tsalolikhin, S.Y., Ed.; Nauka: Saint-Petersburg, Russia, 2004; pp. 11–252. (In Russian) [Google Scholar]
- Chernyshev, A.V. Systematics of genera Naiads (Bivalvia, Unionida) of the Russian Far East. Bull. Russ. Far East Malacol. Soc. 2004, 8, 5–16. (In Russian) [Google Scholar]
- Vinarski, M.V.; Kantor, Y.I. Analytical Catalogue of Fresh and Brackish Water Molluscs of Russia and Adjacent Countries; A.N. Severtsov Institute of Ecology and Evolution: Moscow, Russia, 2016; pp. 1–545. [Google Scholar]
- Bolotov, I.N.; Kondakov, A.V.; Konopleva, E.S.; Vikhrev, I.V.; Aksenova, O.V.; Aksenov, A.S.; Bespalaya, Y.V.; Borovskoy, A.V.; Danilov, P.P.; Dvoryankin, G.A.; et al. Integrative taxonomy, biogeography and conservation of freshwater mussels (Unionidae). Sci. Rep. 2020, 10, 3072. [Google Scholar] [CrossRef]
- Lopes-Lima, M.; Hattori, A.; Kondo, T.; Lee, J.H.; Kim, S.K.; Shirai, A.; Hayashi, H.; Usui, T.; Sakuma, K.; Toriya, T.; et al. Freshwater mussels (Bivalvia: Unionidae) from the Rising Sun (Far East Asia): Phylogeny, systematics and distribution. Mol. Phylogen. Evol. 2020, 146, 106755. [Google Scholar] [CrossRef]
- Sano, I.; Saito, T.; Ito, S.; Ye, B.; Uechi, T.; Seo, T.; Do, V.T.; Kimura, K.; Hirano, T.; Yamazaki, D.; et al. Resolving species-level diversity of Beringiana and Sinanodonta mussels (Bivalvia: Unionidae) in the Japanese archipelago using genome-wide data. Mol. Phyl. Evol. 2022, 175, 107563. [Google Scholar] [CrossRef] [PubMed]
- Bogatov, V.V. Large Bivalve Molluscs of Russia’s Fresh Waters; Dalnauka: Vladivostok, Russia, 2022; pp. 1–288. (In Russian) [Google Scholar]
- Graf, D.L. Palearctic freshwater mussel (Mollusca: Bivalvia: Unionoidae) diversity and the Comparatory Method as a species concept. Proc. Acad. Nat. Sci. Phila. 2007, 156, 71–88. [Google Scholar] [CrossRef]
- Bogatov, V.V.; Neretina, T.V.; Anisimova, A.S.; Abdrakhmanov, A. Evaluation of the Applicability of the Comparatory Method for Species Diagnosis of Unionidae (Bivalvia) by Genetic Analysis. Dokl. Biol. Sci. 2018, 482, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Zatravkin, M.N.; Bogatov, V.V. Large Bivalve Mollusks of the Fresh and Brackish Waters of the Far East of the USSR; Publishing House of the FEB AS USSR: Vladivostok, Russia, 1987; pp. 1–152. (In Russian) [Google Scholar]
- Sayenko, E.M.; Shedko, M.B.; Kholin, S.K. Morphology and some peculiarities of the biology of glochidia of the mollusk genus Beringiana (Bivalvia, Unionidae) of Kamchatka and the Northern Kurils. Vestn. Zool. 2001, 35, 59–68. (In Russian) [Google Scholar]
- Sayenko, E.M. Morphological peculiarities of the glochidia (Bivalvia, Anodontinae) in the Russian Far East island populations. In Proceedings of the Vladimir Ya. Levanidov’s Biennial Memorial Meetings, Vladivostok, Russia, 19–21 March 2003; Volume 2, pp. 166–171. (In Russian). [Google Scholar]
- Prozorova, L.A. Mollusks. In Red Data Book of the Chukotka Autonomous District. Volume 1. Animals; Kondratiev, A.V., Litovka, D.I., Eds.; Teksotel LLC: N. Novgorod, Russia, 2022; pp. 12–21. (In Russian) [Google Scholar]
- Klishko, O. Shell Shape Variability in Mollusks (Bivalvia, Margaritiferidae, Unionoidae) and Their Real Species Diversity. Int. Indep. Sci. J. 2020, 2020, 3–15. [Google Scholar]
- Prozorova, L.A. Beringiana chereshnevi. In Red Book of the Chukchi Autonomous District, V. 1; Chereshnev, I.A., Ed.; Wild North: Magadan, Russia, 2008; pp. 21–31. (In Russian) [Google Scholar]
- Zotin, A.A.; Popov, I.Y. Individual growth of Anodonta beringiana (Unionidae, Bivalvia) in postlarval ontogenesis. Russ. J. Dev. Biol. 2019, 50, 189–193. [Google Scholar] [CrossRef]
- Sayenko, E.M. New data on glochidia of anodontins Beringiana and Kunashiria (Unionidae, Bivalvia). In Proceedings of the Vladimir Ya. Levanidov’s Biennial Memorial Meetings, Vladivostok, Russia, 22–24 March 2021; Volume 9, pp. 151–158. (In Russian). [Google Scholar]
- Shekhovtsov, S.V.; Derzhinsky, Y.A.; Poluboyarova, T.V.; Golovanova, E.V.; Peltek, S.E. Phylogeography and genetic lineages of Aporrectodea rosea (Lumbricidae, Annelida). Eur. J. Soil. Biol. 2020, 99, 103191. [Google Scholar] [CrossRef]
- Folmer, O.; Hoeh, W.R.; Black, M.B.; Vrijenhoek, R.C. Conserved primers for PCR amplification of mitochondrial DNA from different invertebrate phyla. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Bandelt, H.J.; Forster, P.; Röhl, A. Median-joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol. 1999, 16, 37–48. [Google Scholar] [CrossRef]
- Excoffier, L.; Laval, G.; Schneider, S. Arlequin (version 3.0): An integrated software package for population genetics data analysis. Evol. Bioinform. 2005, 1, 117693430500100003. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Rosen, O.V. Terrestrial and freshwater mollusks collected by the Kamchatka expedition of F. P. Ryabushinsky in 1908–1909. Yearly Zool. Mus. Acad. Sci. USSR 1927, 27, 261–274. (In Russian) [Google Scholar]
- Vvedenskaya, T.L.; Ulatov, A.V.; Bonk, T.V. Ecological state of Lake Kultuchnoye (east coast, Kamchatka). In Preservation of the Biodiversity of Kamchatka and Adjacent Seas; Bugaev, V.F., Ed.; Kamchatpress: Petropavlovsk-Kamchatsky, Russia, 2013; pp. 72–91. (In Russian) [Google Scholar]
- Gorovaya, O.Y.; Butorina, T.E. Parasitofauna of lake-river Dolly (Salvelinus malma) from Lake Dalnee in Kamchatka. Sci. Work. Dalrybvtuz 2007, 19, 174–181. (In Russian) [Google Scholar]
- Vvedenskaya, T.; Ulatov, A.; Koval, O. Some hydrobiological information about Khalaktyrskoye Lake (Eastern Kamchatka). In Preservation of the Biodiversity of Kamchatka and Adjacent Seas; Bugaev, V.F., Ed.; Kamchatpress: Petropavlovsk-Kamchatsky, Russia, 2017; pp. 54–58. (In Russian) [Google Scholar]
- Middendorff, A.T. Voyages in the Extreme North and East of Siberia during the Years 1843 and 1844. V. 2; Imperial Academy of Sciences: Saint-Petersburg, Russia, 1851; pp. 163–465. [Google Scholar]
- Andreev, A.V. Wetlands of Russia. Volume 4. Wetlands of the North-East of Russia; Wetland International: Moscow, Russia, 2001; pp. 1–296. (In Russian) [Google Scholar]
- Khamenkova, E.V. Trophical relations of fishes of Kisi lake of Ola river basin (Magadan region). In Proceedings of the Vladimir Ya. Levanidov’s Biennial Memorial Meetings, Vladivostok, Russia, 21–23 March 2011; Volume 5, pp. 563–570. (In Russian). [Google Scholar]
- Ioganson, V.E.; Kuznetsov, A.S.; Deev, G.N.; Boytsov, Y.A.; Tereshchenko, K.I.; Zhukova, V.N.; Chernyshova, M.R. Rivers: Power sources and runoff regime. In North of the Far East; Shilo, N.A., Ed.; Nauka: Moscow, Russia, 1970; pp. 186–203. (In Russian) [Google Scholar]
- Magritsky, D.V. Peculiarities of changes in the heat runoff of the rivers of the North-east of the Asian part of Russia and the assessment of its value. In Dynamics and Interaction of the Earth’s Geospheres. Part 2; Vershinin, D.A., Ed.; Publishing House TSNTI: Tomsk, Russia, 2021; pp. 57–60. (In Russian) [Google Scholar]
- Derzhavin, A.N. Winter trip to the Kuril lake. In Kamchatka Expedition of Fyodor Pavlovich Ryabushinsky, Equipped with the Assistance of the Russian Geographical Society. Issue 1; Schmidt, P.Y., Ed.; Ryabushinsky: Moscow, Russia, 1916; pp. 246–278. [Google Scholar]
- Avise, J.C. Phylogeography: The History and Formation of Species; Harvard University Press: Cambridge, MA, USA, 2000; pp. 1–464. [Google Scholar]
- Sergeeva, I.S.; Bolotov, I.N.; Bespalaya, Y.V.; Makhrov, A.A.; Bukhanova, A.L.; Artamonova, V.S. Freshwater pearl mussels of the genus Margaritifera (Mollusca: Bivalvia) described as M. elongata (Lamarck, 1819) and M. borealis (Westerlund, 1871) should be classified with M. margaritifera (Linnaeus, 1758). Biol. Bull. 2008, 35, 102–105. [Google Scholar] [CrossRef]
- Klishko, O.K. Pearl mussels of the genus Dahurinaia (Bivalvia, Margaritiferidae): Differently sized groups of Margaritifera dahurica Middendorff, 1850. Biol. Bull. 2014, 41, 434–443. [Google Scholar] [CrossRef]
- Makhrov, A.A.; Artamonova, V.S. Instability Stabilized: Mechanisms of Evolutionary Stasis and Genetic Diversity Accumulation in Fishes and Lampreys from Environments with Unstable Abiotic Factors. Contemp. Probl. Ecol. 2020, 13, 370–381. [Google Scholar] [CrossRef]
- Zieritz, A.; Hoffman, J.I.; Amos, W.; Aldridge, D.C. Phenotypic plasticity and genetic isolation-by-distance in the freshwater mussel Unio pictorum (Mollusca: Unionoida). Evol. Ecol. 2010, 24, 923–938. [Google Scholar] [CrossRef]
- Inoue, K.; Hayes, D.M.; Harris, J.L.; Christian, A.D. Phylogenetic and morphometric analyses reveal ecophenotypic plasticity in freshwater mussels Obovaria jacksoniana and Villosa arkansasensis (Bivalvia: Unionidae). Ecol. Evol. 2013, 3, 2670–2683. [Google Scholar] [CrossRef]
- Zając, K.; Zając, T.; Ćmiel, A. What can we infer from the shell dimensions of the thick-shelled river mussel Unio crassus? Hydrobiologia 2018, 810, 415–431. [Google Scholar] [CrossRef]
- Wu, R.; Liu, X.; Guo, L.; Zhou, C.; Ouyang, S.; Wu, X. DNA barcoding, multilocus phylogeny, and morphometry reveal phenotypic plasticity in the Chinese freshwater mussel Lamprotula caveata (Bivalvia: Unionidae). Ecol. Evol. 2022, 12, e9035. [Google Scholar] [CrossRef]
- Chereshnev, I.A. Biological Diversity of Freshwater Ichthyofauna of the North-East of Russia; Dalnauka: Vladivostok, Russia, 1996; pp. 1–198. (In Russian) [Google Scholar]
- Ager, T.A. Late Quaternary vegetation and climate history of the central Bering land bridge from St. Michael Island, western Alaska. Quarter. Res. 2003, 60, 19–32. [Google Scholar] [CrossRef]
- Baranova, Y.P.; Biske, S.F. North-East of the USSR; Nauka: Moscow, Russia, 1964; pp. 1–304. (In Russian) [Google Scholar]
- Hopkins, D.M. Quaternary marine transgression in Alaska. In The Bering Land Bridge; Hopkins, D.M., Ed.; Stanford University Press: Stanford, CA, USA, 1967; pp. 47–90. [Google Scholar]
- Pugachev, O.N. Parasites of Freshwater Fishes of North-East Asia; Publishing House of the Zoological Institute: Leningrad, Russia, 1984; pp. 1–154. (In Russian) [Google Scholar]
- Nakhodkin, N.A.; Nakhodkina, F.N. A river that flows into two oceans. Nauka Tekhnika Yakutii 2020, 1, 82–87. (In Russian) [Google Scholar]
- Chereshnev, I.A. Biogeography of Freshwater Fish Fauna in the Russian Far East; Dalnauka: Vladivostok, Russia, 1998; pp. 1–131. (In Russian) [Google Scholar]
- Koreysha, M.M. Modern Glaciation of the Suntar-Khayat Ridge; USSR Academy of Sciences Press: Moscow, Russia, 1963; pp. 1–170. (In Russian) [Google Scholar]
- Butorina, T.E. Ecological analysis of the parasitic fauna of the charrs (Salvelinus) of the Kamchatka River. In Population Biology and Taxonomy of Salmonids; Konovalov, S.M., Ed.; Institute of Marine Biology: Vladivostok, Russia, 1980; pp. 65–81. (In Russian) [Google Scholar]
- Treasurer, G.W.; Turnbull, T. The pathology and seawater performance of farmed Atlantic salmon infected with glochidia of Margaritifera margaritifera. J. Fish Biol. 2000, 57, 858–866. [Google Scholar] [CrossRef]
- Vikhrev, I.V.; Ieshko, E.P.; Kondakov, A.V.; Mugue, N.S.; Bovykina, G.V.; Efremov, D.A.; Bulakhov, A.G.; Tomilova, A.A.; Yunitsyna, O.A.; Bolotov, I.N. Postglacial expansion routes and mitochondrial genetic diversification of the freshwater pearl mussel in Europe and North America. Diversity 2022, 14, 477. [Google Scholar] [CrossRef]
- De Cicco, A.L. Long-distance movements Anadromous Dolly Varden between Alaska and the USSR. Arctic 1992, 45, 120–123. [Google Scholar]
- Lindberg, G.U. Large Fluctuations in Ocean Level during the Quaternary Period; Nauka: Leningrad, Russia, 1972; pp. 1–548. (In Russian) [Google Scholar]
- Esin, E.V.; Bocharova, E.S.; Mugue, N.S.; Markevich, G.N. Occurrence of sympatric charr groups, Salvelinus, Salmonidae, in the lakes of Kamchatka: A legacy of the last glaciations. J. Fish Biol. 2017, 91, 628–644. [Google Scholar] [CrossRef]
- Osinov, A.G.; Volkov, A.A.; Alekseyev, S.S.; Sergeev, A.A.; Oficerov, M.V.; Kirillov, A.F. On the origin and phylogenetic position of Arctic charr (Salvelinus alpinus complex, Salmonidae) from Lake Cherechen’ (middle Kolyma River basin): Controversial genetic data. Polar Biol. 2017, 40, 777–786. [Google Scholar] [CrossRef]
- Esin, E.V.; Markevich, G.N. Evolution of the Charrs, Genus Salvelinus (Salmonidae). 1. Origins and Expansion of the Species. J. Ichthyol. 2018, 58, 187–203. [Google Scholar] [CrossRef]
- Oleinik, A.G.; Kukhlevsky, A.D.; Skurikhina, L.A. The Relationships of the Charrs Salvelinus sp. 4 (Salmoniformes: Salmonidae) from Lake Nachikinskoe, Kamchatka, as Inferred from Complete Mitochondrial Genome Analysis. Russ. J. Mar. Biol. 2022, 48, 55–59. [Google Scholar] [CrossRef]
- Oleinik, A.G.; Skurikhina, L.A.; Kukhlevsky, A.D.; Bondar, E.I. On the origin of endemic stone charr in the Kamchatka River basin. Hydrobiologia 2019, 840, 21–33. [Google Scholar] [CrossRef]
- Osinov, A.G.; Senchukova, A.L.; Mugue, N.S.; Pavlov, S.D.; Chereshnev, I.A. Speciation and genetic divergence of three species of charr from ancient Lake El’gygytgyn (Chukotka) and their phylogenetic relationships with other representatives of the genus Salvelinus. Biol. J. Linn. Soc. 2015, 116, 63–85. [Google Scholar] [CrossRef]
- Esin, E.V.; Markevich, G.N.; Zlenko, D.V.; Shkil, F.N. Thyroid-Mediated Metabolic Differences Underlie Ecological Specialization of Extremophile Salmonids in the Arctic Lake El’gygytgyn. Front. Ecol. Evol. 2021, 9, 715110. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).