Comparative Study on Enhanced Photocatalytic Activity of Visible Light-Active Nanostructures for Degradation of Oxytetracycline and COD Removal of Licorice Extraction Plant Wastewater
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of L-Asparagine-TiO2, L-Histidine-TiO2 and L-Methionine-TiO2
2.3. Catalysts Characterization
2.4. Photocatalytic Activity Experiments
2.5. Experimental Design Methodology
3. Results and Discussion
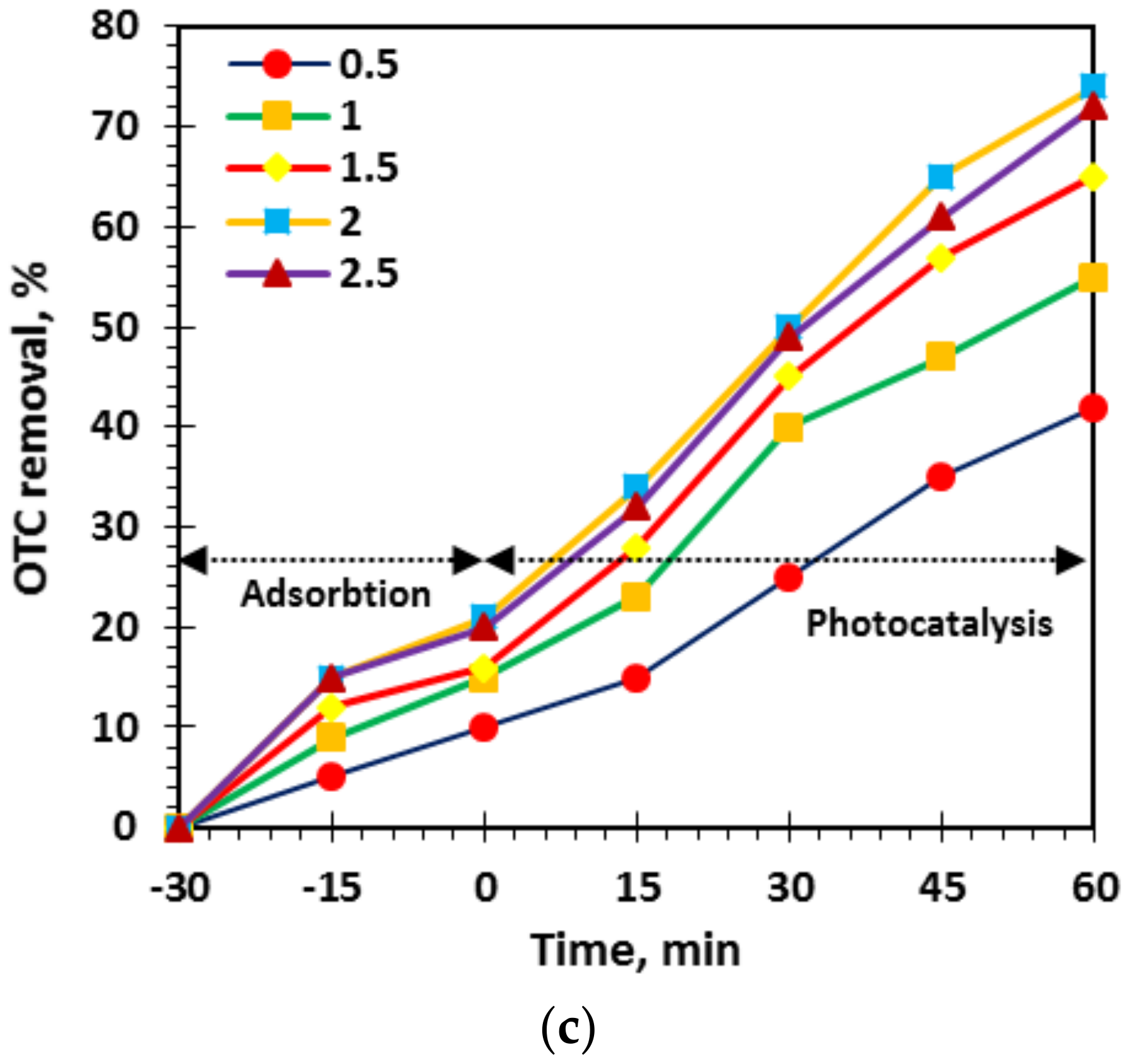
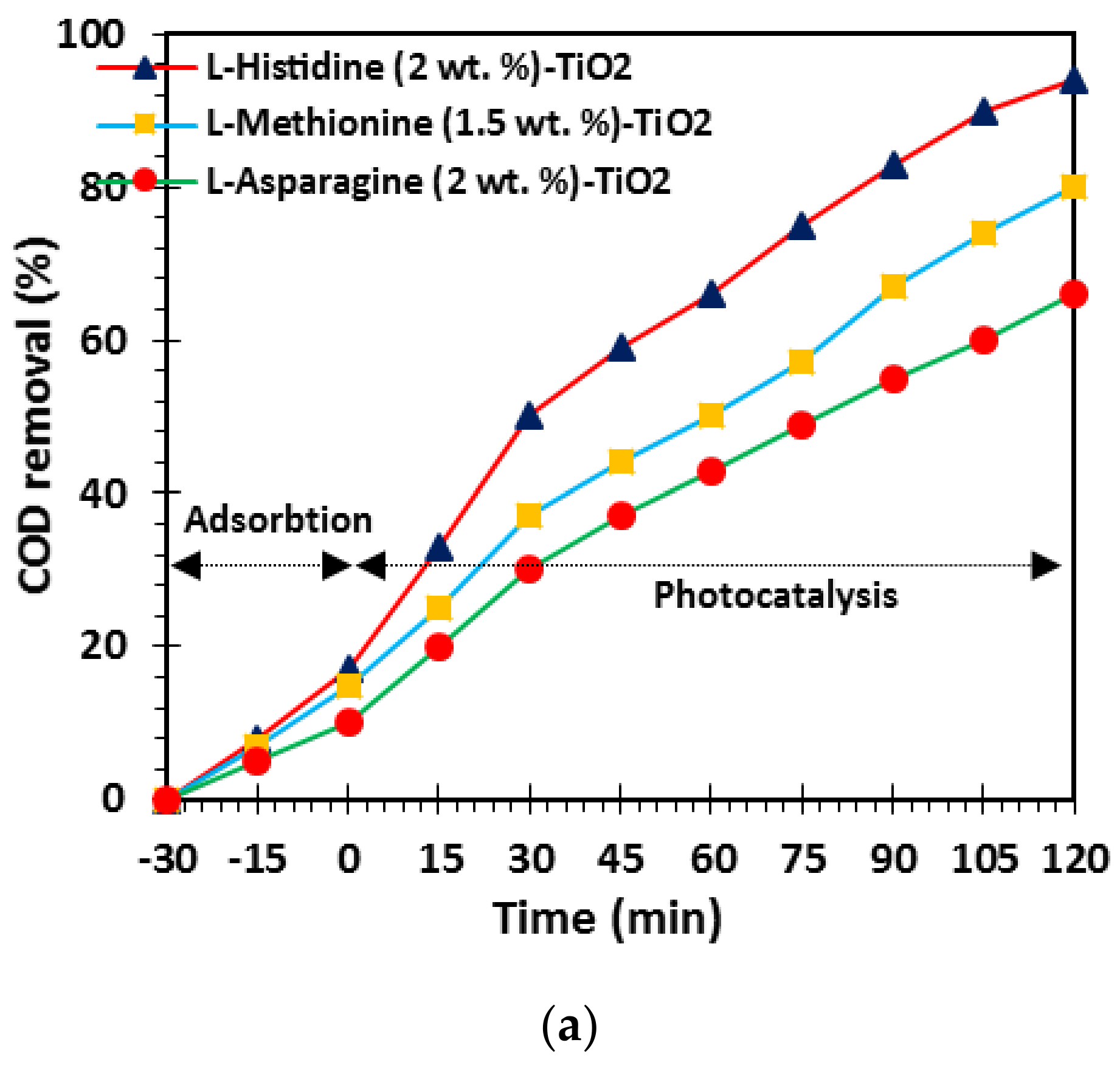
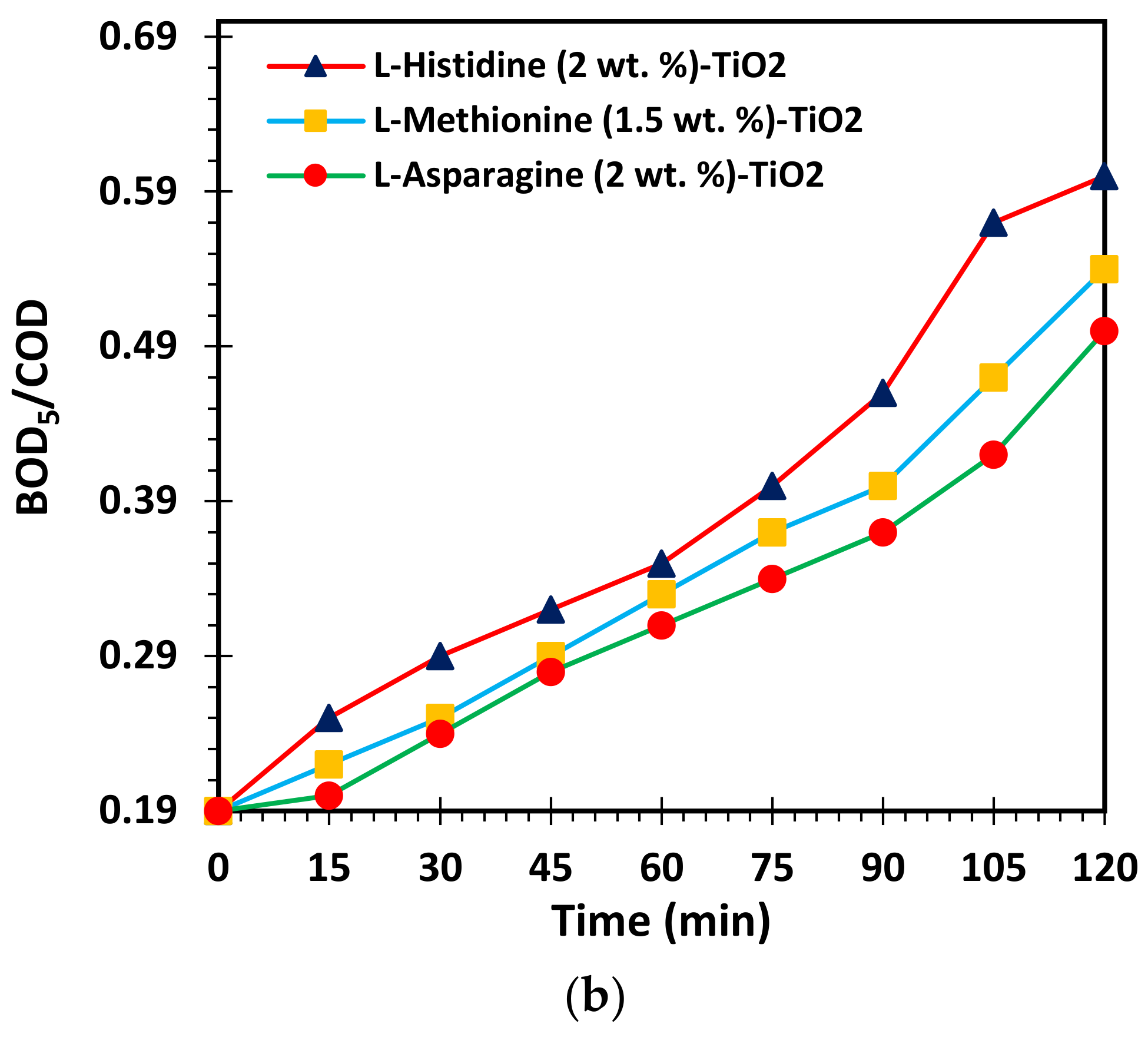
3.1. Optimization of L-Asparagine, L-Histidine and L-Methionine Loading in TiO2 Network
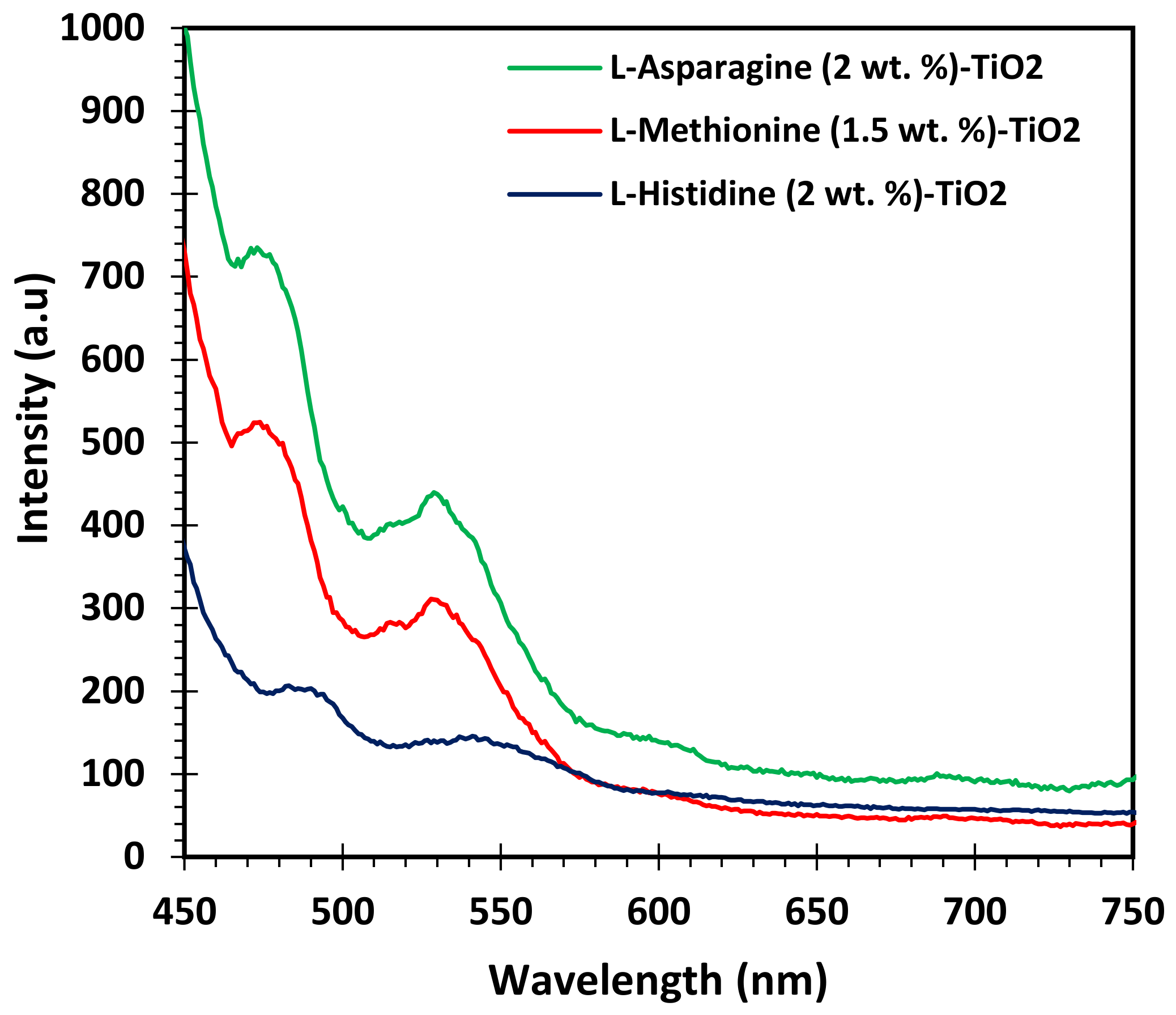
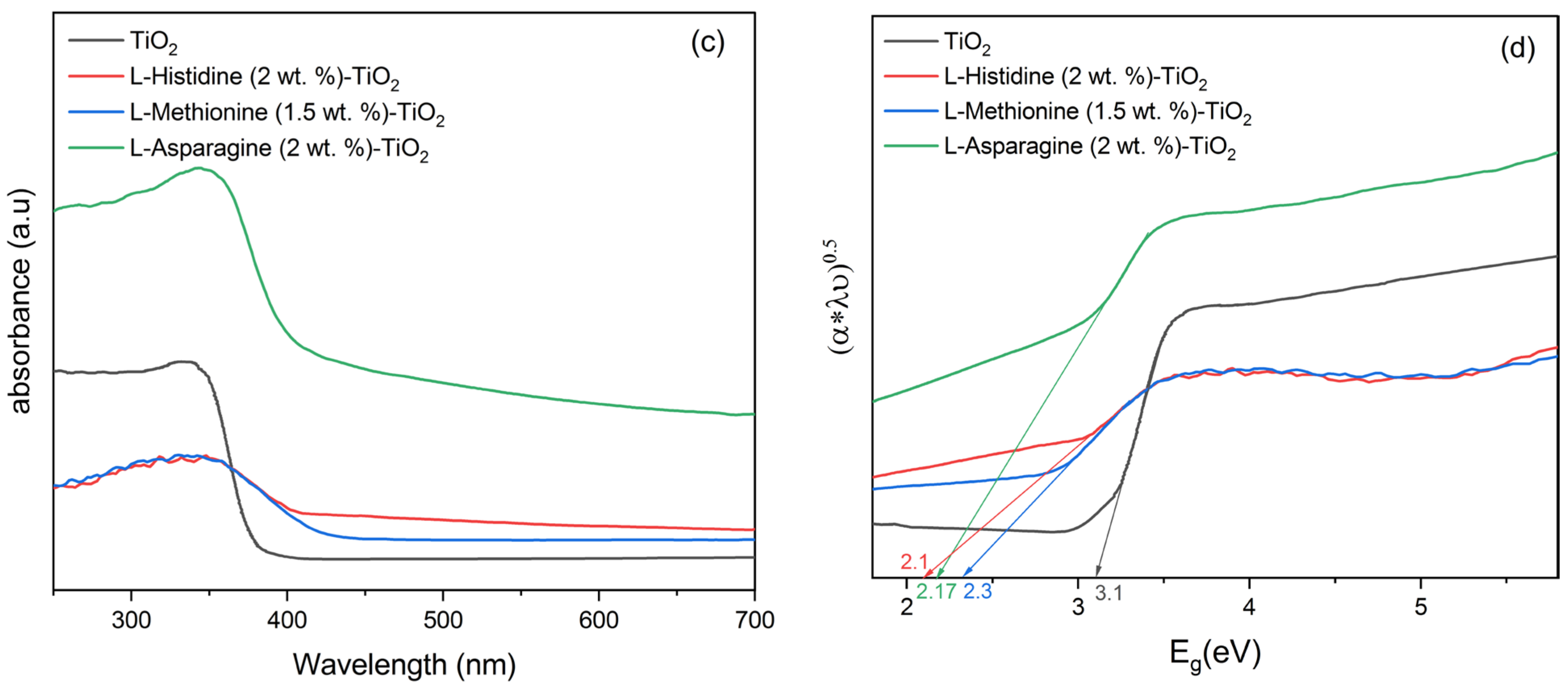
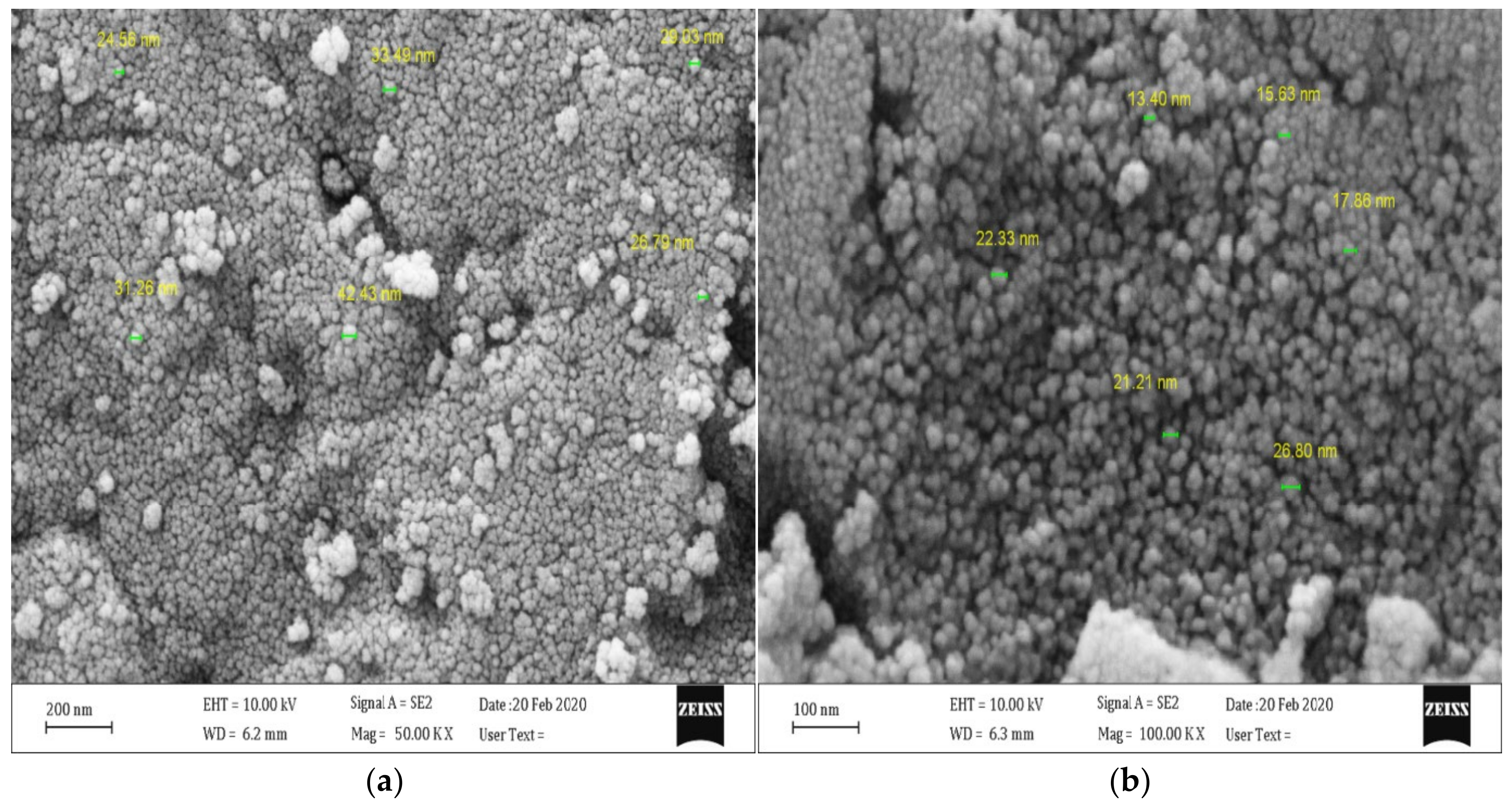
3.2. Characterization of Catalysts
3.3. Statistical Analysis and Modeling for Photodegradation of OTC and COD by L-Histidine (2 wt.%)-TiO2
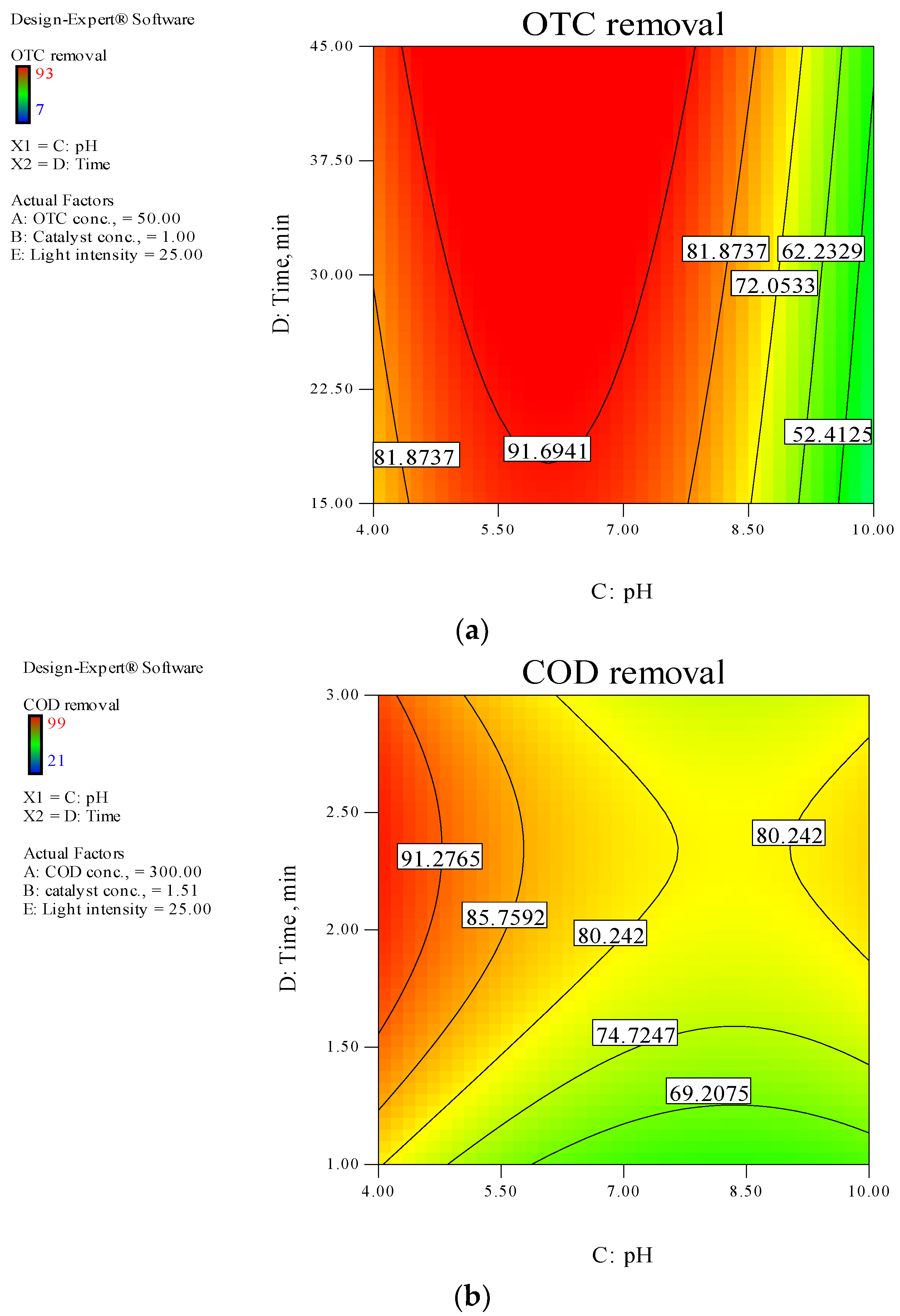
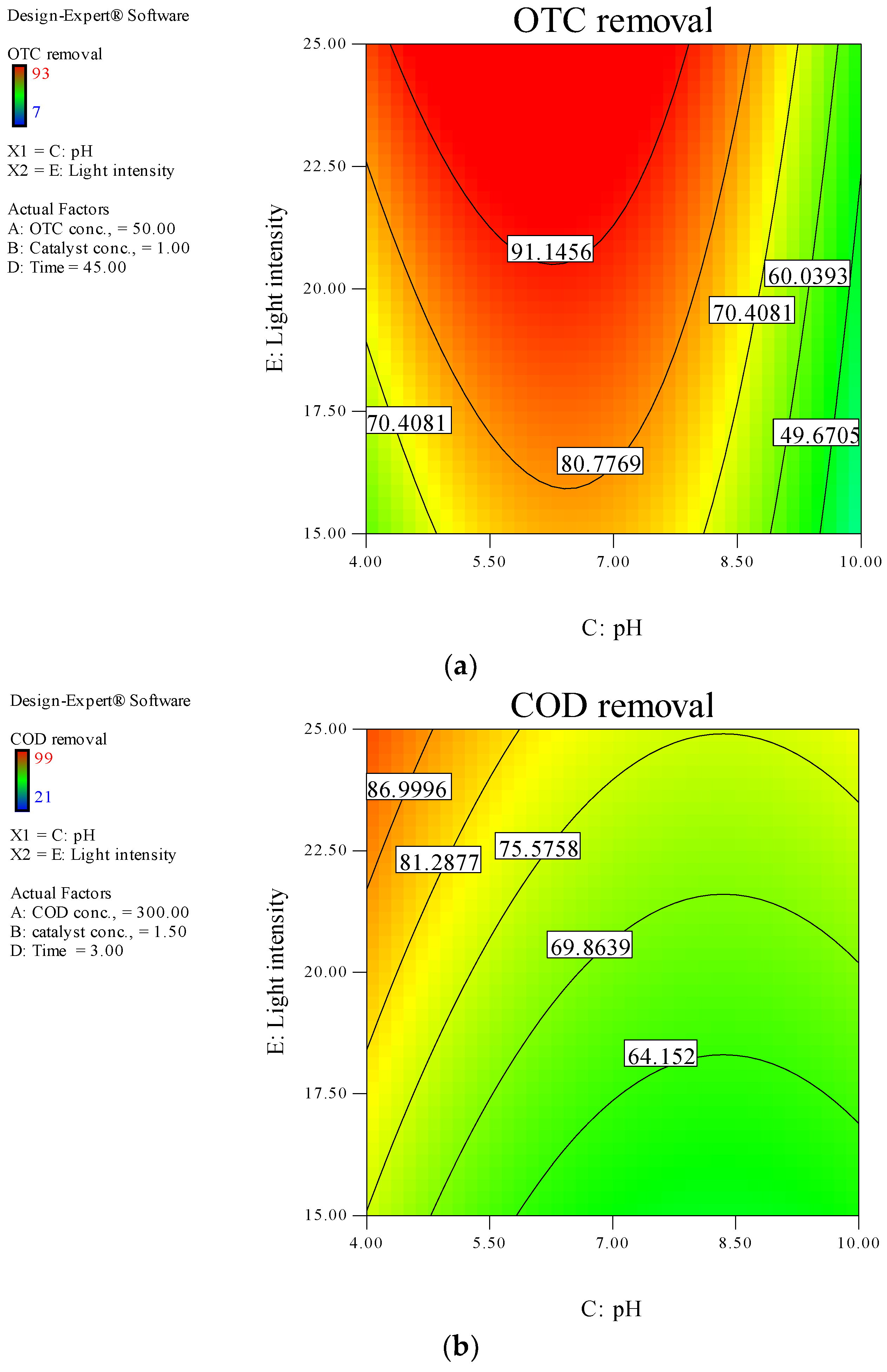
3.4. Photodegradation Modeling and Optimization of Independent Variables
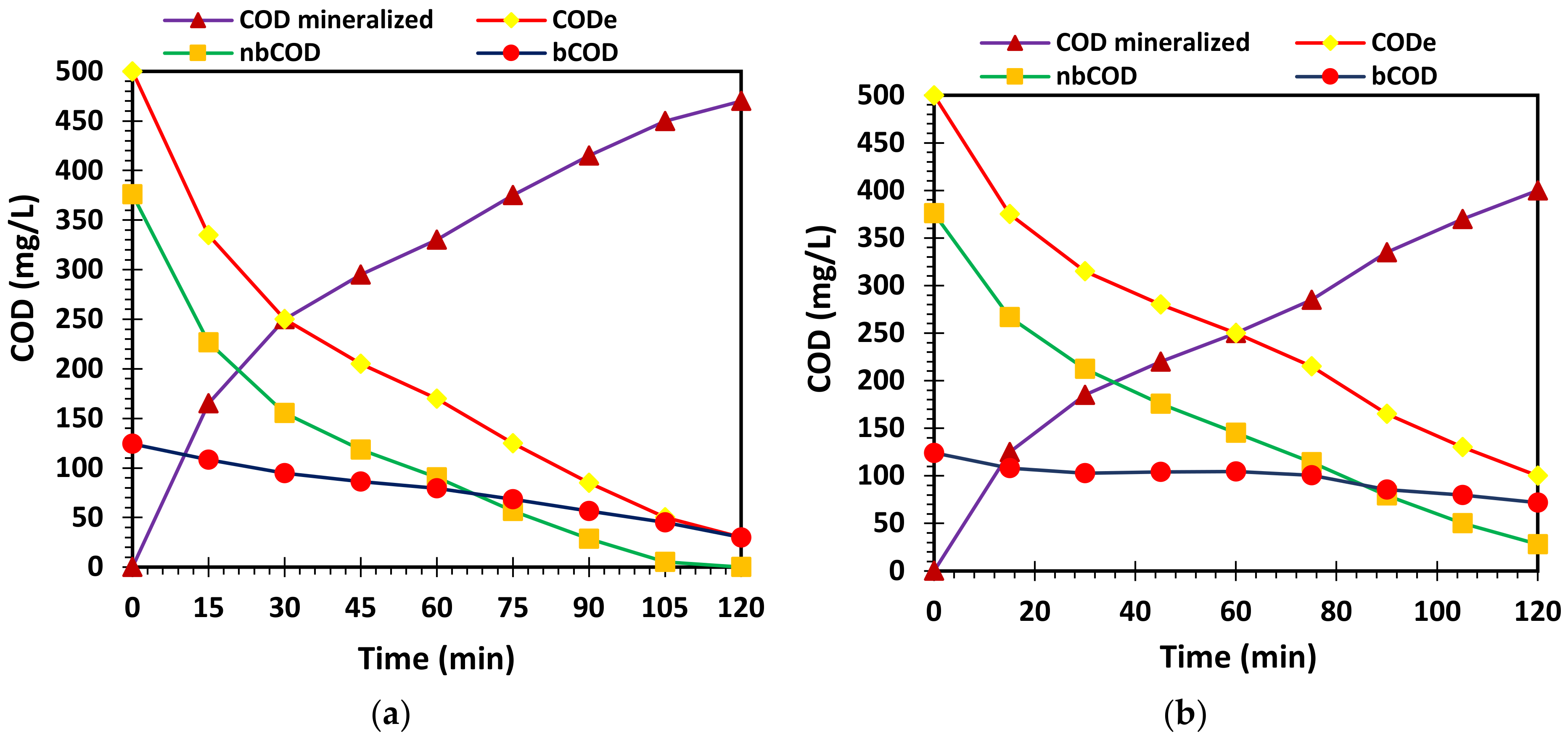
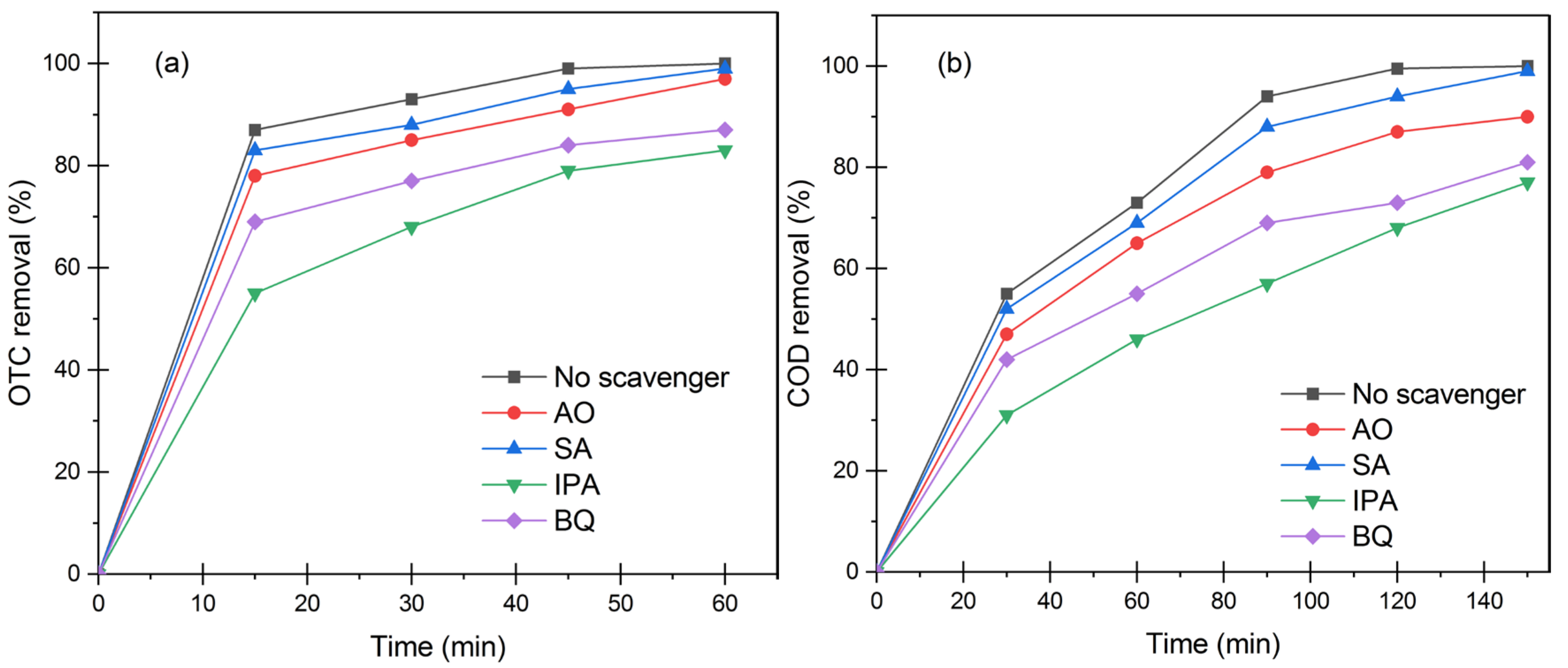
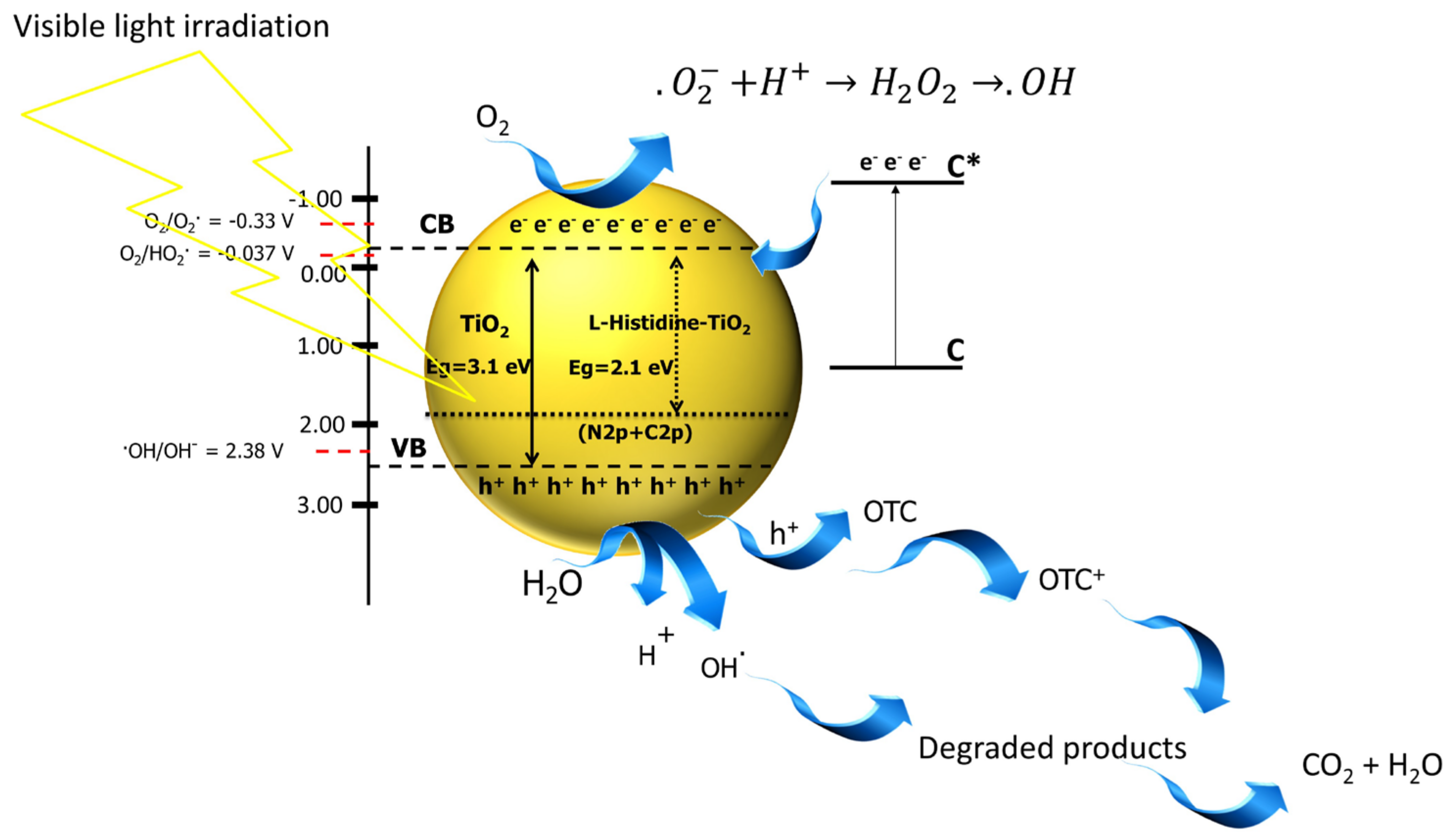
3.5. Photocatalytic Mechanism of L-Histidine (2 wt.%)-TiO2
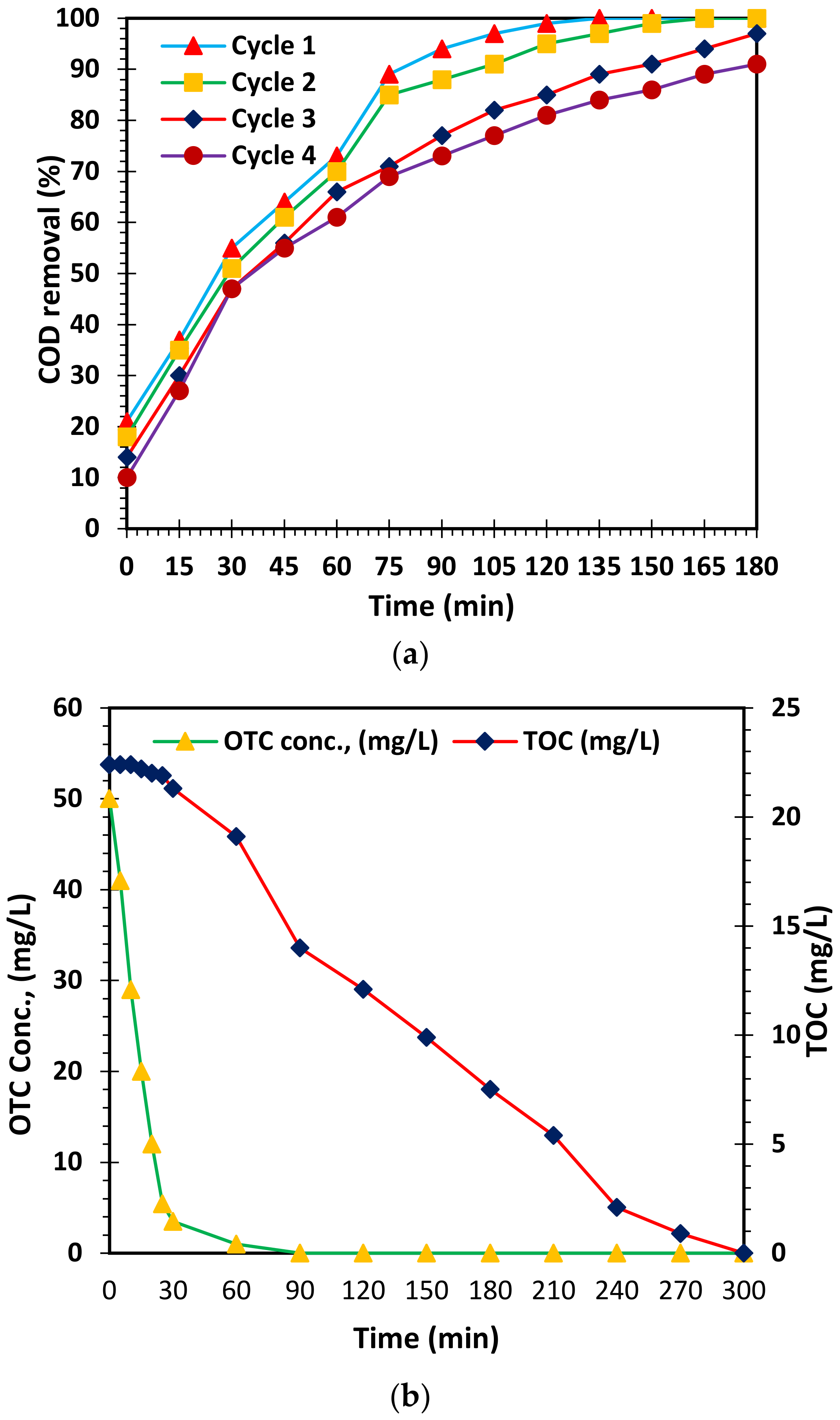
3.6. Reusability Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramanayaka, S.; Sarkar, B.; Cooray, A.T.; Ok, Y.S.; Vithanage, M. Halloysite nanoclay supported adsorptive removal of oxytetracycline antibiotic from aqueous media. J. Hazard. Mater. 2020, 384, 121301. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Chen, Z.; Wu, G.; Wu, Q.; Zhang, F.; Niu, Z.; Hu, H.-Y. Characteristics of water quality of municipal wastewater treatment plants in China: Implications for resources utilization and management. J. Clean. Prod. 2016, 131, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Zangeneh, H.; Zinatizadeh, A.A.; Zinadini, S.; Feyzi, M.; Bahnemann, D.W. Preparation ultrafine L-Methionine (C, N, S triple doped)-TiO2-ZnO nanoparticles and their photocatalytic performance for fouling alleviation in PES nanocomposite membrane. Compos. Part B Eng. 2019, 176, 107158. [Google Scholar] [CrossRef]
- Li, J.; Tian, J.-J.; Wang, L.-G.; Chang, C.-T. Photocatalytic hydrogen production and photodegradation of oxytetracycline by Cu-CdS composites. Mater. Lett. 2018, 217, 243–246. [Google Scholar] [CrossRef]
- Mahmoodi, M.; Rafiee, E.; Eavani, S. Photocatalytic removal of toxic dyes, liquorice and tetracycline wastewaters by a mesoporous photocatalyst under irradiation of different lamps and sunlight. J. Environ. Manag. 2022, 313, 115023. [Google Scholar] [CrossRef]
- Liu, C.; Mao, S.; Shi, M.; Hong, X.; Wang, D.; Wang, F.; Xia, M.; Chen, Q. Enhanced photocatalytic degradation performance of BiVO4/BiOBr through combining Fermi level alteration and oxygen defect engineering. Chem. Eng. J. 2022, 449, 137757. [Google Scholar] [CrossRef]
- Xiao, Y.; Sun, X.; Chen, J.; Zhao, S.; Jiang, C.; Yang, L.; Cheng, L.; Cao, S. Simultaneous formation of a C/N-TiO2 hollow photocatalyst with efficient photocatalytic performance and recyclability. Chin. J. Catal. 2019, 40, 765–775. [Google Scholar] [CrossRef]
- Zhao, C.; Deng, H.; Li, Y.; Liu, Z. Photodegradation of oxytetracycline in aqueous by 5A and 13X loaded with TiO2 under UV irradiation. J. Hazard. Mater. 2010, 176, 884–892. [Google Scholar] [CrossRef]
- Zhao, C.; Pelaez, M.; Duan, X.; Deng, H.; O’Shea, K.; Fatta-Kassinos, D.; Dionysiou, D.D. Role of pH on photolytic and photocatalytic degradation of antibiotic oxytetracycline in aqueous solution under visible/solar light: Kinetics and mechanism studies. Appl. Catal. B Environ. 2013, 134, 83–92. [Google Scholar] [CrossRef]
- Vaiano, V.; Sacco, O.; Sannino, D.; Ciambelli, P. Photocatalytic removal of spiramycin from wastewater under visible light with N-doped TiO2 photocatalysts. Chem. Eng. J. 2015, 261, 3–8. [Google Scholar] [CrossRef]
- Kooravand, S.; Goshadrou, A.; Hatamipour, M.S. Enhanced ethanol production from Glycyrrhiza glabra residue by fungus Mucor hiemalis. Ind. Crops Prod. 2017, 108, 767–774. [Google Scholar] [CrossRef]
- Andrio, D.; Asmura, J.; Yenie, E.; Putri, K. Enhancing BOD5/COD ratio co-substrate tofu wastewater and cow dung during ozone pretreatment. In MATEC Web of Conferences; EDP Sciences: Les Ulis, France, 2019. [Google Scholar]
- Parsaa, Z.; Motevassel, M.; Khosravi-Nikou, M.R.; Jorfi, S.; Ghasemi, B. Biodegradability enhancement of azo dye Direct Orange-26 using UV/Fenton-like process: Optimization using response surface methodology. Desalination Water Treat. 2017, 81, 233–241. [Google Scholar] [CrossRef]
- Dai, Y.; Liu, Y.; Kong, J.; Yuan, J.; Sun, C.; Xian, Q.; Yang, S.; He, H. High photocatalytic degradation efficiency of oxytetracycline hydrochloride over Ag/AgCl/BiVO4 plasmonic photocatalyst. Solid State Sci. 2019, 96, 105946. [Google Scholar] [CrossRef]
- Zangeneh, H.; Zinatizadeh, A.A.; Zinadini, S.; Rafiee, E.; Feyzi, M.; Bahnemann, D.W. A novel L-Histidine (C, N) codoped-TiO2-CdS nanocomposite for efficient visible photo-degradation of recalcitrant compounds from wastewater. J. Hazard. Mater. 2019, 369, 384–397. [Google Scholar] [CrossRef]
- Liu, C.; Mao, S.; Shi, M.; Wang, F.; Xia, M.; Chen, Q.; Ju, X. Peroxymonosulfate activation through 2D/2D Z-scheme CoAl-LDH/BiOBr photocatalyst under visible light for ciprofloxacin degradation. J. Hazard. Mater. 2021, 420, 126613. [Google Scholar]
- Azzam, E.M.; Fathy, N.A.; El-Khouly, S.M.; Sami, M. Enhancement the photocatalytic degradation of methylene blue dye using fabricated CNTs/TiO2/AgNPs/Surfactant nanocomposites. J. Water Process Eng. 2019, 28, 311–321. [Google Scholar] [CrossRef]
- Wang, J.; Fan, C.; Ren, Z.; Fu, X.; Qian, G.; Wang, Z. N-doped TiO2/C nanocomposites and N-doped TiO2 synthesised at different thermal treatment temperatures with the same hydrothermal precursor. Dalton Trans. 2014, 43, 13783–13791. [Google Scholar] [CrossRef]
- Chen, W.; Liu, T.-Y.; Huang, T.; Liu, X.-H.; Duan, G.-R.; Yang, X.-J.; Chen, S.-M. A novel yet simple strategy to fabricate visible light responsive C, N-TiO2/gC3N4 heterostructures with significantly enhanced photocatalytic hydrogen generation. RSC Adv. 2015, 5, 101214–101220. [Google Scholar] [CrossRef]
- Cheng, X.; Yu, X.; Xing, Z. Synthesis and characterization of C–N–S-tridoped TiO2 nano-crystalline photocatalyst and its photocatalytic activity for degradation of rhodamine B. J. Phys. Chem. Solids 2013, 74, 684–690. [Google Scholar] [CrossRef]
- Wang, N.; Li, X.; Yang, Y.; Zhou, Z.; Shang, Y.; Zhuang, X.; Zhang, T. Two-stage calcination composite of Bi2O3-TiO2 supported on powdered activated carbon for enhanced degradation of sulfamethazine under solar irradiation. J. Water Process Eng. 2020, 35, 101220. [Google Scholar] [CrossRef]
- Sushma, C.; Kumar, S.G. C–N–S tridoping into TiO2 matrix for photocatalytic applications: Observations, speculations and contradictions in the codoping process. Inorg. Chem. Front. 2017, 4, 1250–1267. [Google Scholar] [CrossRef]
- Wan, N.; Huang, Y.; Ho, W.; Zhang, L.; Zou, Z.; Lee, S. Oxygen vacancy-mediated efficient electron-hole separation for CNS-tridoped single crystal black TiO2 (B) nanorods as visible-light-driven photocatalysts. Appl. Surf. Sci. 2018, 457, 287–294. [Google Scholar] [CrossRef]
- Eskandari, P.; Amarloo, E.; Zangeneh, H.; Rezakazemi, M.; Zamani, M.R.; Aminabhavi, T. Photocatalytic activity of visible-light-driven L-Proline-TiO2/BiOBr nanostructured materials for dyes degradation: The role of generated reactive species. J. Environ. Manag. 2023, 326, 116691. [Google Scholar] [CrossRef] [PubMed]
- Dolatshah, M.; Zinatizadeh, A.A.; Zinadini, S.; Zangeneh, H. Preparation, characterization and performance assessment of antifouling L-Lysine (C, N codoped)-TiO2/WO3-PES photocatalytic membranes: A comparative study on the effect of blended and UV-grafted nanophotocatalyst. J. Environ. Chem. Eng. 2022, 10, 108658. [Google Scholar] [CrossRef]
- Zangeneh, H.; Zinatizadeh, A.A.; Zinadini, S.; Nazari, S.; Sibali, L.; McKay, T. Highly efficient azo dye degradation in a photocatalytic rotating disc reactor with deposited l-histidine-TiO2-CdS. Mater. Sci. Semicond. Process. 2022, 152, 107071. [Google Scholar] [CrossRef]
- Amaro-Medina, B.M.; Martinez-Luevanos, A.; Soria-Aguilar, M.D.J.; Sanchez-Castillo, M.A.; Estrada-Flores, S.; Carrillo-Pedroza, F.R. Efficiency of Adsorption and Photodegradation of Composite TiO2/Fe2O3 and Industrial Wastes in Cyanide Removal. Water 2022, 14, 3502. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Y.; Ho, W.; Zhang, L.; Zou, Z.; Lee, S. Biomolecule-controlled hydrothermal synthesis of C–N–S-tridoped TiO2 nanocrystalline photocatalysts for NO removal under simulated solar light irradiation. J. Hazard. Mater. 2009, 169, 77–87. [Google Scholar] [CrossRef]
- Xue, X.; Wang, Y.; Yang, H. Preparation and characterization of boron-doped titania nano-materials with antibacterial activity. Appl. Surf. Sci. 2013, 264, 94–99. [Google Scholar] [CrossRef]
- Yang, B.; Park, H.-D.; Won Hong, S.; Lee, S.-H.; Parke, J.-A.; Choi, J.-W. Photocatalytic degradation of microcystin-LR and anatoxin-a with presence of natural organic matter using UV-light emitting diodes/TiO2 process. J. Water Process Eng. 2020, 34, 101163. [Google Scholar] [CrossRef]
- Liu, C.-F.; Perng, T.-P. Fabrication and band structure of Ag3PO4–TiO2 heterojunction with enhanced photocatalytic hydrogen evolution. Int. J. Hydrog. Energy 2020, 45, 149–159. [Google Scholar] [CrossRef]
- Mei, P. The enhanced photodegradation of bisphenol A by TiO2/C3N4 composites. Environ. Res. 2020, 182, 109090. [Google Scholar] [CrossRef]
- Farhadian, N. Chitosan modified N, S-doped TiO2 and N, S-doped ZnO for visible light photocatalytic degradation of tetracycline. Int. J. Biol. Macromol. 2019, 132, 360–373. [Google Scholar] [CrossRef]
- Shayegan, Z.; Haghighat, F.; Lee, C.-S. Carbon-doped TiO2 film to enhance visible and UV light photocatalytic degradation of indoor environment volatile organic compounds. J. Environ. Chem. Eng. 2020, 8, 104162. [Google Scholar] [CrossRef]
- Ba-Abbad, M.M. Synthesis and catalytic activity of TiO2 nanoparticles for photochemical oxidation of concentrated chlorophenols under direct solar radiation. Int. J. Electrochem. Sci. 2012, 7, 4871–4888. [Google Scholar]
- Zangeneh, H.; Mousavi, S.A.; Eskandari, P. Comparison the visible photocatalytic activity and kinetic performance of amino acids (non-metal doped) TiO2 for degradation of colored wastewater effluent. Mater. Sci. Semicond. Process. 2022, 140, 106383. [Google Scholar] [CrossRef]
- Mousavi, S.A.; Farrokhi, F.; Kianirad, N.; Falahi, F. Degradation of aniline from aqueous solution by Fenton process: Modeling and optimization. Desalination Water Treat. 2018, 125, 68–74. [Google Scholar] [CrossRef]
- Mousavi, S.A.; Vasseghian, Y.; Bahadori, A. Evaluate the performance of Fenton process for the removal of methylene blue from aqueous solution: Experimental, neural network modeling and optimization. Environ. Prog. Sustain. Energy 2020, 39, e13126. [Google Scholar] [CrossRef]
- Mousavi, S.A.; Nazari, S. Applying Response Surface Methodology to Optimize the Fenton Oxidation Process in the Removal of Reactive Red 2. Pol. J. Environ. Stud. 2017, 26, 765–772. [Google Scholar] [CrossRef]
- Samy, M.; Ibrahim, M.G.; Gar Alalm, M.; Fujii, M.; Ookawara, S.; Ohno, T. Photocatalytic degradation of trimethoprim using S-TiO2 and Ru/WO3/ZrO2 immobilized on reusable fixed plates. J. Water Process Eng. 2020, 33, 101023. [Google Scholar] [CrossRef]
- Pereira, J.H.; Vilar, V.J.; Borges, M.T.; González, O.; Esplugas, S.; Boaventura, R.A. Photocatalytic degradation of oxytetracycline using TiO2 under natural and simulated solar radiation. Sol. Energy 2011, 85, 2732–2740. [Google Scholar] [CrossRef]
- Hassandoost, R. Hierarchically structured ternary heterojunctions based on Ce3+/Ce4+ modified Fe3O4 nanoparticles anchored onto graphene oxide sheets as magnetic visible-light-active photocatalysts for decontamination of oxytetracycline. J. Hazard. Mater. 2019, 376, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Jin, H.; Shen, A.; Deng, L.; Shi, J.; Xue, X.; Guo, Y.; Liu, Y.; Liang, X. Purification of high-purity glycyrrhizin from licorice using hydrophilic interaction solid phase extraction coupled with preparative reversed-phase liquid chromatography. J. Chromatogr. B 2017, 1040, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Surenjan, A. Synthesis, characterization and performance of visible light active C-TiO2 for pharmaceutical photodegradation. J. Environ. Chem. Eng. 2017, 5, 757–767. [Google Scholar] [CrossRef]
- Kumar, A.; Khan, M.; Zeng, X.; Lo, I. Development of g-C3N4/TiO2/Fe3O4@ SiO2 heterojunction via sol-gel route: A magnetically recyclable direct contact Z-scheme nanophotocatalyst for enhanced photocatalytic removal of ibuprofen from real sewage effluent under visible light. Chem. Eng. J. 2018, 353, 645–656. [Google Scholar] [CrossRef]
- Dugandžić, A.M. Effect of inorganic ions, photosensitisers and scavengers on the photocatalytic degradation of nicosulfuron. J. Photochem. Photobiol. A Chem. 2017, 336, 146–155. [Google Scholar] [CrossRef]
- Huangch, S.; Chen, C.; Tsai, H.; Shaya, J.; Lu, C. Photocatalytic degradation of thiobencarb by a visible light-driven MoS2 photocatalyst. Sep. Purif. Technol. 2018, 197, 147–155. [Google Scholar] [CrossRef]
- Liu, C.; Mao, S.; Wang, H. Peroxymonosulfate-assisted for facilitating photocatalytic degradation performance of 2D/2D WO3/BiOBr S-scheme heterojunction. Chem. Eng. J. 2022, 430, 132806. [Google Scholar] [CrossRef]
- Fakhravar, S.; Farhadian, M.; Tangestaninejad, S. Excellent performance of a novel dual Z-scheme Cu2S/Ag2S/BiVO4 heterostructure in metronidazole degradation in batch and continuous systems: Immobilization of catalytic particles on α-Al2O3 fiber. Appl. Surf. Sci. 2020, 505, 144599. [Google Scholar] [CrossRef]
- Li, W.; Xie, L.; Zhou, L.; Ochoa-Lozano, J.; Li, C.; Chai, X. A systemic study on Gd, Fe and N co-doped TiO2 nanomaterials for enhanced photocatalytic activity under visible light irradiation. Ceram. Int. 2020, 46, 24744–24752. [Google Scholar] [CrossRef]
- Chen, D.; Jiang, Z.; Geng, J.; Wang, Q.; Yang, D. Carbon and nitrogen co-doped TiO2 with enhanced visible-light photocatalytic activity. Ind. Eng. Chem. Res. 2007, 46, 2741–2746. [Google Scholar] [CrossRef]





| Wastewater Properties | Initial Conditions before Treatment |
|---|---|
| Color | Brown |
| COD (mg/L) | 700–800 |
| BOD5/COD ratio (mg/L) | 0.17–0.19 |
| pH | 5.7–6.4 |
| TSS (mg/L) | 120–250 |
| Factors | Levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| OTC | |||
| A: [OTC] (mg/L) | 50 | 75 | 100 |
| B: [Catalyst] (g/L) | 0.5 | 1 | 1.5 |
| C: pH | 4 | 7 | 10 |
| D: Irradiation time (min) | 15 | 30 | 45 |
| E: Light Intensity (W/cm2) | 15 | 20 | 25 |
| LEPW | |||
| A: [COD] (mg/L) | 300 | 500 | 700 |
| B: [Catalyst] (g/L) | 1 | 1.5 | 2 |
| C: pH | 4 | 7 | 10 |
| D: Irradiation time (min) | 60 | 120 | 180 |
| E: Light Intensity (W/cm2) | 15 | 20 | 25 |
| Pseudo First Order Model | K1 | R2 |
|---|---|---|
| L-Histidine-TiO2 | 0.0251 | 0.977 |
| L-Methionine-TiO2 | 0.0149 | 0.983 |
| L-Asparagine-TiO2 | 0.0111 | 0.977 |
| Pseudo Second Order Model | k2 | R2 |
| L-Histidine-TiO2 | 0.0108 | 0.824 |
| L-Methionine-TiO2 | 0.0082 | 0.902 |
| L-Asparagine-TiO2 | 0.0080 | 0.947 |
| Source | Sum of Squares | df | F-Value | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| LEPW | OTC | LEPW | OTC | LEPW | OTC | LEPW | OTC | |
| Model | 12,391.4 | 27,990.10 | 8 | 8 | 49.06 | 129.72 | <0.0001 | <0.0001 |
| A: [dye] | 3790.6 | 2647.06 | 1 | 1 | 120.05 | 98.14 | <0.0001 | <0.0001 |
| B: [catalyst] | 1882.6 | 995.76 | 1 | 1 | 59.62 | 36.92 | <0.0001 | <0.0001 |
| C: pH | 1794.4 | 6250.62 | 1 | 1 | 56.83 | 231.75 | <0.0001 | <0.0001 |
| D: Irradiation time | 1284.7 | 984.97 | 1 | 1 | 40.96 | 36.52 | <0.0001 | <0.0001 |
| E: light Intensity | 2542.2 | 3790.62 | 1 | 1 | 80.52 | 140.54 | <0.0001 | <0.0001 |
| CE | - | 399.03 | - | 1 | - | 14.79 | - | 0.0004 |
| A2 | 181.8 | - | 1 | - | 5.76 | - | 0.0210 | - |
| B2 | - | 178.29 | - | 1 | - | 6.61 | - | 0.0139 |
| C2 | 189.2 | 2951.64 | 1 | - | 5.99 | 109.44 | 0.0187 | <0.0001 |
| D2 | 230.7 | - | 1 | 1 | 7.31 | 9.99 | 0.0100 | - |
| Residual | 1294.5 | 1105.82 | 41 | 42 | ||||
| Lack of Fit | 1127 | 876.94 | 34 | 35 | 1.39 | 0.79 | 0.3457 | 0.7047 |
| Pure Error | 167.5 | 228.88 | 7 | 7 | ||||
| LEPW: R2 = 0.91, adjusted R2 = 0.9, Std. Dev = 5.62, Adeq precision = 33.06, C.V.% = 8.92, Lack of fit = 0.34 | ||||||||
| OTC: R2 = 0.96, adjusted R2 = 0.95, Std. Dev = 5.19, Adeq precision = 39.67, C.V.% = 10.38, Lack of fit = 0.7 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zangeneh, H.; Mousavi, S.A.; Eskandari, P.; Amarloo, E.; Farghelitiyan, J.; Zamani, M.R. Comparative Study on Enhanced Photocatalytic Activity of Visible Light-Active Nanostructures for Degradation of Oxytetracycline and COD Removal of Licorice Extraction Plant Wastewater. Water 2023, 15, 290. https://doi.org/10.3390/w15020290
Zangeneh H, Mousavi SA, Eskandari P, Amarloo E, Farghelitiyan J, Zamani MR. Comparative Study on Enhanced Photocatalytic Activity of Visible Light-Active Nanostructures for Degradation of Oxytetracycline and COD Removal of Licorice Extraction Plant Wastewater. Water. 2023; 15(2):290. https://doi.org/10.3390/w15020290
Chicago/Turabian StyleZangeneh, Hadis, Seyyed Alireza Mousavi, Parisa Eskandari, Ehsan Amarloo, Javad Farghelitiyan, and Mohammad Reza Zamani. 2023. "Comparative Study on Enhanced Photocatalytic Activity of Visible Light-Active Nanostructures for Degradation of Oxytetracycline and COD Removal of Licorice Extraction Plant Wastewater" Water 15, no. 2: 290. https://doi.org/10.3390/w15020290





