Microplastics in Landfill Leachate: A Comprehensive Review on Characteristics, Detection, and Their Fates during Advanced Oxidation Processes
Abstract
:1. Introduction
2. The Detection of Microplastics in Leachate
2.1. Microplastic Sampling
2.2. Separation of Microplastics from Leachate
2.2.1. Sieving and Filtration
2.2.2. Purification
2.3. Identification and Characterization
2.3.1. SEM
2.3.2. FT-IR
| Identification Method | Findings | Microplastic Size | References |
|---|---|---|---|
| SEM | Observe the surface morphology and texture characteristics of plastic particles | 1 μm~1 mm | [19,23,43] |
| EDS | Qualitative and quantitative analysis of different elements in plastic products | <1 mm | [24,48,50] |
| FE-SEM | Reduced sample handling processing allows high-quality microplastic images to be obtained at low voltages | <100 nm | [19,51] |
| FTIR | Used to identify microplastic types | Smaller particles down to 20 μm | [19,45] |
| ATR-FTIR | Isolate the sample from the water to characterize it. Suitable for the analysis of large microplastic products | Microplastics less than 50 μm | [19,52] |
| Micro-FTIR | Suitable for small particle size microplastics | Microplastics less than 10 μm | [19] |
| FPA-FTIR | Microplastic information can be obtained quickly in a short period, making it suitable for detecting smaller particles | Microplastics less than 10 μm | [19,45] |
| Raman | Provides structural information about polymers. | This method can detect microplastics with a size down to 1 μm | [23,45] |
| Pyrolysis-GC–MS | Identify the chemical properties of microplastics | The shape, size, and color of the microplastics do not affect this method | [45] |
2.3.3. Raman
2.3.4. Pyrolysis-GC–MS
2.4. Quality Control
2.4.1. Contamination Control during Sampling
2.4.2. Quality Control in the Separation of Microplastic
3. Microplastic Characteristics in Leachate
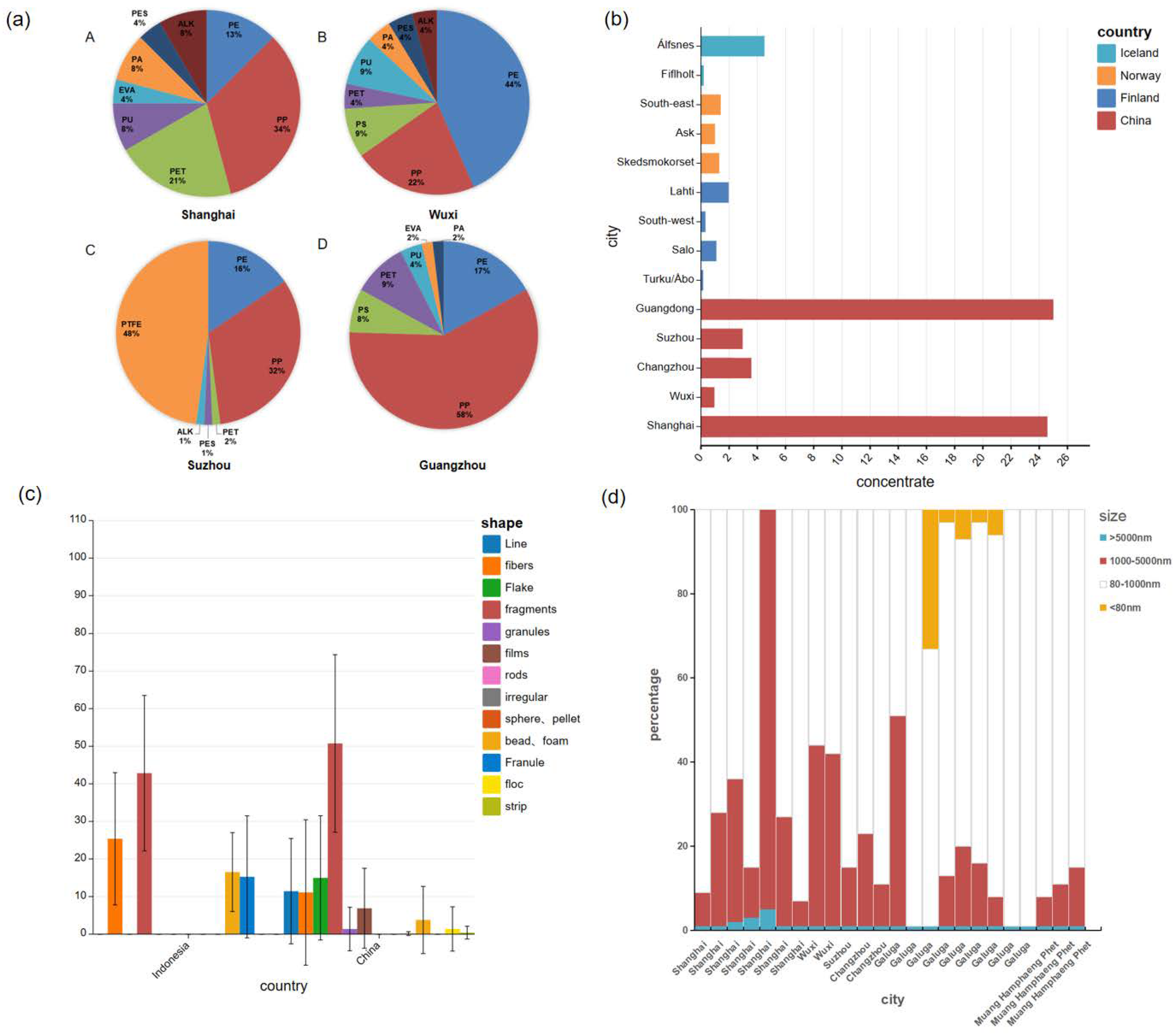
3.1. Microplastics Type
3.2. Microplastics Concentration
3.3. Microplastics Shape
3.4. Microplastics Size
4. Removal of Microplastics by Advanced Oxidation Techniques
4.1. Photodegradation
4.1.1. Photochemical Oxidation
4.1.2. Photocatalytic Oxidation
4.2. Fenton/Fenton-like Systems
4.3. Ozonation
4.4. Activates Persulfates
5. Conclusions and Future Perspectives
- (1)
- Microplastics in landfill leachate were only detected in several countries; more studies worldwide should be investigated. The relationship between microplastics in leachate and plastics waste can be built based on more data, which can predict the microplastic emission from landfill leachate.
- (2)
- Standardization of leachate sampling, quality control, and characterization of microplastics in leachate, especially for smaller micro- or nanoplastics. The methods in other environmental media may not be suitable for leachate with a complex composition.
- (3)
- Due to the small particle size and pores of microplastics, they inevitably become carriers of other pollutants in the leachate, which increases the difficulty of removing other contaminants. The adsorption of microplastics to other pollutants in the leachate has not been well studied, which is critical for the subsequent degradation of microplastics in leachate.
- (4)
- At present, membrane technology is widely used in leachate treatment. However, its microplastics will only be intercepted and cannot be degraded. At the same time, the microplastics in it will also cause membrane blockage. How to effectively remove it from the leachate is still an important issue.
- (5)
- Advanced oxidation technology is a promising method for treating microplastics in leachate in the future, but there is limited relevant research on the degradation process of microplastics in leachate.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lebreton, L.C.M.; Van Der Zwet, J.; Damsteeg, J.W.; Slat, B.; Andrady, A.; Reisser, J. River plastic emissions to the world’s oceans. Nat. Commun. 2017, 8, 15611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vijayakumar, V. Personal Protection Prior to Preoperative Assessment—Little more an anaesthesiologist can do to prevent SARS-CoV-2 transmission and COVID-19 infection. Ain-Shams J. Anesthesiol. 2020, 121, 1–2. [Google Scholar] [CrossRef]
- Shen, M.; Zeng, Z.; Song, B.; Yi, H.; Hu, T.; Zhang, Y.; Zeng, G.; Xiao, R. Neglected microplastics pollution in global COVID-19: Disposable surgical masks. Sci. Total Environ. 2021, 790, 148130. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Kumar, D.; Yoo, C.G.; Gitsov, I.; Majumder, E.L.W. Conversion and removal strategies for microplastics in wastewater treatment plants and landfills. Chem. Eng. J. 2021, 406, 126715. [Google Scholar] [CrossRef]
- Law, K.L. The United States’ contribution of plastic waste to land and ocean. Sci. Adv. 2020, 6, eabd0288. [Google Scholar] [CrossRef]
- Luan, X.; Cui, X.; Zhang, L.; Chen, X.; Li, X.; Feng, X.; Chen, L.; Liu, W.; Cui, Z. Dynamic material flow analysis of plastics in China from 1950 to 2050. J. Clean. Prod. 2021, 327, 129492. [Google Scholar] [CrossRef]
- Ozbay, G.; Jones, M.; Gadde, M.; Isah, S.; Attarwala, T. Design and Operation of Effective Landfills with Minimal Effects on the Environment and Human Health. J. Environ. Public Health 2021, 6921607. [Google Scholar] [CrossRef]
- Qin, F.; Du, J.; Gao, J.; Liu, G.; Song, Y.; Yang, A.; Wang, H.; Ding, Y.; Wang, Q. Bibliometric Profile of Global Microplastics Research from 2004 to 2019. Int. J. Environ. Res. Public Health 2020, 17, 5639. [Google Scholar] [CrossRef]
- Tirkey, A.; Upadhyay, L.S.B. Microplastics: An overview on separation, identification and characterization of microplastics. Mar. Pollut. Bull. 2021, 170, 112604. [Google Scholar] [CrossRef]
- Nguyen, B.; Claveau-Mallet, D.; Hernandez, L.M.; Xu, E.G.; Farner, J.M.; Tufenkji, N. Separation and Analysis of Microplastics and Nanoplastics in Complex Environmental Samples. Acc. Chem. Res. 2019, 52, 858–866. [Google Scholar] [CrossRef]
- Sun, C.; Ding, J.; Gao, F. Methods for microplastic sampling and analysis in the seawater and fresh water environment. Methods Enzym. 2021, 648, 27–45. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.; Paul Chen, J. Microplastics in freshwater systems: A review on occurrence, environmental effects, and methods for microplastics detection. Water Res. 2018, 137, 362–374. [Google Scholar] [CrossRef]
- Simongini, C.; Pucetaite, M.; Serranti, S.; Van Praagh, M.; Hammer, E.; Bonifazi, G. Microplastics identification in landfill leachates by different spectroscopic techniques. Detritus 2022, 202218, 58–69. [Google Scholar] [CrossRef]
- He, P.; Chen, L.; Shao, L.; Zhang, H.; Lu, F. Municipal solid waste (MSW) landfill: A source of microplastics? -Evidence of microplastics in landfill leachate. Water Res. 2019, 159, 38–45. [Google Scholar] [CrossRef]
- Xu, Z.; Sui, Q.; Li, A.; Sun, M.; Zhang, L.; Lyu, S.; Zhao, W. How to detect small microplastics (20–100 mum) in freshwater, municipal wastewaters and landfill leachates? A trial from sampling to identification. Sci. Total Environ. 2020, 733, 139218. [Google Scholar] [CrossRef]
- Narevski, A.C.; Novakovic, M.I.; Petrovic, M.Z.; Mihajlovic, I.J.; Maodus, N.B.; Vujic, G.V. Occurrence of bisphenol A and microplastics in landfill leachate: Lessons from South East Europe. Environ. Sci. Pollut. Res. Int. 2021, 28, 42196–42203. [Google Scholar] [CrossRef]
- Kabir, M.S.; Zhao, R.; Zhang, L.; Wang, H.; Luster-Teasley, S. Sources, Occurrence, and Removal of Microplastic/Nanoplastic in landfill leachate: A Comprehensive Review. ChemRxiv 2022. in preprint. [Google Scholar] [CrossRef]
- Wan, Y.; Chen, X.; Liu, Q.; Hu, H.; Wu, C.; Xue, Q. Informal landfill contributes to the pollution of microplastics in the surrounding environment. Environ. Pollut. 2022, 293, 118586. [Google Scholar] [CrossRef]
- Mariano, S.; Tacconi, S.; Fidaleo, M.; Rossi, M.; Dini, L. Micro and Nanoplastics Identification: Classic Methods and Innovative Detection Techniques. Front Toxicol. 2021, 3, 636640. [Google Scholar] [CrossRef]
- Praagh, M.V.; Hartman, C.; Brandmyr, E. Microplastics in Landfill Leachates in the Nordic Countries; Digitala Vetenskapliga Arkivet: Copenhagen, Denmark, 2019; p. 2018557. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry; John Wiley & Sons: Chichester, UK, 2006; Volume 12, pp. 10–11. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J. Investigation of microplastics in aquatic environments: An overview of the methods used, from field sampling to laboratory analysis. TrAC Trends Anal. Chem. 2018, 108, 195–202. [Google Scholar] [CrossRef]
- Adhikari, S.; Kelkar, V.; Kumar, R.; Halden, R.U. Methods and challenges in the detection of microplastics and nanoplastics: A mini-review. Polym. Int. 2022, 71, 543–551. [Google Scholar] [CrossRef]
- Fries, E.; Dekiff, J.H.; Willmeyer, J.; Nuelle, M.T.; Ebert, M.; Remy, D. Identification of polymer types and additives in marine microplastic particles using pyrolysis-GC/MS and scanning electron microscopy. Environ. Sci. Process. Impacts 2013, 15, 1949–1956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iyare, P.U.; Ouki, S.K.; Bond, T. Microplastics removal in wastewater treatment plants: A critical review. Environ. Sci. Water Res. Technol. 2020, 6, 2664–2675. [Google Scholar] [CrossRef]
- Ma, B.; Xue, W.; Hu, C.; Liu, H.; Qu, J.; Li, L. Characteristics of microplastic removal via coagulation and ultrafiltration during drinking water treatment. Chem. Eng. J. 2019, 359, 159–167. [Google Scholar] [CrossRef]
- Guo, Y.; Liang, H.; Bai, L.; Huang, K.; Xie, B.; Xu, D.; Wang, J.; Li, G.; Tang, X. Application of heat-activated peroxydisulfate pre-oxidation for degrading contaminants and mitigating ultrafiltration membrane fouling in the natural surface water treatment. Water Res. 2020, 179, 115905. [Google Scholar] [CrossRef]
- Shen, M.; Xiong, W.; Song, B.; Zhou, C.; Almatrafi, E.; Zeng, G.; Zhang, Y. Microplastics in landfill and leachate: Occurrence, environmental behavior and removal strategies. Chemosphere 2022, 305, 135325. [Google Scholar] [CrossRef]
- Sun, J.; Dai, X.; Wang, Q.; Van Loosdrecht, M.C.M.; Ni, B.J. Microplastics in wastewater treatment plants: Detection, occurrence and removal. Water Res. 2019, 152, 21–37. [Google Scholar] [CrossRef]
- Cerne, O.; Junestedt, C. Landfill leachate sampling techniques. Linnaeus Eco-Tech 2019, 34, 279–281. [Google Scholar] [CrossRef]
- Stock, F.; Kochleus, C.; Bänsch-Baltruschat, B.; Brennholt, N.; Reifferscheid, G. Sampling techniques and preparation methods for microplastic analyses in the aquatic environment—A review. TrAC Trends Anal. Chem. 2019, 113, 84–92. [Google Scholar] [CrossRef]
- Lv, L.; Yan, X.; Feng, L.; Jiang, S.; Lu, Z.; Xie, H.; Sun, S.; Chen, J.; Li, C. Challenge for the detection of microplastics in the environment. Water Environ. Res. 2021, 93, 5–15. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, Z.; Wu, D.; Zhan, L.; Shi, H.; Xie, B. Occurrence of microplastics in landfill systems and their fate with landfill age. Water Res. 2019, 164, 114968. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, Y.; Peng, C.; Wang, P.; Lu, Y.; He, X.; Wang, L. Comparison of Detection Methods of Microplastics in Landfill Mineralized Refuse and Selection of Degradation Degree Indexes. Env. Sci. Technol. 2021, 55, 13802–13811. [Google Scholar] [CrossRef]
- Kroon, F.; Motti, C.; Talbot, S.; Sobral, P.; Puotinen, M. A workflow for improving estimates of microplastic contamination in marine waters: A case study from North-Western Australia. Environ. Pollut. 2018, 238, 26–38. [Google Scholar] [CrossRef]
- Crichton, E.M.; Noël, M.; Gies, E.A.; Ross, P.S. A novel, density-independent and FTIR-compatible approach for the rapid extraction of microplastics from aquatic sediments. Anal. Methods 2017, 9, 1419–1428. [Google Scholar] [CrossRef]
- Lares, M.; Ncibi, M.C.; Sillanpaa, M.; Sillanpaa, M. Occurrence, identification and removal of microplastic particles and fibers in conventional activated sludge process and advanced MBR technology. Water Res. 2018, 133, 236–246. [Google Scholar] [CrossRef]
- Zhang, Z.; Su, Y.; Zhu, J.; Shi, J.; Huang, H.; Xie, B. Distribution and removal characteristics of microplastics in different processes of the leachate treatment system. Waste Manag. 2021, 120, 240–247. [Google Scholar] [CrossRef]
- Araujo, C.F.; Nolasco, M.M.; Ribeiro, A.M.P.; Ribeiro-Claro, P.J.A. Identification of microplastics using Raman spectroscopy: Latest developments and future prospects. Water Res. 2018, 142, 426–440. [Google Scholar] [CrossRef]
- Lambert, S.; Wagner, M. Formation of microscopic particles during the degradation of different polymers. Chemosphere 2016, 161, 510–517. [Google Scholar] [CrossRef]
- Maes, T.; Jessop, R.; Wellner, N.; Haupt, K.; Mayes, A.G. A rapid-screening approach to detect and quantify microplastics based on fluorescent tagging with Nile Red. Sci. Rep. 2017, 7, 44501. [Google Scholar] [CrossRef] [Green Version]
- Tagg, A.S.; Sapp, M.; Harrison, J.P.; Ojeda, J.J. Identification and Quantification of Microplastics in Wastewater Using Focal Plane Array-Based Reflectance Micro-FT-IR Imaging. Anal. Chem. 2015, 87, 6032–6040. [Google Scholar] [CrossRef] [Green Version]
- Cao, B.; Wan, S.; Wang, Y.; Guo, H.; Ou, M.; Zhong, Q. Highly-efficient visible-light-driven photocatalytic H2 evolution integrated with microplastic degradation over MXene/ZnxCd1-xS photocatalyst. J. Colloid Interface Sci. 2022, 605, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Huppertsberg, S.; Knepper, T.P. Instrumental analysis of microplastics-benefits and challenges. Anal. Bioanal. Chem. 2018, 410, 6343–6352. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-L.; Thomas, K.V.; Luo, Z.; Gowen, A.A. FTIR and Raman imaging for microplastics analysis: State of the art, challenges and prospects. TrAC Trends Anal. Chem. 2019, 119, 115629. [Google Scholar] [CrossRef]
- Fu, W.; Min, J.; Jiang, W.; Li, Y.; Zhang, W. Separation, characterization and identification of microplastics and nanoplastics in the environment. Sci. Total Environ. 2020, 721, 137561. [Google Scholar] [CrossRef] [PubMed]
- Bogner, A.; Jouneau, P.H.; Thollet, G.; Basset, D.; Gauthier, C. A history of scanning electron microscopy developments: Towards “wet-STEM” imaging. Micron 2007, 38, 390–401. [Google Scholar] [CrossRef]
- Deng, L.; Cai, L.; Sun, F.; Li, G.; Che, Y. Public attitudes towards microplastics: Perceptions, behaviors and policy implications. Resour. Conserv. Recycl. 2020, 163, 105096. [Google Scholar] [CrossRef]
- Sun, F.; Meade, E.D.; O’Dowd, N.P. Strain gradient crystal plasticity modelling of size effects in a hierarchical martensitic steel using the Voronoi tessellation method. Int. J. Plast. 2019, 119, 215–229. [Google Scholar] [CrossRef]
- Yuan, Z.; Nag, R.; Cummins, E. Human health concerns regarding microplastics in the aquatic environment—From marine to food systems. Sci. Total Env. 2022, 823, 153730. [Google Scholar] [CrossRef]
- Enyoh, C.E.; Wang, Q.; Chowdhury, T.; Wang, W.; Lu, S.; Xiao, K.; Chowdhury, M.A.H. New Analytical Approaches for Effective Quantification and Identification of Nanoplastics in Environmental Samples. Processes 2021, 911, 2086. [Google Scholar] [CrossRef]
- Kappler, A.; Fischer, M.; Scholz-Bottcher, B.M.; Oberbeckmann, S.; Labrenz, M.; Fischer, D.; Eichhorn, K.J.; Voit, B. Comparison of mu-ATR-FTIR spectroscopy and py-GCMS as identification tools for microplastic particles and fibers isolated from river sediments. Anal. Bioanal. Chem. 2018, 410, 5313–5327. [Google Scholar] [CrossRef]
- Scopetani, C.; Esterhuizen-Londt, M.; Chelazzi, D.; Cincinelli, A.; Setala, H.; Pflugmacher, S. Self-contamination from clothing in microplastics research. Ecotoxicol. Environ. Saf 2020, 189, 110036. [Google Scholar] [CrossRef]
- Centre, E.C.J.R. Guidance on Monitoring of Marine Litter in European Seas. MSFD Tech. Subgr. Mar. Litter 2013, 2749, 25009. [Google Scholar] [CrossRef]
- Pagter, E.; Frias, J.; Nash, R. Microplastics in Galway Bay: A comparison of sampling and separation methods. Mar. Pollut. Bull. 2018, 135, 932–940. [Google Scholar] [CrossRef]
- Gerdts, G. Methodology Used for the Detection and Identification of Microplastics—A Critical Appraisal. Mar. Anthropog. Litter 2015, 201–227. [Google Scholar] [CrossRef] [Green Version]
- Yuan, C.; Almuhtaram, H.; McKie, M.J.; Andrews, R.C. Assessment of microplastic sampling and extraction methods for drinking waters. Chemosphere 2022, 286, 131881. [Google Scholar] [CrossRef]
- Tanaka, M.; Kataoka, T.; Nihei, Y. Variance and precision of microplastic sampling in urban rivers. Environ. Pollut. 2022, 310, 119811. [Google Scholar] [CrossRef]
- Crawford, C.B.; Quinn, B. Microplastic Separation Techniques. In Microplastic Pollutants; Elsevier: Amsterdam, The Netherlands, 2017; pp. 203–218. [Google Scholar]
- Prata, J.C.; Da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T. Methods for sampling and detection of microplastics in water and sediment: A critical review. TrAC Trends Anal. Chem. 2019, 110, 150–159. [Google Scholar] [CrossRef]
- Claessens, M.; De Meester, S.; Van Landuyt, L.; De Clerck, K.; Janssen, C.R. Occurrence and distribution of microplastics in marine sediments along the Belgian coast. Mar. Pollut. Bull. 2011, 62, 2199–2204. [Google Scholar] [CrossRef]
- Claessens, M.; Van Cauwenberghe, L.; Vandegehuchte, M.B.; Janssen, C.R. New techniques for the detection of microplastics in sediments and field collected organisms. Mar. Pollut. Bull. 2013, 70, 227–233. [Google Scholar] [CrossRef]
- Imhof, H.K.; Ivleva, N.P.; Schmid, J.; Niessner, R.; Laforsch, C. Contamination of beach sediments of a subalpine lake with microplastic particles. Curr. Biol. 2013, 23, R867–R868. [Google Scholar] [CrossRef] [Green Version]
- Campanale, C.; Savino, I.; Pojar, I.; Massarelli, C.; Uricchio, V.F. A Practical Overview of Methodologies for Sampling and Analysis of Microplastics in Riverine Environments. Sustainability 2020, 12, 6755. [Google Scholar] [CrossRef]
- Doyle, M.J.W.; Watson, N.M.; Bowlin, S.B. Occurrence of Plastic Micro-Debris in the Southern California Current System; Center for Marine Biodiversity and Conservation: San Diego, CA, USA, 2009; Volume 50, p. 20095. Available online: http://escholarship.org/uc/item/34w4g0s0 (accessed on 13 November 2022).
- Sun, J.; Zhu, Z.R.; Li, W.H.; Yan, X.; Wang, L.K.; Zhang, L.; Jin, J.; Dai, X.; Ni, B.J. Revisiting Microplastics in Landfill Leachate: Unnoticed Tiny Microplastics and Their Fate in Treatment Works. Water Res. 2021, 190, 116784. [Google Scholar] [CrossRef] [PubMed]
- Cordova, M.R.; Riani, E. Micro-and mesoplastics release from the Indonesian municipal solid waste landfill leachate to the aquatic environment: Case study in Galuga Landfill Area, Indonesia. Mar. Pollut. Bull. 2021, 163, 111986. [Google Scholar] [CrossRef]
- Bilgin, M.; Yurtsever, M.; Karadagli, F. Microplastic removal by aerated grit chambers versus settling tanks of a municipal wastewater treatment plant. J. Water Process Eng. 2020, 38, 101604. [Google Scholar] [CrossRef]
- Antunes, J.; Frias, J.; Sobral, P. Microplastics on the Portuguese coast. Mar. Pollut. Bull. 2018, 131, 294–302. [Google Scholar] [CrossRef]
- Li, P.; Wang, X.; Su, M.; Zou, X.; Duan, L.; Zhang, H. Characteristics of Plastic Pollution in the Environment: A Review. Bull. Environ. Contam. Toxicol. 2021, 107, 577–584. [Google Scholar] [CrossRef]
- Westerhoff, P.; Prapaipong, P.; Shock, E.; Hillaireau, A. Antimony leaching from polyethylene terephthalate (PET) plastic used for bottled drinking water. Water Res. 2008, 42, 551–556. [Google Scholar] [CrossRef]
- Sogancioglu, M.; Yel, E.; Ahmetli, G. Pyrolysis of waste high density polyethylene (HDPE) and low density polyethylene (LDPE) plastics and production of epoxy composites with their pyrolysis chars. J. Clean. Prod. 2017, 165, 369–381. [Google Scholar] [CrossRef]
- Yang, S.; Zhou, M.; Chen, X.; Hu, L.; Xu, Y.; Fu, W.; Li, C. A comparative review of microplastics in lake systems from different countries and regions. Chemosphere 2022, 286, 131806. [Google Scholar] [CrossRef]
- Haque, F.; Fan, C. Prospect of microplastic pollution control under the “New normal” concept beyond COVID-19 pandemic. J. Clean. Prod. 2022, 367, 133027. [Google Scholar] [CrossRef]
- Priya, A.K.; Jalil, A.A.; Dutta, K.; Rajendran, S.; Vasseghian, Y.; Qin, J.; Soto-Moscoso, M. Microplastics in the environment: Recent developments in characteristic, occurrence, identification and ecological risk. Chemosphere 2022, 298, 134161. [Google Scholar] [CrossRef]
- Rezania, S.; Park, J.; Md Din, M.F.; Mat Taib, S.; Talaiekhozani, A.; Kumar Yadav, K.; Kamyab, H. Microplastics pollution in different aquatic environments and biota: A review of recent studies. Mar. Pollut. Bull. 2018, 133, 191–208. [Google Scholar] [CrossRef]
- Doyle, M.J.; Watson, W.; Bowlin, N.M.; Sheavly, S.B. Plastic particles in coastal pelagic ecosystems of the Northeast Pacific ocean. Mar. Environ. Res. 2011, 71, 41–52. [Google Scholar] [CrossRef]
- Azizi, N.; Nasseri, S.; Nodehi, R.N.; Jaafarzadeh, N.; Pirsaheb, M. Evaluation of conventional wastewater treatment plants efficiency to remove microplastics in terms of abundance, size, shape, and type: A systematic review and Meta-analysis. Mar. Pollut. Bull. 2022, 177, 113462. [Google Scholar] [CrossRef]
- Hamidian, A.H.; Ozumchelouei, E.J.; Feizi, F.; Wu, C.; Zhang, Y.; Yang, M. A review on the characteristics of microplastics in wastewater treatment plants: A source for toxic chemicals. J. Clean. Prod. 2021, 295, 126480. [Google Scholar] [CrossRef]
- Napper, I.E.; Thompson, R.C. Release of synthetic microplastic plastic fibres from domestic washing machines: Effects of fabric type and washing conditions. Mar. Pollut. Bull. 2016, 112, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Al-Salem, S.M.; Lettieri, P.; Baeyens, J. Recycling and recovery routes of plastic solid waste (PSW): A review. Waste Manag. 2009, 29, 2625–2643. [Google Scholar] [CrossRef]
- Geyer, R. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3. [Google Scholar] [CrossRef]
- Browne, M.A. Spatial Patterns of Plastic Debris along Estuarine Shorelines. Environ. Sci. Technol. 2010, 44, 3404–3409. [Google Scholar] [CrossRef]
- Upadhyay, K.; Bajpai, S. Microplastics in Landfills: A Comprehensive Review on Occurrence, Characteristics and Pathways to the Aquatic Environment. Nat. Environ. Pollut. Technol. 2021, 20, 1–40. [Google Scholar] [CrossRef]
- Alavian Petroody, S.S.; Hashemi, S.H.; Van Gestel, C.A.M. Factors affecting microplastic retention and emission by a wastewater treatment plant on the southern coast of Caspian Sea. Chemosphere 2020, 261, 128179. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Xie, Y.; Wang, J. Microplastic degradation methods and corresponding degradation mechanism: Research status and future perspectives. J. Hazard. Mater. 2021, 418, 126377. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Jin, T.; Zou, T.; Xu, L.; Xi, B.; Xu, D.; He, J.; Xiong, L.; Tang, C.; Peng, J. Current progress on plastic/microplastic degradation: Fact influences and mechanism. Environ. Pollut. 2022, 304, 119159. [Google Scholar] [CrossRef] [PubMed]
- Bacha, A.-U.-R.; Nabi, I.; Zhang, L. Mechanisms and the Engineering Approaches for the Degradation of Microplastics. ACS EST Eng. 2021, 1, 1481–1501. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, X.; Chen, R.; Liu, P.; Liang, W.; Wang, J.; Teng, M.; Wang, X.; Gao, S. Wastewater treatment plants act as essential sources of microplastic formation in aquatic environments: A critical review. Water Res. 2022, 221, 118825. [Google Scholar] [CrossRef]
- Wang, C.; Xian, Z.; Jin, X.; Liang, S.; Chen, Z.; Pan, B.; Wu, B.; Ok, Y.S.; Gu, C. Photo-aging of polyvinyl chloride microplastic in the presence of natural organic acids. Water Res. 2020, 183, 116082. [Google Scholar] [CrossRef]
- Miao, F.; Liu, Y.; Gao, M.; Yu, X.; Xiao, P.; Wang, M.; Wang, S.; Wang, X. Degradation of polyvinyl chloride microplastics via an electro-Fenton-like system with a TiO2/graphite cathode. J. Hazard. Mater. 2020, 399, 713–715. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, J.; Chen, Z.; Yu, Z.; Xue, J.; Luan, T.; Chen, S.; Zhou, S. Mechanisms of polystyrene microplastic degradation by the microbially driven Fenton reaction. Water Res. 2022, 223, 118979. [Google Scholar] [CrossRef]
- He, G.-J.; Zheng, T.-T.; Ke, D.-M.; Cao, X.-W.; Yin, X.-C.; Xu, B.-P. Impact of rapid ozone degradation on the structure and properties of polypropylene using a reactive extrusion process. RSC Adv. 2015, 5, 44115–44120. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Ding, J.; Song, Z.; Yang, B.; Zhang, C.; Guan, B. Degradation of nano-sized polystyrene plastics by ozonation or chlorination in drinking water disinfection processes. Chem. Eng. J. 2022, 427, 131690. [Google Scholar] [CrossRef]
- Amelia, D.; Karamah, E.F.; Mahardika, M.; Syafri, E.; Rangappa, S.M.; Siengchin, S.; Asrofi, M. Effect of advanced oxidation process for chemical structure changes of polyethylene microplastics. In Proceedings of the Research, Invention, and Innovation Congress—Materials Science, Bangkok, Thailand, 4–5 August 2022; 2022; 52, pp. 2501–2504. [Google Scholar] [CrossRef]
- Zhu, K.; Jia, H.; Sun, Y.; Dai, Y.; Zhang, C.; Guo, X.; Wang, T.; Zhu, L. Long-term phototransformation of microplastics under simulated sunlight irradiation in aquatic environments: Roles of reactive oxygen species. Water Res. 2020, 173, 115564. [Google Scholar] [CrossRef]
- Ho, W.K.; Law, J.C.; Zhang, T.; Leung, K.S. Effects of Weathering on the Sorption Behavior and Toxicity of Polystyrene Microplastics in Multi-solute Systems. Water Res. 2020, 187, 116419. [Google Scholar] [CrossRef]
- Mao, R.; Lang, M.; Yu, X.; Wu, R.; Yang, X.; Guo, X. Aging mechanism of microplastics with UV irradiation and its effects on the adsorption of heavy metals. J. Hazard. Mater. 2020, 393, 122515. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Li, Y.; Li, J.; Liu, Y.; Xia, S.; Zhao, J. Effects of exposure of polyethylene microplastics to air, water and soil on their adsorption behaviors for copper and tetracycline. Chem. Eng. J. 2021, 404, 126412. [Google Scholar] [CrossRef]
- Song, Y.K.; Hong, S.H.; Jang, M.; Han, G.M.; Jung, S.W.; Shim, W.J. Combined Effects of UV Exposure Duration and Mechanical Abrasion on Microplastic Fragmentation by Polymer Type. Environ. Sci. Technol. 2017, 51, 4368–4376. [Google Scholar] [CrossRef]
- Na, S.H.; Kim, M.J.; Kim, J.T.; Jeong, S.; Lee, S.; Chung, J.; Kim, E.J. Microplastic removal in conventional drinking water treatment processes: Performance, mechanism, and potential risk. Water Res. 2021, 202, 117417. [Google Scholar] [CrossRef]
- Cai, L.; Wang, J.; Peng, J.; Wu, Z.; Tan, X. Observation of the degradation of three types of plastic pellets exposed to UV irradiation in three different environments. Sci. Total Environ. 2018, 628-629, 740–747. [Google Scholar] [CrossRef]
- Liu, P.; Li, H.; Wu, J.; Wu, X.; Shi, Y.; Yang, Z.; Huang, K.; Guo, X.; Gao, S. Polystyrene microplastics accelerated photodegradation of co-existed polypropylene via photosensitization of polymer itself and released organic compounds. Water Res. 2022, 214, 118209. [Google Scholar] [CrossRef]
- Liu, L.; Xu, M.; Ye, Y.; Zhang, B. On the degradation of (micro)plastics: Degradation methods, influencing factors, environmental impacts. Sci. Total Environ. 2022, 806, 151312. [Google Scholar] [CrossRef]
- Mukherjee, A.G.; Wanjari, U.R.; Bradu, P.; Patil, M.; Biswas, A.; Murali, R.; Renu, K.; Dey, A.; Vellingiri, B.; Raja, G. Elimination of microplastics from the aquatic milieu: A dream to achieve. Chemosphere 2022, 303, 135232. [Google Scholar] [CrossRef]
- Qi, K.; Cheng, B.; Yu, J.; Ho, W. Review on the improvement of the photocatalytic and antibacterial activities of ZnO. J. Alloy. Compd. 2017, 727, 792–820. [Google Scholar] [CrossRef]
- Taylor, C.M.; Ramirez-Canon, A.; Wenk, J.; Mattia, D. Enhancing the photo-corrosion resistance of ZnO nanowire photocatalysts. J. Hazard. Mater. 2019, 378, 120799. [Google Scholar] [CrossRef] [PubMed]
- Tofa, T.S.; Kunjali, K.L.; Paul, S.; Dutta, J. Visible light photocatalytic degradation of microplastic residues with zinc oxide nanorods. Environ. Chem. Lett. 2019, 17, 1341–1346. [Google Scholar] [CrossRef] [Green Version]
- Llorente-Garcia, B.E.; Hernandez-Lopez, J.M.; Zaldivar-Cadena, A.A.; Siligardi, C.; Cedillo-Gonzalez, E.I. First Insights into Photocatalytic Degradation of HDPE and LDPE Microplastics by a Mesoporous N-TiO(2)Coating: Effect of Size and Shape of Microplastics. Coatings 2020, 107, 658. [Google Scholar] [CrossRef]
- Fadli, M.H.; Ibadurrohman, M.; Slamet, S. Microplastic Pollutant Degradation in Water Using Modified TiO2 Photocatalyst Under UV-Irradiation. IOP Conf.Ser. Mater. Sci. Eng. 2021, 1011, 012055. [Google Scholar] [CrossRef]
- Yan, Q.; Lian, C.; Huang, K.; Liang, L.; Yu, H.; Yin, P.; Zhang, J.; Xing, M. Constructing an Acidic Microenvironment by MoS2 in Heterogeneous Fenton Reaction for Pollutant Control. Angew. Chem. Int. Ed. Engl. 2021, 60, 17155–17163. [Google Scholar] [CrossRef]
- Lang, M.; Yu, X.; Liu, J.; Xia, T.; Wang, T.; Jia, H.; Guo, X. Fenton aging significantly affects the heavy metal adsorption capacity of polystyrene microplastics. Sci. Total Environ. 2020, 722, 137762. [Google Scholar] [CrossRef]
- Liu, P.; Qian, L.; Wang, H.; Zhan, X.; Lu, K.; Gu, C.; Gao, S. New Insights into the Aging Behavior of Microplastics Accelerated by Advanced Oxidation Processes. Environ. Sci. Technol. 2019, 53, 3579–3588. [Google Scholar] [CrossRef]
- Liu, B.; Jiang, Q.; Qiu, Z.; Liu, L.; Wei, R.; Zhang, X.; Xu, H. Process analysis of microplastic degradation using activated PMS and Fenton reagents. Chemosphere 2022, 298, 134220. [Google Scholar] [CrossRef]
- Hidayaturrahman, H.; Lee, T.G. A study on characteristics of microplastic in wastewater of South Korea: Identification, quantification, and fate of microplastics during treatment process. Mar. Pollut. Bull. 2019, 146, 696–702. [Google Scholar] [CrossRef]
- Sun, P.; Liu, X.; Zhang, M.; Li, Z.; Cao, C.; Shi, H.; Yang, Y.; Zhao, Y. Sorption and leaching behaviors between aged MPs and BPA in water: The role of BPA binding modes within plastic matrix. Water Res. 2021, 195, 116956. [Google Scholar] [CrossRef]
- Liu, P.; Lu, K.; Li, J.; Wu, X.; Qian, L.; Wang, M.; Gao, S. Effect of aging on adsorption behavior of polystyrene microplastics for pharmaceuticals: Adsorption mechanism and role of aging intermediates. J. Hazard Mater. 2020, 384, 121193. [Google Scholar] [CrossRef]
- Jin, X.; Zhang, S.; Yang, S.; Zong, Y.; Xu, L.; Jin, P.; Yang, C.; Hu, S.; Li, Y.; Shi, X.; et al. Behaviour of ozone in the hybrid ozonation-coagulation (HOC) process for ibuprofen removal: Reaction selectivity and effects on coagulant hydrolysis. Sci. Total Environ. 2021, 794, 148685. [Google Scholar] [CrossRef]
- Wang, J.; Lou, Y.; Xu, C.; Song, S.; Liu, W. Magnetic lanthanide oxide catalysts: An application and comparison in the heterogeneous catalytic ozonation of diethyl phthalate in aqueous solution. Sep. Purif. Technol. 2016, 159, 57–67. [Google Scholar] [CrossRef]
- Zafar, R.; Park, S.Y.; Kim, C.G. Surface modification of polyethylene microplastic particles during the aqueous-phase ozonation process. Environ. Eng. Res. 2020, 26, 200410–200412. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, N. Mechanistic implications of plastic degradation. Polym. Degrad. Stab. 2008, 93, 561–584. [Google Scholar] [CrossRef]
- Ozen, B.F.; Mauer, L.J.; Floros, J.D. Effects of ozone exposure on the structural, mechanical and barrier properties of select plastic packaging films. Packag. Technol. Sci. 2002, 15, 301–311. [Google Scholar] [CrossRef]
- Zafar, R.; Park, S.Y.; Kim, C.G. Comparison of surficial modification of micro-sized polyethylene in between by UV/O3 and UVO submerged system. Environ. Eng. Res. 2021, 27, 210020–210028. [Google Scholar] [CrossRef]
- Mandal, P.; Dubey, B.K.; Gupta, A.K. Review on landfill leachate treatment by electrochemical oxidation: Drawbacks, challenges and future scope. Waste Manag. 2017, 69, 250–273. [Google Scholar] [CrossRef]
- Liu, G.; Sun, Y.; Lu, H.; Ju, R.; Zhu, L.; Kang, K. Research progress in activated persulfate technology. Ind. Water Treat. 2012, 3212, 6–10. [Google Scholar]
- Chen, G.; Wu, G.; Li, N.; Lu, X.; Zhao, J.; He, M.; Yan, B.; Zhang, H.; Duan, X.; Wang, S. Landfill leachate treatment by persulphate related advanced oxidation technologies. J. Hazard. Mater. 2021, 418, 126355. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.; Gao, J.; Dionysiou, D.D.; Liu, C.; Zhou, D. Activation of persulfate by quinones: Free radical reactions and implication for the degradation of PCBs. Environ. Sci. Technol. 2013, 47, 4605–4611. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.-T.; Zhang, Y.; Teng, Y.; Fan, M. Sulfate Radical and Its Application in Decontamination Technologies. Crit. Rev. Environ. Sci. Technol. 2014, 45, 1756–1800. [Google Scholar] [CrossRef]
- Soubh, A.; Baghdadi, M.; Abdoli, M.; Aminzadeh, B. Activation of Persulfate Using an Industrial Iron-Rich Sludge as an Efficient Nanocatalyst for Landfill Leachate Treatment. Catalysts 2018, 85, 218. [Google Scholar] [CrossRef] [Green Version]
- Karimipourfard, D.; Eslamloueyan, R.; Mehranbod, N. Novel heterogeneous degradation of mature landfill leachate using persulfate and magnetic CuFe2O4/RGO nanocatalyst. Process Saf. Environ. Prot. 2019, 131, 212–222. [Google Scholar] [CrossRef]
- Luo, H.; Zeng, Y.; Zhao, Y.; Xiang, Y.; Li, Y.; Pan, X. Effects of advanced oxidation processes on leachates and properties of microplastics. J. Hazard. Mater. 2021, 413, 125342. [Google Scholar] [CrossRef]
- Ouyang, Z.; Li, S.; Zhao, M.; Wangmu, Q.; Ding, R.; Xiao, C.; Guo, X. The aging behavior of polyvinyl chloride microplastics promoted by UV-activated persulfate process. J. Hazard. Mater. 2022, 424, 127461. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Wang, H.; Huang, Q.; Yang, C.; Wang, L.; Lou, Z.; Zhou, Q.; Wang, T.; Ning, C. Microplastics in Landfill Leachate: A Comprehensive Review on Characteristics, Detection, and Their Fates during Advanced Oxidation Processes. Water 2023, 15, 252. https://doi.org/10.3390/w15020252
Wang L, Wang H, Huang Q, Yang C, Wang L, Lou Z, Zhou Q, Wang T, Ning C. Microplastics in Landfill Leachate: A Comprehensive Review on Characteristics, Detection, and Their Fates during Advanced Oxidation Processes. Water. 2023; 15(2):252. https://doi.org/10.3390/w15020252
Chicago/Turabian StyleWang, Lan, Hui Wang, Qiujie Huang, Changfu Yang, Luochun Wang, Ziyang Lou, Qian Zhou, Tiantian Wang, and Chengqi Ning. 2023. "Microplastics in Landfill Leachate: A Comprehensive Review on Characteristics, Detection, and Their Fates during Advanced Oxidation Processes" Water 15, no. 2: 252. https://doi.org/10.3390/w15020252
APA StyleWang, L., Wang, H., Huang, Q., Yang, C., Wang, L., Lou, Z., Zhou, Q., Wang, T., & Ning, C. (2023). Microplastics in Landfill Leachate: A Comprehensive Review on Characteristics, Detection, and Their Fates during Advanced Oxidation Processes. Water, 15(2), 252. https://doi.org/10.3390/w15020252






