Influence of Environmental Variables on Biochemical Biomarkers in the Amphipod Monoporeia affinis from the Gulf of Riga (Baltic Sea)
Abstract
:1. Introduction
2. Materials and Methods
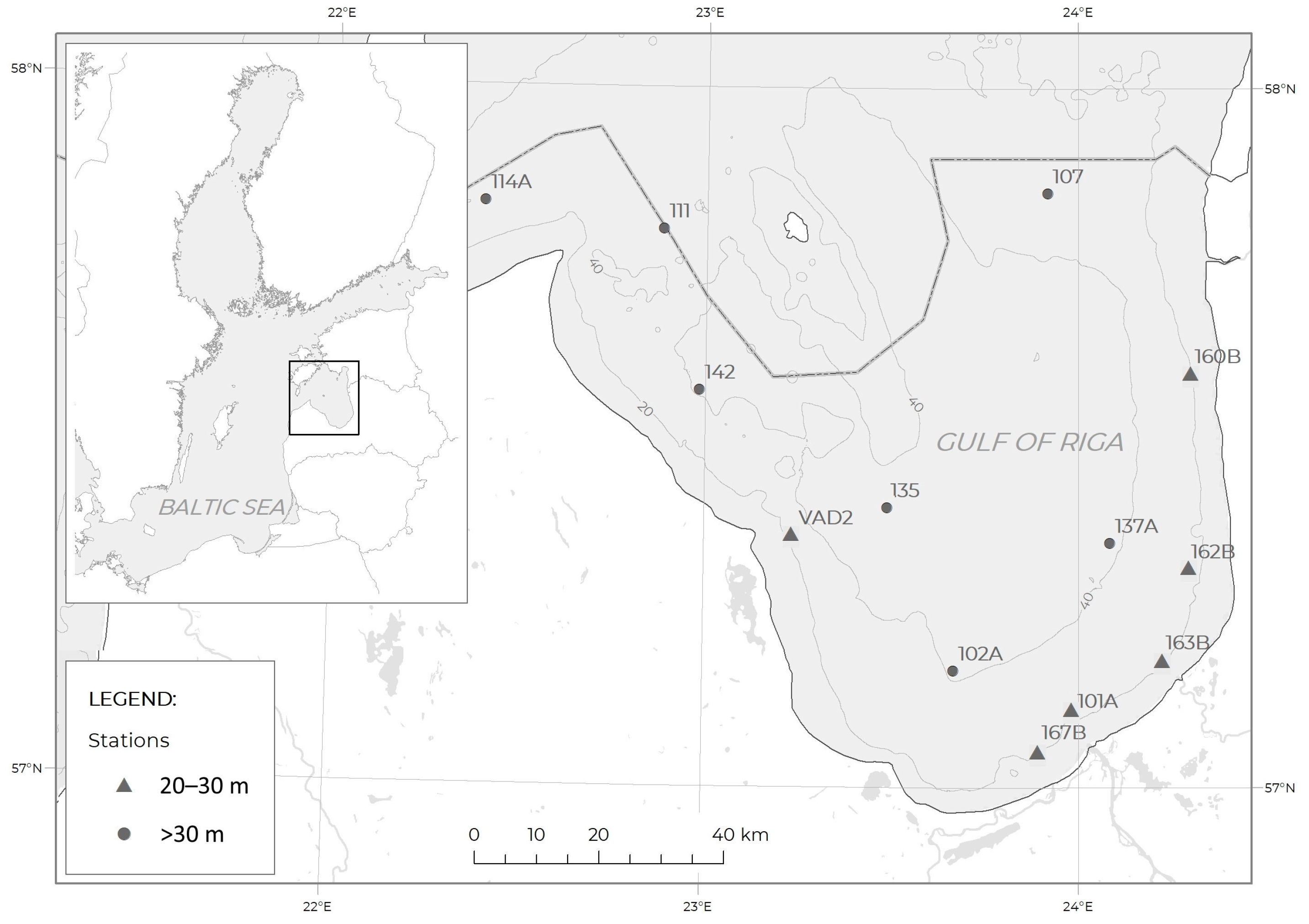
2.1. Study Area
2.2. Sampling
2.3. Sample Preparation and Biomarker Analyses
2.4. Statistical Analysis
3. Results
3.1. Environmental Factors
3.2. Seasonal Variability in Biomarkers at the Coastal Stations
3.3. Biochemical Biomarkers at the Deep-Water Stations
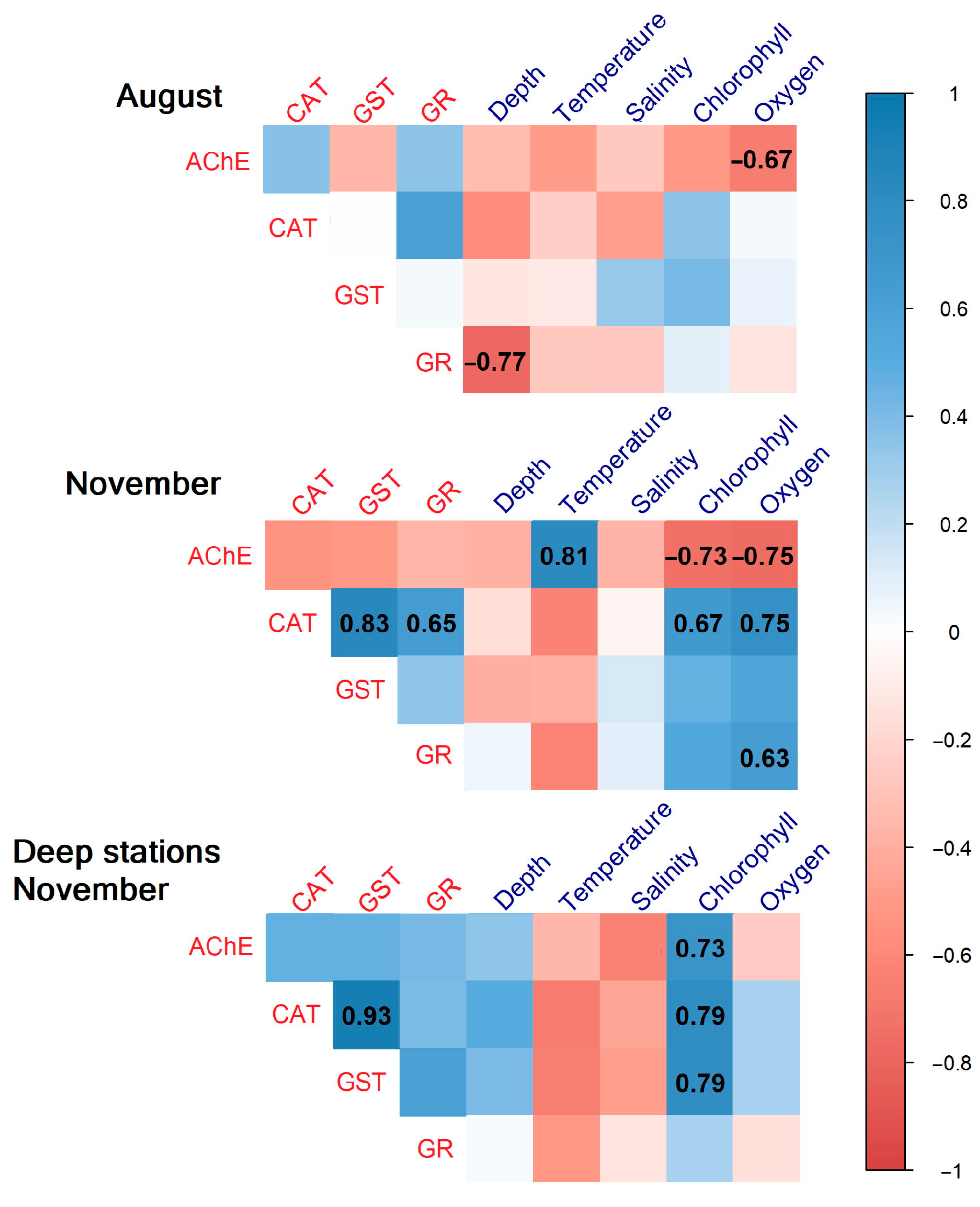
3.4. Correlation Analysis
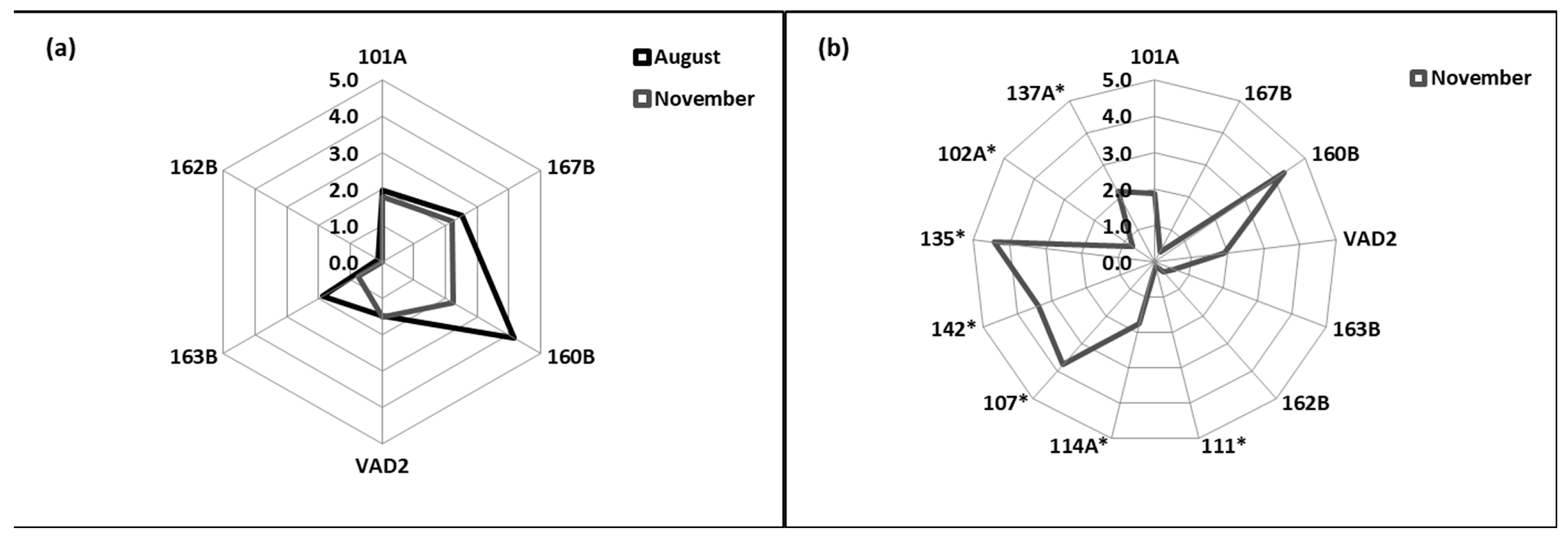
3.5. Integrated Biomarker Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bashir, I.; Lone, F.A.; Bhat, R.A.; Mir, S.A.; Dar, Z.A.; Dar, S.A. Concerns and Threats of Contamination on Aquatic Ecosystems. In Bioremediation and Biotechnology; Springer: Cham, Switzerland, 2020; pp. 1–26. [Google Scholar] [CrossRef] [Green Version]
- Hook, S.E.; Gallagher, E.P.; Batley, G.E. The Role of Biomarkers in the Assessment of Aquatic Ecosystem Health. Integr. Environ. Assess. Manag. 2014, 10, 327–341. [Google Scholar] [CrossRef] [Green Version]
- Smit, M.G.D.; Bechmann, R.K.; Hendriks, A.J.; Skadsheim, A.; Larsen, B.K.; Baussant, T.; Bamber, S.; Sanni, S. Relating biomarkers to whole-organism effects using species sensitivity distributions: A pilot study for marine species exposed to oil. Environ. Toxicol. Chem. 2009, 28, 1104–1109. [Google Scholar] [CrossRef]
- Lionetto, M.G.; Caricato, R.; Giordano, M.E. Pollution Biomarkers in Environmental and Human Biomonitoring. Open Biomark. J. 2019, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Stohs, S.J.; Bagchi, D. Oxidative mechanisms in the toxicity of metal ions. Free Radic. Biol. Med. 1995, 18, 321–336. [Google Scholar] [CrossRef] [Green Version]
- Turja, R.; Sanni, S.; Stankevičiūtė, M.; Andreikėnaitė Butrimavičienė, L.; Devier, M.-H.; Budzinski, H.; Lehtonen, K. Biomarker responses and accumulation of polycyclic aromatic hydrocarbons in Mytilus trossulus and Gammarus oceanicus during exposure to crude oil. Environ. Sci. Pollut. Res. 2020, 27, 15498–15514. [Google Scholar] [CrossRef] [Green Version]
- Vranković, J.; Živić, M.; Radojević, A.; Perić-Mataruga, V.; Todorović, D.; Marković, Z.; Živić, I. Evaluation of oxidative stress biomarkers in the freshwater gammarid Gammarus dulensis exposed to trout farm outputs. Ecotoxicol. Environ. Saf. 2018, 163, 84–95. [Google Scholar] [CrossRef]
- Umar, A.M.; Aisami, A. Acetylcholinesterase Enzyme (AChE) as a Biosensor and Biomarker for Pesticides: A Mini Review. Bull. Environ. Sci. Sustain. Manag. 2020, 4, 7–12. [Google Scholar] [CrossRef]
- Kankaanpää, H.; Leiniö, S.; Olin, M.; Sjövall, O.; Meriluoto, J.; Lehtonen, K.K. Accumulation and depuration of cyanobacterial toxin nodularin and biomarker responses in the mussel Mytilus edulis. Chemosphere 2007, 68, 1210–1217. [Google Scholar] [CrossRef]
- Lehtonen, K.K.; Kankaanpää, H.; Leiniö, S.; Sipiä, V.O.; Pflugmacher, S.; Sandberg-Kilpi, E. Accumulation of nodularin-like compounds from the cyanobacterium Nodularia spumigena and changes in acetylcholinesterase activity in the clam Macoma balthica during short-term laboratory exposure. Aquat. Toxicol. 2003, 64, 461–476. [Google Scholar] [CrossRef]
- Lionetto, M.G.; Caricato, R.; Giordano, M.E. Pollution Biomarkers in the Framework of Marine Biodiversity Conservation: State of Art and Perspectives. Water 2021, 13, 1847. [Google Scholar] [CrossRef]
- Whiteley, N.M.; Rastrick, S.P.S.; Lunt, D.H.; Rock, J. Latitudinal variations in the physiology of marine gammarid amphipods. J. Exp. Mar. Biol. Ecol. 2011, 400, 70–77. [Google Scholar] [CrossRef]
- Podlesińska, W.; Dąbrowska, H. Amphipods in estuarine and marine quality assessment—A review. Oceanologia 2019, 61, 179–196. [Google Scholar] [CrossRef]
- Gorokhova, E.; Löf, M.; Reutgard, M.; Lindström, M.; Sundelin, B. Exposure to contaminants exacerbates oxidative stress in amphipod Monoporeia affinis subjected to fluctuating hypoxia. Aquat. Toxicol. 2013, 127, 46–53. [Google Scholar] [CrossRef]
- Gorokhova, E.; Löf, M.; Halldórsson, H.P.; Tjärnlund, U.; Lindström, M.; Elfwing, T.; Sundelin, B. Single and combined effects of hypoxia and contaminated sediments on the amphipod Monoporeia affinis in laboratory toxicity bioassays based on multiple biomarkers. Aquat. Toxicol. 2010, 99, 263–274. [Google Scholar] [CrossRef]
- Lehtonen, K.K. Seasonal variations in the physiological condition of the benthic amphipods Monoporeia affinis and Pontoporeia femorata in the Gulf of Riga (Baltic Sea). Aquat. Ecol. 2004, 38, 441–456. [Google Scholar] [CrossRef]
- Sundelin, B.; Eriksson Wiklund, A.-K. Malformations in embryos of the deposit-feeding amphipod Monoporeia affinis in the Baltic Sea. Mar. Ecol. Prog. Ser. 1998, 171, 165–180. [Google Scholar] [CrossRef]
- Löf, M.; Sundelin, B.; Bandh, C.; Gorokhova, E. Embryo aberrations in the amphipod Monoporeia affinis as indicators of toxic pollutants in sediments: A field evaluation. Ecol. Indic. 2016, 60, 18–30. [Google Scholar] [CrossRef]
- HELCOM. Reproductive Disorders: Malformed Embryos of Amphipods. HELCOM Supplementaryindicator Report. 2018, pp. 1–23, ISSN 2343-2543. Available online: https://helcom.fi/wp-content/uploads/2019/08/Reproductive-disorders-malformed-embryos-of-amphipods-HELCOM-supplementary-indicator-2018.pdf (accessed on 3 January 2023). [CrossRef]
- Barda, I.; Purina, I.; Rimsa, E.; Balode, M. Seasonal dynamics of biomarkers in infaunal clam Macoma balthica from the Gulf of Riga (Baltic Sea). J. Mar. Syst. 2014, 129, 150–156. [Google Scholar] [CrossRef]
- Braghirolli, F.M.; Oliveira, M.R.; Oliveira, G.T. Seasonal variability of metabolic markers and oxidative balance in freshwater amphipod Hyalella kaingang (Crustacea, Amphipoda). Ecotoxicol. Environ. Saf. 2016, 130, 177–184. [Google Scholar] [CrossRef]
- Leiniö, S.; Lehtonen, K.K. Seasonal variability in biomarkers in the bivalves Mytilus edulis and Macoma balthica from the northern Baltic Sea. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2005, 140, 408–421. [Google Scholar] [CrossRef]
- Benito, D.; Ahvo, A.; Nuutinen, J.; Bilbao, D.; Saenz, J.; Etxebarria, N.; Lekube, X.; Izagirre, U.; Lehtonen, K.K.; Marigómez, I.; et al. Influence of season-depending ecological variables on biomarker baseline levels in mussels (Mytilus trossulus) from two Baltic Sea subregions. Sci. Total Environ. 2019, 689, 1087–1103. [Google Scholar] [CrossRef]
- Sheehan, D.; Power, A. Effects of seasonality on xenobiotic and antioxidant defence mechanisms of bivalve molluscs. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 1999, 123, 193–199. [Google Scholar] [CrossRef]
- Berezina, N.A.; Strode, E.; Lehtonen, K.K.; Balode, M.; Golubkov, S.M. Sediment quality assessment using Gmelinoides fasciatus and Monoporeia affinis (Amphipoda, Gammaridea) in the northeastern Baltic Sea. Crustaceana 2013, 86, 780–801. [Google Scholar] [CrossRef]
- Putna, I.; Strode, E.; Barda, I.; Purina, I.; Rimša, E.; Jansons, M.; Balode, M.; Strake, S. Sediment quality of the ecoregion Engure, Gulf of Riga, assessed by using ecotoxicity tests and biomarker responses. Proc. Latv. Acad. Sciences. Sect. B. Nat. Exact Appl. Sci. 2014, 68, 20–30. [Google Scholar] [CrossRef] [Green Version]
- Strode, E.; Jansons, M.; Purina, I.; Balode, M.; Berezina, N.A. Sediment quality assessment using survival and embryo malformation tests in amphipod crustaceans: The Gulf of Riga, Baltic Sea AS case study. J. Mar. Syst. 2017, 172, 93–103. [Google Scholar] [CrossRef]
- Strode, E.; Balode, M. Toxico-resistance of Baltic amphipod species to heavy metals. Crustaceana 2013, 86, 1007–1024. [Google Scholar] [CrossRef]
- Ojaveer, E. Large-scale processes in the ecosystem of the Gulf of Riga. In Ecosystem of the Gulf of Riga between 1920 and 1990; Ojaveer, E., Ed.; Estonian Academy Publishers: Tallinn, Estonia, 1995; pp. 268–277. [Google Scholar]
- Skudra, M.; Lips, U. Characteristics and inter-annual changes in temperature, salinity and density distribution in the Gulf of Riga. Oceanologia 2017, 59, 37–48. [Google Scholar] [CrossRef] [Green Version]
- Berzinsh, V. Dynamics of hydrological parameters of the Gulf of Riga. In Ecosystem of the Gulf of Riga between 1920 and 1990; Ojaveer, E., Ed.; Estonian Academy: Tallinn, Estonia, 1995; pp. 8–32. [Google Scholar]
- Raudsepp, U. Interannual and Seasonal Temperature and Salinity Variations in the Gulf of Riga and Corresponding Saline Water Inflow From the Baltic Proper. Hydrol. Res. 2001, 32, 135–160. [Google Scholar] [CrossRef]
- Yurkovskis, A. Long-term land-based and internal forcing of the nutrient state of the Gulf of Riga (Baltic Sea). J. Mar. Syst. 2004, 50, 181–197. [Google Scholar] [CrossRef]
- Eglīte, E.; Lavrinovičs, A.; Müller-Karulis, B.; Aigars, J.; Poikāne, R. Nutrient turnover at the hypoxic boundary: Flux measurements and model representation for the bottom water environment of the Gulf of Riga, Baltic Sea. Oceanologia 2014, 56, 711–735. [Google Scholar] [CrossRef] [Green Version]
- Kotta, J.; Lauringson, V.; Martin, G.; Simm, M.; Kotta, I.; Herkül, K.; Ojaveer, H. Gulf of Riga and Pärnu Bay. In Ecology of Baltic Coastal Waters; Schiewer, U., Ed.; Ecological Studies; Springer: Berlin/Heidelberg, Germany, 2008; pp. 217–243. ISBN 978-3-540-73523-6. [Google Scholar]
- Viška, M.; Soomere, T. Simulated and observed reversals of wave-driven alongshore sediment transport at the eastern Baltic Sea coast. Baltica 2013, 26, 145–156. [Google Scholar] [CrossRef] [Green Version]
- Claiborne, A. Catalase Activity. In CRC Handbook of Methods for Oxygen Radical Research; CRC Press: Boca Raton, FL, USA, 1985; ISBN 978-1-351-07292-2. [Google Scholar]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Smith, I.K.; Vierheller, T.L.; Thorne, C.A. Assay of glutathione reductase in crude tissue homogenates using 5,5’-dithiobis(2-nitrobenzoic acid). Anal. Biochem. 1988, 175, 408–413. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Beliaeff, B.; Burgeot, T. Integrated biomarker response: A useful tool for ecological risk assessment. Environ. Toxicol. Chem. 2002, 21, 1316–1322. [Google Scholar] [CrossRef] [PubMed]
- Broeg, K.; Lehtonen, K.K. Indices for the assessment of environmental pollution of the Baltic Sea coasts: Integrated assessment of a multi-biomarker approach. Mar. Pollut. Bull. 2006, 53, 508–522. [Google Scholar] [CrossRef] [Green Version]
- Jemec, A.; Drobne, D.; Tisler, T.; Sepcic, K. Biochemical biomarkers in environmental studies-lessons learnt from enzymes catalase, glutathione S-transferase and cholinesterase in two crustacean species. Environ. Sci. Pollut. Res. 2010, 17, 571–581. [Google Scholar] [CrossRef]
- Verlecar, X.N.; Jena, K.B.; Chainy, G.B.N. Seasonal variation of oxidative biomarkers in gills and digestive gland of green-lipped mussel Perna viridis from Arabian Sea. Estuar. Coast. Shelf Sci. 2008, 76, 745–752. [Google Scholar] [CrossRef]
- Löf, M.; Sundelin, B.; Liewenborg, B.; Bandh, C.; Broeg, K.; Schatz, S.; Gorokhova, E. Biomarker-enhanced assessment of reproductive disorders in Monoporeia affinis exposed to contaminated sediment in the Baltic Sea. Ecol. Indic. 2016, 63, 187–195. [Google Scholar] [CrossRef]
- Jacobson, T.; Prevodnik, A.; Sundelin, B. Combined effects of temperature and a pesticide on the Baltic amphipod Monoporeia affinis. Aquat. Biol. 2008, 1, 269–276. [Google Scholar] [CrossRef] [Green Version]
- Eriksson Wiklund, A.K.; Sundelin, B. Impaired reproduction in the amphipods Monoporeia affinis and Pontoporeia femorata as a result of moderate hypoxia and increased temperature. Mar. Ecol. Prog. Ser. 2001, 222, 131–141. [Google Scholar] [CrossRef] [Green Version]
- Chainy, G.B.N.; Paital, B.; Dandapat, J. An Overview of Seasonal Changes in Oxidative Stress and Antioxidant Defence Parameters in Some Invertebrate and Vertebrate Species. Scientifica 2016, 2016, 6126570. [Google Scholar] [CrossRef] [Green Version]
- Glippa, O.; Engström-Öst, J.; Kanerva, M.; Rein, A.; Vuori, K. Oxidative stress and antioxidant defense responses in Acartia copepods in relation to environmental factors. PLoS ONE 2018, 13, e0195981. [Google Scholar] [CrossRef] [Green Version]
- Taddei, A.; Räsänen, K.; Burdon, F.J. Size-dependent sensitivity of stream amphipods indicates population-level responses to chemical pollution. Freshw. Biol. 2021, 66, 765–784. [Google Scholar] [CrossRef]
- Sroda, S.; Cossu-Leguille, C. Seasonal variability of antioxidant biomarkers and energy reserves in the freshwater gammarid Gammarus roeseli. Chemosphere 2011, 83, 538–544. [Google Scholar] [CrossRef]
- Wiklund, A.-K.E.; Sundelin, B.; Rosa, R. Population decline of amphipod Monoporeia affinis in Northern Europe: Consequence of food shortage and competition? J. Exp. Mar. Biol. Ecol. 2008, 367, 81–90. [Google Scholar] [CrossRef]
- Luna Acosta, A.; Bustamante, P.; Godefroy, J.; Fruitier-Arnaudin, I.; Thomas-Guyon, H. Seasonal variation of pollution biomarkers to assess the impact on health status of juvenile Pacific oysters Crassostrea gigas exposed in situ. Environ. Sci. Pollut. Res. 2010, 17, 999–1008. [Google Scholar] [CrossRef]
- Rousi, H.; Laine, A.O.; Peltonen, H.; Kangas, P.; Andersin, A.-B.; Rissanen, J.; Sandberg-Kilpi, E.; Bonsdorff, E. Long-term changes in coastal zoobenthos in the northern Baltic Sea: The role of abiotic environmental factors. ICES J. Mar. Sci. 2013, 70, 440–451. [Google Scholar] [CrossRef]


| Year | Month | Station | Depth [m] | Temp. [°C] | Absolute Salinity [ppt] | Chlorophyll a [mg/m3] | Oxygen [mg/L] |
|---|---|---|---|---|---|---|---|
| 2020 | August | 101A | 22 | 7.2 | 5.8 | 0.9 | 4.0 |
| 2021 | 22 | 3.3 | 6.1 | 1.8 | 5.9 | ||
| 2020 | November | 22 | 10.9 | 6.0 | 1.6 | 9.5 | |
| 2021 | 23 | 8.4 | 6.0 | 3.7 | 11.1 | ||
| 2020 | August | 167B | 21 | 6.4 | 5.8 | 0.8 | 4.7 |
| 2021 | 21 | 3.2 | 6.2 | 1.6 | 6.4 | ||
| 2020 | November | 21 | 10.8 | 5.9 | 2.0 | 9.7 | |
| 2021 | 22 | 8.4 | 6.0 | 3.0 | 11.1 | ||
| 2020 | August | 163B | 22 | 12.1 | 5.9 | 0.9 | 6.4 |
| 2021 | 22 | 18.4 | 5.9 | 2.2 | 6.9 | ||
| 2020 | November | 22 | 10.8 | 5.9 | 2.1 | 9.7 | |
| 2021 | 21 | 8.5 | 5.9 | 3.0 | 11.1 | ||
| 2020 | August | 162B | 25 | 8.9 | 6.0 | 0.8 | 4.0 |
| 2021 | 25 | 3.9 | 6.1 | 1.5 | 6.8 | ||
| 2020 | November | 25 | 10.7 | 5.9 | 1.9 | 9.6 | |
| 2021 | 24 | 8.3 | 5.9 | 4.0 | 11.3 | ||
| 2020 | August | VAD2 | 26 | 5.5 | 5.9 | 0.9 | 4.6 |
| 2021 | 26 | 3.0 | 6.2 | 1.5 | 8.3 | ||
| 2020 | November | 26 | 10.3 | 5.9 | 2.3 | 9.8 | |
| 2021 | 25 | 7.7 | 6.1 | 3.0 | 11.1 | ||
| 2020 | August | 160B | 22 | 10.1 | 5.9 | 0.9 | 2.9 |
| 2020 | November | 22 | 10.4 | 5.7 | 2.4 | 9.9 | |
| 2021 | November | 22 | 7.9 | 5.9 | 3.0 | 11.5 | |
| 2021 | November | 107 | 32 | 8.4 | 6.0 | 2.7 | 11.2 |
| 2021 | November | 111 | 38 | 8.8 | 6.5 | 2.0 | 10.7 |
| 2021 | November | 114A | 33 | 8.6 | 6.8 | 1.7 | 10.9 |
| 2021 | November | 142 | 42 | 8.2 | 6.3 | 2.3 | 10.7 |
| 2021 | November | 135 | 45 | 8.2 | 6.1 | 2.6 | 5.3 |
| 2021 | November | 102A | 42 | 5.3 | 6.2 | 1.6 | 4.0 |
| 2021 | November | 137A | 42 | 9.0 | 6.0 | 2.2 | 10.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strode, E.; Barda, I.; Suhareva, N.; Kolesova, N.; Turja, R.; Lehtonen, K.K. Influence of Environmental Variables on Biochemical Biomarkers in the Amphipod Monoporeia affinis from the Gulf of Riga (Baltic Sea). Water 2023, 15, 248. https://doi.org/10.3390/w15020248
Strode E, Barda I, Suhareva N, Kolesova N, Turja R, Lehtonen KK. Influence of Environmental Variables on Biochemical Biomarkers in the Amphipod Monoporeia affinis from the Gulf of Riga (Baltic Sea). Water. 2023; 15(2):248. https://doi.org/10.3390/w15020248
Chicago/Turabian StyleStrode, Evita, Ieva Barda, Natalija Suhareva, Natalja Kolesova, Raisa Turja, and Kari K. Lehtonen. 2023. "Influence of Environmental Variables on Biochemical Biomarkers in the Amphipod Monoporeia affinis from the Gulf of Riga (Baltic Sea)" Water 15, no. 2: 248. https://doi.org/10.3390/w15020248
APA StyleStrode, E., Barda, I., Suhareva, N., Kolesova, N., Turja, R., & Lehtonen, K. K. (2023). Influence of Environmental Variables on Biochemical Biomarkers in the Amphipod Monoporeia affinis from the Gulf of Riga (Baltic Sea). Water, 15(2), 248. https://doi.org/10.3390/w15020248







