Hydrogeochemical Characteristics, Water Quality, and Human Health Risks of Groundwater in Wulian, North China
Abstract
:1. Introduction
2. Materials and Methods
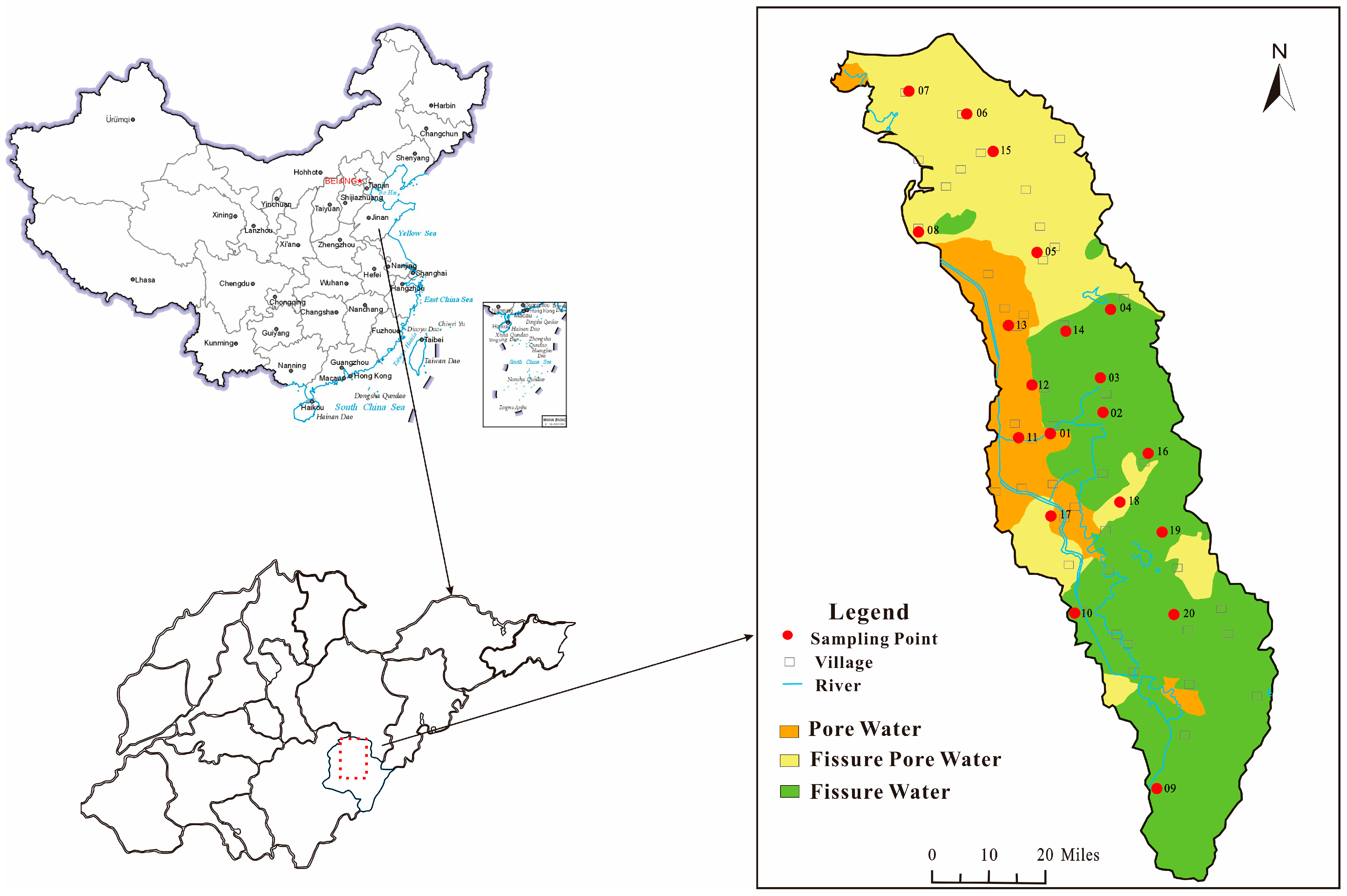
2.1. Study Area
2.2. Sample Collection and Measurements
2.3. Analysis Methods
2.3.1. Analysis of Hydrochemical Composition
2.3.2. Water Quality Analysis
2.3.3. Health Risk Assessment
2.3.4. Source Identification of Nitrate
3. Results and Discussion
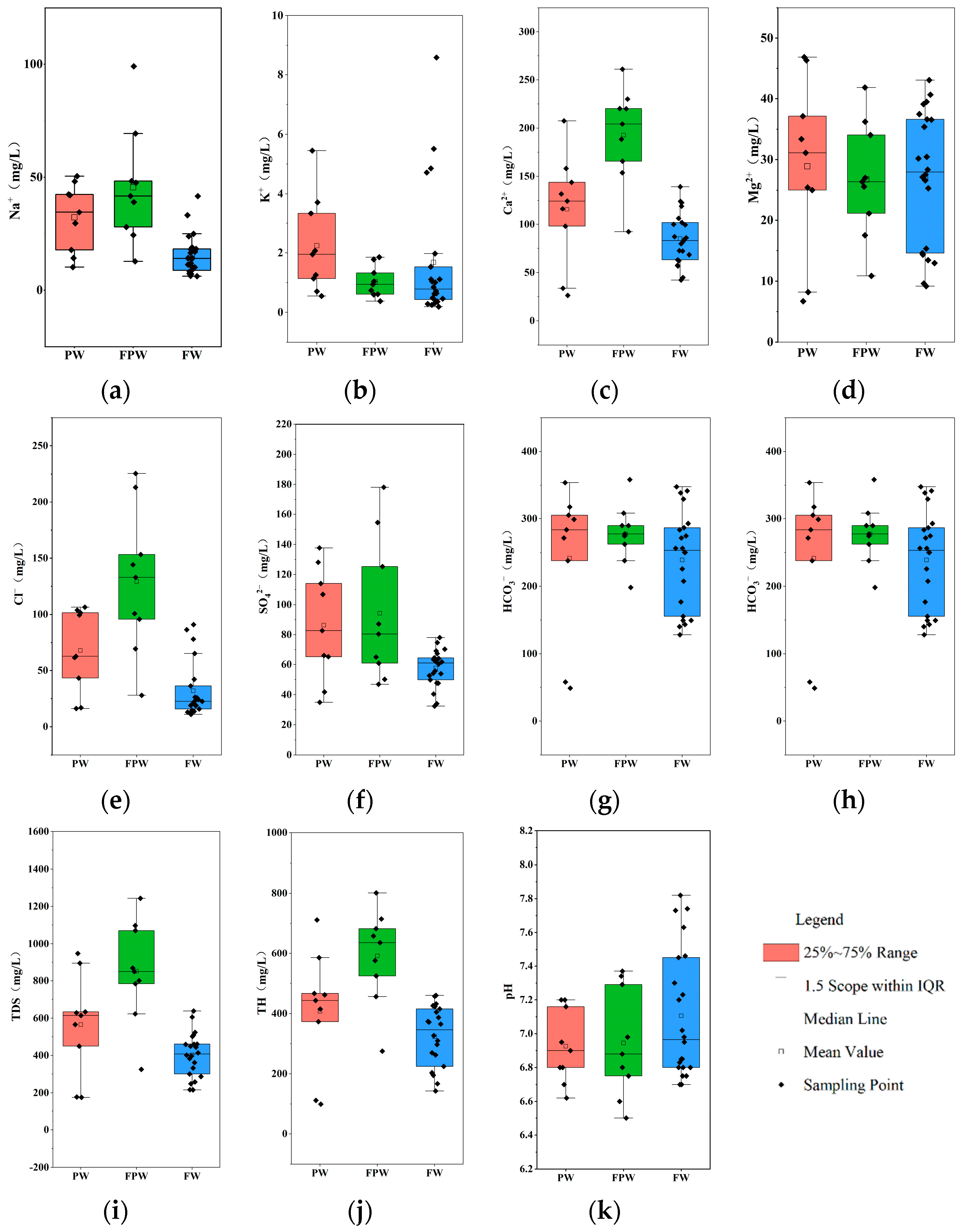
3.1. Groundwater Chemical Characteristics
3.2. Hydrochemical Types
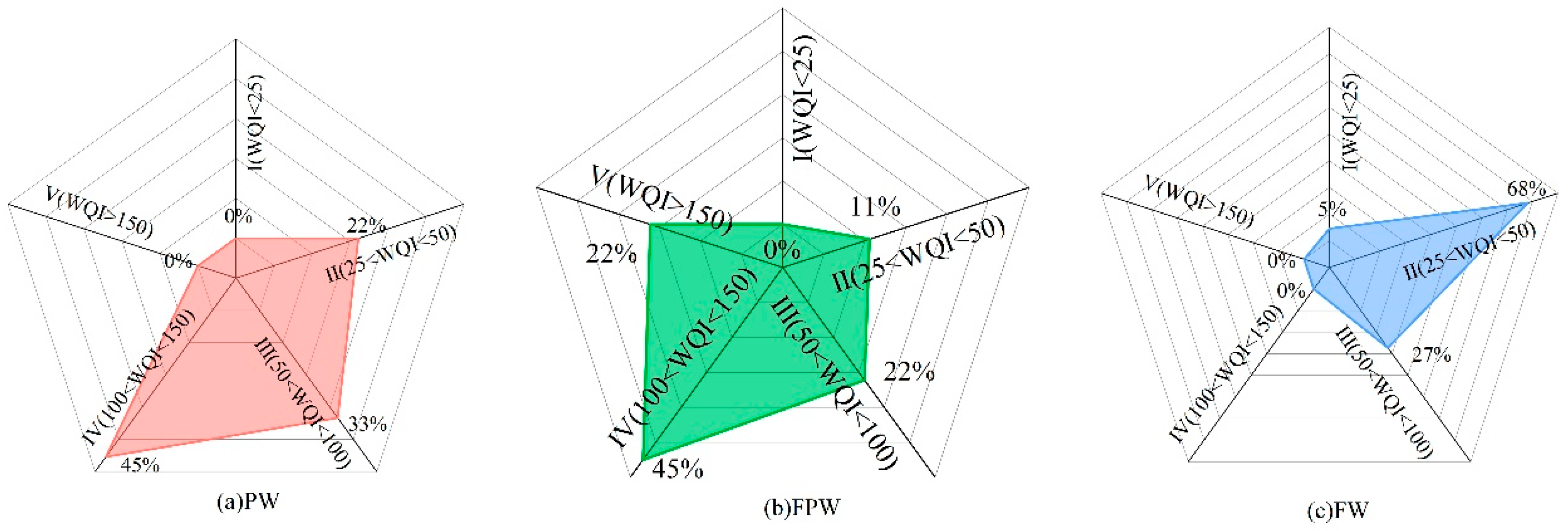
3.3. Groundwater Quality Assessment
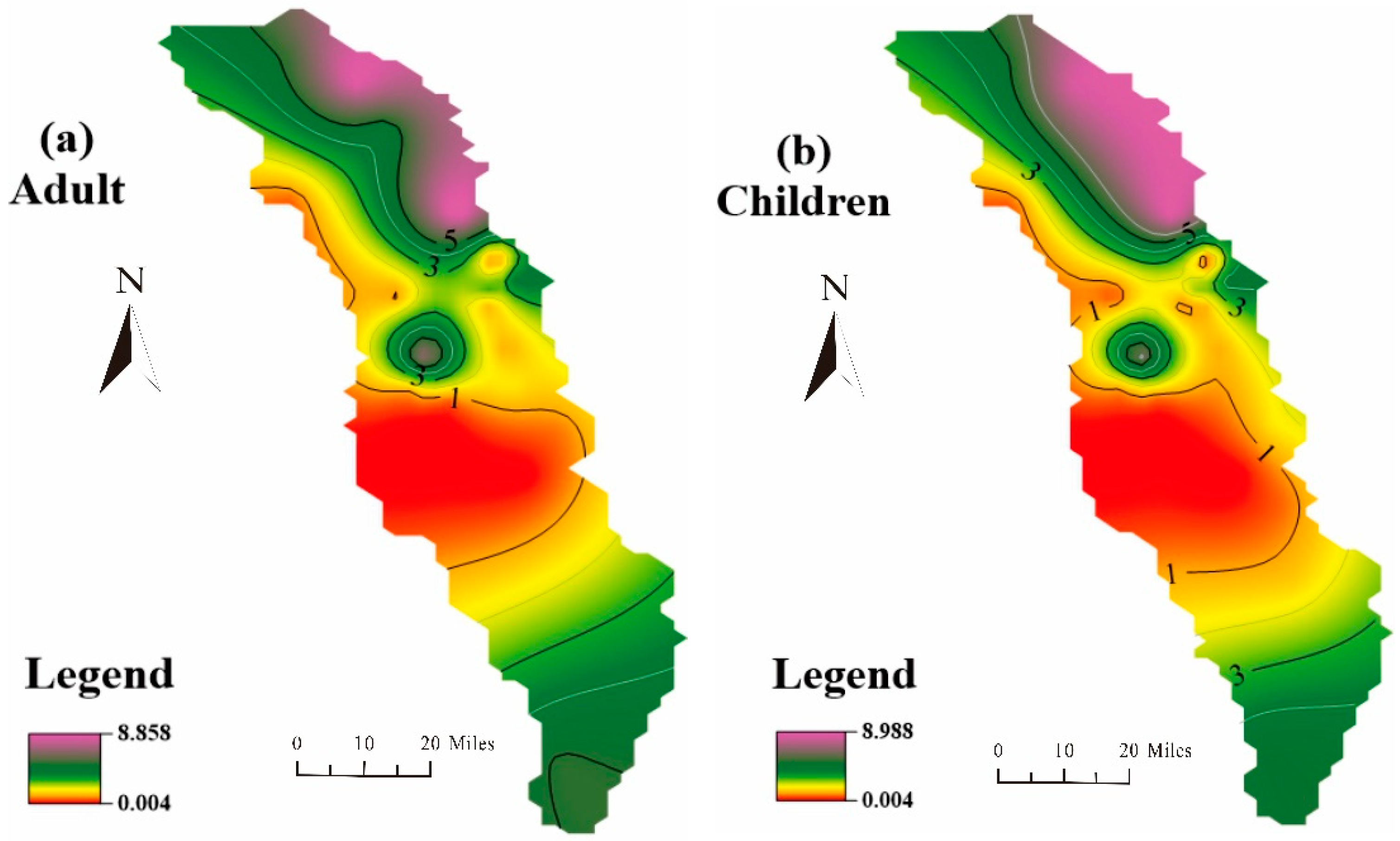
3.4. Health Risk Assessment
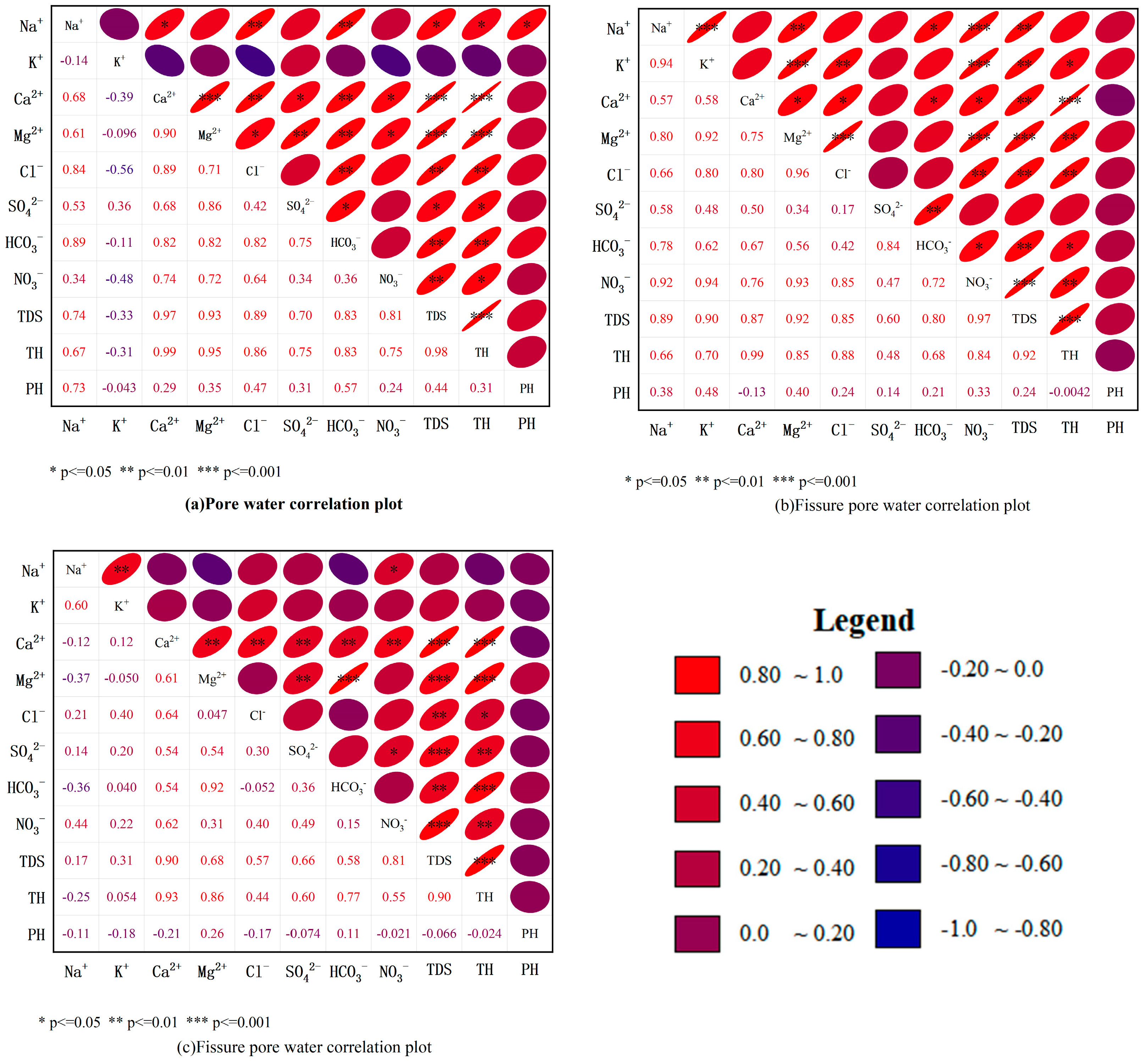
3.5. Correlation Analysis
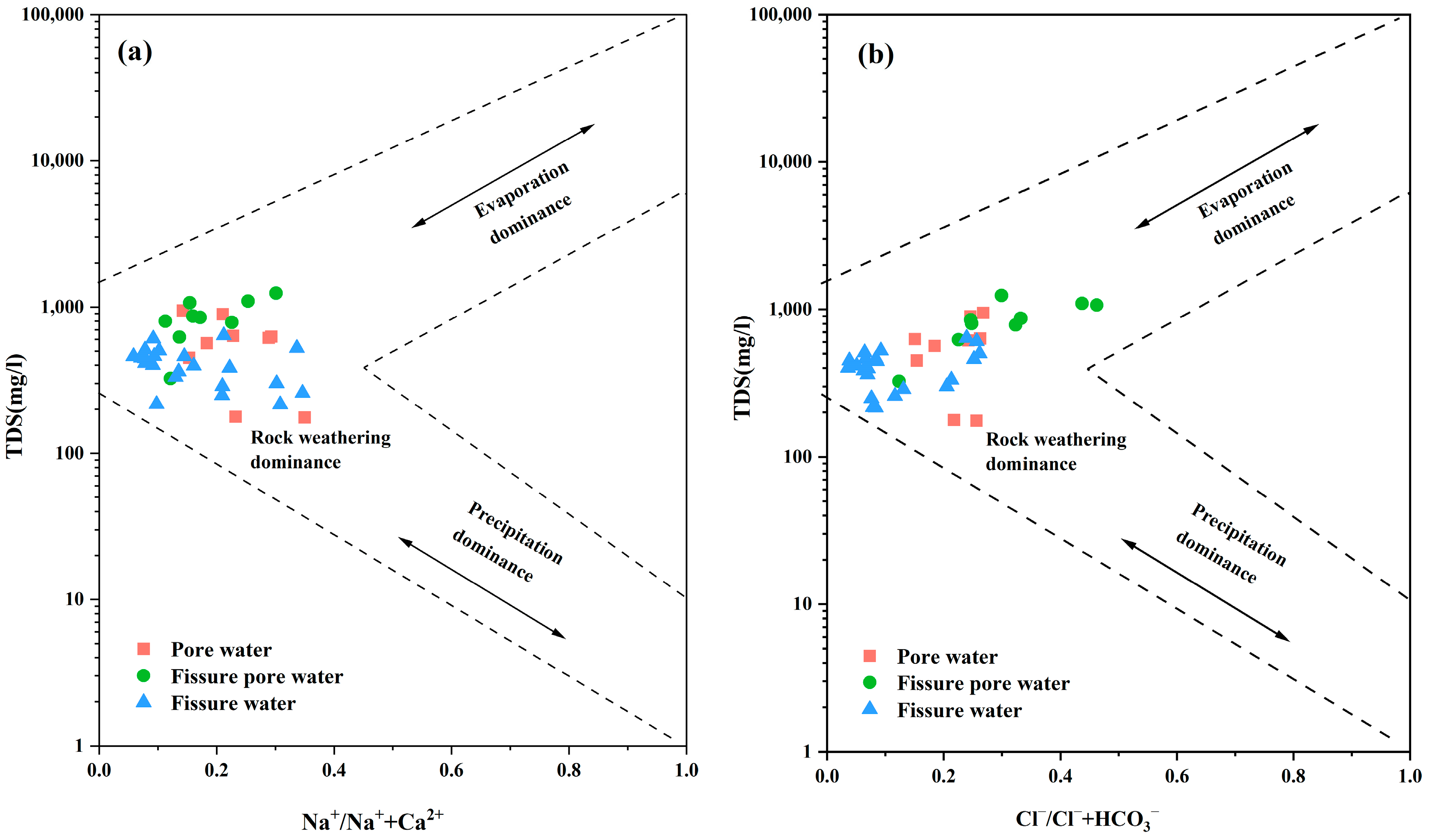
3.6. Gibbs Analysis of Controlling Factors of Hydrochemical Characteristics of Groundwater
3.7. Ion Ratio Analysis
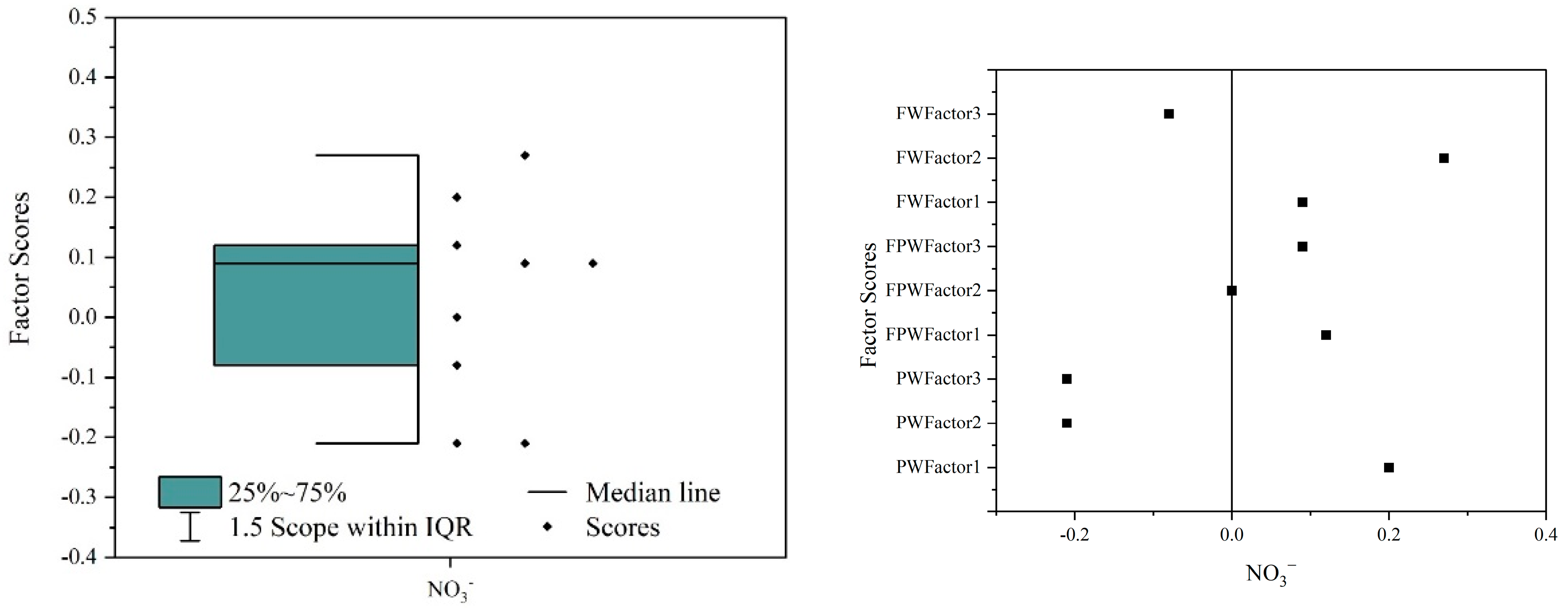
3.8. Source Identification of Nitrate Ion
3.9. Suggestions on Groundwater Development and Pollution Control in the Study Area
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, D.; Wu, J.H.; Wang, Y.H.; Ji, Y.J. Finding High-Quality Groundwater Resources to Reduce the Hydatidosis Incidence in the Shiqu County of Sichuan Province, China: Analysis, Assessment, and Management. Expo. Health 2020, 12, 307–322. [Google Scholar] [CrossRef]
- Elsayed, S.; Gad, M.; Masoud, M.; Osta, M.; Alqarawy, A. Groundwater Suitability for Drinking and Irrigation Using Water Quality Indices and Multivariate Modeling in Makkah Al-Mukarramah Province, Saudi Arabia. Water 2022, 14, 483. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Li, P.Y.; Chen, M.J.; Dong, Z.H.; Lu, C.Y. Groundwater quality for potable and irrigation uses and associated health risk in southern part of Gu’an County, North China Plain. Environ. Geochem. Health 2021, 43, 813–835. [Google Scholar] [CrossRef]
- Gad, M.; Saleh, A.; Hussein, H.; Farouk, M.; Elsayed, S. Appraisal of Surface Water Quality of Nile River Using Water Quality Indices, Spectral Signature and Multivariate Modeling. Water 2022, 14, 1131. [Google Scholar] [CrossRef]
- Yu, F.; Zhou, D.; Li, Z.; Li, X. Hydrochemical Characteristics and Hydrogeochemical Simulation Research of Groundwater in the Guohe River Basin (Henan Section). Water 2022, 14, 1461. [Google Scholar] [CrossRef]
- Li, P.Y.; Wu, J.H. Sustainable living with risks: Meeting the challenges. Hum. Ecol. Risk Assess. 2019, 25, 1–10. [Google Scholar] [CrossRef]
- Liu, J.T.; Gao, Z.J.; Wang, M.; Li, Y.Z.; Yu, C.; Shi, M.J.; Zhang, H.Y.; Ma, Y.Y. Hydrochemical and isotopic characteristics of surface water in the Lhasa River basin. Arab. J. Geosci. 2019, 12, 520. [Google Scholar] [CrossRef]
- Hua, K.; Jun, X.; Li, S.J.; Li, Z. Analysis of hydrochemical characteristics and their controlling factors in the Fen River of China. Sustain. Cities Soc. 2019, 52, 101827. [Google Scholar] [CrossRef]
- Yousefi, M.; Ghoochani, M.; Mahvi, A. Health risk assessment to fluoride in drinking water of rural residents living in the Poldasht city, Northwest of Iran. Ecotoxicol. Environ. Saf. 2017, 148, 426–430. [Google Scholar] [CrossRef]
- He, S.; Wu, J.H. Hydrogeochemical Characteristics, Groundwater Quality, and Health Risks from Hexavalent Chromium and Nitrate in Groundwater of Huanhe Formation in Wuqi County, Northwest China. Expo. Health 2019, 11, 125–137. [Google Scholar] [CrossRef]
- Narsimha, A.; Qian, H. Groundwater quality evaluation using water quality index (WQI) for drinking purposes and human health risk (HHR) assessment in an agricultural region of Nanganur, south India. Ecotoxicol. Environ. Saf. 2019, 176, 153–161. [Google Scholar] [CrossRef]
- Ahmed, I.; Tariq, N.; Muhery, A. Hydrochemical characterization of groundwater to align with sustainable development goals in the Emirate of Dubai, UAE. Environ. Earth Sci. 2019, 78, 44. [Google Scholar] [CrossRef]
- Eslami, F.; Yaghmaeian, K.; Mohammadi, A.; Salari, M.; Faraji, M. An integrated evaluation of groundwater quality using drinking water quality indices and hydrochemical characteristics: A case study in Jiroft, Iran. Environ. Earth Sci. 2019, 78, 314. [Google Scholar] [CrossRef]
- Li, P.Y.; Tian, R.; Xue, C.Y.; Wu, J.H. Progress, opportunities, and key fields for groundwater quality research under the impacts of human activities in China with a special focus on western China. Environ. Sci. Pollut. Res. 2017, 24, 13224–13234. [Google Scholar] [CrossRef]
- Liu, J.T.; Gao, Z.J.; Wang, M.; Li, Y.Z.; Ma, Y.Y.; Shi, M.J.; Zhang, H.Y. Study on the dynamic characteristics of groundwater in the valley plain of Lhasa City. Environ. Earth Sci. 2018, 77, 646. [Google Scholar] [CrossRef]
- Yuan, Y.Y.; Liu, Y.L.; Luo, K.L.; Shahid, M.Z. Hydrochemical characteristics and a health risk assessment of the use of river water and groundwater as drinking sources in a rural area in Jiangjin District, China. Environ. Earth Sci. 2020, 79, 160. [Google Scholar] [CrossRef]
- Qian, H.; Chen, J.; Howard, K. Assessing groundwater pollution and potential remediation processes in a multi-layer aquifer system. Environ. Pollut. 2020, 263, 114669. [Google Scholar] [CrossRef] [PubMed]
- Gharbi, A.; Ibn Ali, Z.; Zairi, M. Groundwater suitability for drinking and agriculture purposes using irrigation water quality index and multivariate analysis: Case of Sidi Bouzid aquifer, central Tunisia. Environ. Earth Sci. 2019, 78, 692. [Google Scholar] [CrossRef]
- Everest, T.; Özcan, H. Applying multivariate statistics for identification of groundwater resources and qualities in NW Turkey. Environ. Monit. Assess. 2019, 191, 47. [Google Scholar] [CrossRef]
- Sajil Kumar, P.J.; James, E.J. Geostatistical and geochemical model-assisted hydrogeochemical pattern recognition along the groundwater flow paths in Coimbatore district, South India. Environ. Dev. Sustain. 2019, 21, 369–384. [Google Scholar] [CrossRef]
- Piper, A.M. A graphic procedure in the geochemical interpretation of water-analyses. EOS Trans. Am. Geophys. Union 1944, 25, 914–928. [Google Scholar] [CrossRef]
- Gibbs, R. Mechanisms Controlling World Water Chemistry. Science 1971, 170, 1088–1090. [Google Scholar] [CrossRef]
- Zhai, Y.Z.; Zheng, F.X.; Zhao, X.B.; Teng, Y.G. Identification of hydrochemical genesis and screening of typical groundwater pollutants impacting human health: A case study in Northeast China. Environ. Pollut. 2019, 252, 1202–1215. [Google Scholar] [CrossRef] [PubMed]
- Abbasnia, A.; Yousefi, N.; Mahvi, A.; Nabizadeh, R.; Radfarad, M.; Yousefi, M.; Alimohammadi, M. Evaluation of groundwater quality using water quality index and its suitability for assessing water for drinking and irrigation purposes: Case study of Sistan and Baluchistan province (Iran). Hum. Ecol. Risk Assess. 2018, 25, 73–82. [Google Scholar] [CrossRef]
- Liu, J.T.; Peng, Y.M.; Li, C.S.; Gao, Z.J.; Chen, S.J. An investigation into the hydrochemistry, quality and risk to human health of groundwater in the central region of Shandong Province, North China. J. Clean. Prod. 2021, 282, 125416. [Google Scholar] [CrossRef]
- Su, H.; Kang, W.D.; Xu, Y.J.; Wang, J.D. Assessing Groundwater Quality and Health Risks of Nitrogen Pollution in the Shenfu Mining Area of Shaanxi Province, Northwest China. Expo. Health 2018, 10, 77–97. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Xu, P.P.; Qian, H. Assessment of Groundwater Quality and Human Health Risk (HHR) Evaluation of Nitrate in the Central-Western Guanzhong Basin, China. Int. J. Environ. Res. Public Health 2019, 16, 4246. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.N.; Han, S.B.; Wang, Y.S.; Wang, Z.; Li, H.X.; Wang, X.Y.; Liu, J.T.; Li, C.S.; Gao, Z.J. Characteristics and Controlling Factors of Groundwater Hydrochemistry in Dongzhi Tableland Area of the Loess Plateau of Eastern Gansu—A Case Study of Ning County Area, North China. Water 2022, 14, 3601. [Google Scholar] [CrossRef]
- Duraisamy, S.; Govindhaswamy, V.; Duraisamy, K.; Krishnaraj, S.; Balasubramanian, A.; Subramani, T. Hydrogeochemical characterization and evaluation of groundwater quality in Kangayam taluk, Tirupur district, Tamil Nadu, India, using GIS techniques. Environ. Geochem. Health 2019, 41, 851–873. [Google Scholar] [CrossRef]
- Song, H.Y.; Liu, J.Q.; Yin, P.; Zhang, Y. Distribution, enrichment and source of heavy metals in Rizhao offshore area, southeast Shandong Province. Mar. Pollut. Bull. 2017, 119, 175–180. [Google Scholar] [CrossRef]
- He, Z.K.; Ma, C.M.; Zhou, A.G.; Qi, H.H.; Liu, C.F.; Cai, H.S.; Zhu, H.C. Using hydrochemical and stable isotopic (δ2H, δ18O, δ11B, and δ37Cl) data to understand groundwater evolution in an unconsolidated aquifer system in the southern coastal area of Laizhou Bay, China. Appl. Geochem. 2018, 90, 129–141. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Hardle, W.; Simar, L. Applied Multivariate Statistical Analysis; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Liu, J.T.; Peng, Y.M.; Li, C.S.; Gao, Z.J.; Chen, S.J. Characterization of the hydrochemistry of water resources of the Weibei Plain, Northern China, as well as an assessment of the risk of high groundwater nitrate levels to human health. Environ. Pollut. 2020, 268, 115947. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.J.; Tang, C.Y.; Song, X.F.; Liu, C.M.; Zhang, Y.H. Identifying the hydrochemical characteristics of rivers and groundwater by multivariate statistical analysis in the Sanjiang Plain, China. Appl. Water Sci. 2016, 6, 169–178. [Google Scholar] [CrossRef] [Green Version]
- Cude, C.G. Oregon water quality index a tool for evaluating water quality management effectiveness. J. Am. Water Resour. Assoc. 2001, 37, 125–137. [Google Scholar] [CrossRef]
- Wu, C.; Wu, X.; Qian, C.; Zhu, G. Hydrogeochemistry and groundwater quality assessment of high fluoride levels in the Yanchi endorheic region, northwest China. Appl. Geochem. 2018, 98, 404–417. [Google Scholar] [CrossRef]
- WHO (World Health Organization). Guidelines for Drinking Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011; Volume 38, pp. 104–108. [Google Scholar]
- Yousefi, M.; Ghalehaskar, S.; Asghari, F.; Ghaderpoury, A.; Dehghani, M.H.; Ghaderpoori, M.; Mohammadi, A. Distribution of fluoride contamination in drinking water resources and health risk assessment using geographic information system, northwest Iran. Regul. Toxicol. Pharmacol. 2019, 107, 104408. [Google Scholar] [CrossRef]
- Chen, J.; Wu, H.; Qian, H. Groundwater Nitrate Contamination and Associated Health Risk for the Rural Communities in an Agricultural Area of Ningxia, Northwest China. Expo. Health 2016, 8, 349–359. [Google Scholar] [CrossRef]
- Li, P.Y.; Qian, H.; Wu, J.H. Conjunctive use of groundwater and surface water to reduce soil salinization in the Yinchuan Plain, North-West China. Int. J. Water Resour. D 2018, 34, 337–353. [Google Scholar] [CrossRef]
- USEPA (US Environmental Protection Agency). Baseline Human Health Risk Assessment Vasquez Boulevard and I-70 Superfund Site; USEPA (US Environmental Protection Agency): Denver, CO, USA, 2001. Available online: https://nepis.epa.gov/Exe/ZyNET.exe/P1006STM.TXT?ZyActionD=ZyDocument&Client=EPA&Index=2000+Thru+2005&Docs=&Query=&Time=&EndTime=&SearchMethod=1&TocRestrict=n&Toc=&TocEntry=&QField=&QFieldYear=&QFieldMonth=&QFieldDay=&IntQFieldOp=0&ExtQFieldOp=0&XmlQuery=&File=D%3A%5Czyfiles%5CIndex%20Data%5C00thru05%5CTxt%5C00000023%5CP1006STM.txt&User=ANONYMOUS&Password=anonymous&SortMethod=h%7C-&MaximumDocuments=1&FuzzyDegree=0&ImageQuality=r75g8/r75g8/x150y150g16/i425&Display=hpfr&DefSeekPage=x&SearchBack=ZyActionL&Back=ZyActionS&BackDesc=Results%20page&MaximumPages=1&ZyEntry=1&SeekPage=x&ZyPURL (accessed on 20 January 2011).
- USEPA. 2004. Available online: http://www.epa.gov/oswer/riskassessment/ragse/pdf/introduction (accessed on 1 July 2004).
- Bempah, C.; Ewusi, A. Heavy metals contamination and human health risk assessment around Obuasi gold mine in Ghana. Environ. Monit. Assess. 2016, 188, 261. [Google Scholar] [CrossRef]
- Narsimha, A.; Rajitha, S. Spatial distribution and seasonal variation in fluoride enrichment in groundwater and its associated human health risk assessment in Telangana State, South India. Hum. Ecol. Risk Assess. 2018, 24, 2119–2132. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Wu, J.H.; Xu, B. Human health risk assessment of groundwater nitrogen pollution in Jinghui canal irrigation area of the loess region, northwest China. Environ. Earth Sci. 2018, 77, 273. [Google Scholar] [CrossRef]
- Bouzourra, H.; Bouhlila, R.; Lakshmanan, E.; Slama, F.; Ouslati, N. Characterizaton of mechanisms and processes of groundwater salinization in irrigated coastal area using statistics, GIS, and hydrogeochemical investigations. Environ. Sci. Pollut. Res. 2015, 22, 2643–2660. [Google Scholar] [CrossRef] [PubMed]
- Li, P.Y.; Wu, J.H.; Tian, R.; He, S.; He, X.D.; Xue, C.Y.; Zhang, K. Geochemistry, Hydraulic Connectivity and Quality Appraisal of Multilayered Groundwater in the Hongdunzi Coal Mine, Northwest China. Mine Water Environ. 2018, 37, 222–237. [Google Scholar] [CrossRef]
- Narsimha, A. Controlling factors and mechanism of groundwater quality variation in semiarid region of South India: An approach of water quality index (WQI) and health risk assessment (HRA). Environ. Geochem. Health 2020, 42, 1725–1752. [Google Scholar] [CrossRef]
- Narsimha, A. Spatial distribution, exposure, and potential health risk assessment from nitrate in drinking water from semi-arid region of South India. Hum. Ecol. Risk Assess. 2018, 26, 310–334. [Google Scholar] [CrossRef]
- Keesari, T.; Saha, D. Groundwater salinization processes: Pitfalls of inferences from Na+/Cl− versus Cl− correlation plots. Environ. Geochem. Health 2021, 43, 949–969. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.L.; Zhang, L.; Fan, W.Y.; Yang, C.; Li, E.H.; Wang, Z. Impact of land use on shallow groundwater quality characteristics associated with human health risks in a typical agricultural area in Central China. Environ. Sci. Pollut. Res. 2021, 28, 1712–1724. [Google Scholar] [CrossRef]
- Jin, Z.F.; Zheng, Q.; Zhu, C.Y.; Wang, Y.; Cen, J.R.; Li, F.L. Contribution of nitrate sources in surface water in multiple land use areas by combining isotopes and a Bayesian isotope mixing model. Appl. Geochem. 2018, 93, 10–19. [Google Scholar] [CrossRef]


| HQ (Min) | HQ (Max) | HQ (Mean) | Proportion of HQ > 1 | ||
|---|---|---|---|---|---|
| Adult | PW | 0.54 | 6.86 | 2.37 | 44% |
| FPW | 0.34 | 9.37 | 5.41 | 89% | |
| FW | 0.004 | 5.13 | 1.19 | 45% | |
| Children | PW | 0.52 | 6.83 | 2.27 | 44% |
| FPW | 0.33 | 8.99 | 5.19 | 89% | |
| FW | 0.004 | 4.92 | 1.14 | 41% |
| Factor 1 | Factor 2 | Factor 3 | Communalities | |
| TDS | 0.99 | 0.1 | 0.99 | |
| NO3− | 0.72 | −0.44 | 0.27 | 0.78 |
| Mg2+ | 0.93 | 0.14 | 0.3 | 0.97 |
| K+ | −0.3 | 0.9 | 0.22 | 0.95 |
| TH | 0.97 | 0.22 | 0.99 | |
| Na+ | 0.82 | 0.21 | −0.49 | 0.95 |
| Cl− | 0.91 | −0.26 | −0.23 | 0.95 |
| Ca2+ | 0.96 | −0.14 | 0.17 | 0.97 |
| HCO3− | 0.9 | 0.27 | −0.18 | 0.92 |
| pH | 0.51 | 0.27 | −0.69 | 0.81 |
| SO42− | 0.73 | 0.58 | 0.34 | 0.98 |
| Eigenvalues | 7.4 | 1.65 | 1.22 | (a) PW |
| Percentage of variance (%) | 67.25 | 15.01 | 11.04 | |
| Cumulative percentage of variance (%) | 67.25 | 82.26 | 93.3 | |
| Factor 1 | Factor 2 | Factor 3 | Communalities | |
| TDS | 1 | 1 | ||
| NO3− | 0.97 | 0.96 | ||
| Mg2+ | 0.94 | 0.15 | −0.29 | 0.99 |
| K+ | 0.92 | 0.32 | 0.94 | |
| TH | 0.91 | −0.36 | −0.17 | 0.98 |
| Na+ | 0.9 | 0.22 | 0.18 | 0.89 |
| Cl− | 0.86 | −0.49 | 0.98 | |
| Ca2+ | 0.84 | −0.5 | −0.12 | 0.97 |
| HCO3− | 0.79 | −0.12 | 0.54 | 0.94 |
| pH | 0.3 | 0.88 | 0.88 | |
| SO42− | 0.59 | −0.16 | 0.66 | 0.93 |
| Eigenvalues | 7.4 | 1.65 | 1.22 | (b) FPW |
| Percentage of variance (%) | 67.25 | 15.01 | 11.04 | |
| Cumulative percentage of variance (%) | 67.25 | 82.26 | 93.3 | |
| Factor 1 | Factor 2 | Factor 3 | Communalities | |
| TDS | 0.97 | 0.18 | 0.98 | |
| NO3− | 0.69 | 0.42 | 0.22 | 0.69 |
| Mg2+ | 0.79 | −0.53 | 0.21 | 0.96 |
| K+ | 0.23 | 0.67 | 0.21 | 0.55 |
| TH | 0.97 | −0.22 | −0.11 | 0.99 |
| Na+ | 0.85 | 0.41 | 0.9 | |
| Cl− | 0.52 | 0.52 | −0.32 | 0.64 |
| Ca2+ | 0.92 | −0.31 | 0.95 | |
| HCO3− | 0.7 | −0.54 | 0.12 | 0.79 |
| pH | −0.37 | 0.75 | 0.71 | |
| SO42− | 0.71 | 0.12 | 0.14 | 0.54 |
| Eigenvalues | 5.15 | 2.44 | 1.11 | (c) FW |
| Percentage of variance (%) | 46.78 | 22.14 | 10.12 | |
| Cumulative percentage of variance (%) | 46.78 | 68.92 | 79.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Zhang, W.; Yang, P.; Feng, J.; Zhang, R.; Gao, Z.; Jin, H.; Song, X.; Gao, X. Hydrogeochemical Characteristics, Water Quality, and Human Health Risks of Groundwater in Wulian, North China. Water 2023, 15, 359. https://doi.org/10.3390/w15020359
Wang M, Zhang W, Yang P, Feng J, Zhang R, Gao Z, Jin H, Song X, Gao X. Hydrogeochemical Characteristics, Water Quality, and Human Health Risks of Groundwater in Wulian, North China. Water. 2023; 15(2):359. https://doi.org/10.3390/w15020359
Chicago/Turabian StyleWang, Min, Wenxiu Zhang, Peng Yang, Jianguo Feng, Ruilin Zhang, Zongjun Gao, Hongjie Jin, Xiaoyu Song, and Xiaobing Gao. 2023. "Hydrogeochemical Characteristics, Water Quality, and Human Health Risks of Groundwater in Wulian, North China" Water 15, no. 2: 359. https://doi.org/10.3390/w15020359
APA StyleWang, M., Zhang, W., Yang, P., Feng, J., Zhang, R., Gao, Z., Jin, H., Song, X., & Gao, X. (2023). Hydrogeochemical Characteristics, Water Quality, and Human Health Risks of Groundwater in Wulian, North China. Water, 15(2), 359. https://doi.org/10.3390/w15020359







