Carotenogenic Activity of Two Hypersaline Greek Dunaliella salina Strains under Nitrogen Deprivation and Salinity Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Strain Isolation
2.2. Experimental Design
2.3. Analytical Procedures
3. Results
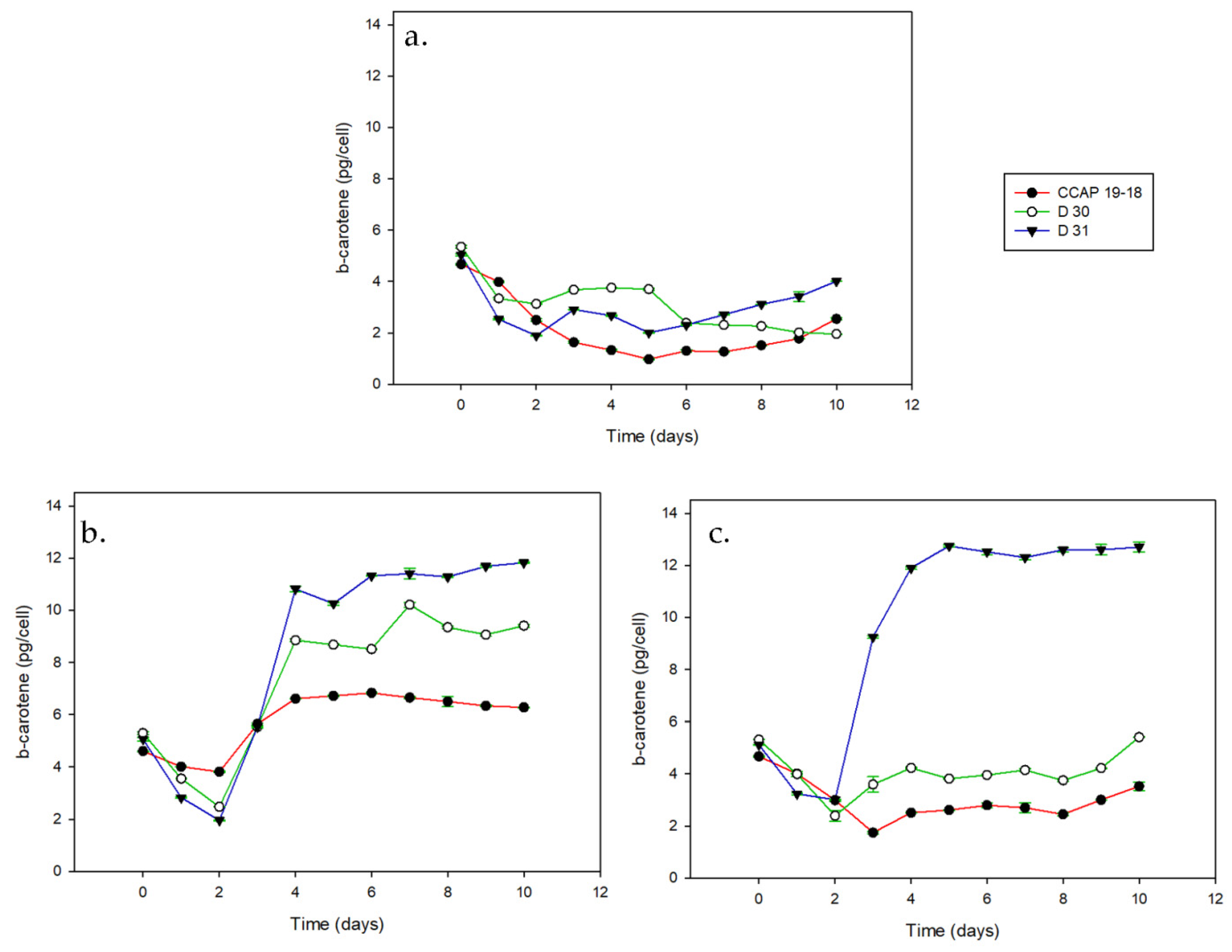
3.1. Growth and Pigments in Daily Basis
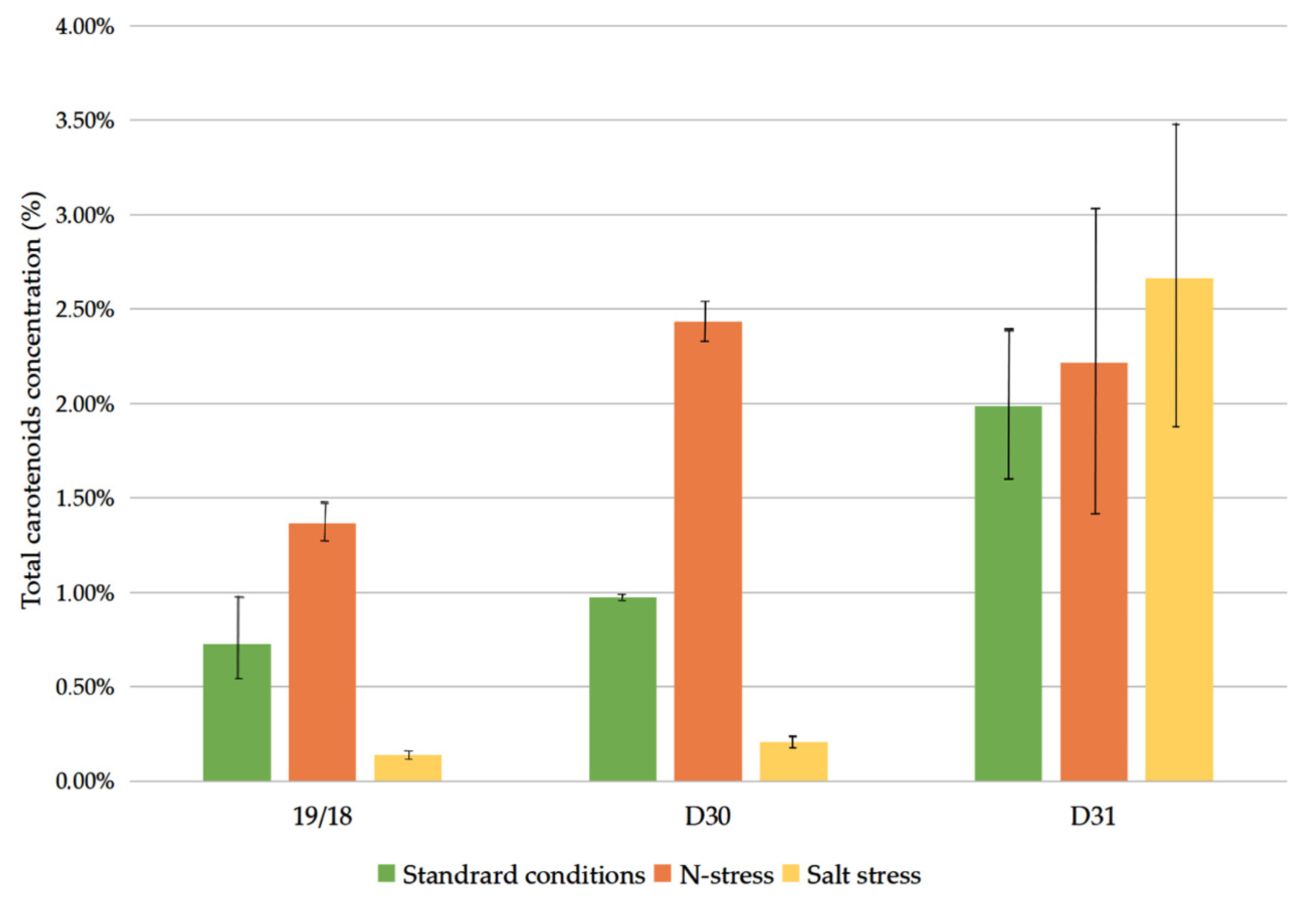
3.2. Carotenoid Content on a Dry Basis
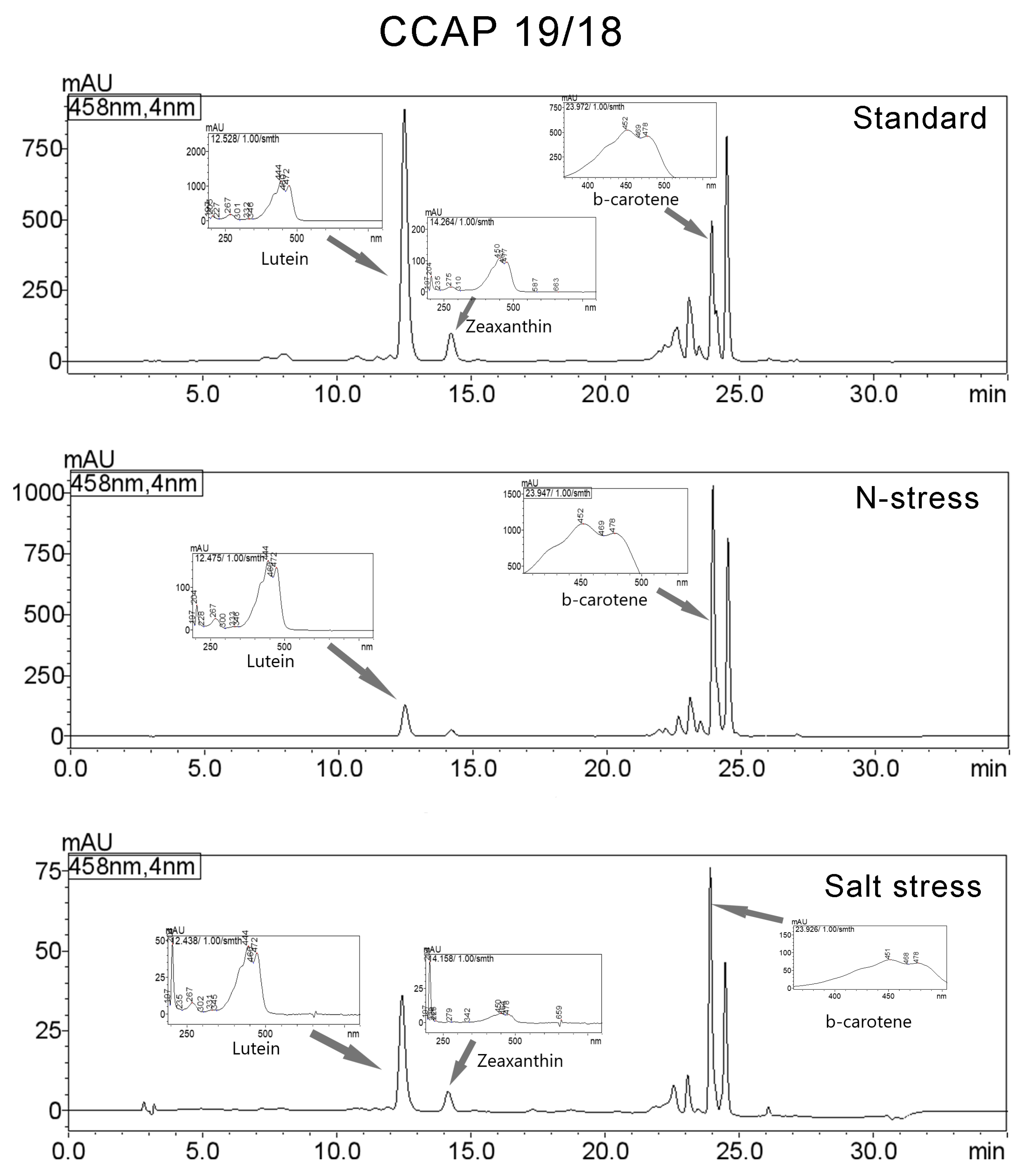
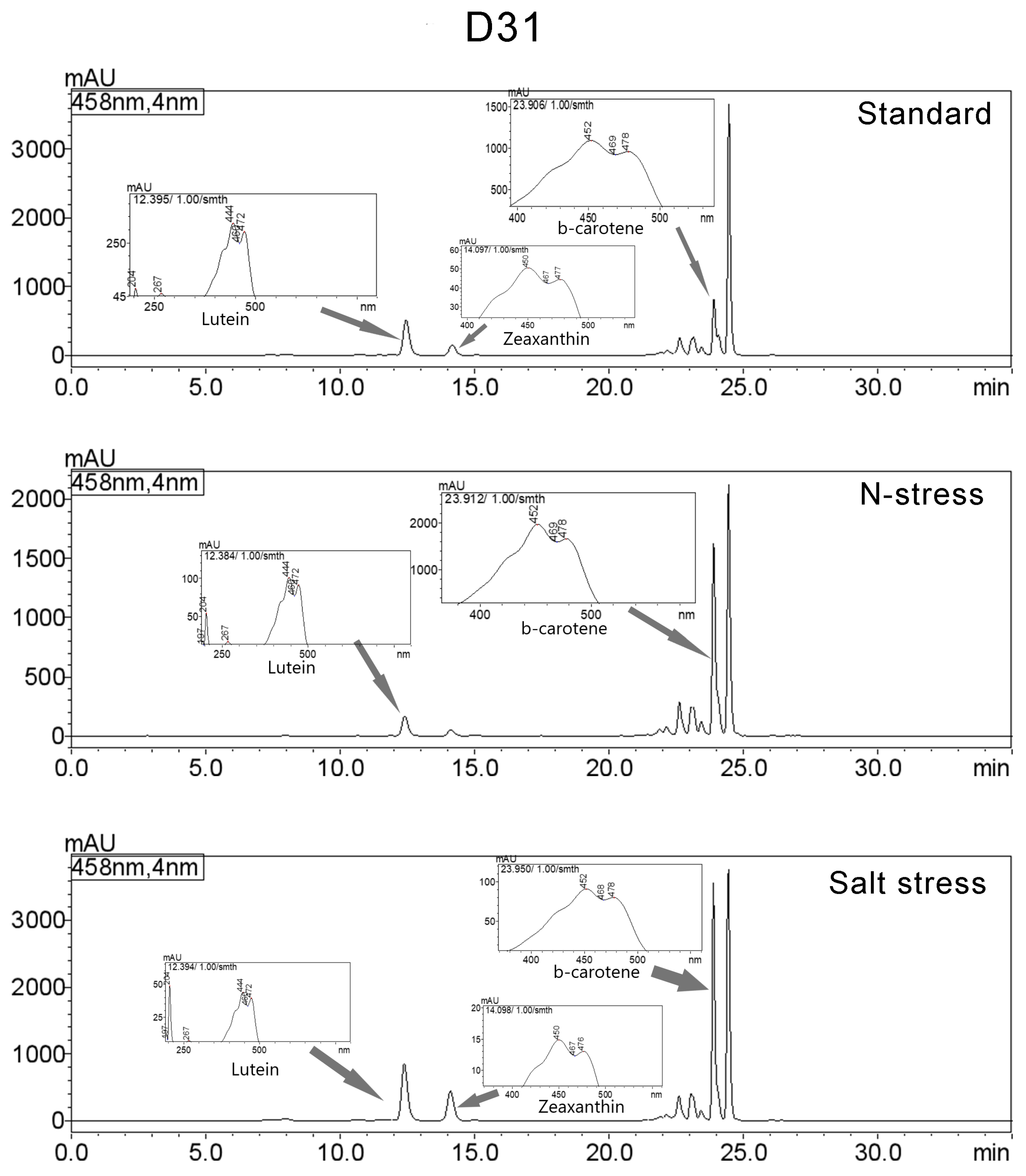
3.3. Carotenoids Composition
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Borowitzka, L.J.; Moulton, T.P.; Borowitzka, M.A. The mass culture of Dunaliella salina for fine chemicals: From laboratory to pilot plant. In Proceedings of the Eleventh International Seaweed Symposium, Developments in Hydrobiology, Qingdao, China, 19–25 June 1983. [Google Scholar] [CrossRef]
- Saha, S.K.; Murray, P. Exploitation of microalgae species for nutraceutical purposes: Cultivation aspects. Fermentation 2018, 4, 46. [Google Scholar] [CrossRef] [Green Version]
- Borowitzka, M.A. Commercial-scale production of microalgae for bioproducts. In Blue Biotechnology: Production and Use of Marine Molecules; La Barre, S., Bates, S.S., Eds.; Wiley-VCH: Weinheim, Germany, 2018; Volume 1, pp. 33–65. [Google Scholar] [CrossRef]
- Winwood, R.J. Recent developments in the commercial production of DHA and EPA rich oils from micro-algae. Ocl 2013, 20, D604. [Google Scholar] [CrossRef] [Green Version]
- Novoveská, L.; Ross, M.E.; Stanley, M.S.; Pradelles, R.; Wasiolek, V.; Sassi, J.F. Microalgal carotenoids: A review of production, current markets, regulations, and future direction. Mar. Drugs 2019, 17, 640. [Google Scholar] [CrossRef] [Green Version]
- Raja, R.; Hemaiswarya, S.; Rengasamy, R. Exploitation of Dunaliella for β-carotene production. Appl. Microbiol. Biotechnol. 2007, 74, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Teodoresco, E.C. Organisation et développement du Dunaliella, nouveau genre de Volvocacée-Polyblepharidée. Beih. Z Bot. Centralbl. 1905, 18, 215–232. [Google Scholar]
- Ben-Amotz, A.; Avron, M. On the factors which determine massive β-carotene accumulation in the halotolerant alga Dunaliella bardawil. Plant. Physiol. 1983, 72, 593–597. [Google Scholar] [CrossRef] [Green Version]
- Hashemi, A.; Moslemi, M.; Pajoum Shariati, F.; Delavari Amrei, H. Beta-carotene production within Dunaliella salina cells under salt stress condition in an indoor hybrid helical-tubular photobioreactor. Can. J. Chem. Eng. 2020, 98, 69–74. [Google Scholar] [CrossRef] [Green Version]
- Lamers, P.P.; Janssen, M.; De Vos, R.C.; Bino, R.J.; Wijffels, R.H. Carotenoid and fatty acid metabolism in nitrogen-starved Dunaliella salina, a unicellular green microalga. J. Biotechnol. 2012, 162, 21–27. [Google Scholar] [CrossRef]
- Borowitzka, M.A.; Siva, C.J. The taxonomy of the genus Dunaliella (Chlorophyta, Dunaliellales) with emphasis on the marine and halophilic species. J. Appl. Phycol. 2007, 19, 567–590. [Google Scholar] [CrossRef]
- Oren, A. The ecology of Dunaliella in high-salt environments. J. Biol. Res. (Thessalon) 2014, 21, 23. [Google Scholar] [CrossRef] [Green Version]
- Massjuk, N.P. Morphology, Systematics, Ecology, Geographic Distribution of Genus Dunaliella Teod, and Perspectives of Its Practical Use; Nauk, Dumka Press: Kiev, Ukraine, 1973. [Google Scholar]
- Ben-Amotz, A.; Avron, M. The role of glycerol in the osmotic regulation of the halophilic alga Dunaliella parva. Plant. Physiol. 1973, 51, 875–878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montazeri-Najafabady, N.; Negahdaripour, M.; Salehi, M.H.; Morowvat, M.H.; Shaker, S.; Ghasemi, Y. Effects of osmotic shock on production of β-carotene and glycerol in a naturally isolated strain of Dunaliella salina. J. Appl. Pharm. Sci. 2016, 8, 160–163. [Google Scholar] [CrossRef] [Green Version]
- Larcher, W. Streß bei Pflanzen. Naturwissenschaften 1987, 74, 158–167. [Google Scholar] [CrossRef]
- Ben-Amotz, A.; Katz, A.; Avron, M. Accumulation of β-carotene in halotolerent algae: Purification and characterization of β-carotene-rich globules from Dunaliella bardawil (Chlorophyceae). J. Phycol. 1982, 18, 529–537. [Google Scholar] [CrossRef]
- Ben-Amotz, A.; Shaish, A.; Avron, M. Mode of action of the massively accumulated β-carotene of Dunaliella bardawil in protecting the alga against damage by excess irradiation. Plant Physiol. 1989, 91, 1040–1043. [Google Scholar] [CrossRef] [Green Version]
- Loeblich, L.A. Action spectra and effect of light-intensity on growth, pigments and photosynthesis in Dunaliella salina. J. Protozool. 1974, 21, 420. [Google Scholar] [CrossRef] [Green Version]
- Loeblich, L.A. Growth limitation of Dunaliella salina by CO2 at high salinity. J. Phycol. 1970, 6, 9. [Google Scholar]
- Shaish, A.; Avron, M.; Pick, U.; Ben-Amotz, A. Are active oxygen species involved in induction of β-carotene in Dunaliella bardawil? Planta. 1993, 190, 363–368. [Google Scholar] [CrossRef]
- Xi, Y.; Kong, F.; Chi, Z. ROS induce β-carotene biosynthesis caused by changes of photosynthesis efficiency and energy metabolism in Dunaliella salina under stress conditions. Front. Bioeng. Biotechnol. 2021, 8, 1447. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, X.; Wang, M.; Zhang, W.; Zhou, B.; Wang, Y. ROS and calcium signaling mediated pathways involved in stress responses of the marine microalgae Dunaliella salina to enhanced UV-B radiation. J. Photochem. Photobiol. B Biol. 2017, 173, 360–367. [Google Scholar] [CrossRef]
- Dring, M.J. Stress resistance and disease resistance in seaweeds: The role of reactive oxygen metabolism. Adv. Bot. Res. 2006, 43, 175–207. [Google Scholar] [CrossRef]
- Tammam, A.A.; Fakhry, E.M.; El-Sheekh, M. Effect of salt stress on antioxidant system and the metabolism of the reactive oxygen species in Dunaliella salina and Dunaliella tertiolecta. Afr. J. Biotechnol. 2011, 10, 3795–3808. [Google Scholar]
- Mueller, L.; Boehm, V. Antioxidant activity of β-carotene compounds in different in vitro assays. Molecules 2011, 16, 1055–1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stahl, W.; Sies, H. Antioxidant activity of carotenoids. Mol. Aspects Med. 2003, 24, 345–351. [Google Scholar] [CrossRef]
- Harvey, P.J.; Ben-Amotz, A. Towards a sustainable Dunaliella salina microalgal biorefinery for 9-cis β-carotene production. Algal Res. 2020, 50, 102002. [Google Scholar] [CrossRef]
- Wolf, L.; Cummings, T.; Müller, K.; Reppke, M.; Volkmar, M.; Weuster-Botz, D. Production of β-carotene with Dunaliella salina CCAP19/18 at physically simulated outdoor conditions. Eng. Life Sci. 2021, 21, 115–125. [Google Scholar] [CrossRef]
- Neves, M.I.; Silva, E.K.; Meireles, M.A. Trends and challenges in the industrialization of natural colorants. Food Public Health 2019, 9, 33–44. [Google Scholar] [CrossRef] [Green Version]
- Kleinegris, D.M.; Janssen, M.; Brandenburg, W.A.; Wijffels, R.H. The selectivity of milking of Dunaliella salina. Mar. Biotechnol. 2010, 12, 14–23. [Google Scholar] [CrossRef] [Green Version]
- Morowvat, M.H.; Ghasemi, Y. Culture medium optimization for enhanced β-carotene and biomass production by Dunaliella salina in mixotrophic culture. Biocatal. Agric. Biotechnol. 2017, 7, 217–223. [Google Scholar] [CrossRef]
- Gallego-Cartagena, E.; Castillo-Ramírez, M.; Martínez-Burgos, W. Effect of stressful conditions on the carotenogenic activity of a Colombian strain of Dunaliella salina. Saudi J. Biol. Sci. 2019, 26, 1325–1330. [Google Scholar] [CrossRef]
- Gómez, P.I.; González, M.A. Genetic polymorphism in eight Chilean strains of the carotenogenic microalga Dunaliella salina Teodoresco (Chlorophyta). Biol. Res. 2001, 34, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Gómez, P.I.; González, M.A. Genetic variation among seven strains of Dunaliella salina (Chlorophyta) with industrial potential, based on RAPD banding patterns and on nuclear ITS rDNA sequences. Aquaculture 2004, 233, 149–162. [Google Scholar] [CrossRef]
- Guevara, M.; Lodeiros, S.; Gómez, O.; Lemus, N.; Núñez, P.; Romero, L.; Vásquez, A.; Rosales, N. Carotenogénesis de cinco cepas del alga Dunaliella sp. (Chlorophyceae) aisladas de lagunas hipersalinas de Venezuela. Rev. Biol. Trop. 2005, 53, 331–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walne, P.R. Studies on the food value of nineteen genera of algae to juvenile bivalves of the genera Ostrea, Crassostrea, Mercenaria and Mytilus. Fish. Invest. Ser. 2 1970, 26, 5. [Google Scholar]
- Guillard, R.R.L.; Hargraves, P.E. Stichochrysis immobilis is a diatom, not a chrysophyte. Phycologia 1993, 32, 234–236. [Google Scholar] [CrossRef]
- Andersen, R.A.; Kawachi, M. Traditional Microalgae Isolation Techniques. In Algal Culturing Techniques; Andersen, R.A., Ed.; Elservier Academic Press: Oxford, UK, 2005; pp. 90–92. [Google Scholar]
- Koletti, K.; Chantzistrountsiou, X.; Ntzouvaras, A.; Tzovenis, I.; Flemetakis, E.; Economou-Amilli, A. Identification and phylogeny of microalgae strains from Greek coastal lagoons (tentative title). A. EconomoU-Amilli, Sector of Ecology & Systematics, Department of Biology, School of Science, National and Kapodistrian University of Athens, Panepistimiopolis Zografou, 15784 Athens, Greece. 2022; manuscript in preparation. [Google Scholar]
- Kleinegris, D.M.; Janssen, M.; Brandenburg, W.A.; Wijffels, R.H. Continuous production of carotenoids from Dunaliella salina. Enzyme Microb. Technol. 2011, 48, 253–259. [Google Scholar] [CrossRef] [Green Version]
- Hashemi, A.; Pajoum Shariati, F.; Delavari Amrei, H.; Heydari Nasab, A. The Effect of Instantaneous and Slow-Release Salt Stress Methods on Beta-Carotene Production within Dunaliella Salina Cells. IJCCE. 2021, 40, 1642–1652. [Google Scholar] [CrossRef]
- He, Q.; Lin, Y.; Tan, H.; Zhou, Y.; Wen, Y.; Gan, J.; Zhang, Q. Transcriptomic profiles of Dunaliella salina in response to hypersaline stress. BMC Genomics 2020, 21, 115. [Google Scholar] [CrossRef]
- Borovkov, A.B.; Gudvilovich, I.N.; Avsiyan, A.L.; Memetshaeva, N.O.A.; Lelekov, A.S.; Novikova, T.M. Production characteristics of Dunaliella salina at two-phase pilot cultivation (Crimea). Turkish J. Fish. Aquat. Sci. 2019, 20, 401–408. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Britton, G.; Liaanen-Jensen, S.; Pfander, H. Carotenoids. Handbook; Springer Science & Business Media: Berlin, Germany, 2004. [Google Scholar] [CrossRef]
- Tukey, J.W. Comparing individual means in the analysis of variance. Biometrics 1949, 99, 114. [Google Scholar] [CrossRef]
- Ben-Amotz, A. Effect of irradiance and nutrient deficiency on the chemical composition of Dunaliella bardawil Ben-Amotz and Avron (Volvocales, Chlorophyta). J. Plant Physiol. 1987, 131, 479–487. [Google Scholar] [CrossRef]
- Lamers, P.P.; van de Laak, C.C.W.; Kaasenbrood, P.S.; Lorier, J.; Janssen, M.; De Vos, R.C.H.; Bino, R.J.; Wijffels, R.H. Carotenoid and fatty acid metabolism in light-stressed Dunaliella salina. Biotechnol. Bioeng. 2010, 106, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Semenenko, V.E.; Abdullayev, A.A. Parametric control of β-carotene biosynthesis in Dunaliella salina cells under conditions of intensive cultivation. Fiz. Rast. 1980, 27, 31–41. [Google Scholar]
- Bonneford, H.; Moelants, N.; Talec, A.; Mayzaud, P.; Bernard, O.; Sciandra, A. Coupling and uncoupling of triglyceride and beta-carotene production by Dunaliella salina under nitrogen limitation and starvation. Biotechnol. Biofuels 2017, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Tang, X.; Kapoore, R.V.; Xu, C.; Vaidyanathan, S. Influence of nutrient status on the accumulation of biomass and lipid in Nannochloropsis salina and Dunaliella salina. Energy Convers. Manag. 2015, 106, 61–72. [Google Scholar] [CrossRef]
- Marín, N.; Morales, F.; Lodeiros, C.; Tamigneaux, E. Effect of nitrate concentration on growth and pigment synthesis of Dunaliella salina cultivated under low illumination and preadapted to different salinities. J. Appl. Phycol. 1998, 10, 405–411. [Google Scholar] [CrossRef]
- Jimenez, C.; Pick, U. Differential reactivity of [beta]-carotene isomers from Dunaliella bardawil toward oxygen radicals. Plant Physiol. 1993, 101, 385–390. [Google Scholar] [CrossRef] [Green Version]
- Zhekisheva, M.; Boussiba, S.; Khozin-Goldberg, I.; Zarka, A.; Cohen, Z. Accumulation of oleic acid in Haematococcus pluvialis (Chlorophyceae) under nitrogen starvation or high light is correlated with that of astaxanthin esters1. J. Phycol. 2002, 38, 325–331. [Google Scholar] [CrossRef] [Green Version]
- Gómez, P.I.; Barriga, A.; Cifuentes, A.S.; Gonzalez, M.A. Effect of salinity on the quantity and quality of carotenoids accumulated by Dunaliella salina (strain CONC-007) and Dunaliella bardawil (strain ATCC 30861) Chlorophyta. Biol. Res. 2003, 36, 185–192. [Google Scholar] [CrossRef] [Green Version]
- Pisal, D.S.; Lele, S.S. Carotenoid production from microalga, Dunaliella salina. Indian J. Biotechnol. 2005, 4, 476–483. [Google Scholar]
- Geider, R.J.; Macintyre, H.L.; Graziano, L.M.; McKay, R.M.L. Responses of the photosynthetic apparatus of Dunaliella tertiolecta (Chlorophyceae) to nitrogen and phosphorus limitation. Eur. J. Phycol. 1998, 33, 315–332. [Google Scholar] [CrossRef]
- Phadwal, K.; Singh, P.K. Effect of nutrient depletion on β-carotene and glycerol accumulation in two strains of Dunaliella sp. Bioresour. Technol. 2003, 90, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Borowitzka, M.A.; Borowitzka, L.J.; Kessly, D. Effects of salinity increase on carotenoid accumulation in the green alga Dunaliella salina. J. Appl. Phycol. 1990, 2, 111–119. [Google Scholar] [CrossRef]
- Cifuentes, A.S.; Gonzalez, M.A.; Parra, O.O. The effect of salinity on the growth and carotenogenesis in two Chilean strains of Dunaliella salina Teodoresco. Biol. Res. 1996, 29, 227–236. [Google Scholar]
- Lv, H.; Cui, X.; Wahid, F.; Xia, F.; Zhong, C.; Jia, S. Analysis of the physiological and molecular responses of Dunaliella salina to macronutrient deprivation. PLoS ONE 2016, 11, e0152226. [Google Scholar] [CrossRef]
- Srinivasan, R.; Mageswari, A.; Subramanian, P.; Suganthi, C.; Chaitanyakumar, A.; Aswini, V.; Gothandam, K.M. Bicarbonate supplementation enhances growth and biochemical composition of Dunaliella salina V-101 by reducing oxidative stress induced during macronutrient deficit conditions. Sci. Rep. 2018, 8, 6972. [Google Scholar] [CrossRef] [Green Version]
- Dale, M.P.; Causton, D.R. Use of the Chlorophyll a/b Ratio as a Bioassay for the Light Environment of a Plant. Funct. Ecol. 1992, 6, 190. [Google Scholar] [CrossRef]
- Mirshekari, M.; Einali, A.; Valizadeh, J. Metabolic changes and activity pattern of antioxidant enzymes induced by salicylic acid treatment in green microalga Dunaliella salina under nitrogen deficiency. J. Appl. Phycol. 2019, 31, 1709–1719. [Google Scholar] [CrossRef]
- Young, E.B.; Beardall, J. Photosynthetic function in Dunaliella tertiolecta (Chlorophyta) during a nitrogen starvation and recovery cycle. J. Phycol. 2003, 5, 897–905. [Google Scholar] [CrossRef]
- Borovkov, A.B.; Gudvilovich, I.N.; Avsiyan, A.L. Scale-up of Dunaliella salina cultivation: From strain selection to open ponds. J. Appl. Phycol. 2020, 32, 1545–1558. [Google Scholar] [CrossRef]
- Fazeli, M.R.; Tofighi, H.; Samadi, N.; Jamalifar, H.; Fazeli, A. Carotenoids accumulation by Dunaliella tertiolecta (Lake Urmia isolate) and Dunaliella salina (ccap 19/18 & wt) under stress conditions. DARU J. Pharm. Sci. 2006, 14, 146–150. [Google Scholar]
- Hadi, M.R.; Shariati, M.; Afsharzadeh, S. Microalgal biotechnology: Carotenoid and glycerol production by Dunaliella sp. algae isolated from the Gave khooni salt marsh, Iran. Biotech. Bioproc. Eng. 2008, 13, 540–544. [Google Scholar] [CrossRef]
- Cifuentes, A.S.; González, M.; Conejeros, M.; Dellarossa, V.; Parra, O. Growth and carotenogenesis in eight strains of Dunaliella salina Teodoresco from Chile. J. Appl. Phycol. 1992, 4, 111–118. [Google Scholar] [CrossRef]
- Fu, W.; Paglia, G.; Magnúsdóttir, M.; Steinarsdóttir, E.A.; Gudmundsson, S.; Palsson, B.Ø.; Andrésson, O.S.; Brynjólfsson, S. Effects of abiotic stressors on lutein production in the green microalga Dunaliella salina. Microb. Cell Factories 2014, 13, 1–9. [Google Scholar] [CrossRef]
- Liang, M.H.; Liang, Z.C.; Chen, H.H.; Jiang, J.G. The bifunctional identification of both lycopene β-and ε-cyclases from the lutein-rich Dunaliella bardawil. Enzyme Microb. Technol. 2019, 131, 109426. [Google Scholar] [CrossRef]
- Del Campo, J.A.; Moreno, J.; Rodríguez, H.; Vargas, M.A.; Rivas, J.; Guerrero, M.G. Carotenoid content of chlorophycean microalgae: Factors determining lutein accumulation in Muriellopsis sp. (Chlorophyta). J. Biotechnol. 2000, 76, 51–59. [Google Scholar] [CrossRef]
- Ho, S.H.; Chan, M.C.; Liu, C.C.; Chen, C.Y.; Lee, W.L.; Lee, D.J.; Chang, J.S. Enhancing lutein productivity of an indigenous microalga Scenedesmus obliquus FSP-3 using light-related strategies. Bioresour. Technol. 2014, 152, 275–282. [Google Scholar] [CrossRef]
- Xie, Y.; Ho, S.H.; Chen, C.N.N.; Chen, C.Y.; Ng, I.S.; Jing, K.J.; Chang, J.S.; Lu, Y. Phototrophic cultivation of a thermo-tolerant Desmodesmus sp. for lutein production: Effects of nitrate concentration, light intensity and fed-batch operation. Bioresour. Technol. 2013, 144, 435–444. [Google Scholar] [CrossRef]
- Kim, S.H.; Liu, K.H.; Lee, S.Y.; Hong, S.J.; Cho, B.K.; Lee, H.; Lee, C.G.; Choi, H.K. Effects of light intensity and nitrogen starvation on glycerolipid, glycerophospholipid, and carotenoid composition in Dunaliella tertiolecta culture. PLoS ONE 2013, 8, e72415. [Google Scholar] [CrossRef] [Green Version]
- Yeh, T.J.; Tseng, Y.F.; Chen, Y.C.; Hsiao, Y.; Lee, P.C.; Chen, T.J.; Chen, C.Y.; Cao, C.Y.; Chang, J.S.; Chen, J.C.; et al. Transcriptome and physiological analysis of a lutein-producing alga Desmodesmus sp. reveals the molecular mechanisms for high lutein productivity. Algal Res. 2017, 21, 103–119. [Google Scholar] [CrossRef]
- Highfield, A.; Ward, A.; Pipe, R.; Schroeder, D.C. Molecular and phylogenetic analysis reveals new diversity of Dunaliella salina from hypersaline environments. J. Mar. Biol. Assoc. UK 2021, 101, 27–37. [Google Scholar] [CrossRef]
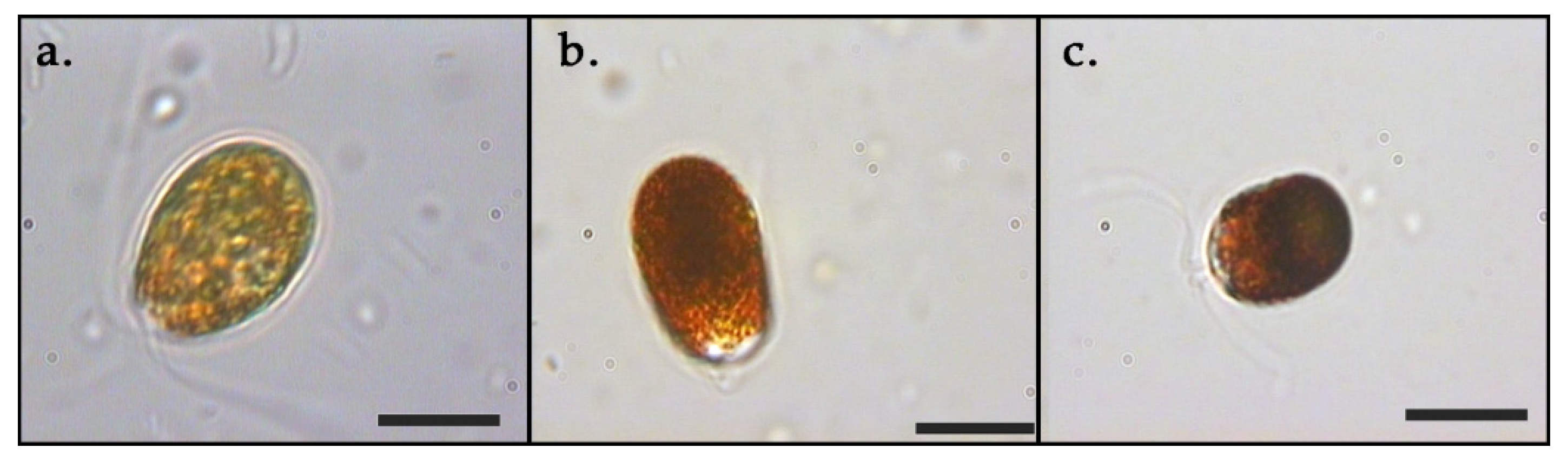
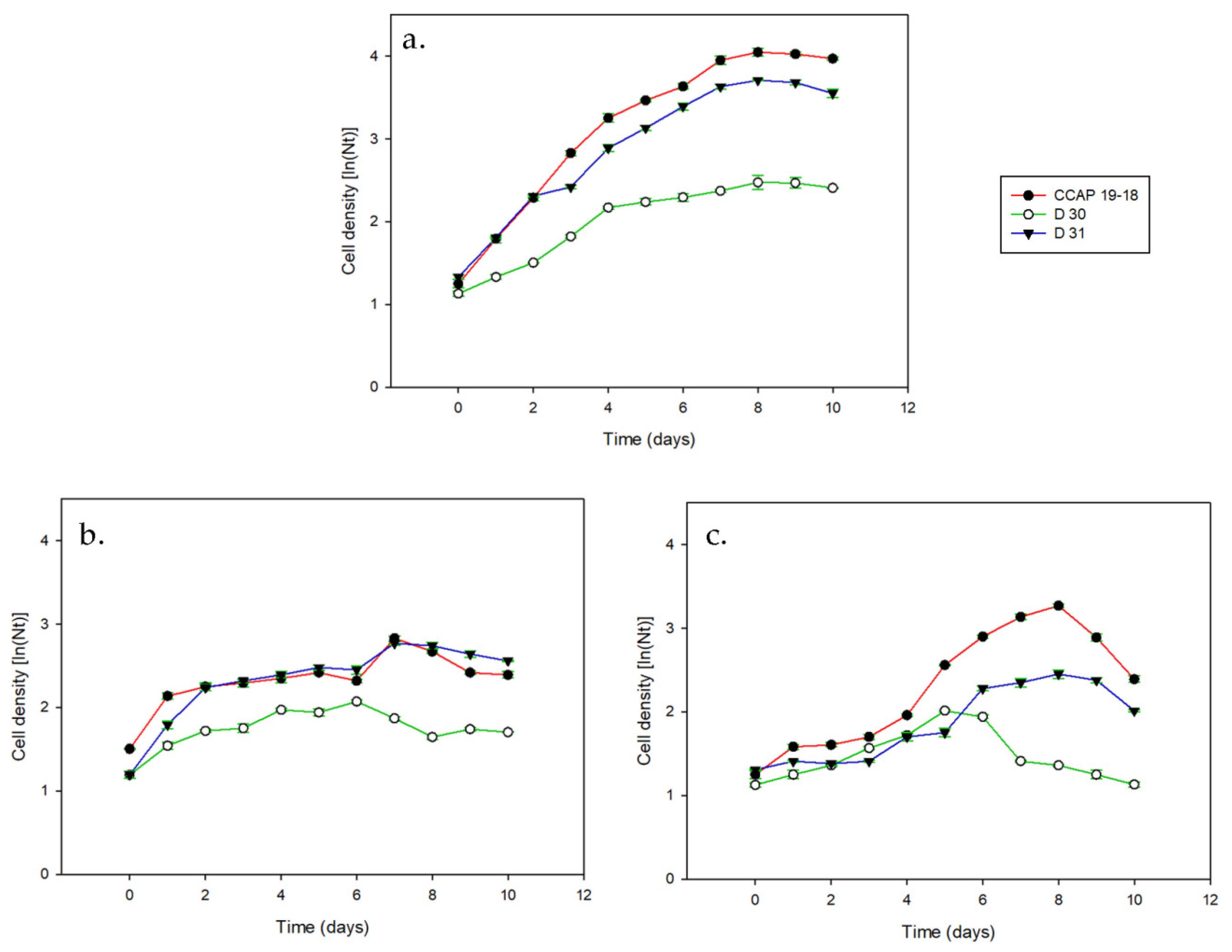
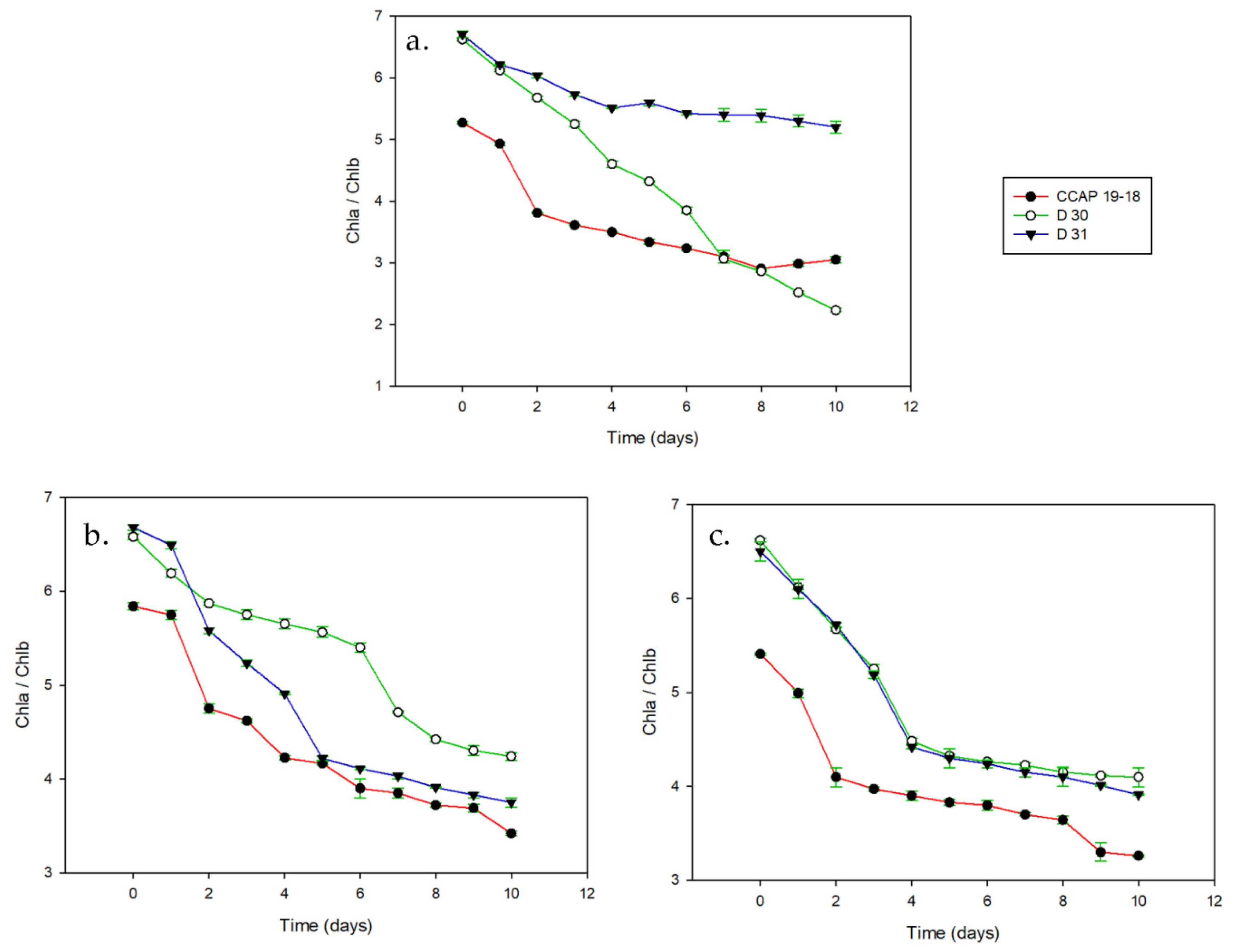
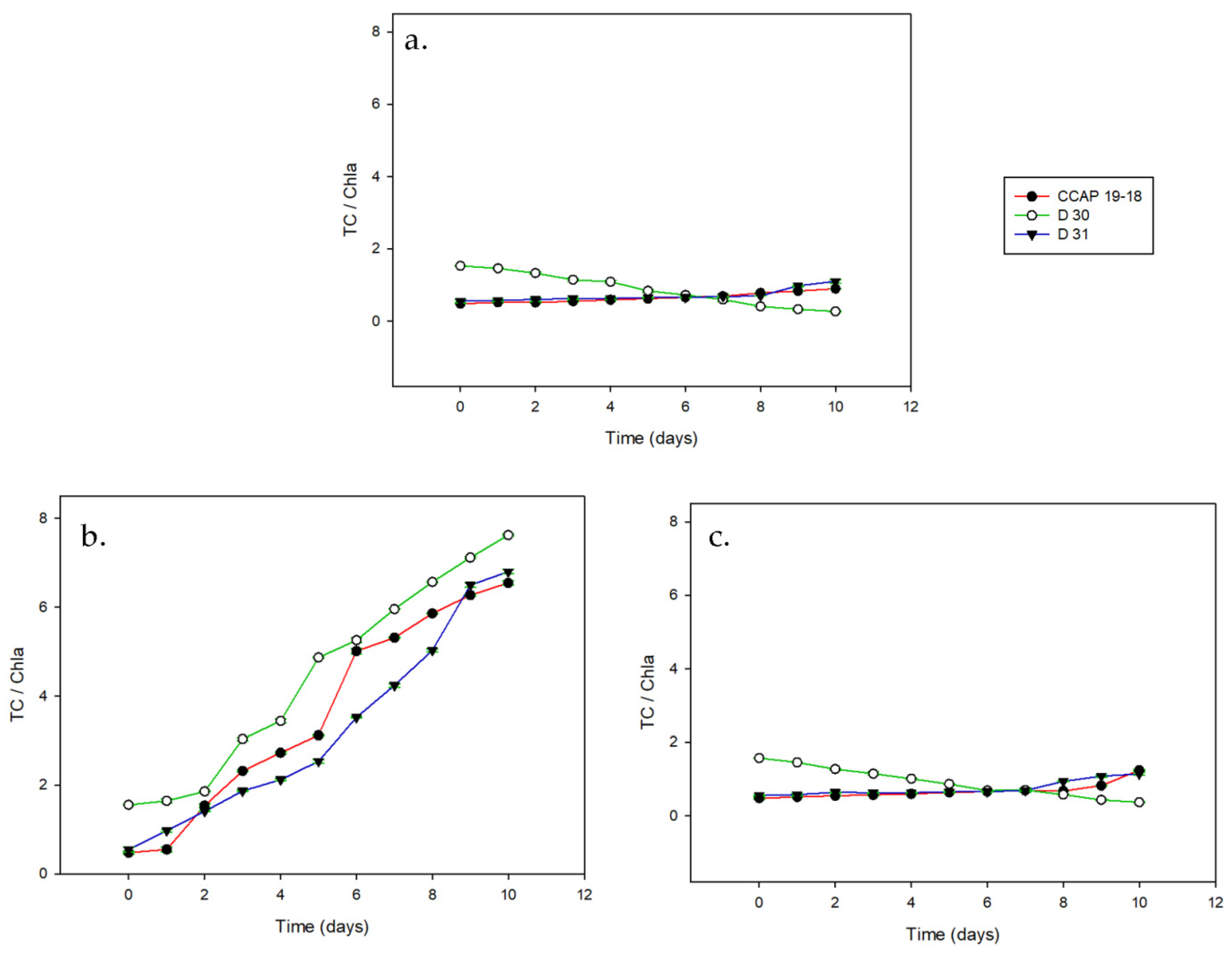

| Strain | μmax (d−1) | ||
|---|---|---|---|
| Standard Conditions | Nitrogen Deprivation | Salinity Shock | |
| CCAP 19\18 | 0.513 a,b (0.010) | 0.197 a,c (0.013) | 0.165 a,b,c (0.007) |
| AthU-Al D30 | 0.255 a (0.006) | 0.188 a,c (0.013) | 0.162 a,b,c (0.017) |
| AthU-Al D31 | 0.390 b (0.004) | 0.288 b (0.011) | 0.112 a,b,c (0.025) |
| Sample | Carotenoids | Ret. Time (min) | Area | Height | Area% | Lamda Max |
|---|---|---|---|---|---|---|
| CCAP 19/18 | ||||||
| Standard | Lutein | 12.528 | 14821899 | 890778 | 36.844 | 444/472/267/205/332 |
| Zeaxanthin | 14.264 | 1826232 | 99707 | 4.54 | 450/477/204/275/663 | |
| β-carotene | 23.972 | 4194955 | 497210 | 10.428 | 452/478/204/273/659 | |
| N-Stress | Lutein | 12.475 | 2086890 | 127377 | 8.285 | 444/472/204/267/333 |
| β-carotene | 23.947 | 9928467 | 1031593 | 39.416 | 452/478/204/273/601 | |
| Salt Stress | Lutein | 12.438 | 610784 | 36318 | 24.293 | 204/444/472/267/331 |
| Zeaxanthin | 14.158 | 108323 | 6245 | 4.308 | 204/450/478/659/225 | |
| β-carotene | 23.926 | 700634 | 77453 | 27.867 | 204/451/478/270/659 | |
| AthuAl D30 | ||||||
| Standard | Lutein | 12.395 | 4238251 | 256546 | 15.098 | 444/472/204/267/333 |
| Zeaxanthin | 14.097 | 806390 | 46474 | 2.873 | 204/450/477/274/659 | |
| β-carotene | 23.906 | 9118255 | 1036987 | 32.483 | 452/478/204/273/659 | |
| N-Stress | Lutein | 12.384 | 1301617 | 79794 | 3.86 | 444/472/204/267/332 |
| Zeaxanthin | 14.08 | 439293 | 25564 | 1.303 | 204/450/476/274/630 | |
| β-carotene | 23.912 | 15360003 | 1846875 | 45.55 | 452/478/204/273/659 | |
| Salt Stress | Lutein | 12.394 | 579670 | 34787 | 20.048 | 204/444/472/267/335 |
| Zeaxanthin | 14.098 | 230176 | 13772 | 7.961 | 204/450/476/273/629 | |
| β-carotene | 23.95 | 782976 | 87623 | 27.079 | 204/452/478/272/659 | |
| AthuAl D31 | ||||||
| Standard | Lutein | 12.457 | 8527333 | 519453 | 14.461 | 444/472/267/204/333 |
| Zeaxanthin | 14.173 | 2669667 | 152510 | 4.527 | 450/477/204/274/659 | |
| β-carotene | 23.908 | 6770635 | 812780 | 11.482 | 452/478/204/273/659 | |
| N-Stress | Lutein | 12.411 | 2735242 | 166483 | 5.774 | 444/472/204/267/333 |
| β-carotene | 23.896 | 15389583 | 1629503 | 32.485 | 451/478/204/273/630 | |
| Salt Stress | Lutein | 12.393 | 13798884 | 850817 | 14.126 | 444/472/267/205/332 |
| Zeaxanthin | 14.105 | 7717916 | 441817 | 7.901 | 450/477/204/274/659 | |
| β-carotene | 23.891 | 27417197 | 3572706 | 28.067 | 449/478/273/204/659 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chantzistrountsiou, X.; Ntzouvaras, A.; Papadaki, S.; Tsirigoti, A.; Tzovenis, I.; Economou-Amilli, A. Carotenogenic Activity of Two Hypersaline Greek Dunaliella salina Strains under Nitrogen Deprivation and Salinity Stress. Water 2023, 15, 241. https://doi.org/10.3390/w15020241
Chantzistrountsiou X, Ntzouvaras A, Papadaki S, Tsirigoti A, Tzovenis I, Economou-Amilli A. Carotenogenic Activity of Two Hypersaline Greek Dunaliella salina Strains under Nitrogen Deprivation and Salinity Stress. Water. 2023; 15(2):241. https://doi.org/10.3390/w15020241
Chicago/Turabian StyleChantzistrountsiou, Xanthi, Alexandros Ntzouvaras, Sofia Papadaki, Amersa Tsirigoti, Ioannis Tzovenis, and Athena Economou-Amilli. 2023. "Carotenogenic Activity of Two Hypersaline Greek Dunaliella salina Strains under Nitrogen Deprivation and Salinity Stress" Water 15, no. 2: 241. https://doi.org/10.3390/w15020241
APA StyleChantzistrountsiou, X., Ntzouvaras, A., Papadaki, S., Tsirigoti, A., Tzovenis, I., & Economou-Amilli, A. (2023). Carotenogenic Activity of Two Hypersaline Greek Dunaliella salina Strains under Nitrogen Deprivation and Salinity Stress. Water, 15(2), 241. https://doi.org/10.3390/w15020241







