Short-Term Meteorological Conditions Explain Cyanobacterial Blooms in a Tropical Reservoir
Abstract
:1. Introduction
2. Materials and Methods
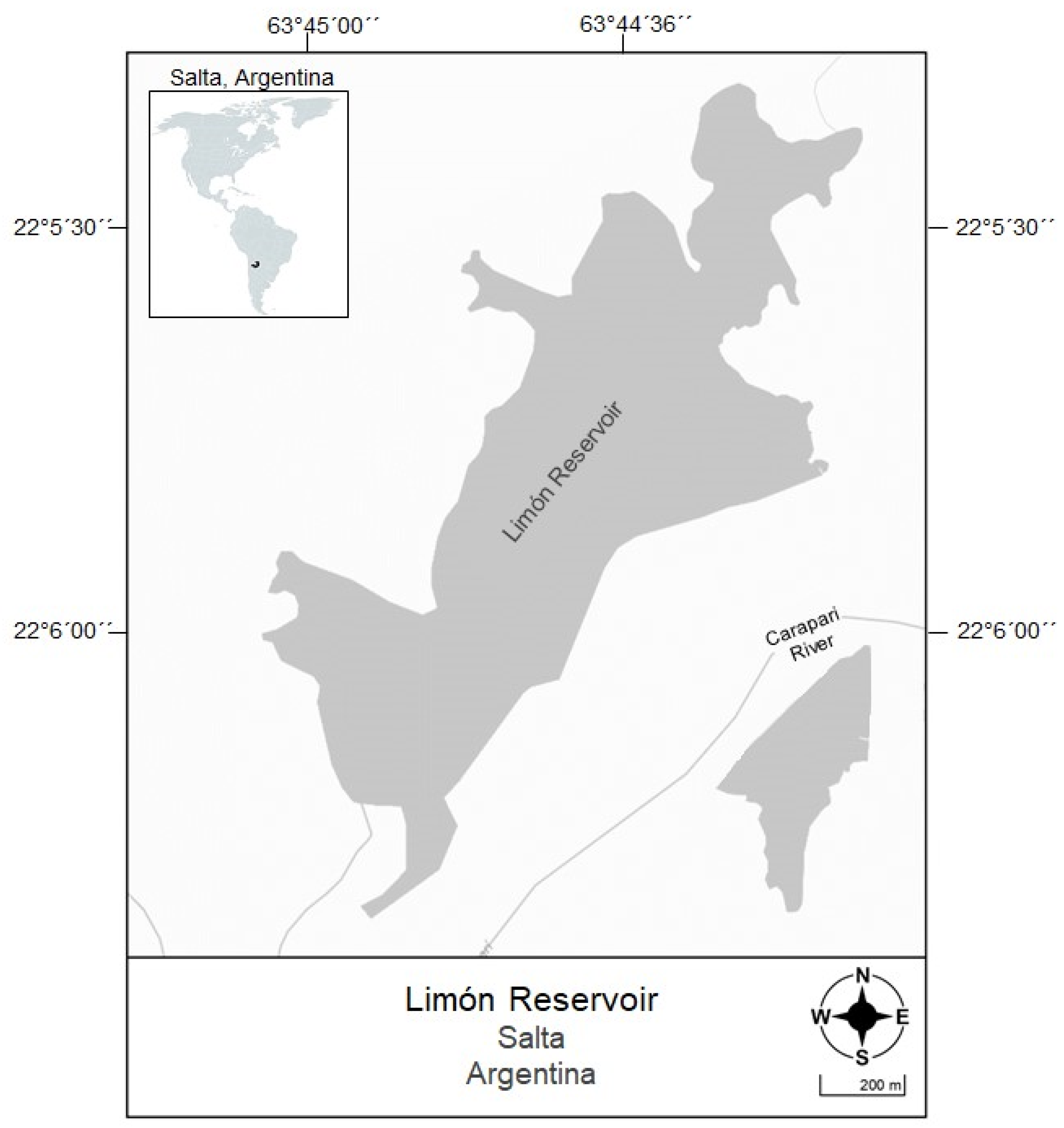
2.1. Study Area
2.2. Sampling and Methodology
2.3. Environmental Parameters
2.4. Phytoplankton and Microscopic Observation
2.5. Morphological Analysis of the 4 Dominant Species of Cyanobacteria
2.6. Statistical Analysis
3. Results
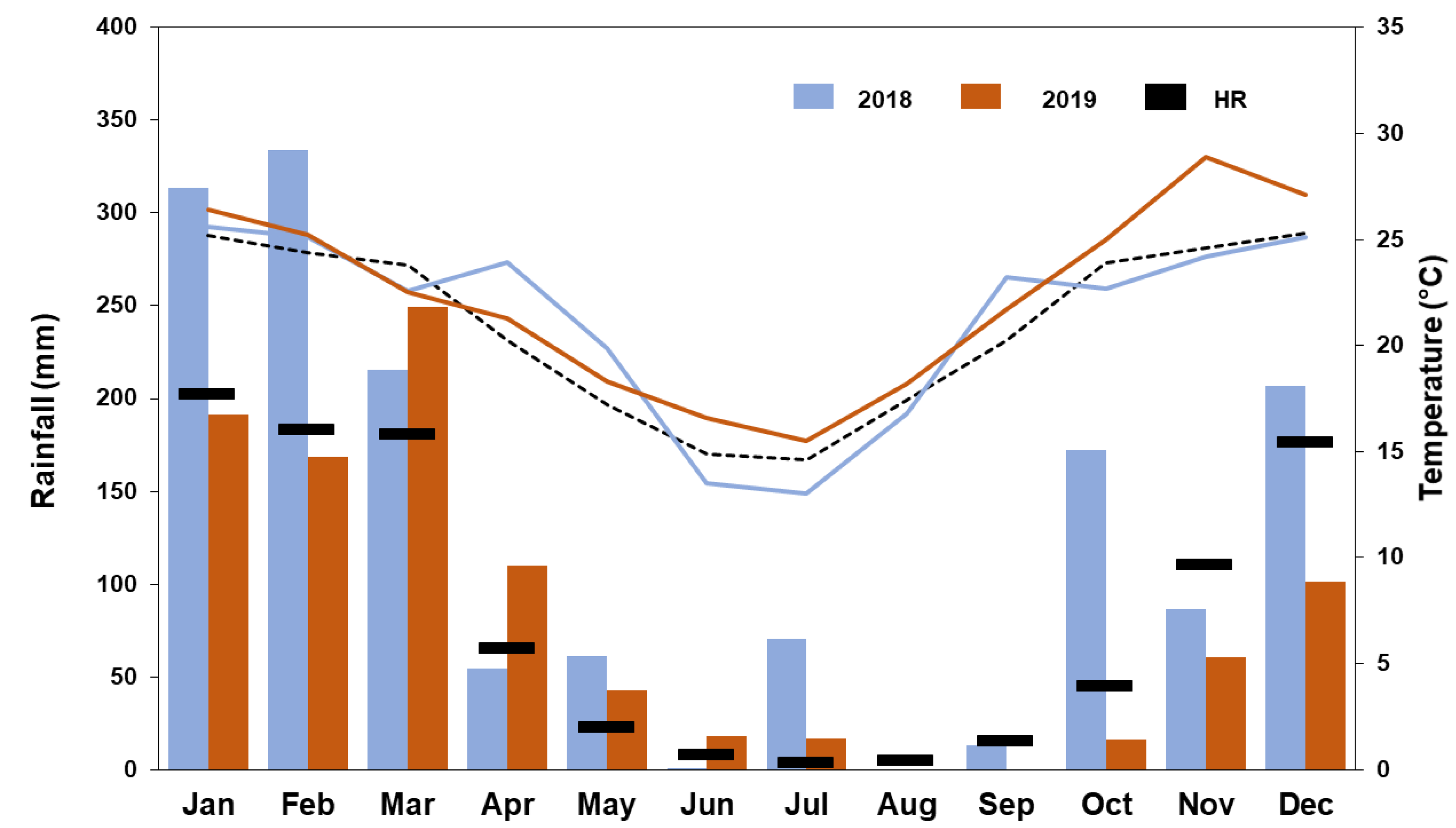
3.1. Meteorological Conditions
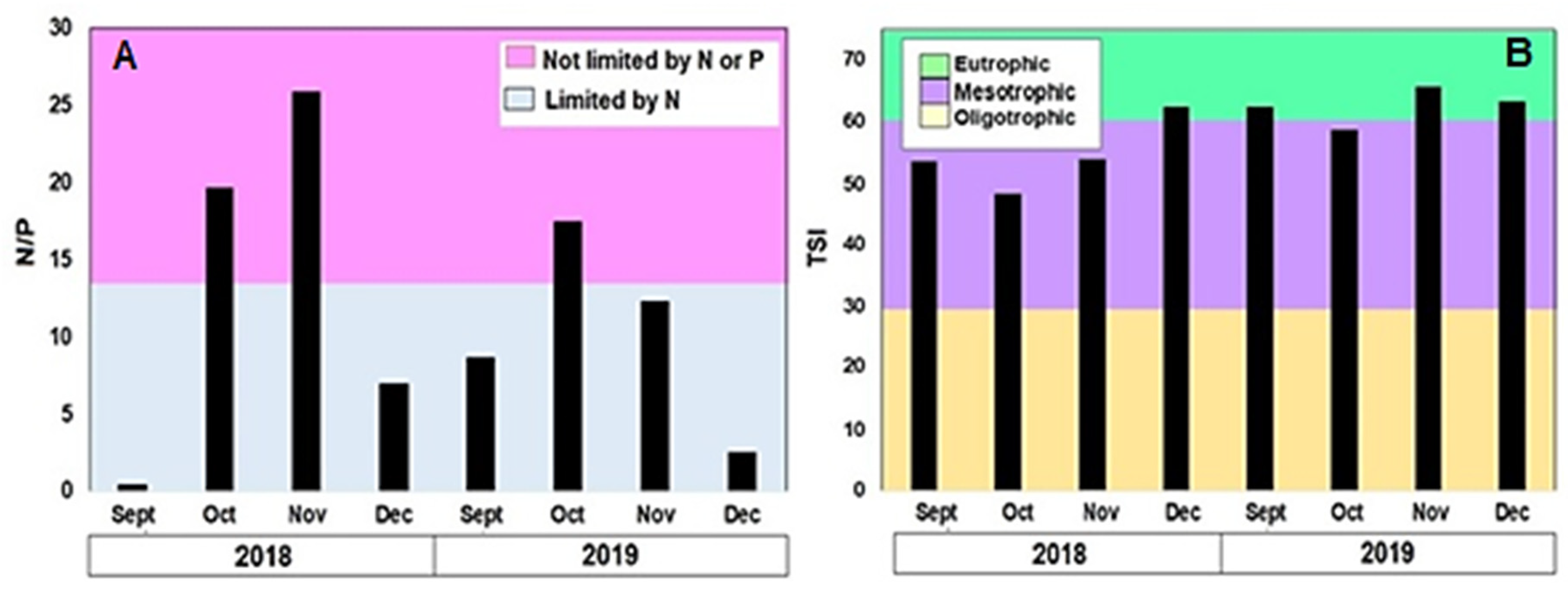
3.2. Physical and Chemical Variables
3.3. Phytoplankton
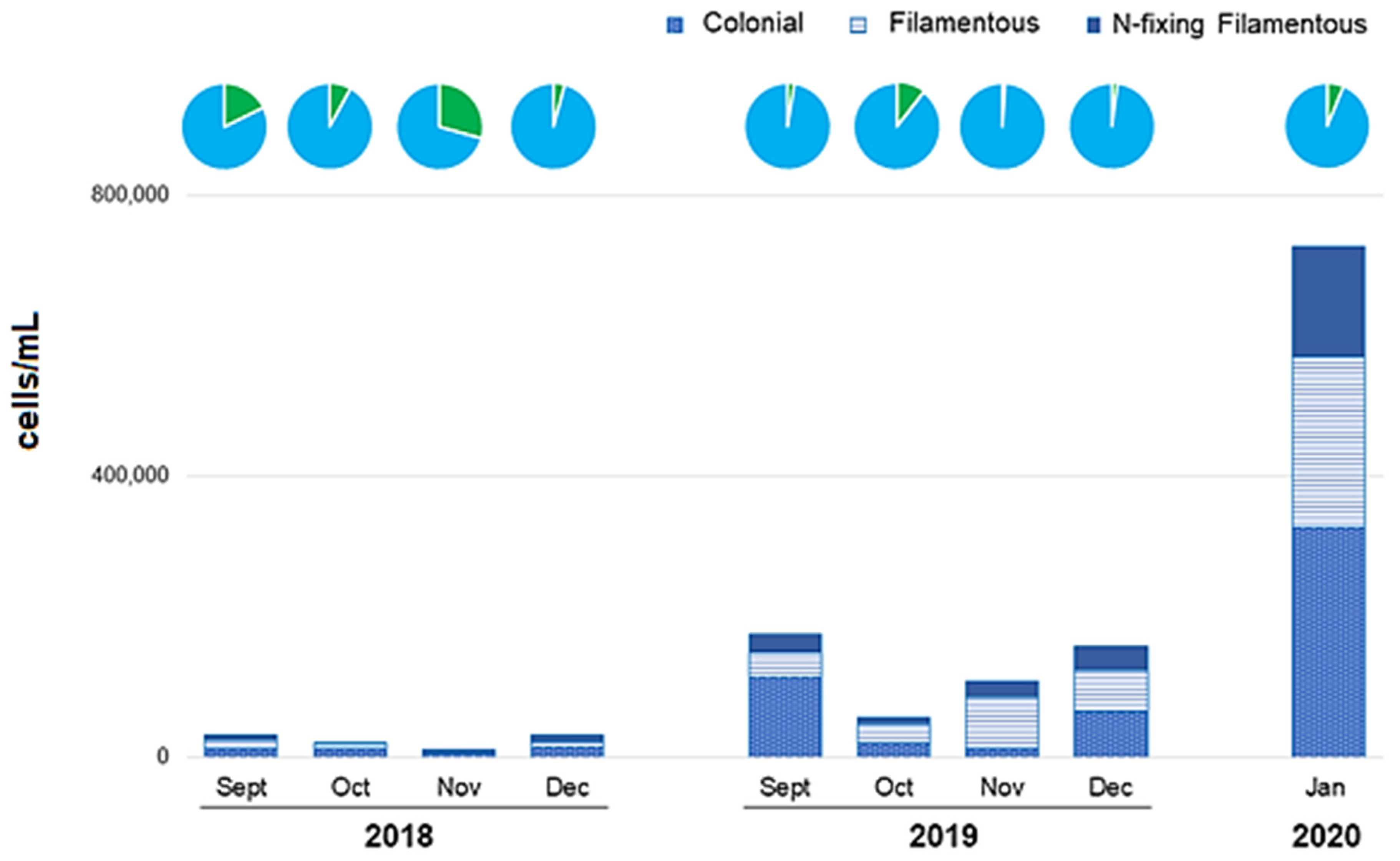
3.4. Cyanobacteria
3.5. Correlation Analysis between Cyanobacterial Abundance and Physical, Chemical, and Metereological Variables
3.6. Blooms
Bloom-Forming Species
3.7. Morphometric Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Carey, C.C.; Ibelings, B.W.; Hoffmann, E.P.; Hamilton, D.P.; Brookes, J.D. Eco-physiological adaptations that favour freshwater cyanobacteria in a changing climate. Water Res. 2012, 46, 1394–1407. [Google Scholar] [CrossRef] [PubMed]
- Winder, M.; Sommer, U. Phytoplankton response to a changing climate. Hydrobiologia 2012, 698, 5–16. [Google Scholar] [CrossRef]
- Nõges, T.; Ghiani, M. Increased nutrient loading and rapid changes in phytoplankton expected with climate change in stratified South European lakes: Sensitivity of lakes with different trophic state and catchment properties. Hydrobiologia 2011, 667, 255–270. [Google Scholar] [CrossRef]
- Giorgi, F.; Lionello, P. Climate change projections for the Mediterranean region. Glob. Planet. Chang. 2008, 63, 90–104. [Google Scholar] [CrossRef]
- Jeppesen, E.; Kronvang, B.; Meeρff, M.; Søndergaard, M.; Hansen, K.M.; Andersen, H.E.; Olesen, J.E. Climate change effects on runoff, catchment phosphorus loading and lake ecological state, and potential adaptations. J. Environ. Qual. 2009, 38, 1930–1941. [Google Scholar] [CrossRef]
- Søndergaard, M.; Jensen, J.P.; Jeppesen, E. Role of sediment and internal loading of phosphorus in shallow lakes. Hydrobiologia 2003, 506, 135–145. [Google Scholar] [CrossRef]
- Özen, A.; Karapınar, B.; Kucuk, İ. Drought-induced changes in nutrient concentrations and retention in two shallow Mediterranean lakes subjected to different degrees of management. Hydrobiologia 2010, 646, 61–72. [Google Scholar] [CrossRef]
- Hallegraeff, G.M. A review of harmful algal blooms and their apparent increase. Phycologia 1993, 32, 79–99. [Google Scholar] [CrossRef] [Green Version]
- Mur, L.R.; Skulberg, O.M.; Utkilen, H. Cyanobacteria in the environment. In Toxic Cyanobacteria in Water: A Guide to Their Public Health, Consequences, Monitoring and Management. St; Chorus, I., Bartram, J., Eds.; Edmundsbury Press: Suffolk, UK, 1999; pp. 15–40. [Google Scholar]
- Aubriot, L.; Bonilla, S.; Kruk, C. Cianobacterias planctónicas: Factores que regulan su crecimiento. In Cianobacterias Planctónicas del Uruguay Manual para la identificación y Medidas de Gestión; Bonilla, S., Ed.; UNESCO: Montevideo, Uruguay, 2009; Volume 16, pp. 5–12. [Google Scholar]
- Mehnert, G.; Rücker, J.; Nicklisch, A.; Leunert, F.; Wiedner, C. Effects of thermal acclimation and photoacclimation on lipophilic pigments in an invasive and a native cyanobacterium of temperate regions. Eur. J. Phycol. 2012, 47, 182–192. [Google Scholar] [CrossRef]
- Kosten, S.; Huszar, V.L.; Mazzeo, N.; Scheffer, M.; Sternberg, L.D.S.; Jeppesen, E. Lake and watershed characteristics rather than climate influence nutrient limitation in shallow lakes. Ecol. Appl. 2009, 19, 1791–1804. [Google Scholar] [CrossRef]
- Fogg, G.E.; Stewart, W.D.P.; Fay, P.; Walsby, A.E. The Blue-Green Algae; Academic Press: London, UK; New York, NY, USA, 1973; p. 459. [Google Scholar]
- Codd, G.A. Blue-green algal toxins: Water-borne hazards to health. Water Public Health 1994, 271–278. [Google Scholar]
- Paerl, H. Nutrient and other environmental controls of harmful cyanobacterial blooms along the freshwater–marine continuum. In Cyanobacterial Harmful Algal Blooms: STATE of the Science and Research Needs; Springer: New York, NY, USA, 2008; pp. 217–237. [Google Scholar]
- Anderson, D.; Glibert, P.; Burkholder, J. Harmful algal blooms and eutrophication: Nutrient sources, composition and consequences. Estuaries 2002, 25, 704–726. [Google Scholar] [CrossRef]
- Van Dolah, F.M. Marine algal toxins: Origins, health effects, and their increased occurrence. Environ. Health Perspect. 2000, 108, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmichael, W. A world overview—One-hundred-twenty-seven years of research on toxic cyanobacteria—Where do we go from here? Cyanobacterial Harmful Algal Bloom. State Sci. Res. Needs 2008, 619, 105–125. [Google Scholar]
- Paerl, H.W.; Huisman, J. Blooms like it hot. Science 2008, 320, 57–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamilton, L.C.; Keim, B.D. Regional variation in perceptions about climate change. Int. J. Climatol 2009, 29, 2348–2352. [Google Scholar]
- World Health Organization. Guidelines for Safe Recreational Water Environments; Coastal and fresh waters: Geneva, Switzerland, 2003; Volume 1.
- Mowe, M.A.; Mitrovic, S.M.; Lim, R.P.; Furey, A.; Yeo, D.C. Tropical cyanobacterial blooms: A review of prevalence, problem taxa, toxins and inuencing environmental factors. J. Limnol. 2015, 74, 205–224. [Google Scholar] [CrossRef] [Green Version]
- Cheung, M.; Liang, Y.; Lee, J. Toxin-producing cyanobacteria in freshwater: A review of the problems, impact on drinking water safety, and efforts for protecting public health. J. Microbiol. 2013, 51, 1–10. [Google Scholar] [CrossRef]
- Vieira-Lanero, R.; Barca, S.; Cobo, M.C.; Cobo, F. Occurrence of Freshwater Cyanobacteria and Bloom Records in Spanish Reservoirs (1981–2017). Hydrobiology 2022, 1, 122–136. [Google Scholar] [CrossRef]
- Sivonen, K.; Jones, G. Cyanobacterial toxins. In Toxic Cyanobacteria in Water: A Guide to Public Health Significance, Monitoring and Management; Chorus, I., Bertram, J., Eds.; The World Health Organization: London, UK, 1999; pp. 44–111. ISBN 0–419–23930–8. [Google Scholar]
- Dyble, J.; Tester, P.A.; Litaker, R.W. Effects of light intensity on cylindrospermopsin production in the cyanobacterial HAB species Cylindrospermopsis raciborskii. Afr. J. Mar. Sci. 2006, 28, 309–312. [Google Scholar] [CrossRef]
- Moschini-Carlos, V.; De Freitas, L.G.; Pompeo, M. Limnological evaluation of water in the Rio Grande and Taquacetuba branches of the Billings Complex (São Paulo, Brazil) and management implications. Ambiente Agua Interdiscip. J. Appl. Sci. 2010, 5, 47–59. [Google Scholar] [CrossRef]
- Paerl, H.W.; Paul, V. Climate change: Links to global expansion of harmful cyanobacteria. Water Res. 2012, 46, 1349–1363. [Google Scholar] [CrossRef] [PubMed]
- Merel, S.; Walker Chicana, D.; Snyder, R.; Baurès, S.; Thomas, O. State of knowledge and concerns on cyanobacterial blooms and cyanotoxins. Environ. Int. 2013, 59, 303–327. [Google Scholar] [CrossRef] [PubMed]
- Machado, L.S.; Santos, L.G.; Lopez-Doval, J.C.; Pompeo, M.L.M.; Moschini, V. Fatores ambientais relacionados à ocorrência de cianobactérias potencialmente tóxicas no reservatorio de Guarapiranga. Rev. Ambiente Água 2016, 11, 810–818. [Google Scholar] [CrossRef] [Green Version]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M.P. Cyanobacterial blooms. Nat. Rev. Microbiol. 2008, 16, 471–483. [Google Scholar] [CrossRef] [PubMed]
- O’Farrell, I.; Motta, C.; Forastier, M.; Polla, W.; Otaño, S.; Meichtry, N.; Devercelli, M.; Lombardo, R. Ecological meta-analysis of bloom-forming planktonic Cyanobacteria in Argentina. Harmful Algae 2019, 83, 1–13. [Google Scholar] [CrossRef]
- Amé, M.; Diaz, M.; Wunderlin, D. Occurrence of toxic cyanobacterial blooms in San Roque reservoir (Córdoba, Argentina): A field and chemometric study. Environ. Toxicol. 2003, 18, 192–201. [Google Scholar] [CrossRef]
- Bazán, R.; Corral, M.; Pagot, M.; Rodríguez, A.; Oroná, C.; Rodríguez, M.I.; Busso, F. Teledetección y modelado numérico para el análisis de la calidad de agua del embalse Los Molinos, Córdoba, Argentina. Rev. Ing. Hidráulica En México 2005, 20, 121–135. [Google Scholar]
- Alvarez Dalinger, F.; Salusso, M.; Moraña, L. Primera caracterización de un reservorio tropical somero en riesgo en el norte de la Argentina. Ecol. Austral 2022, 32, 542–554. [Google Scholar] [CrossRef]
- Vidaurre, A.; Alvarez Dalinger, F.S.; Moraña, L.B.; Salusso, M.M. Cianobacterias en un embalse subtropical de la provincia de Salta (Argentina). Boletín De La Sociedad Argentina De Botánica 2018, 53, 543–549. [Google Scholar] [CrossRef]
- APHA; AWWA; WEF. Standard Methods for the Examination of Water and Wastewater, 21st ed.; APHA. Standard Method 10200H: Washington, DC, USA, 2005. [Google Scholar]
- Cabrera Silva, S. Estimación de clorofila a y feopigmentos. Una revisión metodológica. In Programa Sobre el Hombre y la Biosfera; UNESCO, Universidad de Chile: Santiago de Chile, Chile, 1984; p. 236. [Google Scholar]
- Carlson, R. A trophic state index for lakes. Limnol. Oceanogr. 1977, 22, 361–369. [Google Scholar] [CrossRef] [Green Version]
- Utermöhl, H. ZurVervollkomrnnungverquantitativenPhytoplankton-Methodic. Mitt. Int. Verein. Limnol. 1958, 9, 138. [Google Scholar]
- Venrick, E. How many cells to count? In Monographs on Oceanographic Methods 6: Phytoplankton Manual; Sournia, A., Ed.; United Nations Educational, Scientific and Cultural Organization: Paris, France, 1978; pp. 167–180. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyano prokaryota 1. TeilChroococcales. In Süβwasserflora von Mitteleuropa; Ettl, H., Gärtner, G., Heynig, H., Mollenhaver, Y.D., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; Volume 19, pp. 1–548. [Google Scholar]
- Komárek, J.; Anagnostidis, K.C. Süsswasserflora von Mitteleuropa Bd. 19/1: Cyanoprokaryota: Teil/Part 1: Chroococcales; Spektrum Akademischer Verlag: Stuttgart, Germany, 2005. [Google Scholar]
- Komárková-Legnerová, J. The systematics and ontogenesis of the genera Ankistrodesmus Corda and Monoraphidium gen. nov. Academia 1969. [Google Scholar]
- Komárek, J.; Kaštovský, J.; Mareš, J.; Johansen, J.R. Taxonomic classification of cyanoprokaryotes (cyanobacterial genera) using a polyphasic approach. Preslia 2014, 86, 295–335. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Ba-cillaríophyceae, 1. Teil: Naviculaceae. In Süβwasserflora von Mitteleuropa; Ettl, H., Gärtner, G., Heynig, H., Mollenhaver, Y.D., Eds.; Springer: Berlin/Heidelberg, Germany, 1986; Volume 2, pp. 1–876. [Google Scholar]
- Krammer, K.; Lange Bertalor, H. Bacillaríophyceae. 3. Teil: Centrales, Fragilariaceae, Eunotiaceae. In Süsswasserflora von Mitteleuropa, G. Fischer, Jena Süsswasserflora von Mitteleuropa Bd. 2/3; Ettl, H., Gerloff, H., Heynig, H., Mollenhauer, D., Eds.; Gustav Fischer Verlag: Stuttgart, Germany, 1991. [Google Scholar]
- Chorus, I.; Welker, M. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management; Taylor & Francis: London, UK, 2021; p. 858. [Google Scholar]
- Chorus, I.; Bartram, J. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Yema, L.; Kremer, C.T.; O’Farrell, I.; de Tezanos Pinto, P. Assessing patterns of morphological and physiological trait variations across heterocytous cyanobacteria at cellular and population levels. Hydrobiologia 2018, 823, 93–107. [Google Scholar] [CrossRef]
- Hillebrand, H.; Dürselen, C.D.; Kirschtel, D.; Pollingher, U.; Zohary, T. Biovolume calculation for pelagic and benthic microalgae. J. Phycol. 1999, 35, 403–424. [Google Scholar] [CrossRef]
- Simon, N.; Cras, A.L.; Foulon, E.; Lemée, R. Diversity and evolution of marine phytoplankton. Comptes Rendus Biologies 2009, 332, 159–170. [Google Scholar] [CrossRef]
- Bonilla, S.; Conde, D. El fitoplancton como descriptor sensible de cambios ambientales en las lagunas costeras de la Reserva Bañados del Este. Semin. Taller Sobre Monit. Ambient. 2000, 31, 1–5. [Google Scholar]
- Loza, S.; Carmenate, M.; Pereiro, Y.; Sánchez, M. Respuesta del fitoplancton ante el impacto antrópico de la zona costera NW de ciudad de La Habana, Cuba. In Proceedings of the III Convención de Medio Ambiente, La Habana, Cuba, July, 2–6 July 2007. [Google Scholar]
- Aguilera, A.; Haakonsson, S.; Martín, M.; Salerno, G.; Echenique, R.O. Bloom-forming cyanobacteria and cyanotoxins in Argentina: A growing health and environmental concern. Limnologica 2017, 69, 103–114. [Google Scholar] [CrossRef]
- Znachor, P.; Jurczak, T.; Komarkova, J.; Jezberová, J.; Mankiewicz, J.; Kaštovská, K.; Zapomělová, E. Summerchanges in cyanobacterial bloom composition and micro-cystin concentration in eutrophic Czech reservoirs. Environ. Toxicol. 2006, 21, 236–243. [Google Scholar] [CrossRef]
- Bormans, M.; Ford, P.; Fabbro, L. Spatial and tem-poral variability in cyanobacterial populations controlledby physical processes. J. Plankton Res. 2005, 27, 61–70. [Google Scholar] [CrossRef] [Green Version]
- Bittencourt-Oliveira, M.C. Detection of potential microcystin-producing cyanobacteria in Brazilian reservoirswith a mcyB molecular marker. Harmful Algae 2003, 2, 51–60. [Google Scholar] [CrossRef]
- Steel, J.A.; Duncan, A. Modelling the ecological aspects of bankside reservoirs and implications for man-agement. Hydrobiologia 1999, 395–396, 133–147. [Google Scholar] [CrossRef]
- Soares, M.C.S.; Huszar, V.L.M.; Miranda, M.N.; Mello, M.M.; Roland, F.; Lürling, M. Dominance in Brazil: Distribution and environmental preferences. Hydrobiologia 2013, 717, 1–12. [Google Scholar] [CrossRef]
- Rigosi, A.; Carey, C.C.; Ibelings, B.W.; Brookes, J.D. The interaction between climate warming and eutrophication to promote cyanobacteria is dependent on trophic state and varies among taxa. Limnol. Oceanogr 2014, 59, 99–114. [Google Scholar] [CrossRef] [Green Version]
- Paerl, H.W.; Fulton, R.S.; Moisander, P.H.; Dyble, J. Harmful freshwater algal blooms, with an emphasis on cyanobacteria. Sci. World J. 2001, 1, 76–113. [Google Scholar] [CrossRef] [Green Version]
- Chellappa, N.T.; Borba, J.M.; Rocha, O. Phytoplankton community and physical-chemical characteristics of water in the public reservoir of Cruzeta, RN, Brazil. Braz. J. Biol. 2008, 68, 477–494. [Google Scholar] [CrossRef] [Green Version]
- Mendes, C.F.; dos Santos Severiano, J.; de Moura, G.C.; dos Santos Silva, R.D.; Monteiro, F.M.; de Lucena Barbosa, J.E. The reduction in water volume favors filamentous cyanobacteria and heterocyst production in semiarid tropical reservoirs without the influence of the N: P ratio. Sci. Total Environ. 2022, 816, 151584. [Google Scholar] [CrossRef]
- Smith, V.H. Low nitrogen to phosphorus ratios favor dominance by blue-green algae in lake phytoplankton. Science 1983, 221, 669–671. [Google Scholar] [CrossRef] [Green Version]
- Downing, J.A.; McCauley, E. The nitrogen: Phosphorus relationship in lakes. Limnol. Oceanogr. 1992, 37, 936–945. [Google Scholar] [CrossRef] [Green Version]
- Dokulil, M.T.; Teubner, K. Cyanobacterial dominance in lakes. Hydrobiologia 2000, 438, 1–12. [Google Scholar] [CrossRef]
- Diaz, M.; Pedrozo, F.; Reynolds, C.; Temporetti, P. Chemical composition and the nitrogen-regulated trophic state of Patagonian lakes. Limnologica 2007, 37, 17–27. [Google Scholar] [CrossRef] [Green Version]
- Sukenik, A.; Hadas, O.; Kaplan, A.; Quesada, A. Invasion of Nostocales (cyanobacteria) to subtropical and temperate freshwater lakes–physiological, regional, and global driving forces. Front. Microbiol. 2012, 3, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiedner, C.; Rücker, J.; Brüggemann, R.; Nixdorf, B. Climate change affects timing and size of populations of an invasive cyanobacterium in temperate regions. Oecologia 2007, 152, 473–484. [Google Scholar] [CrossRef]
- Briand, J.F.; Leboulanger, C.; Humbert, J.F.; Bernard, C.; Dufour, P. Cylindrospermopsis raciborskii (cyanobacteria) invasión at mid-latitudes: Selection, wide physiological tolerance or global warming? J. Phycol. 2004, 40, 231–238. [Google Scholar] [CrossRef]
- Mehnert, G.; Leunert, F.; Cirés, S.; Klaus, D.; Jöhnk, J.R.; Brigitte, N.; Claudia, W. Competitiveness of invasive and native cyanobacteria from temperate freshwaters under various light and temperature conditions. J. Plankton Res. 2010, 32, 1009–1021. [Google Scholar] [CrossRef]
- Sarthou Suarez, F.V. Floraciones de Cianobacterias: Efectos de la Eutrofización y la Variabilidad Climática; Universidad de la República: Montevideo, Uruguay, 2016. [Google Scholar]
- O’Farrell, I.; Vinocur, A.; de Tezanos Pinto, P. Longterm study of bloom-forming cyanobacteria in a highly fluctuating vegetated floodplain lake: A morpho-functional approach. Hydrobiologia 2015, 752, 91–102. [Google Scholar] [CrossRef]
- Yema, L.; Litchman, E.; de Tezanos Pinto, P. The role of heterocytes in the physiology and ecology of bloom-forming harmful cyanobacteria. Harmful Algae 2016, 60, 131–138. [Google Scholar] [CrossRef]
- Dolman, A.M.; Ru¨cker, J.; Pick, F.; Fastner, J.; Rohrlack, T.; Mischke, U.; Wiedner, C. Cyanobacteria and cyanotoxins: The influence of nitrogen versus phosphorus. PLoS ONE 2012, 7, e38757. [Google Scholar] [CrossRef]
- Kaplan-Levy, R.N.; Hadas, O.; Summers, M.; Rücker, J.; Sukenik, A. Akinetes: Dormant cells of cyanobacteria. In Dormancy and Resistance in Harsh Environments; Lubzens, E., Cerda, J., Clark, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 5–27. [Google Scholar]
- Pérez, R.; Forchhammer, K.; Salerno, G.; Maldener, I. Clear differences in metabolic and morphological adaptations of akinetes of two Nostocales living in different habitats. Microbiology 2016, 162, 214–223. [Google Scholar] [CrossRef]
- Argueta, C.; Yuksek, K.; Patel, R.; Summers, M.L. Identification of Nostoc punctiforme akinete-expressed genes using differential display. Mol. Microbiol. 2006, 61, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Argueta, C.; Summers, M.L. Characterization of a model system for the study of Nostoc punctiforme akinetes. Arch. Microbiol. 2005, 183, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, J.M.; Herdman, M.; Stewart, W.D. Akinetes of the cyanobacterium Nostoc PCC 7524: Macromolecular composition, structure and control of differentiation. Microbiology 1979, 115, 273–287. [Google Scholar] [CrossRef] [Green Version]
- Lozano, V.L. Hidden impacts of environmental stressors on freshwater communities could be revealed at lower concentrations by correlation of abundances network analyses: An example with herbicides glyphosate, 2,4-D, and their mixtures. Austral Ecol. 2022. [Google Scholar] [CrossRef]

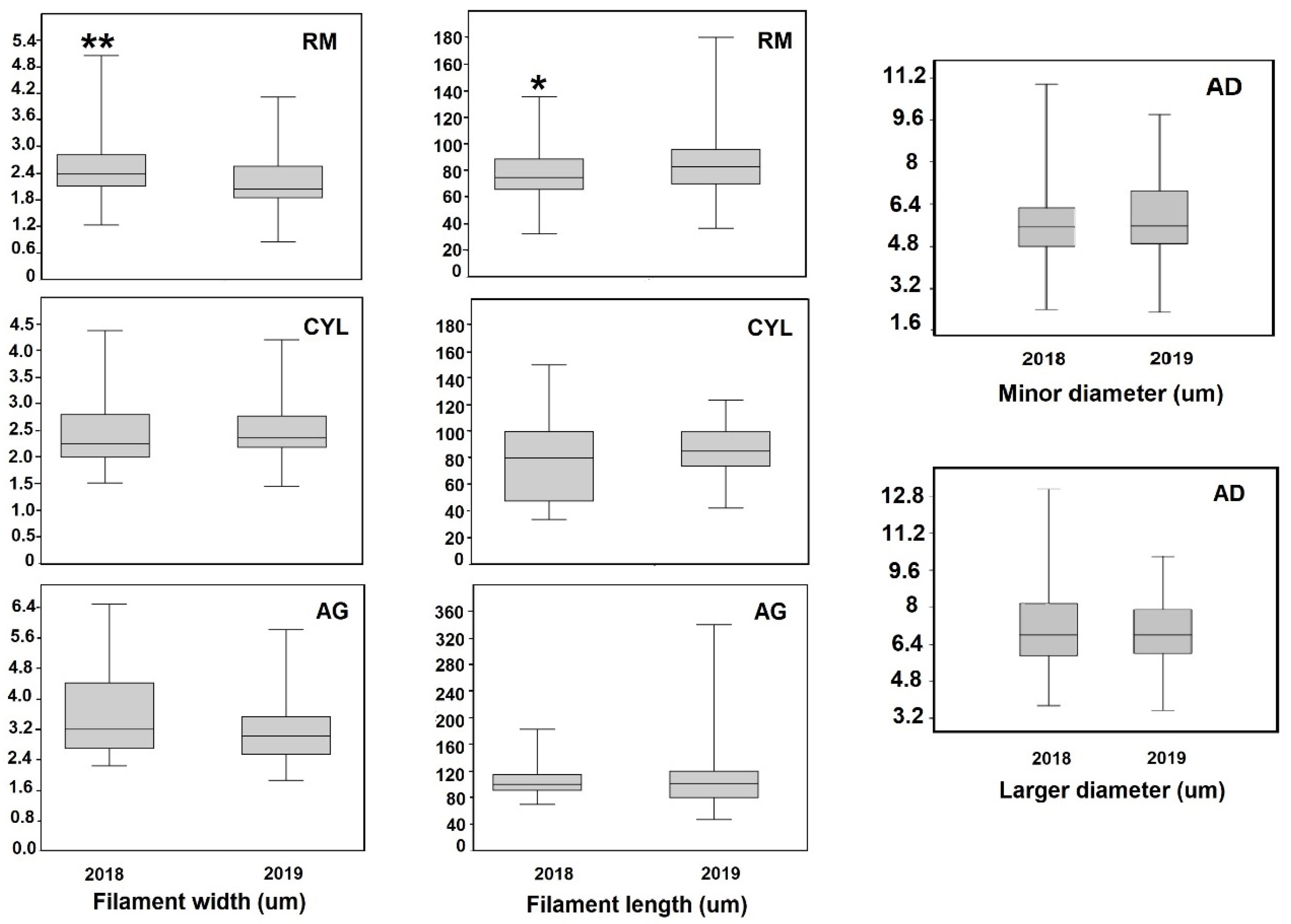
| 2018 | 2019 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Unit | Mean | SD | Min | Max | Mean | SD | Min | Max | |
| Secchi | M | 1.88 | 0.68 | 1 | 2.75 | 1.21 | 0.21 | 1 | 1.5 |
| pH | 7.01 | 0.17 | 6.74 | 7.21 | 7.58 | 0.79 | 6.4 | 8.06 | |
| E.C. | µS/cm | 634.13 | 31.01 | 593 | 682.8 | 644.7 | 25.28 | 617 | 678 |
| Turbidity | NTU | 4.14 | 2.35 | 1.87 | 7.82 | 3.38 | 1.76 | 2.2 | 6 |
| Alkalinity | mg CaCO3/L | 79.87 | 25.83 | 33 | 108 | 221.48 | 12.73 | 203.04 | 230.86 |
| Hardness | mg CaCO3/L | 505.65 | 648.43 | 222 | 1787.62 | 199.97 | 66.2 | 141.64 | 260.38 |
| N-NO2 | mg/L | 0.01 | 0.003 | 0.001 | 0.01 | 0.01 | 0.003 | 0.003 | 0.01 |
| N-NO3 | mg/L | 0.9 | 0.88 | 0.001 | 2.4 | 0.3 | 0.08 | 0.2 | 0.4 |
| N-NH3 | mg/L | 0.75 | 0.90 | 0.20 | 2.10 | 0.38 | 0.04 | 0.32 | 0.41 |
| NH4 | mg/L | 0.44 | 0.14 | 0.26 | 0.58 | 0.48 | 0.05 | 0.41 | 0.42 |
| SIN | mg/L | 1.21 | 0.82 | 0.6 | 2.07 | 0.68 | 0.05 | 0.61 | 0.73 |
| SRP | mg/L | 0.44 | 0.74 | 0.03 | 1.54 | 0.12 | 0.12 | 0.04 | 0.3 |
| N/P | 24.47 | 33.55 | 0.14 | 88.87 | 10.2 | 6.32 | 2.44 | 17.49 | |
| COD | Mg O2/L | 206.3 | 204.5 | 63 | 616.46 | 139.78 | 67.81 | 90.77 | 240.14 |
| CL-a | µg/L | 11.04 | 10.92 | 3.78 | 33.05 | 9.48 | 2.85 | 5.58 | 12.04 |
| In-situ water temperature | °C | 23.92 | 2.59 | 18.8 | 26 | 30.03 | 5.69 | 25 | 35.1 |
| Total Cyanobacteria | Non-Heterocytous Filament | Colonial | Heterocytous Cyanobacteria | ||
|---|---|---|---|---|---|
| Physical and chemical | pH | 0.080 | 0.330 | −0.030 | 0.250 |
| Electrical conductivity | −0.350 | −0.180 | −0.400 | −0.430 | |
| Turbidity | 0.600 | 0.580 | 0.600 | 0.630 | |
| Total solids | −0.700 | −0.530 | −0.730 | −0.670 | |
| Alkalinity | 0.680 | 0.830 | 0.570 | 0.700 | |
| Carbonates | 0.470 | 0.670 | 0.420 | 0.450 | |
| Bicarbonates | 0.710 | 0.580 | 0.720 | 0.670 | |
| Hardness | −0.320 | −0.550 | −0.130 | −0.430 | |
| Calcium | −0.300 | −0.280 | −0.300 | −0.470 | |
| Magnesium | −0.330 | −0.600 | −0.110 | −0.310 | |
| COD | 0.220 | 0.130 | 0.230 | 0.180 | |
| Dissolved oxygen | −0.320 | −0.170 | −0.500 | −0.400 | |
| Chlorophyll a | 0.780 | 0.700 | 0.680 | 0.830 | |
| Secchi Disk | −0.840 | −0.860 | −0.730 | −0.900 | |
| Nutrients | Nitrites | 0.680 | 0.510 | 0.790 | 0.770 |
| Nitrates | −0.120 | −0.300 | 0.030 | −0.060 | |
| Ammonium | −0.020 | 0.160 | −0.140 | −0.180 | |
| SIN | 0.000 | −0.130 | 0.120 | 0.000 | |
| PSRP | 0.220 | 0.190 | 0.240 | 0.200 | |
| N/P | −0.150 | −0.150 | −0.170 | −0.150 | |
| Temperature | Water | 0.670 | 0.720 | 0.580 | 0.770 |
| Sampling Date | 0.780 | 0.870 | 0.670 | 0.870 | |
| 14 d | 0.200 | 0.380 | 0.020 | 0.230 | |
| 30 d | 0.350 | 0.520 | 0.200 | 0.480 | |
| 6 months | 0.97 | 0.90 | 0.92 | 0.83 | |
| 8 months | 0.770 | 0.800 | 0.720 | 0.850 | |
| Rainfall | Sampling Date | −0.430 | −0.520 | −0.340 | −0.550 |
| 14 d | −0.440 | −0.450 | −0.410 | −0.380 | |
| 30 d | −0.230 | −0.250 | −0.180 | −0.080 | |
| 6 months | 0.05 | 0.03 | 0.18 | −0.20 | |
| 8 months | −0.700 | −0.680 | −0.620 | −0.620 |
| Specie | M | Meteorological Variable | R (Spearman) | p-Value |
|---|---|---|---|---|
| AG | W/L | Water temperature | 0.72 | 0.0427 |
| AG | W/L | Average T °C (30 days) | 0.72 | 0.0427 |
| AD | W/L | Daily rain | 0.69 | 0.0395 |
| CYL | Width | Accumulated rain (30 days) | −0.85 | 0.0034 |
| CYL | Width | Accumulated rain (30 days) | −0.91 | 0.0007 |
| CYL | Width | Accumulated rain (14 days) | −0.69 | 0.0401 |
| CYL | W/L | Average T °C (14 days) | 0.78 | 0.0267 |
| RM | Length | Accumulated rain (8 months) | −0.73 | 0.0381 |
| RM | W/L | Accumulated rain (8 months) | −0.8 | 0.0237 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarez Dalinger, F.S.; Lozano, V.L.; Borja, C.N.; Moraña, L.B.; María Mónica, S. Short-Term Meteorological Conditions Explain Cyanobacterial Blooms in a Tropical Reservoir. Water 2023, 15, 302. https://doi.org/10.3390/w15020302
Alvarez Dalinger FS, Lozano VL, Borja CN, Moraña LB, María Mónica S. Short-Term Meteorological Conditions Explain Cyanobacterial Blooms in a Tropical Reservoir. Water. 2023; 15(2):302. https://doi.org/10.3390/w15020302
Chicago/Turabian StyleAlvarez Dalinger, Florencia Soledad, Verónica Laura Lozano, Claudia Nidia Borja, Liliana Beatriz Moraña, and Salusso María Mónica. 2023. "Short-Term Meteorological Conditions Explain Cyanobacterial Blooms in a Tropical Reservoir" Water 15, no. 2: 302. https://doi.org/10.3390/w15020302
APA StyleAlvarez Dalinger, F. S., Lozano, V. L., Borja, C. N., Moraña, L. B., & María Mónica, S. (2023). Short-Term Meteorological Conditions Explain Cyanobacterial Blooms in a Tropical Reservoir. Water, 15(2), 302. https://doi.org/10.3390/w15020302





